Abstract
Objective:
Although depression and anxiety often have distinct etiologies, they frequently co-occur in adolescence. Recent initiatives have underscored the importance of developing new ways of classifying mental illness based on underlying neural dimensions that cuts across traditional diagnostic boundaries. Accordingly, the aim of the study was to clarify reward-related neural circuitry that may characterize depressed-anxious youth.
Method:
The Boston Adolescent Neuroimaging of Depression and Anxiety Human Connectome Project tested group differences regarding subcortical volume and nucleus accumbens activation during an incentive processing task among 14–17-year-old adolescents presenting with a primary depressive and/or anxiety disorder (n=129) or no lifetime history of mental disorders (n=64). Additionally, multimodal modeling examined predictors of depression and anxiety symptom change over a 6-month follow-up period.
Results:
Our findings highlighted considerable convergence. Relative to healthy youth, depressed-anxious adolescents exhibited reduced nucleus accumbens volume and activation following reward receipt. These findings remained when removing all medicated participants (~59% of depressed-anxious youth); subgroup analyses comparing anxious-only, depressed-anxious, and healthy youth also were largely consistent. Multimodal modeling showed that only structural alterations predicted depressive symptoms over time.
Conclusion:
Multimodal findings highlight alterations within nucleus accumbens structure and function that characterize depressed-anxious adolescents. In the current hypothesis-driven analyses, only reduced nucleus accumbens volume, however, predicted depressive symptoms over time. An important next step will be to clarify why structural alterations impact reward-related processes and associated symptoms.
Keywords: anhedonia, subcortical volume, striatum, connectomics, internalizing disorders
Introduction
Although depression and anxiety disorders often have distinct etiologies, they frequently co-occur in adolescence.1–3 Anxiety symptoms typical emerge in childhood, and for many children, predict subsequent anxiety and/or depressive symptoms during adolescence. In other instances, depression symptoms emerge early in childhood and persist into adolescence.4 Notably, as many as 75% of youth with depression experience comorbid anxiety disorders,5–7 and among youth with anxiety, 10–15% report depression,5,8,9 with many more reporting subsyndromal depressive symptoms.10 Given the substantial overlap of depression and anxiety during adolescence,3 researchers have begun to investigate shared vulnerability factors implicated in the etiopathophysiology of depression and anxiety,11,12 including our current Human Connectome initiative,13,14 which focused on clarifying the neural circuitry of depression and anxiety by probing putative reward-related deficits.15,16
Decades of research have characterized the structural and functional neural systems that give rise to reward-related behavioral alterations, which directly affect approach tendencies in youth with depression and anxiety.16,17 This builds on a foundation of single cell recording research in primates. For example, Schultz18 demonstrated that receipt of unpredicted reward was associated with increased dopaminergic neuron firing in the midbrain and striatum, which corresponded to greater phasic dopamine within these regions. Conversely, trials in which expected reward was omitted resulted in the suppression of dopamine neuron firing. Human research has confirmed striatal dopamine release among healthy individuals following reward receipt19 and also linked specific striatal nuclei to distinct aspects of reward processing: nucleus accumbens – reward pleasure, caudate – reward learning, and putamen – reward prediction.20 Whereas individuals with depression generally exhibit hypoactivation within striatal regions across these reward processes,21 patterns within anxious patients are more variable (eg, hypoactivation following reward receipt22 vs hyperactivation to anticipation of reward23). Whether these neurofunctional differences are driven by comorbid presentations of depression and anxiety, which are exceedingly common,1–3 remains unclear.
Depression and anxiety disorders are characterized by differences in subcortical brain structure24–27—many of which may bear directly on these reward-related processes affected by dopaminergic projections within the nigrostriatal and mesolimbic pathways.15,28,29 The nigrostriatal pathway terminates in the caudate and putamen, and the mesolimbic pathway projects to the ventral striatum, hippocampus, and amygdala. Structural alterations within these regions have been consistently implicated in adolescent depression and anxiety disorders. Among adolescents and adults, depression has been linked to structural differences in the amygdala,30–32 hippocampus,33–40 and striatum36,41–46; yet, these findings may be related to antidepressant medication use,37,47 depression recurrence,24 and episode duration.48 A recent study, however, showed that children at high-risk for depression based on a parental history exhibited reduced nucleus accumbens and putamen volumes even prior to depression onset, suggesting that these differences may play a role in depression risk and onset.25 Comparatively less research has investigated structural alterations in anxiety disorders, however, across social anxiety, generalized anxiety, and panic disorder, there is evidence of reduced amygdala26,27,49–51 and hippocampus volumes.52,53 Notably, research probing comorbid depression and anxiety among adults also has implicated striatal alterations.54 Taken together, there is evidence of subcortical volume alterations in depression (ie, nucleus accumbens, putamen) and anxiety disorders (ie, amygdala, hippocampus), but further research is needed to clarify differences in adolescents reporting comorbid depression and anxiety, particularly given the high rates of co-occurrence during a peak period of onset.5–9
For several decades, depression (eg,15,20,55,56) and anxiety (eg,16,23,56–58) research has investigated functional neural correlates of blunted reward processing, which may reflect anhedonia—a transdiagnostic (endo)phenotype typified by a diminished experience of pleasure.59–61 Specifically, the ventral striatum, and the nucleus accumbens in particular, is a core hub of the appetitive-motivational system and receives dopaminergic inputs from the substantia nigra and ventral tegmental area, which directly affect reward-related consummatory behaviors.62 Adolescent depression is characterized by reduced nucleus accumbens activation following reward receipt (relative to loss),63,64 which was partly corroborated in a recent meta-analysis including depressed youth and adults.17 Several research groups also have focused on reward functioning in the context of behavioral inhibition—a transdiagnostic temperament factor reflecting heightened fear response to unfamiliar experiences and situations, which has been implicated in a range of anxiety disorders.16,65 When completing an ecologically valid social reward task, there was a blunting of caudate response following peer acceptance in behaviorally inhibited versus non-inhibited youth.22 Among individuals diagnosed with social anxiety disorder, however, there was reduced activation in the nucleus accumbens following rewarding feedback compared to healthy controls.66 Collectively, there is compelling evidence linking blunted nucleus accumbens response following reward receipt among depressed youth,63,64 and although more limited, initial research in anxiety has highlighted blunting within the dorsal and ventral striatum.22,66
Goals of the Present Study
Given the substantial co-occurrence of depression and anxiety in youth,5–9 the current study sought to clarify reward-related neural circuitry that may characterize depressed-anxious youth. Through the Human Connectome initiative,13,14 multimodal neuroimaging data were acquired from healthy and depressed-anxious adolescents allowing us to test the following hypotheses. First, prior research in depression and anxiety has implicated differences in subcortical brain structure.24–27 Although research on comorbid depression and anxiety is limited,54,67 particularly in adolescents, we hypothesized that relative to healthy adolescents, depressed-anxious youth would exhibit reduced nucleus accumbens, putamen, amygdala, and hippocampal volumes. Second, capitalizing on prior Human Connectome research showing robust nucleus accumbens activation following reward receipt during an incentive processing task,68,69 we tested whether depressed-anxious adolescents showed reduced reward-related activation in the accumbens compared to healthy youth. Last, multimodal models were used to integrate and test the relative influence of clinical, structural, and functional data in predicting depression and anxiety symptoms at 6-month follow-up.
Method
Participants
The Boston Adolescent Neuroimaging of Depression and Anxiety Human Connectome Project collected clinical, neuropsychological, and multimodal MRI data from adolescents presenting with a primary depressive and/or anxiety disorder (ie, generalized anxiety, separation anxiety, social anxiety, specific phobia, agoraphobia, panic disorder) or no lifetime history of DSM-5 mental disorders (ie, healthy controls) (for detailed procedures see13,14; for symptom overlap see Figure S1, available online). Participants were recruited through flyers, internet advertisements (eg, Facebook, Instagram), and public transport postings within the greater Boston area. Parents and adolescents completed a phone screen prior to in-lab assessments at one of three sites: Boston University, the Massachusetts General Hospital, or McLean Hospital. Approximately the same number of participants were recruited at each site, and all participants were scanned at the Martinos Center for Biomedical Imaging at the Massachusetts General Hospital. Inclusion criteria included: ages 14–17-years and English fluency. Exclusion criteria included: IQ<85, any neurodevelopmental disorder, bipolar disorder, psychotic disorders, MRI contraindication, premature birth, serious medication conditions, history of serious head injury (ie, loss of consciousness for >30 minutes), hospitalization for neurological or cardiovascular diseases. To improve the generalizability of our findings, ADHD diagnosis, which is often comorbid in depressed-anxious cases70, and psychotropic medication use were not exclusionary within the depressed-anxious group.
