Abstract
Background
Perinatal human immunodeficiency virus type 1 (HIV-1) continues to occur due to barriers to effective antiretroviral prevention that might be mitigated by long-acting broadly neutralizing monoclonal antibodies (bNAbs).
Methods
An extended half-life bNAb, VRC01LS, was administered subcutaneously at 80 mg/dose after birth to HIV-1–exposed, nonbreastfed (cohort 1, n = 10) and breastfed (cohort 2, n = 11) infants. Cohort 2 received a second dose (100 mg) at 12 weeks. All received antiretroviral prophylaxis. VRC01LS levels were compared to VRC01 levels determined in a prior cohort.
Results
Local reactions (all grade ≤2) occurred in 67% and 20% after dose 1 and dose 2, respectively. The weight-banded dose (mean 28.8 mg/kg) of VRC01LS administered subcutaneously achieved a mean (standard deviation) plasma level of 222.3 (71.6) µg/mL by 24 hours and 44.0 (11.6) µg/mL at week 12, prior to dose 2. The preestablished target of ≥50 µg/mL was attained in 95% and 32% at weeks 8 and 12, respectively. The terminal half-life was 37–41 days. VRC01LS level after 1 dose was significantly greater (P <.002) than after a VRC01 dose (20 mg/kg). No infants acquired HIV-1.
Conclusions
VRC01LS was well tolerated with pharmacokinetics that support further studies of more potent long-acting bNAbs as adjunct treatment with antiretrovirals to prevent infant HIV-1 transmission.
Keywords: broadly neutralizing antibodies, VRC01LS, VRC01, neonates, HIV-1, monoclonal antibodies, bNAb, perinatal HIV-1 transmission, pharmacokinetics, passive immunization, HIV-1 prevention
Extended half-life broadly neutralizing antibody (bNAb), VRC01LS, administered to HIV-1–exposed infants has favorable pharmacokinetic parameters. These results support further evaluation of broad and potent bNAbs for prevention of HIV-1 transmission in infants as an adjunct to antiretrovirals.
Antiretroviral therapy (ART) administered to pregnant and breastfeeding women with human immunodeficiency virus type 1 (HIV-1) infection and antiretroviral (ARV) prophylaxis for their infants has resulted in dramatic decreases in HIV-1 transmission [1]. Despite this, an estimated 150 000 new infant infections occurred in 2019 (World Health Organization Data and Statistics, https://www.who.int/hiv/data/en/). Transmission continues due to several reasons, including late diagnosis of maternal infection, incomplete adherence, infection with resistant virus, and breast-milk transmission, especially among women who acquire HIV while breastfeeding [2]. To eliminate perinatal HIV transmission, additional strategies are needed [3]. Passive immunization with HIV-1–specific broadly neutralizing monoclonal antibodies (bNAbs) could be an effective adjunct to perinatal HIV-1 ARV prophylaxis [4].
The broadly neutralizing antibody VRC01 is specific for the HIV-1 CD4 binding site [5, 6] shown to block transmission of HIV-1 in animal models [7]. VRC01 is well tolerated when administered intravenously or subcutaneously (SC) to adults [8, 9], has antiviral activity in adults with HIV-1 viremia [10], and has no reactivity with human tissue [11]. We described safety, tolerability, and pharmacokinetic (PK) parameters of VRC01, administered SC as single and monthly injections to HIV-1–exposed infants at increased risk of HIV-1 infection enrolled in a prior cohort in this study [12]. Recent trials of VRC01 in women and men at risk of HIV-1 demonstrate that prophylaxis with VRC01 given every 8 weeks reduces sexual transmission of HIV-1, though only for viral isolates that are VRC01 sensitive [13], providing proof-of-concept for passive immunization for HIV-1 [14].
Long-acting bNAbs make delivery more feasible. VRC01 has been engineered by site-directed mutagenesis to introduce the LS mutation (M428L/N434S) resulting in VRC01LS, which demonstrates a 2.5- to 3-fold increase in half-life in adults compared to VRC01 [15]. Pharmacokinetic parameters in adults suggest that VRC01LS may achieve plasma levels for 3 or more months after a single dose. We therefore extended our study of HIV-1 bNAbs in infants to evaluate safety, tolerability, and PK parameters of VRC01LS.
MATERIALS AND METHODS
Participants
The International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) P1112 study (ClinicalTrials.gov identifier NCT02256631) is an open-label, dose-escalating, phase 1, multicenter study conducted in the United States (US) and Africa, evaluating VRC01 (previously reported [12]), and VRC01LS (results described here). Eligible infants were born to women with HIV-1, ≥36 weeks’ gestation, and ≥2 kg birth weight. Cohort 1 enrolled nonbreastfed infants at increased risk of HIV-1, defined as maternal ART initiated after the second trimester or interrupted for >14 days during the third trimester; maternal detectable HIV-1 RNA at ≤30 days of delivery; rupture of membranes >12 hours; or 2 ARV-class-resistant virus. Cohort 2 enrolled breastfed infants. The target enrollment was 10 (±2) per cohort.
Cohort 1 received a single dose of VRC01LS 80 mg (in 0.8 mL) SC administered <72 hours after birth. Cohort 2 received an initial dose of VRC01LS 80 mg SC within 5 days of birth, followed by a second dose of 100 mg (in 1.0 mL) SC at age 12 weeks if breastfeeding continued. All infants received standard-of-care ARV prophylaxis. The cohorts opened sequentially. Safety stopping rules were predefined as death or a grade 4 adverse event (AE) probably or definitely attributable to VRC01LS or ≥2 of the first 6 infants immunized having a grade 3 or higher AE at least possibly related to VRC01LS (excluding neutropenia, anemia, and hyperbilirubinemia). Safety stopping rule criteria were not met.
