Abstract
In many patients, ostensible idiopathic attention deficit-hyperactivity disorder (ADHD) may actually stem from covert prenatal alcohol exposure (PAE), a treatment-relevant distinction. This study attempted a receiver-operator characteristic (ROC) classification of children with ADHD into those with PAE (ADHD+PAE) and those without (ADHD−PAE) using neurobehavioral instruments alongside magnetic resonance spectroscopy (MRS) and diffusion tensor imaging (DTI) of supraventricular brain white matter. Neurobehavioral, MRS, and DTI endpoints had been suggested by prior findings. Participants included children aged 8–13 years, 23 with ADHD+PAE, 19 with familial ADHD−PAE, and 28 typically developing (TD) controls. With area-under-the-curve (AUC) >0.90, the Conners 3 Parent Rating Scale Inattention (CIn) and Hyperactivity/Impulsivity (CHp) scores and the Behavioral Regulation Index (BRI) of the Behavior Rating Inventory of Executive Function (BRIEF2) excellently distinguished the clinical groups from TD, but not from each other (AUC<0.70). Combinations of MRS glutamate (Glu) and N-acetyl-compounds (NAA) and DTI mean diffusivity (MD), axial diffusivity (AD), radial diffusivity (RD), and fractional anisotropy (FA) yielded “good” (AUC>0.80) discrimination. Neuroimaging combined with CIn and BRI achieved AUC 0.72 and AUC 0.84, respectively. But neuroimaging combined with CHp yielded 14 excellent combinations with AUC≥0.90 (all p<0.0005), the best being Glu·AD·RD·CHp/(NAA·FA) (AUC 0.92, sensitivity 1.00, specificity 0.82, p<0.0005). Using Cho in lieu of Glu yielded AUC 0.83. White-matter microstructure and metabolism may assist efforts to discriminate ADHD etiologies and to detect PAE, beyond the ability of commonly used neurobehavioral measures alone.
Keywords: Fetal alcohol spectrum disorder, attention deficit hyperactivity disorder, magnetic resonance spectroscopy, diffusion tensor imaging, white matter
Introduction
Prenatal alcohol exposure (PAE) affects up to 5% of US children (May et al. 2018). ADHD is common in PAE (Mattson et al. 2019, O’Connor 2014). Since, however, PAE often goes unrecognized, patients who have ADHD due to PAE (ADHD+PAE) are frequently misdiagnosed as having ADHD without PAE (ADHD−PAE) due to familial or other causes (Glass et al. 2014, Rasmussen 2005, Wozniak et al. 2019). This has consequences as ADHD+PAE and ADHD−PAE may represent distinct subtypes of ADHD (Coles et al. 1997; Mattson et al. 2019). In particular, ADHD+PAE is less responsive to stimulants (Doig et al. 2008, O’Malley & Nanson 2002; Peadon et al. 2009, Snyder et al. 1997). This is a major clinical issue. It is also a research issue. The clinical ADHD populations from which many studies draw their participants contain a good proportion of patients with occult PAE (Coles 2001). Yet, few studies of ADHD screen for PAE. Some findings in these studies that are actually due to PAE may therefore be attributed to familial or other causes. Thus, there are clinical and research needs to distinguish ADHD+PAE from ADHD−PAE. Differential diagnosis, however, can be challenging. The PAE criterion of maternal alcohol consumption during pregnancy often cannot be established when the birth mother is unavailable (Chernoff et al. 1994) or reluctant to admit drinking while pregnant (CDC 2004). Many children with PAE lack the characteristic (but not essential) facial stigmata of the condition (Mattson et al. 2011, 2013; Wozniak et al. 2019). It would be beneficial to develop means of detecting PAE that rely neither on stigmata nor birth-mother informants.
Neurobehavioral measures may open a path to this goal. Several studies hint that the proportion of inattentive vs. hyperactive/impulsive symptoms varies between ADHD+PAE and ADHD−PAE (Brown et al. 1991, Coles et al.1997, O’Malley & Nanson 2002, Raldiris et al. 2018, Roebuck et al. 1999) some finding greater inattention, others greater hyperactivity in ADHD+PAE. Abnormal executive function is characteristic of fetal alcohol spectrum disorders (FASD; Glass et al. 2014, Mattson et al. 2013) and Nguyen et al. (2014) found that the Behavior Rating Inventory of Executive Function (BRIEF) discriminated between ADHD+PAE and ADHD−PAE. Our research (Schonfeld et al. 2006, 2009) particularly associated the BRIEF Behavioral Regulation Index (BRI) with PAE and poor treatment response. Thus, certain neurobehavioral scales may aide classification.
Neuroimaging offers a second path to identifying PAE. Our previous study (O’Neill et al. 2019) found that proton magnetic resonance spectroscopy (MRS) and diffusion tensor imaging (DTI) distinguished ADHD+PAE from ADHD−PAE. Receiver-operator characteristic (ROC) analysis using MRS choline-containing compounds (Cho) in the corona radiata discriminated the two conditions with area-under-the-curve (AUC) of 0.76 (“fair”). We also observed lower values of the DTI index fractional anisotropy (FA) in PAE. Other investigators reported effects on MRS glutamate (Glu; du Plessis et al. 2014, Howells et al. 2016) or N-acetyl-compounds (NAA; Cortese et al. 2006, du Plessis et al. 2014, Fagerlund et al. 2006) in FASD or in ADHD (O’Neill et al. 2013), while others found effects involving the DTI indices mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) in FASD (Wozniak & Muetzel 2011) or ADHD (Liston et al. 2011), including ADHD screened for PAE (Lawrence et al. 2013). We hypothesized that combinations of neuroimaging measures might elevate the power to differentiate ADHD+PAE from ADHD−PAE above previous observations.