Two-hundred participants completed the baseline clinical assessment; however, 7 participants did not complete the scan and were excluded. Structural MRI (sMRI) analyses included 193 participants (Healthy=64, Depressed-Anxious=129). For functional MRI (fMRI) analyses, 9 additional depressed-anxious participants were excluded (did not complete 2 runs of the task=6, poor task performance=3), leaving 184 participants (Healthy=64, Depressed-Anxious=120). For sMRI (Table 1) and fMRI (Table S1, available online) analyses, there were no significant group differences in sex (non-significant trend), pubertal status (non-significant trend), handedness, ethnicity, IQ, or family income, but the depressed-anxious group was slightly older and exhibited greater head motion. Depressed-anxious participant clinical characteristics are summarized in Table 2.
Table 1.
Participant, Clinical, and Scanner Characteristics
| Healthy (n=64) | Depressed-anxious (n=129) | t/χ2 | p | |
|---|---|---|---|---|
| Participant characteristics | ||||
| Age | 15.19 (0.83) | 15.47 (0.84) | 2.18 | .03 |
| Sex: male (%) | 29 (45.3%) | 40 (31.0%) | 3.21 | .07 |
| Pubertal status: stage (SD) | 3.38 (0.58) | 3.52 (0.56) | 3.19 | .07 |
| Handedness: right (%) | 51 (79.7) | 101 (78.3) | 0.001 | .97 |
| Race: White (%) | 41 (75.9) | 70 (84.3) | 1.01 | .32 |
| IQ | 117.97 (14.44) | 114.45 (15.19) | −1.57 | .12 |
| Income: 100k+ (%) | 44 (69.84%) | 77 (62.6%) | 0.67 | .41 |
| Reporter parent education: Advanced degree (%) | 36 (57.14%) | 58 (46.77%) | 1.41 | .24 |
| Either parent education: Advanced degree (%) | 46 (73.02%) | 74 (61.16%) | 2.07 | .15 |
| Symptoms | ||||
| Depression symptoms | 4.70 (4.61) | 24.65 (14.62) | 14.10 | <.001 |
| General anxiety symptoms | 2.05 (1.79) | 7.16 (3.66) | 13.01 | <.001 |
| Social anxiety symptoms | 6.48 (4.44) | 15.23 (6.17) | 11.24 | <.001 |
| Scanner parameters | ||||
| sMRI – mean motion (mm) | 0.11 (0.05) | 0.14 (0.05) | 2.60 | .01 |
| fMRI – mean motion (mm) | 0.09 (0.03) | 0.10 (0.04) | 2.18 | .05 |
Note. Characteristics presented for the sample of participants with structural data available for analysis (n=193); Table S1, available online, presents these characteristics for the subset with IPT (Incentive Processing Task) data; Depression Symptoms = Mood and Feelings Questionnaire (MFQ); General Anxiety Symptoms = Revised Child Anxiety and Depression Scale (RCADS); Social Anxiety Symptoms = Revised Child Anxiety and Depression Scale; n=1 missing IQ, replaced with sample mean; n=1 missing MFQ scores and RCADS scores; Pubertal Status group comparison reflects a chi-square test.
Table 2.
Current Mental Disorders and Medication Use Among Adolescents Diagnosed with Depressive and/or Anxiety Disorders (n=129)
| Category | N (%) |
|---|---|
| Current mental disorder | |
| Depressive disorder | 57 (44.2%) |
| Anxiety disorder | 118 (91.5%) |
| Comorbid depression and anxiety disorders | 46 (35.7%) |
| ADHD (or other/unspecified ADHD) | 36 (27.9%) |
| Eating disorder (anorexia, binge eating) | 3 (2.3%) |
| PTSD | 4 (3.1%) |
| OCD or related (excoriation, hoarding, trichotillomania, body dysmorphia) | 19 (14.7%) |
| Disruptive disorder (ODD, IED) | 10 (7.8%) |
| Substance use disorder | 2 (1.6%) |
| Any comorbidity (beyond primary depression and/or anxiety) | 49 (38.0%) |
| Current medication | |
| Antidepressant medication | 61 (47.3%) |
| ADHD medication | 27 (20.9%) |
| Other psychiatric medication | 12 (9.3%) |
| Any psychiatric medication | 76 (58.9%) |
Note. Depressive disorder = major depressive disorder (n=51), dysthymia (n=2), depression NOS (n=4), anxiety disorder = generalized anxiety disorder (n=70), social phobia (n=64), separation anxiety (n=7), panic disorder (n=15), agoraphobia (n=8), specific phobia (n=23), PTSD = posttraumatic stress disorder, ADHD = attention-deficit/hyperactivity disorder, IED = intermittent explosive disorder, OCD = obsessive-compulsive disorder, ODD = oppositional defiant disorder
Procedure
During the first study visit, parents provided informed consent and adolescents assented. Participants were administered clinical and neurophysiological assessments (see detailed procedures13). Demographic data were obtained via parent report, along with adolescents’ current psychiatric medication use. Adolescents completed two subtests (Verbal Comprehension and Perceptual Reasoning) of the Wechsler Abbreviated Scale of Intelligence71 to estimate normative Full-Scale IQ. IQ was missing for one participant and was replaced by the sample mean. Pubertal status was determined based on 5-item (1–5 Likert scale) adolescent-report of their relative physical development reflecting primary- and secondary-sex characteristics.72 Scores were averaged across items and rounded. As most participants received a score of 4+ (n=97), scores were binarized as 4+ vs. <4 (stage 1 n=1; stage 2 n=4; stage 3 n=90; missing n=1). The Chapman Handedness Inventory73 assessed adolescents’ lateral-hand dominance; these data were binarized to reflect right-handedness or not. For the second study visit, neuroimaging data were acquired at the Martinos Center. Participants also completed a 6-month follow-up assessment of current depression and anxiety symptoms. Participants were remunerated $70 for the baseline clinical assessment, $50/hour for the MRI, and $25 for the 6-month follow-up.
Clinical Assessment Protocol
Each adolescent-parent dyad completed the Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version (K-SADS74) to assess lifetime mental disorders. Diagnostic criteria were adapted to assess DSM-5 disorders (see13). Inter-rater reliability for anxiety and depression diagnoses assessed in a randomly selected subset (~10%) was in the moderate to substantial range13 and exceeded inter-rater agreement for the DSM-5 field trials for depression and anxiety.75 Adolescents completed the Mood and Feelings Questionnaire (MFQ76), assessing depression symptom severity, which showed excellent internal consistency at baseline (α=.96) and 6-month follow-up (α=.95). Adolescents also completed the Revised Child Anxiety and Depression Scale (RCADS77). The primary subscales of interest characterized generalized anxiety (baseline α=.87; 6-month α=.87) and social anxiety symptoms (baseline α=.91; 6-month α=.92). Secondary analyses also tested cross-sectional relationships with adolescent self-report RCADS77 panic symptoms (α=.91), trait anxiety (State-Trait Anxiety Inventory-Trait Subscale78; α=.91), anhedonia symptoms (SHAPS79; α=.82), and Behavioral Inhibition and Behavioral Activation System80 scores (Inhibition subscale: α=.84; Drive subscale α=.72; Fun-Seeking subscale: α=.58; Reward Responsiveness subscale: α=.74).
Incentive Processing Task (IPT)
Participants completed two runs (2min 52s per run; 5min 44s total) of a block-design IPT to probe neural processing of monetary rewards.68,69 Before the task, participants were reminded that their responses would result in winning or losing actual money. On each trial, participants saw a question mark cue (1.5s) indicating that they should guess via index- or middle-finger button press whether a to-be-revealed number (between 1–9) was greater than or less than 5 (Figure S2, available online). Participants then received an image (1s) of the actual number and visual feedback regarding whether they guessed correctly. Feedback was pseudo-randomized and not contingent on actual performance. Feedback consisted of reward trials (green, upward-facing arrow and “+$1”, indicating that participants had $1 added to their task winnings), punishment trials (red, downward-facing arrow and “−$0.50”, indicating that the participants had $0.50 taken from their task winnings), or neutral trials (grey, double-headed, horizontal arrow if the number that was revealed was 5). This was followed by a 1s inter-trial interval. Trials were arranged in blocks of 8 trials (28s) interleaved with 15s fixation blocks within a given run. Block conditions were balanced and pseudo-randomized across each run. Blocks consisted of either a majority reward or for majority punishment blocks, such that 6/8 trials were reward trials interspersed with 1 neutral and 1 punishment trial, 2 neutral trials, or 2 punishment trials, or vice versa for majority punishment blocks. Participants responding to fewer than 70% of trials or showing greater than 90% response bias for a single-button response were excluded.