VRC01LS was administered by slow SC push in the thigh using a BD Safety-Lok infusion set with a 25 G × 0.75-inch needle, 7 inches of tubing, and a luer-lock adapter. VRC01LS was administered as 1 or 2 injections determined by site investigators. Infants were observed for 4 hours after the first dose and for 1 hour after the second dose. Safety assessment schedule is in Supplementary Table. During the 30 days after VRC01LS injection, all signs/symptoms/diagnoses and laboratory AEs were collected; beyond 30 days after injection through week 96, grade 2 or higher signs and symptoms and grade 1 or higher laboratory evaluations were collected. Toxicities were graded according to the Division of AIDS Table for Grading the Severity of Maternal and Pediatric Adverse Events (corrected version 2.1, July 2017). Assessments included local reactions (injection site pain, swelling, redness, or bruising); systemic reactions (such as irritability, fever, or lethargy); and other AEs, which included any abnormality not fitting into 1 of the above categories. Injection site pain was graded using a study-specific scale [12].
Study Drug
VRC01LS (VRC-HIVMAB080-00-AB) is a recombinant human immunoglobulin G1 antibody produced in a Chinese hamster ovary cell line in accordance with current Good Manufacturing Practice regulations. VRC01LS was manufactured at the National Institute of Allergy and Infectious Diseases Vaccine Research Center Clinical Material Program operated by Leidos Biomedical Research, Frederick, Maryland, as a 100 mg/mL solution and provided in single-dose vials. VRC01LS was stored at a temperature range of –45°C to –10°C (–49°F to 14°F).
Regulatory
The study was approved by each site’s institutional review board. Women provided written informed consent for their own and their infants’ participation. Human research participation guidelines of the US Department of Health and Human Services and those of the investigators’ institutions were followed.
Laboratory
Hematology, chemistries, and HIV-1 testing were performed at clinical laboratories. VRC01LS quantification in plasma was performed with a Beckman Biomek–based automation platform. The anti-idiotype 5C9 monoclonal antibody was added to Immulon-4HXB microtiter overnight prior to blocking. Three-fold dilutions, from 1:100 to 1:218 700, were analyzed in duplicate. Anti-human IgG1 conjugated with horseradish peroxidase and TMB (3,5′, 5,5′-tetra-methylbenzidine) substrate was used to develop the reaction. Sulfuric acid was then added to halt color development. Plates were read within 30 minutes at 450 nm with a Molecular Devices Paradigm plate reader. Sample concentrations were quantified using a linear regression of a standard curve of VRC01LS covering a range between 5 and 125 ng/mL after dilutions. Screening for VRC01LS anti-idiotypic antibodies in plasma was performed by a electrochemiluminescence bridging assay as previously described [9].
Analysis
Descriptive summaries of baseline characteristics and AEs are presented by cohort. PK data for cohort 2 infants who received only dose 1 are grouped with cohort 1 data. Plasma VRC01LS concentrations were analyzed by noncompartmental methods. Maximum concentration (Cmax), time of maximum concentration (Tmax), and concentrations at weeks 4 (C4WK), 8 (C8WK), 12 (C12WK), and 24 (C24WK) were taken directly from observed data. The terminal slope, λz, was determined from nonlinear regression of the log-linear portion of the concentration–time curve and terminal half-life calculated as 0.693/λz. The area under the concentration–time curve (AUC) was calculated for the first 84 days (AUC0-84D) and complete AUC0-∞ using the linear trapezoidal method. Concentrations below the quantification limit were set equal to 0 for the AUC calculations. For those with concentrations at the final PK sample (Clast), the AUC after Clast was estimated as Clast/λz. Infants who discontinued PK sampling at week 12 or earlier were excluded from the AUC and half-life calculations. Data were analyzed using SAS version 9.4 software (SAS Institute, Cary, North Carolina) and R version 3.2.1 software with R Studio (version 1.2.456). VRC01LS PK parameters are compared in a post hoc analysis to our previously published data for single-dose VRC01 (20 mg/kg) administered SC to infants enrolled in a prior cohort in this study [12]. The infants receiving VRC01 met the same eligibility criteria, enrolled at the same sites, and had PK assays performed in the same laboratory as the infants receiving VRC01LS.
RESULTS
Accrual
Twenty-one infants enrolled between July 2017 and January 2018 at 8 sites; 10 in cohort 1 and 11 in cohort 2. Baseline demographics are in Table 1. Seven infants enrolled in the US and 14 enrolled in Africa. The median time from birth to VRC01LS administration was 2 days in both cohorts. All infants received ARV prophylaxis. No infants acquired HIV-1 based on nucleic acid testing on 6 occasions through week 36 with 5 additional occasions though week 96 for cohort 2 (Supplementary Table).
Table 1.