The present study sought such superior discrimination by adding the Conners 3 Parent Rating Scale (Conners et al. 2011) Inattention (CIn) and Hyperactivity/Impulsivity (CHp) subscales and the BRIEF2 BRI to MRS and DTI metrics in brain white matter. We also implemented improvements over O’Neill et al. (2019), including narrower participant age-range, more stringent diagnosis, ADHD−PAE restricted to familial etiology, broader sampling of white matter, 3-T rather than 1.5-T MRI, 32-channel rather than single-channel headcoil, 20-ms rather than 30-ms MRS echo-time, and 98–99- rather than 6-direction DTI. The goal was to identify combinations of CIn, CHp, and/or BRI with Glu, NAA, Cho, FA, MD, AD, and RD that would best segregate ADHD+PAE and familial ADHD−PAE participants from each other and from TD controls.
Methods in Brief
For further details (including Study Design and Neurobehavioral Assessments), see supplementary materials.
Participants and Clinical Assessments
Participants (Table 1) comprised 23 children with DSM-5 ADHD and prenatal alcohol exposure (ADHD+PAE), 19 with familial ADHD without prenatal alcohol exposure (ADHD−PAE), and 28 TD control children with neither ADHD nor PAE. PAE was assessed using the modified Institute of Medicine criteria (Hoyme et al. 2016, O’Connor et al. 2019). All children were between the ages of 8 and 13 with IQs ≥70. Children in the ADHD−PAE group had to have one or more first-degree relatives with diagnosed ADHD. A positive family history was required in order to compare two groups each with a well-defined etiology of ADHD. Note that this does exclude patients with ADHD but neither PAE nor a positive family history.
Table 1.
Demographics, clinical characteristics, and current psychotropic medications
| ADHD+PAE | ADHD-PAE | TD | |
|---|---|---|---|
|
| |||
| n | 23 | 19 | 28 |
| #males | 16 | 12 | 13 |
| Age | 9.7 ± 1.6*††† | 10.7 ± 0.9 | 11.3 ± 1.6 |
| Race/ethnicity | 5 white | 12 white | 14 white |
| 3 black | 1 black | 0 black | |
| 5 Latino | 1 Latino | 7 Latino | |
| 1 Asian | 2 Asian | 1 Asian | |
| 3 other | 0 other | 1 other | |
| 6 mixed | 3 mixed | 5 mixed | |
| Mother’s education, years | 15.7 ± 2.0 | 17.2 ± 3.3 | 17.6 ± 4.3 |
| WASI FSIQ | 94.9 ± 12.8**††† | 107.5 ± 11.5† | 116.7 ± 15.6 |
| Conners (CIn) | 80.1 ± 13.0††† | 81.7 ± 8.8††† | 47.6 ± 10.3 |
| Conners (CHp) | 86.3 ± 5.2**††† | 76.9 ±13.3††† | 49.4 ± 10.6 |
| BRIEF2 BRI | 70.7 ± 8.7††† | 67.3 ± 10.5††† | 42.2 ± 4.3 |
| Current | 10 none | 14 none | 28 none |
| psychotropics, | 10 stimulants | 4 stimulants | 0 stimulants |
| #participants | 1 antidepressants | 3 antidepressants | 0 antidepressants |
| 1 mood stabilizers | 1 mood stabilizers | 0 mood stabilizers | |
| 4 antipsychotics | 1 antipsychotics | 0 antipsychotics | |
| 5 noradrenergics | 1 noradrenergics | 0 noradrenergics | |
| 0 other | 0 other | 0 other | |
| Exposure to other | 1 tobacco | 1 tobacco | 3 tobacco |
| teratogens, | 4 marijuana | 1 marijuana | 0 marijuana |
| #participants | 4 methamphetamine | 0 methamphetamine | 0 methamphetamine |
| 0 cocaine | 0 cocaine | 0 cocaine | |
| 1 barbiturates | 0 barbiturates | 0 barbiturates | |
| 0 opioids | 0 opioids | 0 opioids | |
| 0 hallucinogens | 0 hallucinogens | 0 hallucinogens | |
| 1 antidepressants | 1 antidepressants | 1 antidepressants | |
| 0 anticonvulsants | 0 anticonvulsants | 1 anticonvulsants | |
| 0 antibiotics | 2 antibiotics | 0 antibiotics | |
| 2 OTC painkillers | 1 OTC painkillers | 2 OTC painkillers | |
p < 0.05
p < 0.01 vs. ADHD−PAE
p < 0.01
p < 0.001 vs. TD.
Mother’s education: proxy for socioeconomic status. FSIQ=full-scale IQ. CIn=Conners 3 Parent Rating Scale Inattention. CHp=Conners 3 Parent Rating Scale Hyperactivity/Impulsivity. BRIEF2 BRI=Behavior Rating Inventory of Executive Function, Behavioral Regulation Index. stimulants=amphetamine, lisdexamphetamine, methylphenidate, dexmethylphenidate, antidepressants=fluoxetine. mood stabilizers=lamotrigine. antipsychotics=aripiprazole, risperidone. noradrenergics=atomoxetine, guanfacine. OTC painkillers=aspirin/acetaminophen/ibuprofen.
Participants were seen for two sessions, the first for diagnostic and neurocognitive testing, the second for MRI. Children in the ADHD groups met DSM-5 criteria for ADHD, any subtype, according to the clinician-administered Schedule for Affective Disorders and Schizophrenia for School-Aged Children Parent Version (K-SADS P; Kaufman et al. 1997), computerized version (Townsend et al. 2020). Full-scale IQ was estimated with the Wechsler Abbreviated Scale of Intelligence (WASI-II; Wechsler 2011). Parent ratings on the Conners 3 Behavior Rating Scale (Conners et al. 2011) were used to evaluate inattentive and hyperactive/impulsive symptoms. Parents completed the BRIEF2 (Gioia et al. 2015) to measure the child’s executive functioning, with the BRI as principal outcome. Children taking stimulants were asked to be off-medication at least 24 hr prior to each session. This time frame represented a balance between complete washout of drug and family burden of managing reemergence of symptoms. (For further details on recruitment, screening, inclusions, and exclusions see supplementary materials.)
