Abstract
Objective:
Hypervitaminosis A is well described but overlooked in chronic kidney disease (CKD) and has been associated with hypercalcemia, contributing to bone mineral disease. Our objective is to assess prevalence of hypervitaminosis A and its association with bone health in an advanced CKD population.
Methods:
We performed a retrospective review of 58 children with CKD 4–5 to examine the association between vitamin A levels and bone health and compared these values between a primarily formula fed (FF) and non-primarily formula fed cohort (NFF).
Results:
Fifty-six of 58 patients (97%) had hypervitaminosis A with mean vitamin A level 1475±597 mcg/dl. When compared to the upper limit of normal (ULN) vitamin A level for age, the FF group’s vitamin A level was 2.9× ULN and the NFF group’s vitamin A level was 2.2× ULN (p=0.02). The mean calcium level was 10.3 mg/dl in the FF group and 9.8 mg/dl in the NFF group (p=0.057). Percent of patients below, within, or above goal PTH range was statistically significant with 15 (62%) of the FF group below goal and 16 (72%) of the NFF cohort above goal (p=0.006).
Conclusions:
We concluded vitamin A and calcium levels are higher in the FF versus the NFF population. FF patients are more likely to have PTH levels below goal range, placing them at risk for adynamic bone disease. We recommend monitoring Vitamin A levels as part of routine nutritional assessments as well as dietary interventions to prevent hypervitaminosis A to improve bone health in late CKD.
Keywords: Vitamin A, CKD, Bone Mineral Disease
Introduction:
Hypervitaminosis A is a well-described but often underrecognized finding in chronic kidney disease (CKD). Elevated levels of vitamin A arise as early as CKD stage 2 (1). Provitamin A carotenoids are derived from plant sources and are less bioavailable than preformed vitamin A retinols from animal sources. Both plant and animal sources are metabolized to a common product, retinal (ROH), which is stored in the liver. In order for ROH to reach its target organs, it is bound to proteins including Retinol Binding Protein (RBP) and transthyretin, and ROH is oxidized to its active form, retinoic acid (RA). RA and RBP are both renally metabolized (2). In patients with CKD, RA and RBP accumulate in serum as glomerular filtration rate (GFR) declines, which is thought to be associated with decreased renal excretion and increased hepatic retinol production (1, 2).
Signs and symptoms of chronic vitamin A toxicity are nonspecific and include ataxia, dry skin, headache, alopecia, bone pain, hepatotoxicity leading to cirrhosis, and hyperlipidemia. In addition, high levels of vitamin A can result in hypercalcemia in patients with CKD, leading to bone mineral disease (1). Animal studies have shown that high levels of vitamin A increase active local bone resorption by increasing the presence of osteoclasts and decreasing the number and activity of osteoblasts (3, 4). In addition, osteoclast activity appears to be stimulated more by vitamin A in the developing skeleton than the mature skeleton which increases calcium levels in the serum (5). There are no evidence-based guidelines for the intake of vitamin A for children with CKD. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) recommendations are based on expert opinion and state that the “intake of vitamin A should be limited to the age appropriate dietary reference intake (DRI)”.
In this study, we report vitamin A levels in a cohort of pediatric patients with advanced CKD and examine the correlation between vitamin A levels and markers of bone health, including calcium, phosphorus, and PTH. Additionally, we assessed differences between a cohort of primarily formula fed patients (FF) versus those that are not primarily formula fed (NFF). While previous studies have examined vitamin A levels based on diet, no study has correlated vitamin A levels with additional markers of bone health among FF and NFF groups. We hypothesized that hypervitaminosis A would be associated with hypercalcemia and low parathyroid hormone (PTH) levels with higher vitamin A levels in the FF group.