Baseline Characteristics
| Characteristic | Treatment Group | ||
|---|---|---|---|
| Cohort 1 (Single Dose)a (n = 10) | Cohort 2 (2 Doses)a,b (n = 11) | Total (N = 21) | |
| Male sex, No. (%) | 6 (60) | 5 (45) | 11 (52) |
| Race/ethnicity, No. (%) | |||
| Black | 8 (80) | 11 (100) | 19 (90) |
| White | 2 (20) | 0 (0) | 2 (10) |
| Hispanic/Latino | 1 (10) | 0 (0) | 1 (5) |
| Infant ARV, No. (%) | |||
| 3TC, ZDV, NVP | 2 (20) | 0 (0) | 2 (10) |
| NVP | 2 (20) | 11 (100) | 13 (62) |
| ZDV | 5 (50) | 0 (0) | 5 (24) |
| ZDV, NVP | 1 (10) | 0 (0) | 1 (5) |
| Age, d, at VRC01LS administration | |||
| Mean (SD) | 2.0 (0.9) | 2.4 (0.8) | 2.2 (0.9) |
| Median (Q1, Q3) | 2 (2, 3) | 2 (2, 3) | 2 (2, 3) |
| Min, max | 0, 3 | 1, 4 | 0, 4 |
| Weight at birth, g | |||
| Mean (SD) | 3123 (534) | 2948 (381) | 3031 (457) |
| Median (Q1, Q3) | 2865 (2750, 3520) | 2920 (2550, 3330) | 2880 (2700, 3330) |
| Min, max | 2535, 4045 | 2500, 3545 | 2500, 4045 |
| Enrollment site, No. (%) | |||
| Africac | 3 (30) | 11 (100) | 14 (67) |
| United States | 7 (90) | 0 (0) | 7 (33) |
Abbreviations: 3TC, lamivudine; ARV, antiretroviral; NVP, nevirapine; Q1, quartile 1; Q3, quartile 3; SD, standard deviation; ZDV, zidovudine.
aDose 1: 80 mg VRC01LS; dose 2: 100 mg VRC01LS.
bCohort 2 required breastfeeding.
cCape Town, South Africa, and Harare, Zimbabwe.
All infants received the initial VRC01LS dose and 10 of 11 infants in cohort 2 received a second dose (1 stopped breastfeeding prior to week 12), demonstrating excellent adherence to the dosing regimen. One participant inadvertently received an incomplete dose 2 and this child’s PK results after week 12 were not included in the summary concentration calculations. Early study discontinuations occurred at weeks 2, 4, 35, and 84 for cohort 1 and week 13 in cohort 2 and were due to loss to follow-up (3 infants) or consent withdrawal (2 infants). One other infant had 1 missed visit.
Safety
All cohort 1 infants received VRC01LS as a single 0.8-mL SC injection. In cohort 2, the dose was split between 2 injection sites for 5 and 6 infants for the initial and second dose respectively, with the remaining cohort 2 infants receiving a single SC injection for both doses. After the first dose, transient, grade 1 local reactions were common, occurring in 60% and 82% of cohort 1 and 2, respectively (Table 2). Most common was erythema/redness (48%), ranging in diameter from 1.0 to 2.1 cm, with resolution at 1 hour in 90%. Injection site edema occurred in 6 infants and induration in 2 infants. All local reactions after the first dose resolved by 24 hours. After the second dose, local reactions were less frequent, occurring in only 2 of 10 (20%) infants. One child had grade 2 erythema and grade 1 induration, resolved by 30 minutes and 24 hours, respectively. The other had grade 2 induration resolved by 24 hours. Injection site tenderness/pain was uncommon, occurring in 2 infants and resolved by 1 and 24 hours. Systemic symptoms temporally correlated with immunization occurred in 1 infant in cohort 1 and in 3 infants in cohort 2. Symptoms, all grade 1, varied among the 4 infants and included increased sleep, decreased appetite, irritability, and in 1 infant, fever.
Table 2.
Summary of Local Reactions
| Characteristic | Cohort 1 | Cohort 2 | Cohort 2 |
|---|---|---|---|
| Single Dose | Initial Dose | Week 12 Dose | |
| Total No. of children | 10 | 11 | 10 |
| Total No. of injection sites | 10 | 16 | 16 |
| Average volume/injection site, mL (min, max) | 0.8 (0.8, 0.8) | 0.6 (0.4, 0.8) | 0.6 (0.3, 1.0) |
| Children with specified reaction, No. (%) | |||
| Any local reactiona | 6 (60) | 9 (82) | 2 (20) |
| Edema | 1 (10) | 5 (45) | … |
| Erythema/redness | 4 (40) | 6 (55) | 1 (10) |
| Induration | 2 (20) | … | 2 (20) |
| Bruising | 1 (10) | … | … |
| Grade average (min, max) | |||
| Any local reactiona | 1.0 (1, 1) | 1.0 (1, 1) | 2.0 (2, 2) |
| Edema | 1.0 (1, 1) | 1.0 (1, 1) | … |
| Erythema/redness | 1.0 (1, 1) | 1.0 (1, 1) | 2.0 (2, 2) |
| Induration | 1.0 (1, 1) | 1.0 (1, 1) | 1.5 (1, 2) |
| Bruising | 1.0 (1, 1) | … | … |
| Size average, cm (min, max) | |||
| Edema | 1.5 (1.5, 1.5) | 1.2 (1.0, 1.3) | … |
| Erythema/redness | 1.9 (1.5, 2.1) | 1.5 (1.0, 2.0) | 3.3 (3.3, 3.3) |
| Induration | 0.6 (0.1, 1.0) | … | 2.8 (2.1, 3.5) |
| Resolved by 1 h, No. (%) | |||
| Any local reactionb | 3 (60) | 8 (89) | 0 (0) |
| Edema | 1 (100) | 5 (100) | … |
| Erythema/redness | 4 (100) | 5 (83) | 1 (100) |
| Induration | 0 (0) | … | 0 (0) |
| Resolved by 4 h, No. (%) | |||
| Any local reactionb | 3 (60) | 8 (89) | … |
| Edema | 1 (100) | 5 (100) | … |
| Erythema/redness | 4 (100) | 5 (83) | … |
| Induration | 0 (0) | … | … |
| Resolved by 24 h, No. (%) | |||
| Any local reactionb | 5 (100) | 9 (100) | 2 (100) |
| Edema | 1 (100) | 5 (100) | … |
| Erythema/redness | 4 (100) | 6 (100) | … |
| Induration | 2 (100) | … | 2 (100) |
aThe values given for grade are the largest grade of any of the reactions reported (edema, erythema, induration, bruising) within that child, whichever group is specified.
bThe values given for percentage resolved by 1, 4, and 24 hours are the longest duration among edema, erythema, and induration (ie, these calculations exclude bruising, which is expected to be prolonged).