MR Acquisition and Post-Processing
MRS (STEAM, TR/TM/TE=2000/20/10 ms, voxels 10×10×10 mm3, 4 excitations) of supraventricular white matter (Fig. S2) and whole-brain DTI (TR/TE=3230/89.2 ms, b=0/1500/3000 s/mm2, voxels 1.5 ×1.5×1.5 mm3) were acquired at 3 T and post-processed in overlapping volumes to yield levels of Glu, NAA, Cho and MD, AD, RD, and FA.
Statistics
ROC curves were plotted for classification variables generated by combining neurobehavioral and neuroimaging variables in supraventricular white matter. Variables combined were restricted to those with a priori potential to discriminate ADHD+PAE from ADHD−PAE. They included CIn, CHp, and BRI and the neuroimaging measures Glu, NAA, Cho, MD, AD, RD, and FA. Combinations were limited to simple multiplication or division: multiplication where higher values were anticipated in ADHD+PAE; division where lower values were expected. Based on the above-cited literature, our pilot (O’Neill et al. 2019), and the uncertain assumption of greater pathology in ADHD+PAE, CIn, CHp, BRI, Glu, MD, AD, and RD served as multiplicands and NAA, Cho, and FA as divisors. An example ROC variable was Glu·MD·RD·CHp/FA with Glu, MD, RD, and CHp as multiplicands and FA as divisor. Combination results are chiefly reported for variables yielding AUC≥0.90 (“excellent”) and p<0.05. For key cases, we present sensitivity, specificity, and numbers of false negatives and false positives. ROC analyses were also performed to discriminate ADHD+PAE and ADHD−PAE from TD. For these cases, abbreviated results are presented as it became apparent that CIn, CHp, and BRI alone readily distinguished both ADHD groups from controls.
For treatment of statistical power and multiple comparisons see supplementary materials.
Results
Participants and Neurobehavioral Assessments
The ADHD+PAE sample (Table 1) had significantly lower age and IQ than the ADHD−PAE and TD samples. Low IQ is a frequent consequence of PAE, the neurobehavioral metrics were age-normed standard scores, and within-groups there were no significant correlations between age or IQ and any MRS or DTI classification measure. Therefore, age and IQ were not covaried in statistical analyses. In particular, IQ was not used as a covariate based on arguments that this is inappropriate for studies of neurodevelopmental disorders (Dennis et al. 2007). Possible psychiatric comorbidities on the K-SADS included: major depressive disorder, dysthymia, disruptive mood dysregulation, mania/hypomania, schizophrenia/psychosis, panic disorder, agoraphobia, separation anxiety, social anxiety, selective mutism, specific phobia, generalized anxiety, obsessive compulsive disorder, oppositional defiant disorder, conduct disorder, and tic disorder. On Fisher’s exact tests, there were no statistically significant differences between the ADHD+PAE and ADHD−PAE groups for any single diagnosis (p>0.05) or for total number of comorbid diagnoses.
ROC Comparisons of ADHD+PAE to ADHD−PAE
Performance of ROC classification of individual participants into ADHD+PAE vs. ADHD−PAE diagnostic categories was examined for neurobehavioral measures, for neuroimaging measures, and for combined neurobehavioral and neuroimaging measures. Both single-variables (e.g., CHp alone, AD alone) and combinations of variables (e.g., CHp·BRI, Glu·AD·RD, Glu·AD·MD·CHp/NAA) were examined.
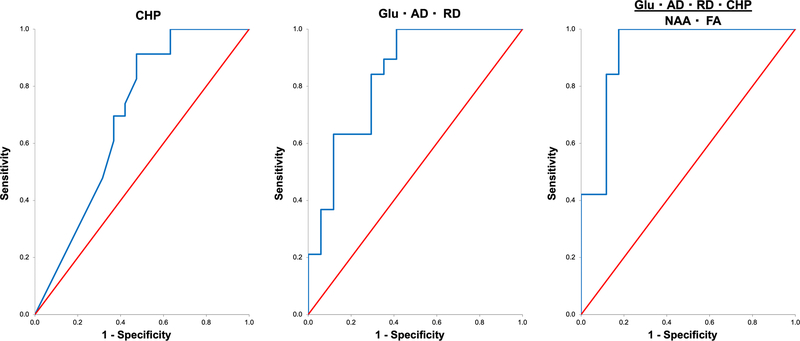
Among single-variable neurobehavioral measures, the CHp (AUC =0.70±0.09, p=0.024, sensitivity=1.00, specificity=0.37; Fig. 1) yielded the best ROC classification of ADHD+PAE vs. ADHD−PAE. It was a poor-to-fair discriminator. Classification performances of the single-variable neurobehavioral measures Conners CIn (AUC=0.44±0.09, p=0.509) and BRI (AUC =0.57±0.09, p=0.455) were both inferior to this. Combinations of CIn, CHp, and BRI did not improve upon the classification achieved with the CHp alone, the best-performing combination being CHp·BRI with AUC=0.65±0.09 (p=0.090 trend).
Fig. 1.
Receiver operator characteristic (ROC) curves for distinguishing children with attention deficit-hyperactivity disorder (ADHD) with prenatal alcohol exposure (ADHD+PAE) from children with familial ADHD without prenatal alcohol exposure (ADHD−PAE) using neurobehavioral and neuroimaging measures, the latter averaged across left and right supraventricular white matter. (Left) the best neurobehavioral discriminator, Conners 3 Parent Rating Scale Hyperactivity/Impulsivity (CHp) had an area under the curve (AUC) of 0.70±0.09 (p=0.024), a “fair” segregation. (Center) the best neuroimaging discriminator was the combination Glu·AD·RD with AUC=0.84±0.07 (p<0.0005), a “good” segregation. (Right) the best neurobehavioral+neuroimaging discriminator was the combination Glu·AD·RD·CHp/(NAA·FA) with AUC=0.92±0.05 (p<0.0005), an “excellent” segregation with sensitivity 1.00 and specificity 0.82. Thus, objective neuroimaging metrics reflecting the microstructure and metabolism of brain white matter enhance the capacity of neurobehavioral scales alone to detect PAE and to distinguish different etiologies of ADHD. Glu=glutamate measured by proton magnetic resonance spectroscopy (MRS); NAA=MRS N-acetyl-compounds; AD=axial diffusivity measured by diffusion tensor imaging (DTI); RD=DTI radial diffusivity; FA=DTI fractional anisotropy.