Methods:
We performed a retrospective chart review of 58 patients to examine vitamin A levels in a pediatric population. The study included all patients with CKD 4 or 5 treated at a pediatric medical center from April 2018 to June 2020. For the purpose of stratification, CKD 4 was defined as patients with a GFR of 15–30 ml/min/1.73m2, and CKD 5 was defined as patients on dialysis or with a GFR <15 ml/min/1.73m2. For non-dialysis dependent patients, GFR was calculated based on the Larsson cystatin c GFR when available (n=16), or by using the Schwartz “bedside” formula (n=3) (8, 9). The FF group consisted of those patients that received 50% or more of their daily caloric needs from enteral formula. The NFF group consisted of patients that received less than 50% of their daily caloric needs from enteral formula. Evaluation of vitamin A levels at a yearly minimum for CKD 4–5 patients is standard of care at our institution and the most recent value in the study timeframe was examined. As a result, we were able to include all patients with CKD 4–5 at our institution in this study. The vitamin A assay was performed through the Hospital Lab via manual double extraction and High-Performance Liquid Chromatography. Reference ranges for vitamin A level were based on the Pediatric Reference Ranges Second Edition AACC Press (10). Calcium, phosphorus, and PTH levels were obtained as part of routine lab work within a month of the Vitamin A level. Patients were stratified by age, gender, CKD stage, primary renal diagnosis, formula type, presence of any residual urine output (UOP), use of growth hormone, dialysis modality, dialysate, and vitamin A containing polyvitamin supplementation. This research study was approved by the institutional review board at the Medical Center. Descriptive statistics were performed for demographic data, vitamin A, calcium, phosphorus, and PTH levels. Calcium and phosphorus levels were based on age-specific normal ranges determined in the KDOQI Clinical Practice Guidelines for Nutrition in Children with Chronic Kidney Disease and are shown in Table 1 (7). The optimal PTH level for pediatric patients with CKD is not known. We used a goal PTH range of 70–100 pg/ml for patients with CKD 4 and 100–300 pg/ml for patients with CKD 5 based on KDOQI and IPDN guideline recommendations (7, 11, 12). We performed independent t-tests comparing vitamin A levels to multiple binary variables. Pearson correlation coefficients were utilized to examine the correlation between vitamin A levels, age and calcium levels. PTH levels were classified as below, within, or above goal, and one-way ANOVA testing was used for analysis of these groups. Statistical analyses were performed using IBM SPSS statistical software (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY). A p-value of <0.05 was considered statistically significant.
Table 1:
KDOQI Clinical Practice Guidelines for Nutrition in Children with Chronic Kidney Disease(6)
| Age | Calcium Level (mg/dl) | Phosphorus Level (mg/dl) |
|---|---|---|
| 0–5 months | 8.7–11.3 | 5.2–8.4 |
| 6–12 months | 8.7–11.0 | 5–7.8 |
| 1–5 years | 9.4–10.8 | 4.5–6.5 |
| 6–12 years | 9.4–10.3 | 3.6–5.8 |
| 13–20 years | 8.8–10.2 | 2.3–4.5 |
KDOQI clinical practice guidelines for calcium and phosphorus levels in children with chronic kidney disease (CKD) based on age(6).
Results:
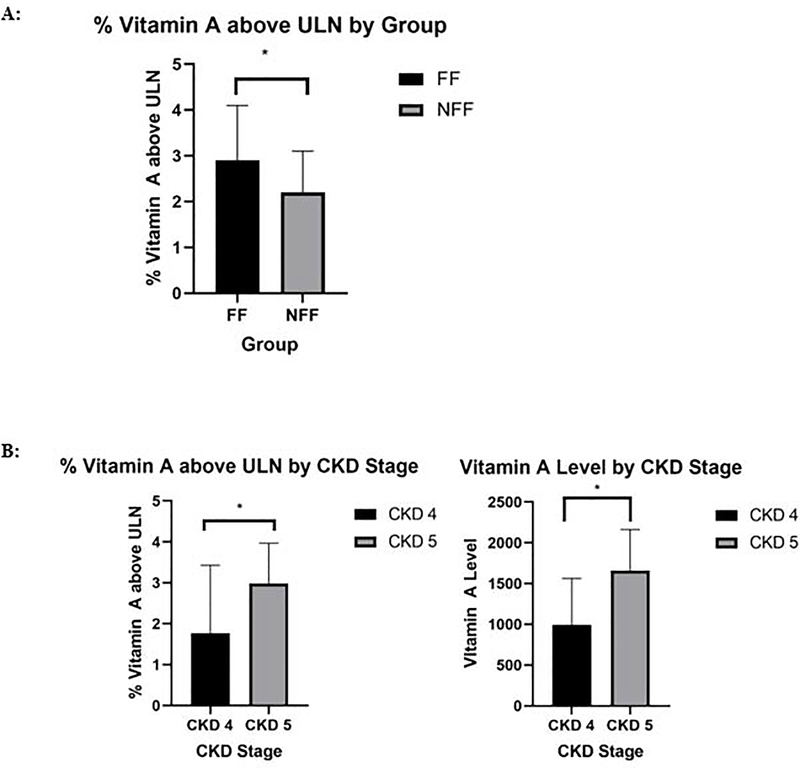
Demographic and biochemical characteristics for the 58 patients included in this study are demonstrated in Table 2. Thirty one of the 36 patients in the FF group received at least 85% of their nutrition via enteral formula (mean 92%; Range 55%-100%). No patient in the NFF group received enteral formula. The FF group was significantly younger with a mean age of 3.6±4.8 years compared to 13.8 ±4 years in the NFF group (p=0.001). Thirty-two patients (55%) were male with 67% male in the FF group and 36% male in the NFF group, which was statistically significant (p=0.024). Sixteen patients (28%) had CKD 4 and 42 patients (72%) had CKD 5. The most frequent cause of CKD was Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) (n=24) followed by Wilm’s tumor associated CKD (n=5). Thirty-nine patients (67%) were on dialysis with 25 patients on peritoneal dialysis (PD) and 14 patients on hemodialysis (HD). There was a significant difference between the FF and NFF groups in terms of dialysis modality with the majority of the FF group on PD and the majority of the NFF on HD (p=0.012). Of the 58 patients, 56 (97%) were found to have elevated levels of vitamin A with a mean vitamin A level of 1475±597 mcg/dl and a median level of 1450 mcg/dl. The mean vitamin A level in the FF group was 1519±637 mcg/dl and 1404±533 mcg/dl in the NFF group (p=0.48). When compared to the upper limit of normal (ULN) vitamin A level for age, there was a statistically significant difference between groups with the FF group’s vitamin A level (2.9 × ULN) and the NFF group’s vitamin A level (2.2 × ULN; p=0.02) (Figure 1).