In the first 30 days after VRC01LS administration, grade 3 events occurred in 1 of 10 participants in cohort 1 and 3 of 11 participants in cohort 2. One participant had grade 3 ABO hemolytic disease with grade 3 hyperbilirubinemia (cohort 1), 2 had grade 3 neutropenia (cohort 2), and 1 had grade 3 hemoglobin (cohort 2), all with alternative explanations. After 30 days, 2 infants in each cohort experienced grade 3 AEs. One infant had bronchiolitis with hypoxia and 3 had anemia and/or neutropenia, none attributed to VRC01LS. There were no grade 4 AEs throughout the study.
Antidrug antibodies were evaluated at weeks 24 and 48 for infants with available plasma. No antidrug antibodies were detected in the 13 infants tested (8 in cohort 1; 5 in cohort 2) with 13 tested at week 24 and 11 of those tested at week 48 (data not shown).
Pharmacokinetics
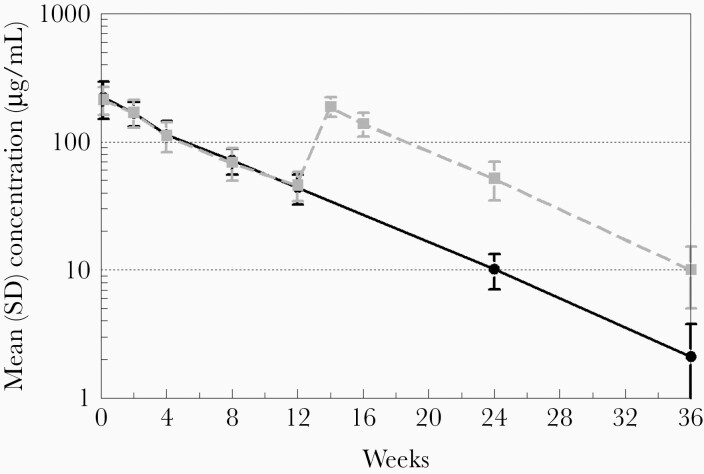
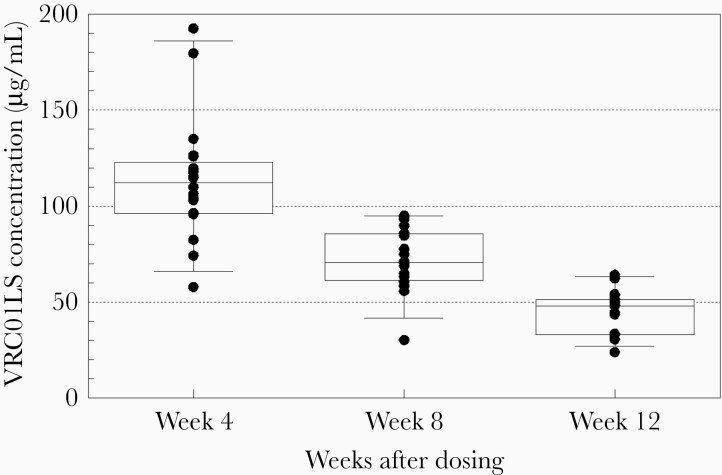
Mean VRC01LS plasma levels following 1 and 2 doses are shown in Figure 1. While the sparse PK sampling precludes precise determination of the peak concentration, VRC01LS was rapidly absorbed following SC administration, with all infants achieving plasma concentrations >100 µg/mL by 24 hours with a mean plasma level of 222.3 (standard deviation [SD], 71.6) µg/mL following the initial dose (VRC01LS mean per kg dose, 28.8 [range, 21.1–34.8] mg/kg) (Figure 1). VRC01LS plasma concentrations exceeding 50 µg/mL, the predefined target, were found in all infants (20/20) at week 4 and in 95% (18/19) at week 8 (Figure 2). At week 12, prior to the second dose, the mean VRC01LS plasma level was 45.0 (SD, 11.6) µg/mL, with 100% (19/19) of infants’ levels exceeding 20 µg/mL, and 32% exceeding 50 µg/mL (Figure 2). Of note, among infants who received a single dose of 80 mg, 67% (6/9) continued to have measurable VRC01LS levels at week 24 with a mean plasma concentration of 10.2 (SD, 3.1) µg/mL (Figure 1). Following dose 2 of 100 mg (VRC01LS mean dose, 17.2 [range, 15.1–21.4] mg/kg) administered at week 12, the mean plasma level at week 14 was 188.6 (SD, 33.1) µg/mL (Figure 1). All infants had concentrations >50 µg/mL and 10 µg/mL through study weeks 16 and 24, respectively. The mean plasma level was 10.1 (SD, 5.1) µg/mL at week 36. The VRC01LS concentrations for dose 1 and dose 2 at 4 and 12 weeks postadministration of were similar, indicating no appreciable accumulation or PK parameter changes with repeat administration or age.
Figure 1.
Pharmacokinetics of VRC01LS after 1 subcutaneous (SC) dose of 80 mg (solid black line) or 2 SC doses (80 mg followed by 100 mg at 12 weeks) (dashed gray line). The plasma pharmacokinetic sampling for VRC01LS (µg/mL) is described in the Methods. Error bars indicate the standard deviation (SD).
Figure 2.