Among single-variable neuroimaging measures, the best classification of ADHD+PAE vs. ADHD−PAE was attained with AD (AUC=0.77±0.08, p<0.0005). This was a fair discriminator. Combinations of neuroimaging variables did improve the classification of ADHD+PAE vs. ADHD−PAE from the “fair” into the “good” range. Thereby, the neuroimaging variable combination with the highest AUC was Glu·AD·RD with AUC=0.84±0.07 (p<0.0005; Fig. 1).
Combinations of neurobehavioral and neuroimaging measures were examined separately for combinations including Cho, combinations including Glu, and combinations including both Cho and Glu. The combination using Cho with the best ADHD+PAE vs. ADHD−PAE classification performance was Cho·AD·MD·RD·CHp/NAA (AUC =0.83±0.07, p<0.0005), a good discriminator. When, however, combinations including Glu were examined, there were 14 combinations that achieved classification with AUC≥0.90 (all p<0.0005; Table 2). These were all excellent discriminators. The best discriminators were Glu·AD·MD·CHp/NAA (AUC=0.93±0.04) and Glu·AD·RD·CHp/(NAA·FA) (AUC=0.92±0.05; Fig. 1)(both p<0.0005). The latter had best classification performance of all combinations tried with sensitivity 1.00 and specificity 0.82 (19/19 true positives, 14/17 true negatives, 3/17 false positives, 0/19 false negatives, and 6 missing). (Missing participants were ones with good MRS and CHp data, but DTI data that failed quality control). Combinations of Cho and Glu did not exceed this performance, the best being Glu·Cho·AD·MD·RD·CHp/(NAA·FA) (AUC=0.87±0.06, p<0.0005).
Table 2.
Combinations of neurobehavioral and neuroimaging variables with ROC discrimination between ADHD+PAE and ADHD-PAE with AUC≥0.90 (“excellent”), (Neuroimaging variables from supraventricular white matter.)
| Combination Variable | AUC ± SE | P |
|---|---|---|
|
| ||
| *Glu-AD-MD-CHp/NAA | 0.93 ± 0.04 | 0.000025 |
| *Glu-AD-RD-CHp/NAA | 0.93 ± 0.04 | 0.000025 |
| *Glu-AD-MD-RD-CHp/NAA | 0.93 ± 0.05 | 0.000025 |
| *Glu-AD-RD-CHp/(NAA-FA) | 0.92 ± 0.05 | 0.000039 |
| *Glu-AD-MD-RD-CHp/(NAA-FA) | 0.92 ± 0.05 | 0.000039 |
| *Glu-MD-RD-CHp/NAA | 0.91 ± 0.05 | 0.00006 |
| *Glu-AD-MD-CHp/(NAA-FA) | 0.91 ± 0.05 | 0.00006 |
| *Glu-MD-RD-CHp/(NAA-FA) | 0.91 ± 0.05 | 0.00006 |
| Glu-AD-CHp/NAA | 0.91 ± 0.05 | 0.00006 |
| Glu-AD-CHp/(NAA-FA) | 0.91 ± 0.05 | 0.00006 |
| Glu-RD-CHp/(NAA-FA) | 0.91 ± 0.05 | 0.00006 |
| Glu-MD-CHp/NAA | 0.90 ± 0.05 | 0.000092 |
| Glu-RD-CHp/NAA | 0.90 ± 0.05 | 0.000092 |
| Glu-MD-CHp/(NAA-FA) | 0.90 ± 0.05 | 0.000092 |
Glu=magnetic resonance spectroscopy (MRS) glutamate. NAA=MRS N-acetyl-compounds. MD=diffusion tensor imaging (DTI) mean diffusivity. AD=DTI axial diffusivity. RD=DTI radial diffusivity. FA=DTI fractional anisotropy. CHp=Conners 3 Parent Rating Scale Hyperactivity/Impulsivity.
In summary, neurobehavioral measures alone yielded, at best, fair (AUC ≤0.70), neuroimaging alone yielded “good” (AUC>0.80), and Glu- (but not Cho-) based combinations of neurobehavioral and neuroimaging measures yielded “excellent” (AUC>0.90) identification of PAE.
ROC Comparisons of ADHD+PAE and ADHD−PAE to TD
CIn, CHp, and BRI alone were each highly effective discriminators between ADHD+PAE and TD (all AUC >0.90, p<0.0005) and between ADHD−PAE and TD (all AUC>0.95, p<0.0005). The best neuroimaging discriminator for ADHD+PAE was AD/NAA (AUC=0.68±0.08, p=0.026); the best for ADHD−PAE was Glu·MD (AUC=0.70±0.08, p=0.034). Given this relative performance, further improvement through combining with neuroimaging was not explored.
Discussion
This study improved upon previous results (O’Neill et al. 2019) that ADHD+PAE was distinguishable from ADHD−PAE by neuroimaging of white matter. The principal findings were: 1) Neurobehavioral measures alone (Conners 3 Inattention and Hyperactivity/Impulsivity-- CIn, CHp, BRIEF2 Behavioral Regulation Index-- BRI) discriminated ADHD+PAE and ADHD−PAE excellently from TD controls, but poorly from each other; 2) Neuroimaging measures alone (e.g., the combination Glu·AD·RD of MRS Glu and DTI AD and RD in supraventricular white matter) yielded “good” discrimination between ADHD+PAE and ADHD−PAE; 3) Aggregating neurobehavioral and neuroimaging measures (e.g., the combination Glu·AD·RD·CHp/(NAA·FA), adding MRS NAA and DTI FA to the measures in the foregoing example) led to “excellent” discrimination of ADHD+PAE vs. ADHD−PAE. Taken together, these findings imply that hyperactive/impulsive symptoms assessed by the Conners 3 combined with neuroimaging-detectable effects in white matter differentiate the familial and prenatal ethanol toxicity etiologies of ADHD very well.