Table 2:
Demographic Characteristics (n=58)
| Total: (n=58) | FF(n=36) | NFF (n=22) | ||
|---|---|---|---|---|
|
| ||||
| Mean Age: | 7.5±6.7 | 3.6±4.8 | 13.8±4.0 | <0.001* |
|
| ||||
| Frequency (n, %) | p-value | |||
|
| ||||
| Sex (Male): | 32 (55) | 24 (67) | 8 (36) | 0.024* |
|
| ||||
| CKD Stage: | 0.967 | |||
| 4 | 16 (28) | 10 (28) | 6 (27) | |
| 5 | 42 (72) | 26 (72) | 16 (73) | |
|
| ||||
| Residual UOP: | 45 (78) | 26 (72) | 19 (86) | 0.332 |
|
| ||||
| Dialysis Dependent: | 39 (67) | 23 (64) | 16 (73) | 0.572 |
|
| ||||
| Dialysis Type: | 0.012* | |||
| PD | 25 (43) | 19 (53) | 6 (27) | |
| HD | 14 (24) | 4 (11) | 10 (46) | |
| No dialysis | 19 (33) | 13 (36) | 6 (27) | |
|
| ||||
| Dianeal Type: | 0.101 | |||
| PD-2 | 8 (14) | 5 (14) | 3 (14) | |
| Low Calcium | 17 (29) | 14 (39) | 3 (14) | |
| No dianeal | 33 (57) | 17 (47) | 16 (72) | |
|
| ||||
| Polyvitamin Supplement: | 6 (10) | 6 (17) | 0 | 0.073 |
|
| ||||
| Hypercalcemic: | 9(16) | 8 (22) | 1 (5) | 0.133 |
|
| ||||
| Mean Calcium Level (mg/dl): | 10.1±1.1 | 10.3 ±1.3 | 9.8±0.7 | 0.057 |
|
| ||||
| Hyperphosphatemic: | 19 (33) | 6 (17) | 13 (59) | 0.001* |
|
| ||||
| Phosphorus levels (mg/dl): | 6.0±1.9 | 5.7±1.9 | 6.5±1.8 | 0.123 |
|
| ||||
| Growth Hormone: | 16 (28) | 13 (36) | 3 (14) | 0.077 |
|
| ||||
| Mean PTH level (pg/ml): | 474±685 | 428±717 | 546±642 | 0.535 |
|
| ||||
| PTH range: | 0.006* | |||
| Below Goal: | 16 (29) | 15 (44) | 1 (5) | |
| Within Goal: | 10 (18) | 5 (15) | 5 (23) | |
| Above Goal: | 30 (53) | 14 (41) | 16 (72) | |
|
| ||||
| Mean Vitamin A level by age (mcg/L): | 1475±597 | 1519±637 | 1404±533 | 0.479 |
| <1 year | 1351±771 | 1351±771 | NA | NA |
| 1–13 years | 1516±505 | 1574±473 | 1329±600 | 0.309 |
| ≥13years | 1515±589 | 1960±838 | 1432±525 | 0.160 |
|
| ||||
| Age group | <0.001* | |||
| <1 year | 14 (24) | 14 (39) | 0 (0) | |
| 1–13 years | 25 (43) | 19 (53) | 6 (27) | |
| ≥13years | 19 (33) | 3 (8) | 16 (73) | |
|
| ||||
| % Vitamin A above ULN | 2.6±1.2 | 2.9±1.2 | 2.2±0.9 | 0.02* |
Demographic characteristics of the study cohort, analyzed according to subgroup. The FF group was younger, predominately male, and more likely to be on PD than the NFF group (p=0.001, 0.024, 0.012). The FF also had more PTH levels below goal range and a greater % Vitamin A above ULN than the NFF group (p=0.006, 0.02). The NFF group was more hyperphosphatemic than the FF group ((p=0.001). FF (formula fed): ≥50% of caloric intake from formula and NFF (not formula fed): <50% of caloric intake from formula. CKD: Chronic Kidney Disease. UOP: Urine Output. PD: Peritoneal Dialysis. HD: Hemodialysis. ULN: Upper Limit of Normal.