VRC01LS concentrations at 4-week intervals after the first dose. Box plot describes median, interquartile range (25th–75th percentiles; box), and 5th–95th percentiles in additional to the individual concentrations.
The terminal slope half-life was determined in 18 participants with >12 weeks of PK samples after their final dose. The terminal slope half-life averaged 39.2 (SD, 5.0) days and was similar following single-dose and 2-dose administration. Mean participant weight increased 90% from 3.0 (SD, 0.5) kg at birth to 5.7 (SD, 0.6) kg at week 12. Additional PK parameters are provided in Table 3.
Table 3.
VRC01LS Pharmacokinetic Measures When Administered as 1 or 2 Subcutaneous Doses to Neonates Born to Women With Human Immunodeficiency Virus
| Pharmacokinetic Parameter | Mean (SD) | |
|---|---|---|
| Dose 1 | Dose 2 | |
| Dose, mg/kg | 28.8 vs 28.5 (4.1)a | 17.2 (2.0) |
| AUC0-∞, µg × d/mL | 11 589 (2064) | NA |
| AUC0-84D, µg × d/mL | 9041 (2047) | 8839 (1891) |
| λ z, per day | 0.0169 (0.0019) | 0.0190 (0.0025) |
| T1/2λz, d | 41.3 (4.3) | 37.1 (4.9) |
| CL/F, mL/d/kg | 2.35 (0.34) | NA |
| Cmax, µg/mL | 227.1 (67.6) | NA |
| Tmax, d | 0.5 (0.75) | NA |
| VRC01LS C4WK, µg/mL | 113.6 (31.0) | 139.3 (29.6) |
| VRC01LS C12WK, µg/mL | 44.0 (11.6) | 52.2 (17.5) |
| VRC01LS C24WK, µg/mL | 10.2 (3.1) | 10.1 (5.1) |
Abbreviations: λ z, terminal slope; AUC, area under the concentration–time curve; C4WK, C12WK, C24WK, concentrations at week 4, week 12, and week 24; CL/F, clearance; Cmax, maximum concentration; NA, not applicable; SD, standard deviation; T1/2, half-life; Tmax, time of maximum concentration.
aThe mean dose in mg/kg for dose 1 in cohort 1 and cohort 2.
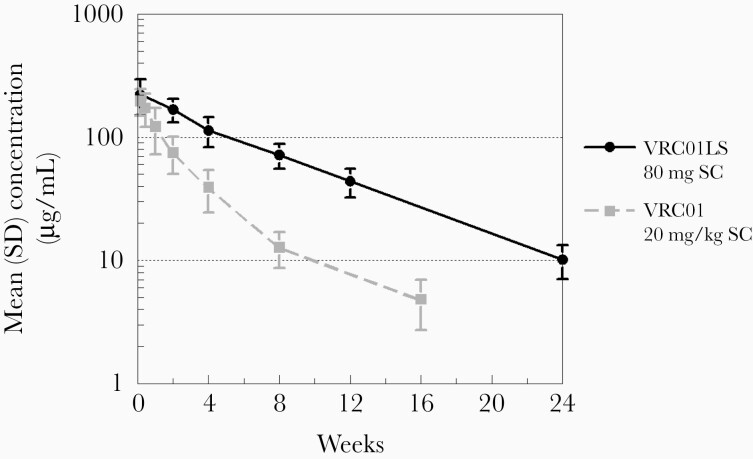
To determine the effect of the LS mutations on the rate of bNAb elimination for VRC01LS relative to VRC01, the mean concentration–time profile for the VRC01LS 80 mg dose (mean, 28.8 mg/kg) was compared to the previously reported profile for VRC01 (dosed at 20 mg/kg) administered to infants enrolled in a prior cohort of this study [12]. Despite lower initial concentrations at day 1, the mean level of VRC01LS was more than double that of VRC01 at 4 weeks postdose and beyond with significant differences at week 4 (P = .0018) and week 8 (P = .0019), demonstrating delayed elimination of VRC01LS compared to VRC01 (Figure 3).
Figure 3.
First-dose VRC01LS (fixed at 80 mg) mean concentration–time profile (solid black line) compared to single-dose VRC01 (20 mg/kg) profile (dashed gray line) determined in the prior stage for this study and previously reported [12]. Error bars indicate the standard deviation. Abbreviations: SC, subcutaneous; SD, standard deviation.
DISCUSSION
This is the first study of an anti–HIV-1 monoclonal antibody with extended half-life in infants. The study assessed safety and PK parameters of VRC01LS administered SC at birth and 12 weeks to infants exposed to HIV-1. A fixed birth dose of 80 mg achieved a mean level of 45 µg/mL at week 12, prior to the second dose. There was no antibody accumulation with the repeat dose and no evidence of age-based changes in antibody clearance. The elimination rate of VRC01LS in infants was significantly slower than that of VRC01, demonstrating PK characteristics compatible with infrequent dosing to maintain bNAb levels during the period of breastfeeding.
VRC01LS was well tolerated. Local injection site reactions were common with the first dose and less common with the second dose; thus, reactions were not exacerbated with repeated dosing. The higher rate of mild local reactions with the first dose might relate to less SC tissue in most neonates relative to later infancy. The frequency and type of local reaction were similar to those observed for VRC01 [12] and consistent with reactions in children receiving SC IgG for antibody deficiency [16, 17]. A few infants had short-lived behavioral alterations temporally associated with VRC01LS doses that might signify systemic reactions; these were mild, self-limited symptoms that are common during infancy, and lacking a control group, it is not possible to determine if these symptoms were due to VRC01LS. Rare infusion reactions are reported in adult clinical trials evaluating VRC01 [8]; no infusion reactions were observed in this study. No safety signals were observed during prolonged exposure to the VRC01LS with levels detectable beyond 36 weeks, in line with findings in the prior study of repeated doses of VRC01 [12]. Acceptability to caregivers was not formally assessed; however, adherence to the VRC01LS dosing was excellent as it was for monthly dosing of VRC01 [12].