The first major finding was that CIn, CHp, and BRI excellently discriminated ADHD+PAE and ADHD−PAE from TD, but poorly from each other. That CIn and CHp differentiated the two clinical groups from controls is not surprising as children were preselected for above-normal inattention and/or hyperactivity/impulsivity. That the BRI provided excellent separation underscores the conviction that executive-function deficits attend FASD and ADHD (Kingdon et al. 2016, Kodituwakku et al. 2001, Peadon & Elliott 2010). Although MRS and DTI effects in white matter are multiply documented in both FASD (Howells et al. 2016, Sherbaf et al. 2019, Wozniak & Muetzel 2011) and ADHD (Chen et al. 2016, Makris et al. 2009, O’Neill et al. 2013), these neuroimaging modalities may not improve upon neurobehavioral instruments in the primary diagnosis of ADHD.
Regarding, however, differential diagnosis of ADHD+PAE vs. ADHD−PAE, the second and third major findings were that neuroimaging provided “good” and neurobehavioral plus neuroimaging measures provided “excellent” classification. The CHp and (in supraventricular white matter) MRS Glu and the DTI indices contributed meaningfully to discrimination. Of MRS neurometabolites, Glu proved a better classifier than Cho.
The Supplementary Introduction (see supplementary materials) discusses how Cho, Glu, and the DTI indices are neurobiologically relevant to and may distinguish between ADHD+PAE and ADHD−PAE. In brief, low levels of white-matter Glu in ADHD−PAE relative to ADHD+PAE and the ability of classification variables containing Glu to distinguish these two conditions are consistent with the notion of hypoglutamatergia specific to familial ADHD−PAE. Clinically, emerging glutamatergic therapies for ADHD might have better prospects in patients with familial ADHD−PAE (Elia et al. 2018). Higher MD, AD, and RD in ADHD+PAE than ADHD−PAE and the ability of variables containing these indices to identify PAE may imply more severe delayed development of white matter in ADHD+PAE. Clinically, microstructural pathology in frontal white matter might represent part of the anatomic substrate of cognitive and motor dysfunction in these children. Diverse therapies in various conditions offer prospects of improving white-matter DTI indices (Bouziane et al. 2019, Carpenter et al. 2019, Chaddock-Heyman et al. 2018, Liu et al. 2020, Tseng et al. 2019) and, with modifications, might be further investigated in different age-groups in ADHD+PAE and ADHD−PAE. Present findings encourage future studies examining glutamatergic (Elia et al. 2020) and white-matter (Chen et al. 2016, van Ewijk et al. 2012) aspects of ADHD. Thereby, investigators should, as far as possible, account for PAE in their recruitment and analyses in anticipation of possibly different findings in ADHD+PAE than ADHD−PAE. The remaining Discussion below focusses on programmatic steps for other future studies that might refine the present classification procedure into a truly practical cost-effective, feasible technique for operational help in actual FASD diagnosis.
A first step in refinement would be replication of present findings in a larger cohort with separate training and cross-validation samples. The latter was precluded in the current study by the modest sample size. Beyond ROC, more sophisticated classification algorithms, such as support vector machine (Veronese et al. 2013), random forest (Ho 1998), linear discriminant analysis (Cohen et al. 2003), quadratic discriminant analysis (Tharwat 2016), logistic regression (Tolles & Meurer 2016), naïve bayes (Domingos & Pazzani 1997), or XGBoost (Chen & Guestrin 2016) should also be investigated. A next step would be to survey Glu, Cho, NAA, MD, AD, RD, and FA in the entire brain, in case superior classification is afforded in other regions. Near whole-brain MRS at high spatial-resolution studies can be acquired in reasonable scan times using techniques like echo-planar spectroscopic imaging (EPSI; Maudsley et al. 2006). Refinement could then follow by developing MRS and DTI acquisition protocols that are shorter and better tolerated by children. For example, single-voxel MRS might replace the multivoxel MRS deployed here, dropping runtime from ~14 to ~3 min. Similarly, DTI runtime could be lowered from the present ~23 min by sampling fewer directions or foregoing repeated reversed phase-encodings. In the present study, all participants were classified for comparison based upon the criteria set forth for PAE (mother’s drinking level) and not based upon whether or not they met criteria for the FAS facial phenotype. Therefore, selection criteria permitted participants to exhibit the facial phenotype or not according to ratings by two clinicians blind to PAE status. After having identified putative brain and behavioral markers of PAE in this sample with known PAE and/or facial features, the next step is to evaluate the ability of these markers to detect differences in a sample with unknown PAE. Comparison with classification methods based on other modalities would be a final step.
Limitations of this study included sample size. Although the sample size in this study is comparable to those in most MRS and DTI studies of ADHD and FASD (reviewed in O’Neill et al. 2013, Liston et al. 2011, Wozniak & Muetzel 2011), present methods should be retested on larger numbers of participants. The ADHD+PAE group was younger than the other two groups. Although no neuroimaging measure varied significantly with age in any group and all neurobehavioral indices were age-normed, ideally all groups would be age-matched. IQ was also lower in the ADHD+PAE group; however, all participants had IQ≥70, unlike some research samples of children meeting criteria for intellectual disability which often include results from extreme cases. Further, lower IQ is a common symptom of PAE and it is not conventional to correct for it (Dennis et al. 2007) and no neuroimaging measure varied significantly with IQ. Use of psychotropic medication was more frequent in the ADHD+PAE than the ADHD−PAE group which is consistent with findings in other studies (O’Connor 2014). Behavioral symptoms often lead to higher (unfortunately often less efficacious) use of drug treatment in ADHD+PAE. The most commonly prescribed drugs in the ADHD+PAE sample were stimulants and participants on stimulants were asked not to take them on testing days. Still, ideally the ADHD−PAE group would be equally medicated. Additionally, the length of time that participants had been treated with medication long-term was not tabulated. Hence, the influence of chronic medication on results is unclear. Some participants had exposure to teratogens other than ethanol (Table 1). This is common in PAE and extremely difficult to exclude. Our cohort had modest frequency of exposure relative to many published samples, but ideally there would be no exposure to other teratogens.