Figure 1:
A: Percent of vitamin A level above the upper limit of normal per group and B: Vitamin A level and % vitamin A above ULN based on stage of CKD. A:* The FF group’s % vitamin A above ULN was higher than that of the NFF group (2.9±1.2 vs 2.2±0.9, p=0.02). B: *CKD 5 was associated with a greater vitamin A level and % vitamin A above ULN than CKD 4 (1661±500 vs 990±572, p=0.001 and 2.99±0.98 vs 1.77±1.16, p=0.001). FF (formula fed): ≥50% of caloric intake from formula and NFF (not formula fed): <50% of caloric intake from formula. ULN: Upper Limit of Normal. CKD: Chronic Kidney Disease.
A total of nine study patients (16%) were hypercalcemic. The mean calcium level was 10.1 mg/dl overall, 10.3 mg/dl in the FF group and 9.8 mg/dl in the NFF group (p=0.057). One patient required treatment with bisphosphonate for severe hypercalcemia. Nineteen patients (33%) were hyperphosphatemic with a statistical difference between the FF (n=6, 17%) and the NFF groups (n=13, 59%) (p=0.001). The mean PTH level was 474 pg/ml ±685 pg/ml overall, 428 ±717 pg/ml in the FF group and 546 ±642 pg/ml in the NFF group (p=0.54). Two FF patients were missing a PTH level within one month of the time that the vitamin A level was obtained and were not included in PTH data. Sixteen patients (29%) had PTH levels below goal range for their stage of CKD. Percent of patients below, within, or above goal PTH range was statistically significant between the FF and NFF groups with 15 (44%) of patients in the FF group below goal range PTH and 16 patients (72%) of the NFF cohort above the goal range for PTH (p=0.006).
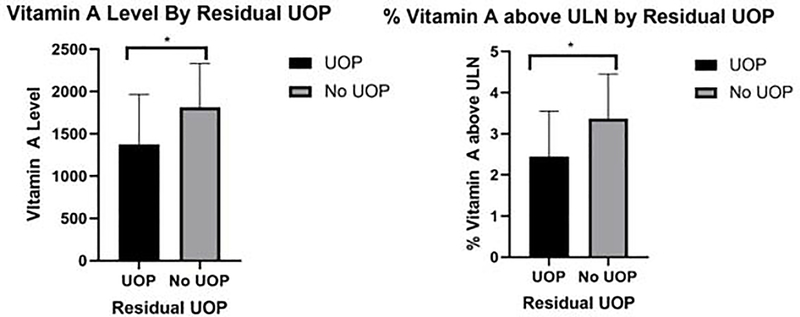
Table 3 displays the correlation between vitamin A and CKD variables. There was a statistical difference between vitamin A measurements and stage of CKD with the CKD 4 group having a mean vitamin A of 990±572 mcg/dl which was 1.77 × ULN while the CKD 5 group had an average vitamin A level of 1661±500 mcg/dl which was 2.99 × ULN (p=0.001, p=0.001 respectively) (Figure 1). There was also a statistical difference between vitamin A measurements and residual UOP with the residual UOP group having a mean vitamin A of 1378±588 mcg/dl which was 2.44 × ULN while the group with no residual UOP had an average vitamin A level of 1816±517 mcg/dl which was 3.37 × ULN (p=0.019, p=0.010, respectively) (Figure 2).
Table 3:
Correlation of Vitamin A level with Hypercalcemia, Hyperphosphatemia, CKD Stage and Residual Urine Output
| Mean Vitamin A Level | % Vitamin A above ULN | |
|---|---|---|
|
| ||
| CKD 4 (N=16) | 990±572 | 1.77±1.16 |
| CKD 5 (N=42) | 1661±500 | 2.99±0.98 |
|
| ||
| p | <0.001* | <0.001* |
|
| ||
| Hypercalcemia (N=9) | 1485±589 | 2.63±1.30 |
| No Hypercalcemia (N=49) | 1425±677 | 2.65±1.15 |
|
| ||
| p | 0.786 | 0.954 |
|
| ||
| Residual UOP (N=45) | 1378±588 | 2.44±1.11 |
| No Residual UOP (N=13) | 1816±517 | 3.37±1.08 |
|
| ||
| p | 0.019* | 0.010* |
|
| ||
| Hyperphosphatemic (N=19) | 1558±466 | 2.62±0.96 |
|
| ||
| Non-Hyperphosphatemic (N=39) | 1436±654 | 2.66±1.26 |
|
| ||
| p | 0.469 | 0.900 |
Vitamin A level and % Vitamin A above ULN stratified based on hypercalcemia, hyperphosphatemia, CKD Stage and residual urine output. CKD 5 was associated with a greater vitamin A level and % vitamin A above ULN than CKD 4 (p=0.001, 0.001). Lack of residual UOP was associated with a greater vitamin A level and % vitamin A above ULN than presence of residual UOP (p=0.019, 0.010). CKD: Chronic Kidney Disease. ULN: Upper Limit of Normal. UOP: Urine Output.