We found differences in VRC01LS PK parameters relative to adults that are pertinent to planning for use of bNAbs during infancy [15]. Following SC administration, absorption appears to be more rapid in infants than adults. Although limited early sample time points prevent precise characterization of the infant Cmax in our study, infant concentrations at 24 hours were higher than the next sample at week 2, whereas in adults following SC administration, the Cmax did not occur until day 9 [15]. After adjusting for per-kilogram dose differences, the 24-hour concentrations after SC administration in infants are 2-fold greater than those in adults (222 vs 106 µg/mL). Rapid absorption is likely important for efficacy in prevention of perinatal/early breastfeeding transmission since exposure is occurring contemporaneously with the initial antibody dose. The enhanced SC absorption supports feasibility for use in infants since this route is more acceptable than intravenous dosing. The half-life of VRC01LS in adults of 64.6 days [15] exceeds the mean half-life of 37–41 days in our study of infants. A major contributor to this difference is infant growth. Between birth and 12 weeks of age, the time of the second dose, the infants’ weights nearly doubled. Thus, a dilution factor related to increase in body size is an important factor in interpreting the half-life in infants. The mechanism of extended half-life associated with the LS mutation is enhanced binding to the neonatal Fc receptor (FcRn) [18, 19]. There are few data on the age-related changes in FcRn expression. Some physiologically based PK models indicate modest increase in weight-normalized FcRn concentrations associated with younger age [20], which may result in slower intrinsic metabolic turnover in infants after adjusting for size, although not sufficient to negate the growth effect [21]. Despite a shorter half-life in infants than adults, VRC01LS half-life is markedly prolonged compared to VRC01 in infants.
We chose to use a weight-banded, fixed dose of VRC01LS to mimic an approach that would be feasible for clinical scale-up in a resource-limited setting. Modeling based on adult PK parameters predicted the week 12 trough to be between 40 and 120 µg/mL, approximating our preestablished target trough of 50 µg/mL. The target trough was chosen at the time of study design based on 91% of a multiclade panel of tier 2 viruses having an inhibitory concentration50 of <50 µg/mL for VRC01 [5, 6]. The weight-banded doses in our study were selected at volumes previously demonstrated to be well tolerated for SC injection and typically given at a single injection site; however, larger SC injection volumes are feasible if a higher trough is desired. Using proportional changes in concentration with a higher initial dose of 100 or 120 mg (approximately 40 mg/kg), we would expect the week 12 trough to be about 55 or 65 µg/mL, approximately 25%–50% higher than the 44 µg/mL trough observed at 12 weeks with the 80-mg dose. Alternatively, dosing with 80 mg at 8-week intervals would also be expected to maintain troughs over 50 µg/mL. Recent adult trial data indicate that serum neutralizing titers, dependent on bNAb potency and serum levels, are predictive of protective efficacy [13]. Our PK data for VRC01LS will be valuable in modeling infant dosing for bNAbs with greater potency that may be considered for future use. The adult efficacy trial of VRC01 also emphasizes that bNAb combinations are likely necessary to achieve sufficient breadth to prevent transmission [13]. The rapid expansion of bNAbs in clinical development provides a pipeline of potential agents targeting multiple epitopes [11, 22–28]. One of these, VRC07-523LS, is currently under study in an infant cohort in our study.
Passive antibody is particularly well suited for prophylaxis of perinatal and breastfeeding HIV transmission given the defined and limited duration of exposure and the small quantities needed for infant doses. Clinical infrastructure exists for injectable agents since childhood vaccines are given at birth through the first year of life. Long-acting agents mitigate the adherence barriers inherent to daily ARV [29, 30]. In areas of high HIV-1 seroincidence during late pregnancy and breastfeeding [31–33], long-acting bNAbs given universally at birth could provide infant prophylaxis not dependent on maternal preexposure prophylaxis. Administration of combination bNAbs to infant macaques early after acute simian human immunodeficiency virus (SHIV) infection eradicated SHIV-infected cells, aborting the infection [34, 35]; bNAbs given early to exposed infants might have a similar effect. Studies of new agents to prevent perinatal HIV-1 have been deterred due to low transmission rates with optimal ARV; however, there are settings with suboptimal ARV use (eg, late start of ARV, ongoing viremia, HIV acquisition during pregnancy or breastfeeding) where transmission may be as high as 8% [36–38], and alternative strategies are needed.
This study is limited by sample size, which precludes precise estimates of the frequency of common AEs, and rare AEs may not be detected. However, the study adds safety data on bNAbs during infancy. Participant demographics in the single- and 2-dose cohorts differ as a result of differing standards for care in different countries. Theoretically, dosing of bNAb within 24–30 hours of life may increase efficacy, particularly for interrupting peripartum transmission [34]. Early dosing in a clinical setting is likely to be feasible, but for this study, enrollment after 24 hours was allowed to facilitate study procedures; therefore, comparisons of PK parameters for ≥24 and <24 hours of life are not possible. However, it is notable that study sites succeeded in giving the majority of the initial doses by day 2 of life, earlier than the end of the allowed window.