In summary and conclusion, combined with the Conners 3 Hyperactivity/Impulsivity score, MRS and DTI of supraventricular white matter yielded excellent (AUC=0.92) discrimination of pediatric ADHD+PAE from familial ADHD−PAE, with perfect (1.00) sensitivity and high (0.82) specificity. This was in contrast to using any of these measures alone. Of particular note the commonly clinically used Conners Hyperactivity/Impulsivity Scale did not accurately differentiate ADHD+PAE from familial ADHD−PAE on its own. At sensitivity 1.00, Conners specificity was only 0.37. Although multiple factors influence response to common psychostimulant treatments in ADHD (Manos 2008), response is frequently inferior for ADHD+PAE than for ADHD−PAE. Odds of spontaneous remission and long-term outcomes are also worse for ADHD+PAE. This implies a need to augment standard instruments like the Conners with more sophisticated diagnostic techniques including imaging. Although intensive inquiry into maternal drinking history is encouraged and resolves diagnostic issues in some cases, for many children with PAE (e.g., the many in foster care) the biological mother cannot be located. Even when located, she may be unwilling or unable to confirm drinking during pregnancy. MRI and MRS techniques are sometimes objected to on grounds of cost. However, the cost for a single scanning session (as conceived here) to establish an early diagnosis, does seem appropriate as it may set a child on a course of appropriate early intervention avoiding ineffective therapies with lifelong negative outcomes. Once the method is well validated, moreover, third-party providers may be persuaded to assume the cost of scans given the estimated life-span cost of medical services (1.6 million, Lupton et al., 2004) associated with prenatal alcohol exposure and possible improvement in health provided by early diagnosis.
Thus, the present approach could open a pathway towards better diagnosis of individuals with prenatal alcohol exposure in the absence of maternal self-report of PAE, other informants, and physical dysmorphologies.
Fig. 1 Receiver operator characteristic (ROC) curves for distinguishing children with attention deficit-hyperactivity disorder (ADHD) with prenatal alcohol exposure (ADHD+PAE) from children with familial ADHD without prenatal alcohol exposure (ADHD−PAE) using neurobehavioral and neuroimaging measures, the latter averaged across left and right supraventricular white matter. (Left) the best neurobehavioral discriminator, Conners 3 Parent Rating Scale Hyperactivity/Impulsivity (CHp) had an area under the curve (AUC) of 0.70±0.09 (p=0.024), a “fair” segregation. (Center) the best neuroimaging discriminator was the combination Glu·AD·RD with AUC=0.84±0.07 (p<0.0005), a “good” segregation. (Right) the best neurobehavioral+neuroimaging discriminator was the combination Glu·AD·RD·CHp/(NAA·FA) with AUC=0.92±0.05 (p<0.0005), an “excellent” segregation with sensitivity 1.00 and specificity 0.82. Thus, objective neuroimaging metrics reflecting the microstructure and metabolism of brain white matter enhance the capacity of neurobehavioral scales alone to detect PAE and to distinguish different etiologies of ADHD. Glu=glutamate measured by proton magnetic resonance spectroscopy (MRS); NAA=MRS N-acetyl-compounds; AD=axial diffusivity measured by diffusion tensor imaging (DTI); RD=DTI radial diffusivity; FA=DTI fractional anisotropy.
Supplementary Material
Funding
This research was supported by NIAAA R01 AA025066 (PIs: O’Connor and Levitt).
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Compliance with ethical standards
Ethical approval The UCLA Institutional Review Board approved all procedures. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. All parents gave informed consent. All children gave assent. Participants were compensated for their time.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Bouziane C, Filatova OG, Schrantee A, Caan MWA, Vos FM, & Reneman L (2019). White matter by diffusion MRI following methylphenidate treatment: A randomized control trial in males with sttention-deficit/hyperactivity disorder. Neuroradiology, 293, 186–192. [DOI] [PubMed] [Google Scholar]
- Brown RT, Coles CD, Smith IE, Platzman KA, Silverstein J, Erickson S, & Falek A (1991). Effects of prenatal alcohol exposure at school age. II. Attention and behavior. Neurotoxicology and Teratology, 13, 369–376. [DOI] [PubMed] [Google Scholar]
- Carpenter KLH, Major S, Tallman C, Chen LW, Franz L, Sun J, Kurtzberg J, Song A, & Dawson G (2019). White matter tract changes associated with clinical improvement in an open-label trial assessing autologous umbilical cord blood for treatment of young children with autism. Stem Cells Translational Medicine, 8, 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, Centers for Disease Control and Prevention. (2004). Fetal alcohol syndrome: Guidelines for referral and diagnosis. National Center on Birth Defects and Developmental Disabilities. Department of Health and Human Services. [Google Scholar]
- Chaddock-Heyman L, Erickson KI, Kienzler C, Drollette ES, Raine LB, Kao S-C, Bensken J, Weisshappel R, Castelli DM, Hillman CH, & Kramer AF (2018). Physical activity increases white matter microstructure in children. Frontiers in Neuroscience, 12, 950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, & Guestrin C (2016). XGBoost: A scalable tree boosting system. arXiv, 1603.02754v3. [Google Scholar]
- Chen L, Hu X, Ouyang L, He N, Liao Y, Liu Q, Zhou M, Wu M, Huang X, & Gong Q (2016). A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention-deficit/hyperactivity disorder. Neuroscience and Biobehavioral Reviews, 68, 838–847. [DOI] [PubMed] [Google Scholar]
- Chernoff R, Combs-Orme T, Risley-Curtiss C, & Heisler A (1994). Assessing the health status of children entering foster care. Pediatrics, 93, 594–601. [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, & Aiken LS (2003). Applied multiple regression/correlation analysis for the behavioural sciences 3rd Edition. NY: Routledge. [Google Scholar]