Figure 2:
Vitamin A level and % vitamin A above ULN based on presence or lack of residual UOP. *Lack of residual UOP was associated with a greater vitamin A level and % vitamin A above ULN than presence of residual UOP (1816±517 vs 1378±588, p=0.019 and 3.37±1.08 vs 2.44±1.11, p=0.010). UOP: Urine Output. ULN: Upper Limit of Normal.
We examined the vitamin A content of the formulas of the 36 FF patients. Eleven patients (31%) had a diet including multiple enteral formulas. The most common enteral formula was Similac® PM 60/40 (n=16), followed by Suplena® (n=6). Five patients received at least some of their nutrition from maternal breast milk (MBM). As demonstrated in Table 4, the vitamin A content of formulas, including renal formulas, varies extensively as does the type of vitamin A in the formula which determines its degree of bioavailability. Retinyl Acetate and vitamin A palmitate are animal-based sources of vitamin A (absorption rates 70–90%). Beta Carotene is a plant-based source of vitamin A (absorption rates 20–50%)(13). Supplemental Table 1 demonstrates the amount of vitamin A if 50% of calories were consumed from each respective formula for three different representative ages.
Table 4:
Vitamin A content per 100 calories
| Product | Form of Vitamin A* | IU | mcg | N= |
|---|---|---|---|---|
|
| ||||
| Similac® PM 60/40**(21) | Vitamin A Palmitate and Beta Carotene | 300 | 90 | 16 |
|
| ||||
| Suplena®**(22) | Vitamin A Palmitate | 176 | 53 | 6 |
|
| ||||
| Nepro®**(23) | Vitamin A Palmitate | 176 | 53 | 2 |
|
| ||||
| Renastart™**(24) | Retinyl Acetate | 91 | 27 | 2 |
|
| ||||
| Pediasure Peptide® 1.0(25) | Vitamin A Palmitate | 250 | 75 | 4 |
|
| ||||
| Elecare Junior®(26) | Vitamin A Palmitate and Beta Carotene | 273 | 82 | 2 |
|
| ||||
| Vivonex TEN®(27) | Vitamin A Palmitate | 253 | 77 | 1 |
|
| ||||
| Real Food Blends®(28) | Beta Carotene | 1 | ||
|
|
|
|||
| -Orange Chicken, Carrots & Brown Rice | 0.087 | 0.052 | ||
|
|
|
|||
| -Salmon, Oats & Squash | 2.27 | 1.36 | ||
|
|
|
|||
| -Quinoa, Kale & Hemp | 83.3 | 50 | ||
|
|
|
|||
| -Beef, Potatoes & Spinach | 17.7 | 10.6 | ||
|
|
|
|||
| -Eggs, Apples & Oats | 47.8 | 28.7 | ||
|
|
|
|||
| -Turkey, Sweet Potatoes & Peaches | 445.8 | 267.5 | ||
|
| ||||
| Whole Milk (Horizon® brand)(29) | Retinol | 185 | 56 | 2 |
|
|
||||
| Mature Breast Milk | Retinol and Beta Carotene | 207 | 82 | 6 |
The vitamin A content of enteral formulas based on 100 calories.
Retinyl Acetate and vitamin A palmitate are animal-based sources of vitamin A (absorption rates 70–90%). Beta Carotene is a plant-based source of vitamin A (absorption rates 20–50%).
Indicates a renal formula(25–33).
Discussion:
In this retrospective study, we evaluated vitamin A level and its association with markers of bone health in patients with CKD 4–5. Additionally, we assessed differences in a cohort that was primarily formula fed and compared this to a cohort that was not primarily formula fed. This is the first study investigating hypervitaminosis A and its relationship to enteral feeding status, calcium, phosphorus and PTH. We identified that nearly all patients with advanced CKD have hypervitaminosis A, independent of enteral feeding status. We also identified that the Vitamin A level is higher in the FF population compared with the NFF population using age-based reference ranges. Our other major finding is that patients who are FF are more likely to have PTH levels below goal range. Conversely, those who are NFF are more likely to have PTH levels above goal range. We also found that patients with CKD 5 and those with no residual UOP have higher levels of vitamin A.