Although maternal ART and infant ARV prophylaxis are highly effective in preventing perinatal HIV-1 transmission, most experts and stakeholders believe that additional interventions, beyond increasing ARV access, are needed to achieve elimination of perinatal HIV-1 transmission [3]. Long-acting agents such as bNAbs are attractive as adherence to daily treatment over the duration of breastfeeding is not achieved by all [29, 30]. This study demonstrates safety and favorable PK parameters for an extended half-life bNAb allowing for dosing at 3-month intervals and supports further evaluation of broad and potent bNAbs for prevention of HIV-1 transmission in infants as an adjunct to ARV.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Members of the protocol team not included in the byline include (in alphabetical order): Dwight Yin, MD, MPH; Grace Aldrovandi, MD, CM; Frederic Bone; Emily Brown, MA; Dale Dayton, RN, CCRA; Bobbie Graham; Christopher Hensel; Benjamin Johnston; Maximina Jokonya; Jonathan Lucas, MPH; Kathryn Myers, BA; Alejandro Reyes Santos; Nicole Tobin, MD; and Bonnie Zimmer. We thank the Vaccine Production Program of the Vaccine Research Center (VRC) and the Vaccine Clinical Materials Program/Leidos Biomedical Research for providing scientific support and investigational Good Manufacturing Practices (GMP) product and for conducting key assays for the assessment of the trial. Individuals at the sites who contributed to the study include (listed in order of number of enrollments and alphabetically for sites with equal enrollments): Family Clinical Research Unit (FAM-CRU): Magdel Rossouw, MBChB; Lindie Rossouw, MBChB; Jeanne Louw, MSc. Harare Family Care: Tichaona Vhembo, MBChB, MPH; Tsungai Patience Mhembere, BPharm, MPH; Petronella Matibe, MSc Sp Ed, BSc Sp Ed. University of Florida Center for HIV/AIDS Research, Education and Service (UF CARES): Saniyyah Mahmoudi, MSN, ARNP; Alexandrea Maldonado; Nizar Maraqa, MD. Bronx-Lebanon Hospital Center: Mahboobullah M Baig, MBBS; Tanya Rogo, MD; Martha Cavallo, CPNP. Johns Hopkins University: Aleisha Collinson-Streng, BSN, RN; Thuy Anderson, BSN, RN; W Christopher Golden, MD; Deborah Persaud, MD. South Florida Children’s Diagnostic and Treatment Center Fort Lauderdale: Ana M Puga, MD; Lisa-Gaye Robinson, MD, MPH; Zulma Eysallenne, BSN; Dayana Leon, RN. Texas Children’s Hospital: Mary E Paul, MD; Chivon McMullen-Jackson, BSN, RN, CCRP; Shelley Buschur, BA, RN, CNM; Mariam Pontifes, CCRP. University of Colorado Denver: Joyce Sung, MD; Carrie Glenny, RN, BSN, MA; Jennifer Dunn, MS, FNP; Kacey Navarro, MS, FNP.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health, all components of the NIH, under award numbers UM1AI068632 (IMPAACT Leadership and Operations Center), UM1AI068616 (IMPAACT Statistical and Data Management Center), and UM1AI106716 (IMPAACT Laboratory Center), and by NICHD contract number HHSN275201800001I. Funding for this study was also provided by the Intramural Research Program of the Vaccine Research Center, NIAID, NIH. This publication resulted in part from research supported by the Duke University Center for AIDS Research, an NIH-funded program (5P30 AI064518 to C. K. C.), and the Colorado Clinical and Translational Science Award from the National Center for Advancing Translational Sciences (award number UL1 TR001082 to E. J. M.).
Potential conflicts of interest. J. R. M. is an inventor on an NIH patent for VRC01LS. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 5 March 2019. Oral abstract 1059
Contributor Information
International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) P1112 Team:
Magdel Rossouw, Lindie Rossouw, Jeanne Louw, Tichaona Vhembo, Tsungai Patience Mhembere, Petronella Matibe, Saniyyah Mahmoudi, Alexandrea Maldonado, Nizar Maraqa, Mahboobullah M Baig, Tanya Rogo, Martha Cavallo, Aleisha Collinson-Streng, Thuy Anderson, W Christopher Golden, Deborah Persaud, Ana M Puga, Lisa-Gaye Robinson, Zulma Eysallenne, Dayana Leon, Mary E Paul, Chivon McMullen-Jackson, Shelley Buschur, Mariam Pontifes, Joyce Sung, Carrie Glenny, Jennifer Dunn, and Kacey Navarro
References
- 1. Abuogi LL, Humphrey JM, Mpody C, et al. Achieving UNAIDS 90-90-90 targets for pregnant and postpartum women in sub-Saharan Africa: progress, gaps and research needs. J Virus Erad 2018; 4:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tippett Barr BA, van Lettow M, van Oosterhout JJ, et al. National estimates and risk factors associated with early mother-to-child transmission of HIV after implementation of option B+: a cross-sectional analysis. Lancet HIV 2018; 5:e688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voronin Y, Jani I, Graham BS, et al. Recent progress in immune-based interventions to prevent HIV-1 transmission to children. J Int AIDS Soc 2017; 20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaebler C, Caskey M. Broadly neutralizing antihuman immunodeficiency virus antibodies in infants: promising new tools for prevention of mother-to-child transmission? J Infect Dis 2020; 222:525–7. [DOI] [PubMed] [Google Scholar]
- 5. Wu X, Yang ZY, Li Y, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 2010; 329:856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou T, Georgiev I, Wu X, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 2010; 329:811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pegu A, Yang Z-Y, Boyington JC, et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med 2014; 6:243ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayer KH, Seaton KE, Huang Y, et al. ; HVTN 104 Protocol Team; and the NIAID HIV Vaccine Trials Network . Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: results of a phase 1 randomized trial. PLoS Med 2017; 14:e1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ledgerwood J, Coates E, Yamshchikov G, et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol 2015; 182:289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lynch RM, Boritz E, Coates EE, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Science Transl Med 2015; 7:319ra206. [DOI] [PubMed] [Google Scholar]