MRI Acquisition and Processing
Comprehensive information on MRI hardware, sequences, and acquisition harmonization with other HCP studies are described elsewhere.14 Briefly, data were collected on a Siemens 3T Prisma MRI using a 64-channel head coil, using 52 head elements. One high-resolution, multi-echo magnetization-prepared rapid gradient echo (MPRAGE) T1w and one high-resolution T2w image were acquired (0.8 mm isotropic voxels, 208 slices, FOV = 256 × 240 × 167 mm, T1w: TR/TE=2400/2.18 ms, T2w: TR/TE=3200/564 ms) along with a vNav setter for prospective motion correction.81 The vNav-enabled sequences estimate motion throughout the structural scans and reacquires/replaces k-space data unduly affected by motion (mean motion was used as a covariate in structural analyses). Task-fMRI images were acquired using 2-D multiband, gradient-recalled echo-planar imaging (2.0 mm isotropic voxels, 72 slices, multiband acceleration factor=8, TR/TE=800/37 ms, flip angle=52°). Task data were acquired in two runs with different phase encoding directions (ie, anterior-posterior [AP], posterior-anterior [PA]).
MRI data were processed per HCP guidelines using the HCP minimally preprocessed pipeline v3.26.1 (github.com/Washington-University/Pipelines/releases/tag/v3.26.1). Briefly, PreFreeSurfer, FreeSurfer, PostFreeSurfer workflows were used to process structural images; gradient nonlinearity distortion correction, bias-field corrections, a high-pass spatial filter, nonlinear transformation and normalization, and segmentation/parcellation were applied. Structural surface registration used the Multimodal Surface Matching algorithm (MSMSulc). Subcortical and intracranial volumes were extracted from this FreeSurfer v6.0 processing. Any volume outliers >3SD from the mean were Winsorized to the next most extreme non-outlier value.
Task fMRI data were processed using fMRIVolume and fMRISurface pipelines; preprocessing steps included gradient nonlinearity distortion correction, rigid-body motion correction, dual-phase encoded spin-echo distortion correction, T1w-alignment, MNI spatial normalization, and BOLD signal intensity normalization by the average, whole-brain time series. Task fMRI data were placed in grayordinate space (registered to 32k_fs_LR mesh). A spatial filter was applied so that higher-noise voxels (greater than .5 SDs above mean coefficient of variation within a 5mm Gaussian neighborhood) were excluded from surface maps. Grayordinate space data were smoothed (2 mm FWHM; in volume-space subcortically and on the mesh cortical surface) on the mesh surface and subjected to a high-pass filter of 0.005 Hz. Participant-level GLMs were modeled in FSL using a double-gamma hemodynamic response function convolved with block boxcar function by type (reward or punishment) and including regressors for head motion parameter estimates. An additional 2 mm smoothing kernel was applied to GLM outputs for a final smoothing of 4 mm. One sample t-tests were run to display group-level activation maps across participants. Contrast activation was extracted from anatomically defined nucleus accumbens ROIs (Harvard-Oxford subcortical atlas) from the volume-space data in the CIFTI format output.
Analysis
Participant characteristics.
Data analysis was performed in Rv3.6.3. Group differences among healthy and depressed-anxious adolescents were tested using t-tests for continuous variables and χ2 tests for categorical variables.
Structural MRI.
Linear regression analyses examined differences between healthy and depressed-anxious participants in subcortical volume, controlling for age, sex, IQ, pubertal status, handedness, ADHD, medication usage, and head motion during the structural scan. Intracranial volume (ICV) was first examined to test group differences in global volume. Although hypotheses focused on specific subcortical regions, we tested all subcortical volumes (left/right average) to ensure specificity of findings while also controlling for ICV and False Discovery Rate (FDR) correcting for 7 tests (ie, amygdala, caudate, hippocampus, nucleus accumbens, pallidum, putamen, thalamus). Standardized regression coefficients (b) are presented. Effect size estimates for group differences, adjusting for covariates, were calculated as Cohen’s d from regression results.82 Follow-up subgroup analyses compared anxiety disorders only (n=72; participants met criteria for generalized anxiety disorder, social phobia, separation anxiety, panic disorder, agoraphobia and/or specific phobia but did not report a depressive disorder), depressed-anxious (n=57; n=11 depressive disorder only and n=48 comorbid diagnoses of depression and anxiety) versus healthy controls (n=64). Sensitivity analyses tested group differences after excluding all medicated depressed-anxious participants. Supplemental analyses examined cortical thickness across Destrieux atlas ROIs.83
Functional MRI.
Given a priori interest in reward-related nucleus accumbens reactivity and focal activity in this area for the Reward-Punishment contrast (Figure S3, available online), we extracted accumbens activation averaged within an anatomically defined ROI for Reward-Baseline, Punishment-Baseline, and Reward-Punishment activity. Linear regression analyses examined group differences in activation, controlling for age, sex, IQ, pubertal status, handedness, ADHD, medication usage as well as well as head motion (average root mean square motion, averaged across runs). Follow-up subgroup and sensitivity analyses excluding medication use were performed. Exploratory whole-brain CIFTI-space analyses were performed in FSL PALM using TFCE (500 iterations, tail acceleration) while controlling for the same covariates.
Multimodal Prediction.
Finally, we tested the additive value of brain volume and IPT brain activation in predicting longitudinal change in symptoms. Specifically, we ran stepwise regression models predicting depression (MFQ) and anxiety symptom (RCADS) scores at the 6-month follow-up. Initial models included baseline symptoms, age, sex, IQ, pubertal status, handedness, ADHD, medication, and head motion during sMRI and fMRI. The second models added average nucleus accumbens volume. The third models added average accumbens Reward-Baseline activation.
Results
Structural MRI data (N=193).
Relative to healthy adolescents (n=64), depressed-anxious participants (n=129) showed slightly smaller ICV (b=−0.34, t(182)=−2.25, p=.03, d=−0.34). Accordingly, ICV was included as a covariate in all subsequent tests examining subcortical volume. Compared to healthy controls, depressed-anxious adolescents had reduced total subcortical gray matter volume (b=−0.22, t(181)=−2.04, p=.04, d=−0.32), controlling for ICV and all covariates. Notably, this effect was observed in individual subcortical regions (Table 3; Figure 1A), including the amygdala and nucleus accumbens (Figure 1B), although only nucleus accumbens volume differences survived FDR corrections for multiple comparisons. In this model (Table S2, available online), larger accumbens volumes related to larger ICV (b=0.46, t(181)=5.86, p<.001) and showed a trend-level non-significant association with medication use (b=0.29, t(181)=1.88, p=.06); no other covariates were significant. Subsequent analyses found that relative to healthy youth, depressed-anxious adolescents had reduced accumbens volume in both the left (b=−0.49, t(181)=−2.89, p=.004) and right hemisphere (b=−0.47, t(181)=−2.89, p=.004), with no significant hemisphere by group interaction (b=−0.09, t(369)=−0.95, p=.34). Further sensitivity analyses indicated that the effect of reduced accumbens volume in the depressed-anxious versus healthy youth remained significant when excluding all depressed-anxious participants reporting medication use (b=−0.42, t(107)=−2.55, p=.012; N=117). Interestingly, reduced accumbens volume correlated with greater depression severity (r=−.23, p<.01), trait anxiety (r=−.22, p<.01), and state anxiety (GAD (r=−.18, p<.05), social (r=−.26, p<.001), and panic symptoms (r=−.21, p<.01); Table S3, available online). Further, greater accumbens volume associated with enhanced motivational drive (r=.17, p<.05) and fun-seeking (r=.18, p<.01) whereas reduced volume correlated with greater behavioral inhibition (r=−.24, p<.05).
Table 3.
Subcortical Volume Differences in Healthy Controls (n=64) versus Adolescents Diagnosed with Depressive and/or Anxiety Disorders (n=129)
| B | t(182) | p | d | % Diff. | |
|---|---|---|---|---|---|
| Thalamus | −0.10 | −0.78 | .44 | −0.12 | 0.96% |
| Caudate | −0.14 | −0.83 | .40 | −0.13 | 1.60% |
| Putamen | −0.19 | −1.20 | .23 | −0.20 | 2.34% |
| Pallidum | −0.22 | −1.51 | .13 | −0.24 | 2.27% |
| Hippocampus | −0.16 | −1.09 | .28 | −0.18 | 1.57% |
| Amygdala | −0.29 | −2.04 | .04 | −0.33 | 3.39% |
| Nucleus accumbens | −0.51 | −3.20 | .001 * | −0.51 | 8.32% |
Note. Results indicate group difference effects from regression models controlling for intracranial volume, age, sex, IQ, pubertal status, handedness, ADHD, medication, and head motion during the T1. B values represent standardized regression coefficients. Negative B, t-statistic, and d values indicate smaller subcortical volumes among adolescents diagnosed with depressive and/or anxiety disorders vs. healthy participants. The % Diff column indicates the percent difference between groups in estimated marginal means of volume. Volumes represent averages across left and right hemispheres. Boldface type indicates significant effects.