We hypothesize that Vitamin A levels are increased for the FF patients as they are receiving higher levels of vitamin A in their enteral formulas than those who are NFF. Previous data shows that patients who are exclusively formula fed are the highest risk population for hypervitaminosis A (1). The vitamin A content of common renal formulas varies extensively which may contribute to this finding. While some renal formulas are low in vitamin A content such as Renastart™ with 91 IU of vitamin A per 100 calories, some renal formulas such as Similac® PM 60/40 have high vitamin A levels with 300 IU vitamin A per 100 calories. Additionally, many renal and non-renal formulas provide vitamin A well above the DRI for age, even when 50% of calories are obtained from the respective formula. Although formula prominent diets are easier to regulate than diets in children who consume solid food, these formulas are often higher in vitamin A content than a regular diet alone (14). Six of the FF patients were on polyvitamin supplementation with the standard dose of 1 ml of Enfamil Poly-Vi-Sol® containing 750 IU of Vitamin A which can exacerbate hypervitaminosis A (15).
Additionally, some of these patients receive some proportion of their enteral nutrition through breast milk. The vitamin A content of breast milk varies based on the diet of the mother, and while it is the best form of nutrition for infants, including the CKD population, it is hard to quantify the exact amount of vitamin A present in breast milk. It has been estimated that mature human breast milk has about 62 μg total Retinol Equivalents(16).
We found that the FF group was more likely to have PTH levels below goal while the NFF group was more likely to have PTH levels above goal. We propose that elevated vitamin A levels increase osteoblastic activity which increases serum calcium levels and suppresses PTH levels causing adynamic bone disease (3). We believe the difference in PTH levels between groups is associated with increased adherence with medication regimens as those who are FF are younger and have more parental control over medications. As the FF population is younger, they are more susceptible to CKD associated mineral bone disease. A PTH level below goal range is associated with adynamic bone disease, characterized by low bone turnover, poor bone mineralization and loss of bone volume with detrimental impacts on growth (17). The incidence of adynamic bone disease in the pediatric dialysis population has been estimated at 25–27% (18–20). In the FF population, the incidence of PTH below goal range was 44%, suggesting these patients may be at increased risk of adynamic bone disease. Due to dysregulation of PTH as well as calcium and phosphorus abnormalities, the fracture risk is 2–3 times higher in the pediatric dialysis population than in the general population (21). Furthermore, dysregulation of PTH, calcium, and phosphorus leads to vascular calcifications with great impact on cardiovascular health, which is the leading cause of death in patients with CKD. Civilibal et al. found that the presence of vascular calcifications in the pediatric CKD population was associated with higher levels of phosphorus, higher calcium-phosphorus product, and higher calcium based phosphorus binder intake (22). As vascular calcifications can present as early as late adolescence, efforts to halt bone disease are of utmost importance for morbidity free longevity.
Additionally, we found that 16% of our patients were hypercalcemic with one patient requiring bisphosphonate treatment. We hypothesized there would be a higher incidence of hypercalcemia in this population. The majority of patients receiving dialysis were on low calcium dialysate at the time vitamin A levels were obtained, mitigating higher rates of hypercalcemia in this population. Because of the prevalence of hypercalcemia in CKD at our institution, low calcium dialysate is standard of care beyond the neonatal period in an attempt to prevent hypercalcemia.
We demonstrated that there is an increase in vitamin A levels in patients without residual UOP. The correlation between residual UOP and lower vitamin A levels is likely explained because vitamin A metabolites are excreted in the urine (1). This finding supports the importance of maintaining residual renal function in patients with advanced CKD in order to enhance excretion of vitamin A and its metabolites.
In terms of limitations, additional studies need to be performed to further distinguish the association between vitamin A levels and hypercalcemia. Our patient population was not large enough to control for additional factors that can contribute to hypercalcemia in CKD including 1,25-OH vitamin D and 25-OH vitamin D levels and supplementation, age, additional comorbidities, dialysate composition, and HD calcium bath. Additionally, vitamin A levels increase post-prandially but there was no requirement in this study for vitamin A levels to be drawn in a fasting state (which would not be feasible to perform in a young patient population). Vitamin A is a fat soluble vitamin but we did not control for BMI although data associating vitamin A level with BMI is inconclusive (23, 24). As this study was retrospective, we were unable to assess vitamin A content in the diet of the NFF group. The generalizability of this study is limited by it being a single center study with a population consisting mainly of very young patients with CKD 5. We plan to conduct a multicenter study to expand this patient cohort and confirm these preliminary findings in a larger patient cohort.
In terms of future directions, measuring maternal breast milk vitamin A levels may provide a way to offer nutritional counseling on optimal vitamin A intake to lactating mothers of our young dialysis patients that receive breast milk exclusively or mixed with renal formula. Also, these data can be utilized to modify vitamin A content of renal formulas. While potassium, sodium, and phosphorus are lower in renal formulas than other pediatric formulas, this is not currently true of vitamin A content in all renal formulas. More research needs to be conducted to evaluate patients with CKD stages 1–3 to determine appropriate and optimum vitamin A monitoring recommendations.