- Coles CD (2001). Fetal alcohol exposure and attention: Moving beyond ADHD. Alcohol Research and Health, 25(3), 199–203. [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, & Smith IE (1997). A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcoholism: Clinical and Experimental Research, 21, 150–161. [PubMed] [Google Scholar]
- Conners CK, Pitkanen J, & Rzepa SR (2011). Conners 3rd Edition (Conners 3; Conners 2008). In Kreutzer JS, DeLuca J, & Caplan B (Eds.). Encyclopedia of clinical neuropsychology. NY: Springer. [Google Scholar]
- Cortese BM, Moore GJ, Bailey BA, Jacobson SW, Delaney-Black V, & Hannigan JH (2006). Magnetic resonance and spectroscopic imaging in prenatal alcohol-exposed children: preliminary findings in the caudate nucleus. Neurotoxicology and Teratology, 28, 597–606. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schac har R, Barnes MA, & Fletcher JM (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society 15(3), 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig J, McLennan JD, & Gibbard WB (2008). Medication effects on symptoms of attention-deficit/hyperactivity disorder in children with fetal alcohol spectrum disorder. Journal of Child and Adolescent Psychopharmacology 18, 365–371. [DOI] [PubMed] [Google Scholar]
- Domingos P, & Pazzani M (1997). On the optimality of the simple Bayesian classifier under zero-one loss. Machine Learning, 29, 103–130. [Google Scholar]
- du Plessis L, Jacobson JL, Jacobson SW, Hess AT, van der Kouwe A, Avison MJ, Molteno CD, Stanton ME, Stanley JA, Peterson BS, & Meintjes EM (2014). An in vivo (1)H magnetic resonance spectroscopy study of the deep cerebellar nuclei in children with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research, 38(5), 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia J, Izaki Y, Ambrosini A, & Hakonarson H (2020). Glutamatergic neurotransmission in ADHD: Neurodevelopment and pharmacological implications. Journal of Pediatrics and Neonatology 2, 1006. [Google Scholar]
- Elia J, Ungal G, Kao C, Ambrosini A, De Jesus-Rosario N, Larsen L, Chiavacci R, Wang T, Kurian C, Titchen K, Sykes B, Hwang S, Kumar B, Potts J, Davis J, Malatack J, Slattery E, Moorthy G, Zuppa A, Weller A, Byrne E, Li YR, Kraft WK, & Hakonarson H (2018). Fasoracetam in adolescents with ADHD and glutamatergic gene network variants disrupting mGluR neurotransmitter signaling. Nature Communications 9(4), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlund A, Heikkinen S, Autti-Rämö I, Korkman M, Timonen M, Kuusi T, Riley EP, & Lundbom N (2006). Brain metabolic alterations in adolescents and young adults with fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research, 30, 2097–2104. [DOI] [PubMed] [Google Scholar]
- Gioia G, Isquith PK, Guy SC, & Kenworthy L (2015). Behavior Rating Inventory of Executive Function. Second Psychological Assessment Resources; Lutz, FL: 2015 [Google Scholar]
- Glass L, Ware AL, & Mattson SN (2014). Neurobehavioral, neurologic, and neuroimaging characteristics of fetal alcohol spectrum disorders. Handbook of Clinical Neurology, 125, 435–462. [DOI] [PubMed] [Google Scholar]
- Ho TK (1998). The random subspace method for constructing decision forests. IEEE Transactions on Pattern Analysis and Machine Intelligence, 20(8), 832–844. [Google Scholar]
- Howells FM, Donald KA, Roos A, Woods RP, Zar HJ, Narr KL, & Stein DJ (2016). Reduced glutamate in white matter of male neonates exposed to alcohol in utero: a 1H-magnetic resonance spectroscopy study. Metabolic Brain Disease, 31, 1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley MA, Marais A, & May PA (2016). Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics, 138(2). 2015–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, & Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kingdon D, Cardoso C, & McGrath JJ (2016). Research Review: Executive function deficits in fetal alcohol spectrum disorders and attention-deficit/hyperactivity disorder – a meta-analysis. Journal of Child Psychology and Psychiatry, 57, 2, 116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodituwakku PW, Kalberg W,, & May PA (2001) The effects of prenatal alcohol exposure on executive functioning. Alcohol Research and Health 25, 192–198. [PMC free article] [PubMed] [Google Scholar]
- Lawrence KE, Levitt JG, Loo SL, Ly R, Yee V, O’Neill J, Alger J, & Narr KL (2013). White matter microstructure in attention-deficit/hyperactivity disorder subjects and their siblings. Journal of the American Academy of Child and Adolescent Psychiatry, 52(4), 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Malter Cohen M, Teslovich T, Levenson D, & Casey BJ (2011). Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biological Psychiatry, 69(12), 1168–1177. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhanga X, Liu Y, Yuan X, Yang L, Zhang R, Zhang X, Wanga X, Xu F, & Zhu C (2020). Early application of caffeine improves white matter development in very preterm infants. Respiratory Physiology & Neurobiology, 281, 103495. [DOI] [PubMed] [Google Scholar]
- Lupton C, Burd L, Harwood R (2004). Cost of fetal alcohol spectrum disorders. American Journal of Medical Genetics, 127C (1), 42–50. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Monuteaux MC, & Seidman LJ (2009). Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Developmental Neuroscience, 31, 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manos MJ (2008). Pharmacologic treatment of ADHD: Road conditions in driving patients to successful outcomes. The Medscape Journal of Medicine, 10(1), 5. [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Bernes GA, Doyle FR (2019). Fetal alcohol spectrum disorders: A review of the neurobehavioral deficits associated with prenatal alcohol exposure. Alcohol Clinical and Experimental Research, 43,1046–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, & Nguyen TT (2011). Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychology Review 21, 81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Adnams CM, Jones KL, Riley EP, & CIFASD. (2013). Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcoholism: Clinical and Experimental Research, 37, 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley AA, Darkazanli A, Alger JR, Hall LO, Schuff N, Studholme C, Yu Y, Ebel A, Frew A, Goldgof D, Gu Y, Pagare R, Rousseau F, Sivasankaran K, Soher BJ, Weber P, Young K, & Zhu X (2006). Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR in Biomedicine 19(4), 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley MA, Kopald D, & Hoyme HE (2018). Prevalence of Fetal Alcohol Spectrum Disorders in 4 US communities. Journal of the American Medical Association, 319(5), 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Glass L, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Jones KL, Riley EP, Mattson SN, & CIFASD (2014). The clinical utility and specificity of parent report of executive function among children with prenatal alcohol exposure. Journal of the International Neuropsychological Society, 20(7), 704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ (2014). Mental health outcomes associated with prenatal alcohol exposure: Genetic and environmental factors. Current Developmental Disorders Reports, DOI 10.1007/s40474-014-0021-7. [DOI] [Google Scholar]
- O’Connor MJ, Portnoff LC, Lebsack-Coleman M, & Dipple KM (2019). Suicide risk in adolescents with fetal alcohol spectrum disorders. Birth Defects Research, 111(12), 822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley KD, & Nanson J (2002). Clinical implications of a link between fetal alcohol spectrum disorder and attention-deficit hyperactivity disorder. The Canadian Journal of Psychiatry 47, 349–354. [DOI] [PubMed] [Google Scholar]
- O Neill J, Levitt JG, & Alger JR (2013). Magnetic resonance spectroscopy studies of attention deficit hyperactivity disorder. In. l ml & Panigrahy A (Eds.), MR spectroscopy of pediatric brain disorders (pp. 229–276). NY: Springer. [Google Scholar]
- O Neill J, O’Connor MJ, Yee V, Ly R, Narr K, Alger JR, & Levitt JG (2019). Differential neuroimaging indices in prefrontal white matter in prenatal alcohol-associated ADHD versus idiopathic ADHD. Birth Defects Research, 111, 797–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peadon E & Elliott EJ (2010). Distinguishing between attention-deficit hyperactivity and fetal alcohol spectrum disorders in children: clinical guidelines. Neuropsychiatric Disease and Treatment, 6, 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peadon E, Rhys-Jones B, Bower C, & Elliott EJ (2009). Systematic review of interventions for children with Fetal Alcohol Spectrum Disorders. BMC Pediatrics, 9, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raldiris TL, Bowers TG, & Towsey C (2018). Comparisons of intelligence and behavior in children with fetal alcohol spectrum disorder and ADHD. Journal of Attention Disorders, 22(10), 959–970. [DOI] [PubMed] [Google Scholar]
- Rasmussen C (2005). Executive functioning and working memory in fetal alcohol spectrum disorder. Alcoholism: Clinical and Experimental Research, 29, 1359–1367. [DOI] [PubMed] [Google Scholar]
- Roebuck T, Mattson SN, & Riley EP (1999). Behavioral and psychosocial profiles of alcohol-exposed children. Alcoholism: Clinical & Experimental Research, 23, 1070–1076. [PubMed] [Google Scholar]
- Schonfeld AM, Paley B,., Frankel F, & O’Connor MJ (2006). Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychology, 12, 439–452. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Paley B,., Frankel F, & O’Connor MJ (2009). ehavioral regulation as a predictor of response to children’s friendship training in children with fetal alcohol spectrum disorders. The Clinical Neuropsychologist, 23, 428–445. [DOI] [PubMed] [Google Scholar]
- Sherbaf FG, Aarabi MH, Yazdi MH, & Haghshomar M (2019). White matter microstructure in fetal alcohol spectrum disorders: A systematic review of diffusion tensor imaging studies. Human Brain Mapping, 40, 1017–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J, Nanson J, Snyder R, & Block G (1997). A study of stimulant medication in children with FAS. In: Streissguth A, Kanter J, editor. Overcoming and Preventing Secondary Disabilities in Fetal Alcohol Syndrome and Fetal Alcohol Effects. Seattle, WA: University of Washington Press; pp. 64–77. [Google Scholar]
- Tharwat A (2016). Linear vs. quadratic discriminant analysis classifier: a tutorial. International Journal of Applied Pattern Recognition 3(2), 145. [Google Scholar]
- Tolles J, & Meurer WJ. (2016). Logistic regression relating patient characteristics to outcomes. JAMA, 316(5), 533–534. [DOI] [PubMed] [Google Scholar]
- Townsend L, Kobak K, Kearney C, Milham M, Andreotti C, Escalera J, Alexander L, Gill MK, Birmaher B, Sylvester R, Rice D, Deep A, & Kaufman J (2020). Development of three Web-based computerized versions of the Kiddie Schedule for Affective Disorders and Schizophrenia Child Psychiatric Diagnostic Interview: Preliminary validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 59(2), 309–325. [DOI] [PubMed] [Google Scholar]
- Tseng C-H, Chien Y-H, Lee N-C, Hsu Y-C, Peng S-F, Tseng W-Y, & Hwu W-L (2019). Gene therapy improves brain white matter in aromatic L-amino acid decarboxylase deficiency. Annals of Neurology, 85, 644–652. [DOI] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, & Oosterlaan J (2012). Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 36, 1093–1106. [DOI] [PubMed] [Google Scholar]
- Veronese E, Castellani U, Peruzzo D, Bellani B, & Brambilla P (2013). Machine learning approaches: From theory to application in schizophrenia. Computational and Mathematical Methods in Medicine, 2013, 867924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2011). Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II). San Antonio: NCS Pearson. [Google Scholar]
- Wozniak JR & Muetzel RL (2011). What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychology Reviews, 21, 133–147. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Riley EP, & Charness MD (2019). Diagnosis, epidemiology, assessment, pathophysiology, and management of fetal alcohol spectrum disorders, Lancet Neurology, 18(8), 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.