FDR-corrected p-value<.05.
Figure 1. Group Differences in Subcortical Volumes and Nucleus Accumbens Activation.
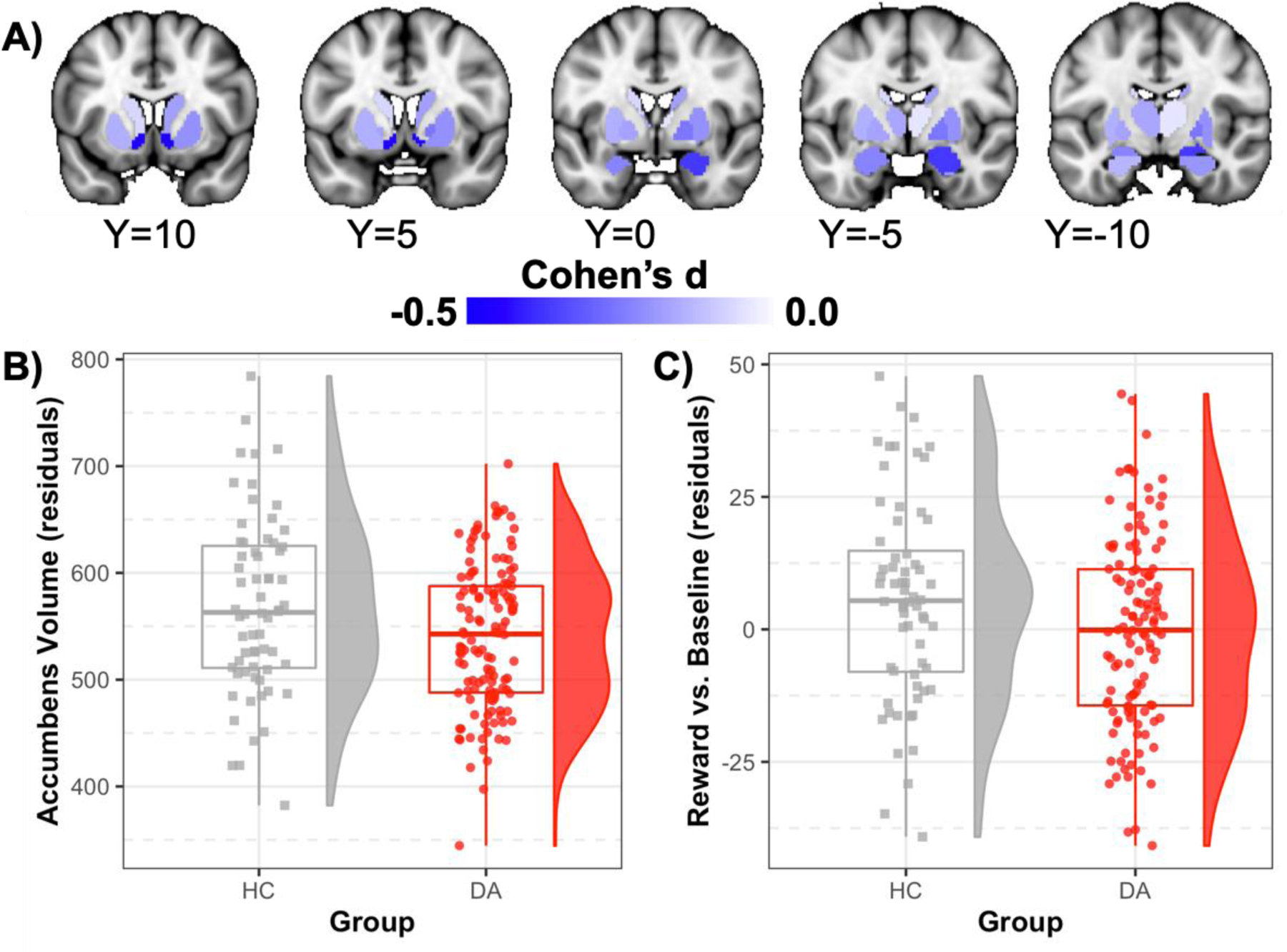
Note. Panel A displays d effect size values for all subcortical regions from the regression models denoted in the main text controlling for intracranial volume, age, sex, IQ, pubertal status, handedness, ADHD, medication, and head motion during the T1. These values are derived from models examining left and right hemisphere volume separately. Negative Cohen’s d values indicate smaller volume among adolescents diagnosed with depressive and/or anxiety disorders versus healthy control subjects. Panel B displays scatterplots, boxplots, and density plots for differences in average accumbens volumes (residualized for all covariates) for healthy (HC; grey squares) and depressed-anxious participants (DA; red circles). Panel C scatterplots, boxplots, and density plots for differences in average accumbens activity to the Reward-Baseline contrast (residualized for all covariates) for healthy (HC; grey squares) and depressed-anxious participants (DA; red circles).
Follow-up subgroup analyses indicated that both the anxiety-only (b=−0.50, t(180)=−3.00, p=.003) and anxious-depressed (b=−0.54, t(180)=−2.80, p=.006) subgroups exhibited comparably lower accumbens volume relative to healthy controls. Interestingly, trend-level reduced amygdala volume was found for the anxiety-only (b=−0.30, t(180)=−2.02, p=.04) but not the depressed-anxious (b=−0.27, t(180)=−1.58, p=.12) subgroup. Group differences in regional cortical thickness are presented in Table S4 and Figure S4, available online).
Functional MRI data (N=184).
Depressed-anxious (n=120) participants exhibited blunted accumbens Reward-Baseline contrast activation compared to healthy controls (n=64), (b=−0.47, t(173)=−2.51, p=.01, d=−0.40; Table S2, available online, Figure 1C). This effect was observed in the right hemisphere (b=−0.50, t(173)=−2.62, p=.01, d=−0.42) with a non-significant trend in the left hemisphere (b=−0.36, t(173)=−1.90, p=.06, d=−0.30). Among all covariates, significant or non-significant trend-level effects were observed such that males (b=0.40, t(173)=2.52, p=.02), right-handedness (b=0.33, t(173)=1.92, p=.06), and comorbid ADHD (b=0.38, t(173)=1.83, p=.07) were characterized by greater accumbens response to Reward-Baseline. We also re-ran the model excluding all participants reporting current medication use, and similarly, we found that depressed-anxious youth reported blunted activation in the accumbens relative to healthy youth (b=−0.47, t(103)=−2.38, p=.019; N=112). Highlighting specificity, no significant between-group effects emerged for Punishment-Baseline (b=−0.15, t(173)=−0.77, p=.44, d=−0.12) or Reward versus Punishment (b=−0.21, t(173)=−1.10, p=.27, d=−0.18) (Figure S5, available online). Accumbens activation for the Reward-Baseline contrast showed no associations with baseline symptom or trait measures (ps>.05; Table S3, available online).
Follow-up subgroup analyses indicated slightly stronger blunting of accumbens Reward-Baseline response among the anxiety-only subgroup (b=−0.50, t(172)=−2.53, p=.01) with a trend-level non-significant effect among the depressed-anxious subgroup (b=−0.41, t(172)=−1.83, p=.07) relative to healthy controls. Exploratory analyses of whole-brain group differences did not pass stringent TFCE correction.
Multimodal Prediction.
Additional analyses tested factors that predicted depression severity at the 6-month follow-up, controlling for baseline symptoms in all models. Of the participants (n=177) with all baseline and both accumbens structure and function data, 32 participants (depressed-anxious=27; healthy controls=5) were missing 6-month follow-up data (remaining n=145). Although specific factors related to attrition (pubertal status, depression symptoms, social anxiety symptoms, fMRI motion; Table S5, available online), these were accounted for in subsequent analyses. In the initial model, baseline depression symptoms were highly predictive of 6-month follow-up depression symptoms (MFQ; b=0.45, t(133)=5.70, p<.001). There was a smaller effect of sex (b=−0.43, t(133)=−2.18, p=.03), and no other covariates emerged as significant predictors. In the second model, we examined the additive predictive power of average nucleus accumbens volume, which significantly improved the prediction of 6-month depressive symptoms (b=-0.18, t(132)=-2.25, p=.03; Figure 2), as smaller volume predicted worsening symptoms at the 6-month follow-up. Notably, accumbens volume remained a significant predictor of depression symptoms (b=−0.17, t(130)=−2.25, p=.03) when also controlling for both generalized and social anxiety symptoms. In the third model, there was no significant added predictive power including Reward-Baseline accumbens activation to the model (b=0.01, t(131)= 0.19, p=.85). Figure 2 includes the incremental R2-values obtained after including each predictor of depressive symptoms. Importantly, the significant structural and non-significant functional accumbens effect remained whether structure preceded or followed functional results in the model building, and the second model (covariates+accumbens volume) exhibited the lowest AIC (346.83) and BIC (388.50) compared to the initial (AIC=350.28; BIC=388.98) and third models (AIC=348.78; BIC=393.44).