While hypervitaminosis A is a recognized comorbidity of CKD, the prevalence and severity can easily go unrecognized. We found that hypervitaminosis A is common in the pediatric population with CKD 4–5. FF patients have higher Vitamin A levels above the ULN than NFF patients, likely because of the content of vitamin A in their formula or breast milk. FF patients also are more likely to have PTH levels below goal, which places them at increased risk for adynamic bone disease. While further research is needed to be conducted on vitamin A levels in pediatric patients with advanced CKD, Vitamin A levels should be considered as part of the routine nutritional assessment in late CKD patients, particularly in those with findings of hypercalcemia and bone disease.
Practical Application:
This data demonstrates that almost all pediatric patients with CKD 4–5 have elevated levels of Vitamin A and that formula fed patients have higher levels of vitamin A than non-formula fed patients. Formula fed patients also have lower PTH levels and non-formula fed patients have higher levels of PTH. Through this data, we advocate for routine monitoring of Vitamin A levels in advanced CKD patients and further evaluation of vitamin A levels of renal formulas.
Supplementary Material
Supplemental Table 1: The vitamin A delivered when 50% of caloric needs are provided by each respective formula for three different representative ages. F=Female. M=Male. *Not recommended to receive 50% of calories from milk(34).
Support and Disclosures:
Disclosures: None
Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Manickavasagar B, McArdle AJ, Yadav P, Shaw V, Dixon M, Blomhoff R, et al. Hypervitaminosis A is prevalent in children with CKD and contributes to hypercalcemia. Pediatr Nephrol. 2015;30(2):317–25. Epub 2014/08/15. doi: 10.1007/s00467-014-2916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jing J, Isoherranen N, Robinson-Cohen C, Petrie I, Kestenbaum BR, Yeung CK. Chronic Kidney Disease Alters Vitamin A Homeostasis via Effects on Hepatic RBP4 Protein Expression and Metabolic Enzymes. Clin Transl Sci. 2016;9(4):207–15. Epub 2016/06/10. doi: 10.1111/cts.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lionikaite V, Henning P, Drevinge C, Shah FA, Palmquist A, Wikstrom P, et al. Vitamin A decreases the anabolic bone response to mechanical loading by suppressing bone formation. FASEB J. 2019;33(4):5237–47. Epub 2019/01/23. doi: 10.1096/fj.201802040R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnicot NA. The local action of vitamin A on bone. J Anat. 1950;84(4):374–87. Epub 1950/10/01. [PMC free article] [PubMed] [Google Scholar]
- 5.Scheven BA, Hamilton NJ. Retinoic acid and 1,25-dihydroxyvitamin D3 stimulate osteoclast formation by different mechanisms. Bone. 1990;11(1):53–9. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 6.Gordon CE, Balk EM, Becker BN, Crooks PA, Jaber BL, Johnson CA, et al. KDOQI US commentary on the KDIGO clinical practice guideline for the prevention, diagnosis, evaluation, and treatment of hepatitis C in CKD. Am J Kidney Dis. 2008;52(5):811–25. Epub 2008/10/31. doi: 10.1053/j.ajkd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Group KW. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis. 2009;53(3 Suppl 2):S11–104. Epub 2009/02/28. doi: 10.1053/j.ajkd.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GJ, Haycock GB, Edelmann CM Jr., Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58(2):259–63. Epub 1976/08/01. [PubMed] [Google Scholar]
- 9.Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64(1):25–30. Epub 2004/03/18. doi: 10.1080/00365510410003723. [DOI] [PubMed] [Google Scholar]
- 10.Soldin SJ. Pediatric Reference Ranges: AACC Press; 1997. 181 p. [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes CKDMBDUWG. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (2011). 2017;7(1):1–59. Epub 2017/07/01. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borzych D, Rees L, Ha IS, Chua A, Valles PG, Lipka M, et al. The bone and mineral disorder of children undergoing chronic peritoneal dialysis. Kidney Int. 2010;78(12):1295–304. Epub 2010/09/03. doi: 10.1038/ki.2010.316. [DOI] [PubMed] [Google Scholar]
- 13.Lemke SL, Dueker SR, Follett JR, Lin Y, Carkeet C, Buchholz BA, et al. Absorption and retinol equivalence of beta-carotene in humans is influenced by dietary vitamin A intake. J Lipid Res. 2003;44(8):1591–600. Epub 2003/06/05. doi: 10.1194/jlr.M300116-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.What We Eat in America [Internet] Food Surveys Research Group. 2017-2018. [Google Scholar]
- 15.Enfamil Poly-Vi-Sol 2020 [cited 2021]. Available from: https://www.enfamil.com/products/enfamil-poly-vi-sol-drops/?gclid=Cj0KCQiArvX_BRCyARIsAKsnTxPmY_Us05B95RdIniQVc-aur-Cltd6v3frx8hKQBESY0ecND2ts7rkaAtvkEALw_wcB.