- 11. Rudicell RS, Kwon YD, Ko SY, et al. ; NISC Comparative Sequencing Program . Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol 2014; 88:12669–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cunningham CK, McFarland EJ, Morrison RL, et al. ; IMPAACT P1112 Team . Safety, tolerability, and pharmacokinetics of the broadly neutralizing human immunodeficiency virus (HIV)-1 monoclonal antibody VRC01 in HIV-exposed newborn infants. J Infect Dis 2020; 222:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corey L, Gilbert PB, Juraska M, et al. ; HVTN 704/HPTN 085 and HVTN 703/HPTN 081 Study Teams . Two randomized trials of neutralizing antibodies to prevent HIV-1 acquisition. N Engl J Med 2021; 384:1003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walker BD. The AMP trials—a glass half full. N Engl J Med 2021; 384:1068–9. [DOI] [PubMed] [Google Scholar]
- 15. Gaudinski MR, Coates EE, Houser KV, et al. ; VRC 606 Study Team . Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med 2018; 15:e1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shapiro R. Subcutaneous immunoglobulin therapy by rapid push is preferred to infusion by pump: a retrospective analysis. J Clin Immunol 2010; 30:301–7. [DOI] [PubMed] [Google Scholar]
- 17. Jolles S, Sleasman JW. Subcutaneous immunoglobulin replacement therapy with Hizentra, the first 20% SCIG preparation: a practical approach. Adv Ther 2011; 28:521–33. [DOI] [PubMed] [Google Scholar]
- 18. Saunders KO. Conceptual approaches to modulating antibody effector functions and circulation half-life. Frontiers Immunol 2019; 10:1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7:715–25. [DOI] [PubMed] [Google Scholar]
- 20. Hardiansyah D, Ng CM. Effects of the FcRn developmental pharmacology on the pharmacokinetics of therapeutic monoclonal IgG antibody in pediatric subjects using minimal physiologically-based pharmacokinetic modelling. MAbs 2018; 10:1144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Nikanjam M, Cunningham CK, et al. Model informed development of VRC01 in newborn infants using a population pharmacokinetics approach. Clin Pharmacol Ther 2021; 109:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gama L, Koup RA. New-generation high-potency and designer antibodies: role in HIV-1 treatment. Annu Rev Med 2018; 69:409–19. [DOI] [PubMed] [Google Scholar]
- 23. Huang J, Kang BH, Ishida E, et al. Identification of a CD4-binding-site antibody to HIV that evolved near-pan neutralization breadth. Immunity 2016; 45:1108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scheid JF, Mouquet H, Ueberheide B, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 2011; 333:1633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walker LM, Huber M, Doores KJ, et al. ; Protocol G Principal Investigators . Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 2011; 477:466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mouquet H, Scharf L, Euler Z, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A 2012; 109:E3268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doria-Rose NA, Bhiman JN, Roark RS, et al. New member of the V1V2-directed CAP256-VRC26 lineage that shows increased breadth and exceptional potency. J Virol 2016; 90:76–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sok D, Burton DR. Recent progress in broadly neutralizing antibodies to HIV. Nat Immunol 2018; 19:1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larsen A, Magasana V, Dinh TH, et al. Longitudinal adherence to maternal antiretroviral therapy and infant nevirapine prophylaxis from 6 weeks to 18 months postpartum amongst a cohort of mothers and infants in South Africa. BMC Infect Dis 2019; 19:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Napyo A, Tylleskär T, Mukunya D, et al. Barriers and enablers of adherence to infant nevirapine prophylaxis against HIV 1 transmission among 6-week-old HIV exposed infants: a prospective cohort study in northern Uganda. PLoS One 2020; 15:e0240529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnson LF, Stinson K, Newell ML, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr 2012; 59:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pintye J, Davey DLJ, Wagner AD, et al. ; PrEP in Pregnancy Working Group . Defining gaps in pre-exposure prophylaxis delivery for pregnant and post-partum women in high-burden settings using an implementation science framework. Lancet HIV 2020; 7:e582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dinh TH, Delaney KP, Goga A, et al. Impact of maternal HIV seroconversion during pregnancy on early mother to child transmission of HIV (MTCT) measured at 4–8 weeks postpartum in South Africa 2011–2012: a national population-based evaluation. PLoS One 2015; 10:e0125525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shapiro MB, Cheever T, Malherbe DC, et al. Single-dose bNAb cocktail or abbreviated ART post-exposure regimens achieve tight SHIV control without adaptive immunity. Nat Commun 2020; 11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hessell AJ, Jaworski JP, Epson E, et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat Med 2016; 22:362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bardeskar NS, Ahir-Bist SP, Mehta PR, Samant-Mavani P, Nanavati R, Mania-Pramanik J. Anti-retroviral therapy failure in HIV-1 infected pregnant women and its associated risk of HIV transmission. Arch Gynecol Obstet 2020; 302:1229–35. [DOI] [PubMed] [Google Scholar]
- 37. Mafaune HW, Sacks E, Chadambuka A, et al. Effectiveness of maternal transmission risk stratification in identification of infants for HIV birth testing: lessons from Zimbabwe. J Acquir Immune Defic Syndr 2020; 84(Suppl 1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moyo F, Haeri Mazanderani A, Murray T, et al. Characterizing viral load burden among HIV-infected women around the time of delivery: findings from four tertiary obstetric units in Gauteng, South Africa. J Acquir Immune Defic Syndr 2020; 83:390–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.