Figure 2. Multimodal Prediction Model Fit.
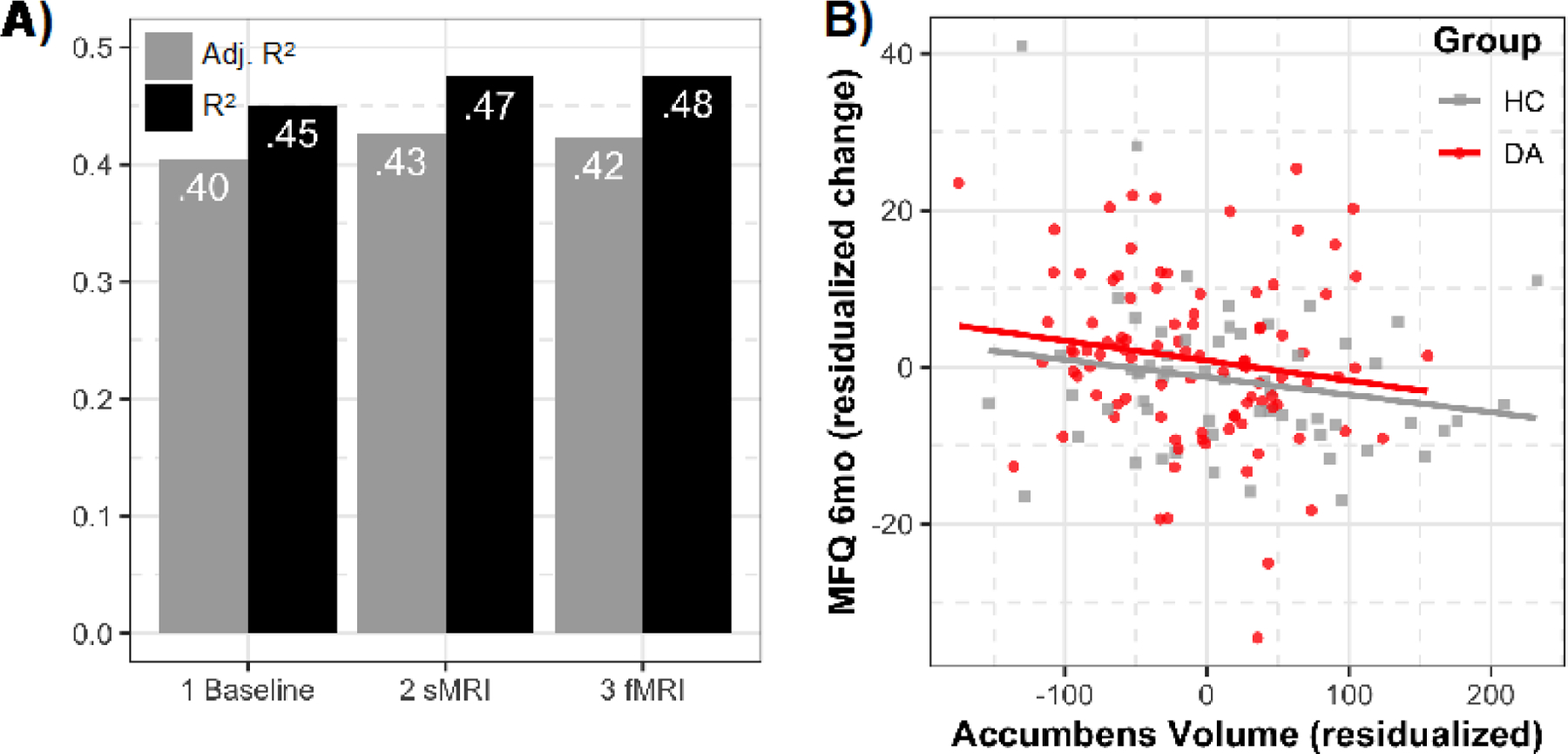
Note. Panel A presents adjusted R2 (gray) and R2 (black) values for the three models tested in the multimodal prediction section predicting MFQ (Mood and Feelings Questionnaire) depression scores at the 6-month follow-up. The first model includes baseline MFQ scores and all covariates. The second model adds average accumbens volumes (sMRI). The third model adds average accumbens Reward vs. Baseline activation (fMRI). Panel B displays the association between residualized accumbens volumes and residualized MFQ (Mood and Feelings Questionnaire) depression scores from the 6-month follow-up, also residualized for all covariates including baseline depression. These represent results from the multimodal prediction model 2 (sMRI) indicating that smaller accumbens volume predict worsening depression symptoms at 6-month follow-up. HC = healthy controls; DA = depressed-anxious.
We also conducted an exploratory LASSO regression (R glmnet package, 10-fold cross-validation) to examine a regularized weighting of the factors in the third model (down-weighting and removing small coefficients to reduce model complexity and collinearity). This method suggested retaining seven variables in predicting 6-month MFQ scores: baseline MFQ, age, sex, puberty, ADHD comorbidity, medication use, and accumbens volume (ie, removing ICV, IQ, handedness, head motion, accumbens function; AIC=339.11, BIC=365.90, R2=.448). Accumbens volume remained a significant predictor in this reduced model (p=.03).
Similarly, we tested multimodal prediction of anxiety symptom severity at the 6-month follow-up (n=143). Neither of the predictors of interest (accumbens volume, accumbens Reward-Baseline activation) significantly predicted RCADS generalized anxiety or social anxiety symptoms at follow-up (ps>.05), controlling for baseline anxiety and other covariates. Of note, amygdala volume also did not predict 6-month symptoms over and above baseline scores (ps>.05; MFQ, RCADS social anxiety, and RCADS generalized anxiety).
Discussion
The Research Domain Criteria initiative has underscored the importance of developing new ways of classifying mental illness based on multimodal assessment of underlying neural dimensions. The Positive Valence Systems, in particular, provide a useful heuristic to probe reward-related constructs that are central to anhedonia. Accordingly, our Human Connectome project sought to characterize potential reward-related biological markers that may cut across traditional diagnostic boundaries among depressed-anxious youth. Our results indicated that relative to healthy participants, depressed-anxious adolescents exhibited reduced nucleus accumbens volumes and activation following reward receipt. Multimodal models suggested that structural alterations, but not reward-related functional activation, prospectively predicted depression symptoms, above and beyond baseline depression and anxiety symptoms.
Impaired Reward Processing and Clinical Course
Although depression and anxiety disorders frequently co-occur,1–3 particularly when accounting for subthreshold symptoms, a substantive corpus of research has focused on identifying neural correlates that are specific to each disorder. Yet, clarifying multimodal biological dimensions for depressed-anxious youth may, ultimately, prove more fruitful for improving clinical outcomes. Our findings highlighted considerable convergence of impaired reward processing, which may stem from structural and functional alterations within the nucleus accumbens. Impairments within the striatum have been linked to anhedonia20,84—a transdiagnostic phenotype that is more commonly associated with depression20 but also more recently, anxiety.85 The presence of anhedonia and associated neural alterations are important to consider given that current medication approaches insufficiently address motivational and reward-processing deficits that characterize anhedonia, and thus, treatment failure is common.86–88 Similarly, in psychotherapy, anhedonia severity is predictive of greater dropout, poor treatment response, and higher rates of recurrence.29,89,90 In light of this evidence, it is unsurprising that anhedonia and associated reward impairments are closely related to suicide behaviors among adolescents.91 More broadly, it suggests that reward alterations may reflect a common factor in psychopathology given known etiological roles in other mental disorders including substance use, bipolar disorder, and psychosis,92 which is consistent with recent research that has identified transdiagnostic neurofunctional markers associated with disruptions in emotional processing.93
Increasingly, taxometric research has demonstrated that examining internalizing disorders as dimensional constructs as opposed to discrete categories defined by the Diagnostic and Statistical Manual could aid research and treatment.94–98 Accordingly, recent psychotherapeutic approaches are modifying targets to align with this shift. For example, a randomized control trial administered a positive affect treatment focused on improving reward sensitivity—directly targeting anhedonia—among depressed-anxious individuals. Patients were recruited based on dimensional depression and anxiety cutoffs (as opposed to diagnoses). Preliminary results for the positive affect treatment—relative to a negative affect treatment, which was a combination of exposure and cognitive behavioral skills—demonstrated reduced depression and anxiety symptoms, improved positive affect, and reduced suicidal ideation.90 Similarly, the unified protocol for transdiagnostic treatment is rooted in the belief that there is considerable symptom overlap across disorders and thus, targets core temperamental characteristics that are common to anxiety, depression, and related disorders.99,100 Overall, as treatment response has not markedly improved in recent decades, particularly in depression,101–103 these innovative approaches afford the promise that the shift toward targeting transdiagnostic markers may optimize clinical outcomes.