- 16.The composition of mature human milk. Report of a Working Party of the Committee on Medical Aspects of Food Policy. Rep Health Soc Subj (Lond) 1977;12:i-xi–1-47. Epub 1977/01/01. [PubMed] [Google Scholar]
- 17.Bakkaloglu SA, Wesseling-Perry K, Pereira RC, Gales B, Wang HJ, Elashoff RM, et al. Value of the new bone classification system in pediatric renal osteodystrophy. Clin J Am Soc Nephrol. 2010;5(10):1860–6. Epub 2010/07/17. doi: 10.2215/CJN.01330210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziolkowska H, Paniczyk-Tomaszewska M, Debinski A, Polowiec Z, Sawicki A, Sieniawska M. Bone biopsy results and serum bone turnover parameters in uremic children. Acta Paediatr. 2000;89(6):666–71. Epub 2000/07/29. [PubMed] [Google Scholar]
- 19.Yalcinkaya F, Ince E, Tumer N, Ensari A, Ozkaya N. Spectrum of renal osteodystrophy in children on continuous ambulatory peritoneal dialysis. Pediatr Int. 2000;42(1):53–7. Epub 2000/03/07. doi: 10.1046/j.1442-200x.2000.01171.x. [DOI] [PubMed] [Google Scholar]
- 20.Andrade MC, Carvalhaes JT, Carvalho AB, Lazarretti-Castro M, Brandao C. Bone mineral density and bone histomorphometry in children on long-term dialysis. Pediatr Nephrol. 2007;22(10):1767–72. Epub 2007/08/08. doi: 10.1007/s00467-007-0546-7. [DOI] [PubMed] [Google Scholar]
- 21.Denburg MR, Kumar J, Jemielita T, Brooks ER, Skversky A, Portale AA, et al. Fracture Burden and Risk Factors in Childhood CKD: Results from the CKiD Cohort Study. J Am Soc Nephrol. 2016;27(2):543–50. Epub 2015/07/04. doi: 10.1681/ASN.2015020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Civilibal M, Caliskan S, Kurugoglu S, Candan C, Canpolat N, Sever L, et al. Progression of coronary calcification in pediatric chronic kidney disease stage 5. Pediatr Nephrol. 2009;24(3):555–63. Epub 2008/11/05. doi: 10.1007/s00467-008-1038-0. [DOI] [PubMed] [Google Scholar]
- 23.Luna RC, do Nascimento CC, Asciutti LS, Franceschini Sdo C, Filizola RG, Diniz Ada S, et al. Relation between glucose levels, high-sensitivity C-reactive protein (hs-CRP), body mass index (BMI) and serum and dietary retinol in elderly in population-based study. Arch Gerontol Geriatr. 2012;54(3):462–8. Epub 2011/07/19. doi: 10.1016/j.archger.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Garcia OP, Ronquillo D, Caamano Mdel C, Camacho M, Long KZ, Rosado JL. Zinc, vitamin A, and vitamin C status are associated with leptin concentrations and obesity in Mexican women: results from a cross-sectional study. Nutr Metab (Lond) 2012;9(1):59. Epub 2012/06/19. doi: 10.1186/1743-7075-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nutrition A. Similac PM 60/40 Available from: https://abbottnutrition.com/similac-pm-60-40.
- 26.Nutrition A. Suplena with CarbSteady. Available from: https://abbottnutrition.com/suplena-with-carbsteady.
- 27.Nutrition A. Nepro. Available from: https://abbottnutrition.com/suplena-with-carbsteady.
- 28.Science VNH. Renastart. Available from: https://www.nestlehealthscience.us/vitaflo-usa/pediatric-renal-disease/renastart.
- 29.Nutrition A. Pediasure Peptide 1.0. Available from: https://abbottnutrition.com/pediasure-peptide-1_0-cal.
- 30.Nutrition A. Elecare Junior. Available from: https://abbottnutrition.com/elecare-jr. [Google Scholar]
- 31.Science NH. Vivonex T. E. N. Available from: https://www.nestlehealthscience.us/vitaflousa/pediatric-renal-disease/renastart.
- 32.Blends RF. Real Food Blends. Various mixtures.
- 33.Organic H. Whole cow’s milk. Available from: https://horizon.com/organic-dairyproducts/organic-milk/.
- 34.Recommended Dietary Allowances: 10th Edition. The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC)1989. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: The vitamin A delivered when 50% of caloric needs are provided by each respective formula for three different representative ages. F=Female. M=Male. *Not recommended to receive 50% of calories from milk(34).