Multimodal Modeling and the Promise of Clinical Translation
Although we were able to leverage multimodal data to characterize depressive and anxious disorders in youth, a key challenge was how to integrate these data in the service of clinical translation. Our findings showed that reduced accumbens volume but not reward-related accumbens activation was cross-sectionally associated with depression and anxiety symptom severity, and interestingly, greater accumbens volume related to both motivational and anticipatory (ie, fun-seeking) processes. Of note, reduced accumbens volume also prospectively predicted depression symptoms over time. These findings have several implications but also raise important questions. For example, it is not evident why reduced accumbens volume matters. One possibility may be that reduced volume translates to fewer available dopamine receptors, which could diminish dopaminergic inputs from the substantia nigra and ventral tegmental area and potentially impact learning (ie, prediction error).18,62 Impaired reward learning and responsiveness may then contribute to treatment non-response given difficulties integrating adaptive coping strategies, thereby contributing to the persistence of debilitating symptoms. Alternatively, it may be that functional processes more readily reflect state-based effects, which are influenced by an adolescent’s current emotions. By contrast, volume alterations may be more trait-like scars stemming from the experience of chronic symptoms, medication use, and early life adversity. Whether the development and use of pharmacological agents that selectively enhance neuromaturation in specific brain regions would normalize reward-related processes implicated in depression and anxiety remains unclear; however, it would seem that clarifying why volume matters may yield important next steps for future interventions.
Although results showed neurofunctional group differences, accumbens activation did not improve the prediction of depressive and anxious symptoms over time. An inherent challenge with assessing reward-related behaviors in adolescents is whether or not the stimuli are in fact rewarding. Within the IPT, correct responses resulted in money earned. That said, money accumulated is a secondary reward that may be experienced and processed differently than immediate receipt of primary rewards. This conceptual issue as to what is rewarding is important to consider in the backdrop of psychometric concerns regarding fMRI data. Namely, there are many commonly used reward tasks, including within the Human Connectome projects, that have demonstrated poor internal consistency and test-rest reliability.104 Our null longitudinal findings with a consummatory reward task may underscore the importance of addressing conceptual and psychometric challenges.
Summary and Future Directions
There are several limitations that should be noted. First, given the inclusion of comorbid depressed-anxious youth, medication use was common. Medication use has known effects on structural37,47 and functional105 neural correlates implicated in reward processing. Additionally, although current medication use was obtained, we did not assess lifetime medication, which may impact neurodevelopment and the course of symptoms over time. We also did not obtain information on lifetime psychosocial treatment, which may impact symptom trajectories. Second, the IPT is a block design, which is well-suited to capture accumbens activation following reward outcomes. However, additional metrics core to reward (eg, anticipation, prediction error) and affective disorders were not tested. Third, although the transdiagnostic sample is a strength as it improves generalizability, it may be that specific disorders differentially impact activation patterns. For example, our results show a non-significant trend that ADHD was related to greater accumbens response in the Reward-Baseline contrast; accordingly, future work may consider the risk-benefit of including ADHD in studies focused on elucidating neural circuitry within internalizing disorders. Similarly, it may be that prodromal bipolar disorder or subthreshold mania symptoms in general could impact our understanding of reward-related activation in depressed-anxious youth. Last, the present study focused on whether accumbens structure and function contributed to the persistence of depression and anxiety symptoms, however, a compelling future direction, which is consistent with RDoC initiatives, would be to derive latent measures of positive or negative valence.
In summary, comorbidity is the rule rather than the exception, and given the recent taxometric realignment,11,12 it is critical to identify brain-behavior markers that cut across traditional diagnostic categories. Accordingly, our multimodal findings highlight alterations—across nucleus accumbens structure and function—that characterize depressed-anxious adolescents. Only reduced nucleus accumbens volume, however, predicted depressive symptoms over time, and thus, an important next step will be to clarify why structural abnormalities impact reward-related processes and associated symptoms.
Supplementary Material
Acknowledgments
This project was supported by the National Institute of Mental Health (NIMH) U01MH108168 (J.D.E.G., S.W.G.). R.P.A. and D.P. were partially supported by R01MH119771 and R56MH121426. D.A.P. was partially supported by R37MH068376 and R01MH101521. N.A.H. received partial support through F32MH114525, P20GM130461(6026), and the Brain and Behavior Research Foundation. A.Y. was partially supported by R01EB021265, U01EB026996, and R56MH121426. J.D.E.G., S.W.G., N.A.H., I.F., and N.L. were partially supported by the Poitras Center for Psychiatric Disorders Research at the McGovern Institute for Brain Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or of any other sponsor.
Disclosure:
Dr. Auerbach has served on the scientific advisory board for Ksana Health. Dr. Hofmann has received financial support from the Alexander von Humboldt Foundation, the National Center for Complementary and Integrative Health, NIMH, and the James S. McDonnell Foundation 21st Century Science Initiative in Understanding Human Cognition - Special Initiative. He has received compensation for his work as editor from Springer Nature and the Association for Psychological Science, and as an advisor from the Palo Alto Health Sciences Otsuka Pharmaceuticals, and for his work as a Subject Matter Expert from John Wiley and Sons, Inc. and SilverCloud Health, Inc. He has received royalties and payments for his editorial work from various publishers. Dr. Pizzagalli has received funding from the Millennium Pharmaceuticals and consulting fees from BlackThorn Therapeutics, Boehreinger Ingelheim, Compass Pathway, Concert Pharmaceuticals, Engrail Therapeutics, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals; one honorarium from Alkermes; and stock options from BlackThorn Therapeutics. Drs. Pagliaccio, Hubbard, Siless, Henin, Gabrieli, Yendiki, Whitfield-Gabrieli, Mss. Frosch, Kremens, Cosby, Mr. Jones, and Ms. Lo have reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry. 2015;54(1):37–44 e32. 10.1016/j.jaac.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Avenevoli S, McLaughlin KA, et al. Lifetime co-morbidity of DSM-IV disorders in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A). Psychol Med. 2012;42(9):1997–2010. 10.1017/S0033291712000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merikangas K, Avenevoli S, Costello J, Koretz D, Kessler RC. National comorbidity survey replication adolescent supplement (NCS-A): I. Background and measures. J Am Acad Child Adolesc Psychiatry. 2009;48(4):367–369. 10.1097/CHI.0b013e31819996f1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen JR, Andrews AR, Davis MM, Rudolph KD. Anxiety and depression during childhood and adolescence: Testing theoretical models of continuity and discontinuity. J Abnorm Child Psychol. 2018;46(6):1295–1308. 10.1007/s10802-017-0370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angold A, Costello EJ, Erkanli A. Comorbidity. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 1999;40(1):57–87. [PubMed] [Google Scholar]
- 6.Avenevoli S, Stolar M, Li J, Dierker L, Merikangas KR. Comorbidity of depression in children and adolescents: Models and evidence from a prospective high-risk family study. Biol Psychiatry. 2001;49(12):1071–1081. 10.1016/s0006-3223(01)01142-8 [DOI] [PubMed] [Google Scholar]
- 7.Yorbik O, Birmaher B, Axelson D, Williamson DE, Ryan ND. Clinical characteristics of depressive symptoms in children and adolescents with major depressive disorder. J Clin Psychiatry. 2004. 10.4088/JCP.v65n1210 [DOI] [PubMed] [Google Scholar]
- 8.Axelson DA, Birmaher B. Relation between anxiety and depressive disorders in childhood and adolescence. Depress Anxiety. 2001;14(2):67–78. 10.1002/da.1048 [DOI] [PubMed] [Google Scholar]
- 9.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837–844. 10.1001/archpsyc.60.8.837 [DOI] [PubMed] [Google Scholar]
- 10.Van Voorhees BW, Melkonian S, Marko M, Humensky J, Fogel J. Adolescents in primary care with sub-threshold depressed mood screened for participation in a depression prevention study: co-morbidity and factors associated with depressive symptoms. The open psychiatry journal. 2010;4:10. [PMC free article] [PubMed] [Google Scholar]
- 11.Brown TA, Naragon-Gainey K. Evaluation of the unique and specific contributions of dimensions of the triple vulnerability model to the prediction of DSM-IV anxiety and mood disorder constructs. Behav Ther. 2013;44(2):277–292. 10.1016/j.beth.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown TA. Temporal course and structural relationships among dimensions of temperament and DSM-IV anxiety and mood disorder constructs. J Abnorm Psychol. 2007;116(2):313. 10.1037/0021-843X.116.2.313 [DOI] [PubMed] [Google Scholar]
- 13.Hubbard NA, Siless V, Frosch IR, et al. Brain Function and Clinical Characterization in the Boston Adolescent Neuroimaging of Depression and Anxiety Study. NeuroImage: Clinical. 2020. 10.1016/j.nicl.2020.102240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siless V, Hubbard NA, Jones R, et al. Image Acquisition and Quality Assurance in the Boston Adolescent Neuroimaging of Depression and Anxiety Study. NeuroImage: Clinical. 2020. 10.1016/j.nicl.2020.102242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auerbach RP, Admon R, Pizzagalli DA. Adolescent depression: stress and reward dysfunction. Harv Rev Psychiatry. 2014;22(3):139–148. 10.1097/HRP.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caouette JD, Guyer AE. Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Developmental cognitive neuroscience. 2014;8:65–76. 10.1016/j.dcn.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keren H, O’Callaghan G, Vidal-Ribas P, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. American Journal of Psychiatry. 2018;175(11):1111–1120. 10.1176/appi.ajp.2018.17101124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. 10.1126/science.275.5306.15 [DOI] [PubMed] [Google Scholar]
- 19.Zald DH, Boileau I, El-Dearedy W, et al. Dopamine transmission in the human striatum during monetary reward tasks. Journal of Neuroscience. 2004;24(17):4105–4112. 10.1523/JNEUROSCI.4643-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Admon R, Pizzagalli DA. Dysfunctional reward processing in depression. Current Opinion in Psychology. 2015;4:114–118. 10.1016/j.copsyc.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyer AE, Benson B, Choate VR, et al. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Dev Psychopathol. 2014;26(1):229. 10.1017/S0954579413000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyer AE, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26(24):6399–6405. 10.1523/JNEUROSCI.0666-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmaal L, Veltman DJ, van Erp TG, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016;21(6):806. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagliaccio D, Alqueza KL, Marsh R, Auerbach RP. Brain volume abnormalities in youth at high risk for depression: adolescent brain and cognitive development study. Journal of the American Academy of Child & Adolescent Psychiatry. 2019. DOI: 10.1016/j.jaac.2019.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milham MP, Nugent AC, Drevets WC, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: A voxel-based morphometry investigation. Biol Psychiatry. 2005;57(9):961–966. DOI: 10.1016/j.biopsych.2005.01.038 [DOI] [PubMed] [Google Scholar]
- 27.Massana G, Serra-Grabulosa JM, Salgado-Pineda P, et al. Amygdalar atrophy in panic disorder patients detected by volumetric magnetic resonance imaging. Neuroimage. 2003;19(1):80–90. DOI: 10.1016/s1053-8119(03)00036-3 [DOI] [PubMed] [Google Scholar]
- 28.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. DOI: 10.1126/science.1100907 [DOI] [PubMed] [Google Scholar]
- 29.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35(3):537–555. DOI: 10.1016/j.neubiorev.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosso IM, Cintron CM, Steingard RJ, Renshaw PF, Young AD, Yurgelun-Todd DA. Amygdala and hippocampus volumes in pediatric major depression. Biol Psychiatry. 2005;57(1):21–26. doi: 10.1016/j.biopsych.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Saleh K, Carballedo A, Lisiecka D, et al. Impact of family history and depression on amygdala volume. Psychiatry Res. 2012;203. doi: 10.1016/j.pscychresns.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 32.Kronenberg G, van Elst LT, Regen F, Deuschle M, Heuser I, Colla M. Reduced amygdala volume in newly admitted psychiatric in-patients with unipolar major depression. J Psychiatr Res. 2009;43(13):1112–1117. doi: 10.1016/j.jpsychires.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 33.MacMaster FP, Kusumakar V. Hippocampal volume in early onset depression. BMC medicine. 2004;2(1):2. doi: 10.1186/1741-7015-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caetano SC, Fonseca M, Hatch JP, et al. Medial temporal lobe abnormalities in pediatric unipolar depression. Neurosci Lett. 2007;427(3):142–147. doi: 10.1016/j.neulet.2007.06.01 [DOI] [PubMed] [Google Scholar]
- 35.Jaworska N, Yücel K, Courtright A, MacMaster FP, Sembo M, MacQueen G. Subgenual anterior cingulate cortex and hippocampal volumes in depressed youth: the role of comorbidity and age. J Affect Disord. 2016;190:726–732. doi: 10.1016/j.jad.2015.10.06 [DOI] [PubMed] [Google Scholar]
- 36.MacMaster FP, Mirza Y, Szeszko PR, et al. Amygdala and hippocampal volumes in familial early onset major depressive disorder. Biol Psychiatry. 2008;63(4):385–390. doi: 10.1016/j.biopsych.2007.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516–1518. doi: 10.1176/appi.ajp.160.8.1516 [DOI] [PubMed] [Google Scholar]
- 38.Frodl T, Meisenzahl EM, Zetzsche T, et al. Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during a 1-year follow-up. J Clin Psychiatry. 2004. doi: 10.4088/jcp.v65n0407 [DOI] [PubMed] [Google Scholar]
- 39.Vasic N, Walter H, Höse A, Wolf RC. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. J Affect Disord. 2008;109(1–2):107–116. DOI: 10.1016/j.jad.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 40.Janssen J, Pol HEH, Lampe IK, et al. Hippocampal changes and white matter lesions in early-onset depression. Biol Psychiatry. 2004;56(11):825–831. DOI: 10.1016/j.biopsych.2004.09.011 [DOI] [PubMed] [Google Scholar]
- 41.Matsuo K, Rosenberg DR, Easter PC, et al. Striatal volume abnormalities in treatment-naive patients diagnosed with pediatric major depressive disorder. J Child Adolesc Psychopharmacol. 2008;18(2):121–131. doi: 10.1089/cap.2007.0026. [DOI] [PubMed] [Google Scholar]
- 42.Arnone D, McKie S, Elliott R, et al. State-dependent changes in hippocampal grey matter in depression. In: Mol Psychiatry. 2012. doi: 10.1038/mp.2012.150 [DOI] [PubMed] [Google Scholar]
- 43.Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138(1–2):9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 44.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30(11):3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parashos IA, Tupler LA, Blitchington T, Krishnan KRR. Magnetic-resonance morphometry in patients with major depression. Psychiatry Research: Neuroimaging. 1998;84(1):7–15. DOI: 10.1016/s0925-4927(98)00042-0 [DOI] [PubMed] [Google Scholar]
- 46.Husain MM, McDonald WM, Doraiswamy PM, et al. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Research: Neuroimaging. 1991;40(2):95–99. DOI: 10.1016/0925-4927(91)90001-7 [DOI] [PubMed] [Google Scholar]
- 47.Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13(11):993. DOI: 10.1038/mp.2008.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34. [PMC free article] [PubMed] [Google Scholar]
- 49.Blackmon K, Barr WB, Carlson C, et al. Structural evidence for involvement of a left amygdala-orbitofrontal network in subclinical anxiety. Psychiatry Research: Neuroimaging. 2011;194(3):296–303. d oi: 10.1016/j.pscychresns.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asami T, Yamasue H, Hayano F, et al. Sexually dimorphic gray matter volume reduction in patients with panic disorder. Psychiatry Research: Neuroimaging. 2009;173(2):128–134. doi: 10.1016/j.pscychresns.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 51.Hayano F, Nakamura M, Asami T, et al. Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry Clin Neurosci. 2009;63(3):266–276. DOI: 10.1111/j.1440-1819.2009.01960.x [DOI] [PubMed] [Google Scholar]
- 52.Gold AL, Steuber ER, White LK, et al. Cortical Thickness and Subcortical Gray Matter Volume in Pediatric Anxiety Disorders. Neuropsychopharmacology. 2017;42(12):2423–2433. DOI: 10.1038/npp.2017.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moon C-M, Kim G-W, Jeong G-W. Whole-brain gray matter volume abnormalities in patients with generalized anxiety disorder: voxel-based morphometry. Neuroreport. 2014;25(3):184–189. doi: 10.1097/WNR.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 54.Zhao K, Liu H, Yan R, et al. Cortical thickness and subcortical structure volume abnormalities in patients with major depression with and without anxious symptoms. Brain and behavior. 2017;7(8):e00754. doi: 10.1002/brb3.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry. 2009;166(6):702–710. doi: 10.1176/appi.ajp.2008.08081201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar P, Goer F, Murray L, et al. Impaired reward prediction error encoding and striatal-midbrain connectivity in depression. Neuropsychopharmacology. 2018;43(7):1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current opinion in neurology. 2005;18(4):411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- 58.Helfinstein SM, Benson B, Perez-Edgar K, et al. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49(3):479–485. doi: 10.1016/j.neuropsychologia.2010.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Auerbach RP, Pagliaccio D, Pizzagalli DA. Toward an improved understanding of anhedonia. JAMA psychiatry. 2019;76(6):571–573. DOI: 10.1001/jamapsychiatry.2018.4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current opinion in psychiatry. 2015;28(1):7. DOI: 10.1097/YCO.0000000000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richey JA, Brewer JA, Sullivan-Toole H, et al. Sensitivity shift theory: A developmental model of positive affect and motivational deficits in social anxiety disorder. Clin Psychol Rev. 2019;72:101756. DOI: 10.1016/j.cpr.2019.101756 [DOI] [PubMed] [Google Scholar]
- 62.Haber SN, Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology. 2010;35(1):4–26. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forbes EE, Ryan ND, Phillips ML, et al. Healthy Adolescents’ Neural Response to Reward: Associations With Puberty, Positive Affect, and Depressive Symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(2):162–172.e165. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vervoort L, Wolters LH, Hogendoorn SM, de Haan E, Boer F, Prins PJM. Sensitivity of Gray’s Behavioral Inhibition System in clinically anxious and non-anxious children and adolescents. Personality and Individual Differences. 2010;48(5):629–633. 10.1016/j.paid.2009.12.021 [DOI] [Google Scholar]
- 66.Becker M, Simon D, Miltner W, Straube T. Altered activation of the ventral striatum under performance-related observation in social anxiety disorder. Psychol Med. 2017;47(14):2502. doi: 10.1017/S0033291717001076 [DOI] [PubMed] [Google Scholar]
- 67.van Tol M-J, van der Wee NJ, van den Heuvel OA, et al. Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry. 2010;67(10):1002–1011. doi: 10.1001/archgenpsychiatry.2010.121 [DOI] [PubMed] [Google Scholar]
- 68.Barch DM, Burgess GC, Harms MP, et al. Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Somerville LH, Bookheimer SY, Buckner RL, et al. The Lifespan Human Connectome Project in Development: A large-scale study of brain connectivity development in 5–21 year olds. Neuroimage. 2018;183:456–468. doi: 10.1016/j.neuroimage.2018.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of child psychology and psychiatry. 1999;40(1):57–87. I: 10.1111/1469-7610.00424 [DOI] [PubMed] [Google Scholar]
- 71.Wechsler D Wechsler Abbreviated Scale of Intelligence. In. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- 72.Taylor S, Whincup P, Hindmarsh P, Lampe F, Odoki K, Cook D. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatric and perinatal epidemiology. 2001;15(1):88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 73.Chapman LJ, Chapman JP. The measurement of handedness. Brain and cognition. 1987;6(2):175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- 74.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. DOI: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- 75.Freedman R, Lewis DA, Michels R, et al. The initial field trials of DSM-5: new blooms and old thorns. In: Am Psychiatric Assoc; 2013. [DOI] [PubMed] [Google Scholar]
- 76.Angold A, Costello EJ, Messer SC, Pickles A. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International journal of methods in psychiatric research. 1995. [Google Scholar]
- 77.de Ross RL, Gullone E, Chorpita BF. The revised child anxiety and depression scale: a psychometric investigation with Australian youth. Behaviour Change. 2002;19(2):90–101. DOI: 10.1375/bech.19.2.90 [DOI] [Google Scholar]
- 78.Spielberger CD, Gorsuch RL, Lushene RE. State-trait anxiety inventory (self-evaluation questionnaire). Consulting Psychololgists Press; 1968. [Google Scholar]
- 79.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167(1):99–103. DOI: 10.1192/bjp.167.1.99 [DOI] [PubMed] [Google Scholar]
- 80.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67(2):319. 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- 81.Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, van der Kouwe AJ. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magnetic resonance in medicine. 2012;68(2):389–399. doi: 10.1002/mrm.23228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cohen J Statistical Power Analysis for the Behavioral Sciences (Vol. 2nd Editio). In: Lawrence Erlbaum Associates, Publishers. 10.1234/12345678; 1998. [DOI] [Google Scholar]
- 83.Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. DOI: 10.1016/j.neuroimage.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Auerbach RP, Pisoni A, Bondy E, et al. Neuroanatomical Prediction of Anhedonia in Adolescents. Neuropsychopharmacology. 2017;42(10):2087–2095. DOI: 10.1038/npp.2017.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Winer ES, Bryant J, Bartoszek G, Rojas E, Nadorff MR, Kilgore J. Mapping the relationship between anxiety, anhedonia, and depression. J Affect Disord. 2017;221:289–296. DOI: 10.1016/j.jad.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl). 2009;205(4):667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nutt D, Demyttenaere K, Janka Z, et al. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol. 2007;21(5):461–471. DOI: 10.1177/0269881106069938 [DOI] [PubMed] [Google Scholar]
- 88.McMakin DL, Olino TM, Porta G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry. 2012;51(4):404–411. doi: 10.1016/j.jaac.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spijker J, Bijl RV, de Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Acta Psychiatr Scand. 2001;103(2):122–130. DOI: 10.1034/j.1600-0447.2001.103002122.x [DOI] [PubMed] [Google Scholar]
- 90.Craske MG, Meuret AE, Ritz T, Treanor M, Dour H, Rosenfield D. Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia. J Consult Clin Psychol. 2019;87(5):457. DOI: 10.1037/ccp0000396 [DOI] [PubMed] [Google Scholar]
- 91.Auerbach RP, Millner AJ, Stewart JG, Esposito EC. Identifying differences between depressed adolescent suicide ideators and attempters. J Affect Disord. 2015;186:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current opinion in psychiatry. 2015;28(1):7–12. 10.1038/npp.2017.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McTeague LM, Rosenberg BM, Lopez JW, et al. Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. American Journal of Psychiatry. 2020;177(5):411–421. 10.1176/appi.ajp.2019.18111271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruscio AM, Borkovec TD, Ruscio J. A taxometric investigation of the latent structure of worry. J Abnorm Psychol. 2001;110(3):413–422. DOI: 10.1037//0021-843x.110.3.413 [DOI] [PubMed] [Google Scholar]
- 95.Olatunji BO, Williams BJ, Haslam N, Abramowitz JS, Tolin DF. The latent structure of obsessive-compulsive symptoms: A taxometric study. Depress Anxiety. 2008;25(11):956–968. DOI: 10.1002/da.20387 [DOI] [PubMed] [Google Scholar]
- 96.Ruscio J, Brown TA, Ruscio AM. A taxometric investigation of DSM-IV major depression in a large outpatient sample: Interpretable structural results depend on the mode of assessment. Assessment. 2009;16(2):127–144. doi: 10.1177/1073191108330065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olatunji BO, Broman-Fulks JJ, Bergman SM, Green BA, Zlomke KR. A taxometric investigation of the latent structure of worry: Dimensionality and associations with depression, anxiety, and stress. Behav Ther. 2010;41(2):212–228. DOI: 10.1016/j.beth.2009.03.00 [DOI] [PubMed] [Google Scholar]
- 98.Kliem S, Beller J, Kroger C, Birowicz T, Zenger M, Brahler E. Dimensional latent structure of somatic symptom reporting in two representative population studies: Results from taxometric analyses. Psychol Assess. 2014;26(2):484–492. DOI: 10.1037/a0035721 [DOI] [PubMed] [Google Scholar]
- 99.Barlow DH, Farchione TJ, Sauer-Zavala S, et al. Unified protocol for transdiagnostic treatment of emotional disorders: Therapist guide. Oxford University Press; 2017. [Google Scholar]
- 100.Barlow DH, Farchione TJ, Bullis JR, et al. The unified protocol for transdiagnostic treatment of emotional disorders compared with diagnosis-specific protocols for anxiety disorders: A randomized clinical trial. JAMA psychiatry. 2017;74(9):875–884. DOI: 10.1001/jamapsychiatry.2017.2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tolin DF. Is cognitive-behavioral therapy more effective than other therapies? A meta-analytic review. Clin Psychol Rev. 2010;30(6):710–720. DOI: 10.1016/j.cpr.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 102.Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognitive therapy and research. 2012;36(5):427–440. doi: 10.1007/s10608-012-9476-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Loerinc AG, Meuret AE, Twohig MP, Rosenfield D, Bluett EJ, Craske MG. Response rates for CBT for anxiety disorders: Need for standardized criteria. Clin Psychol Rev. 2015;42:72–82. DOI: 10.1016/j.cpr.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 104.Elliott ML, Knodt AR, Ireland D, et al. What is the test-retest reliability of common task-fMRI measures? New empirical evidence and a meta-analysis. BioRxiv. 2020;681700(10.1101):681700. doi: 10.1177/0956797620916786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abler B, Grön G, Hartmann A, Metzger C, Walter M. Modulation of frontostriatal interaction aligns with reduced primary reward processing under serotonergic drugs. Journal of Neuroscience. 2012;32(4):1329–1335. DOI: 10.1523/JNEUROSCI.5826-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


