Abstract
Background
The effect of malaria infection on the immunogenicity of the recombinant vesicular stomatitis virus–Zaire Ebola virus envelope glycoprotein (GP) vaccine (rVSVΔG-ZEBOV-GP) (ERVEBO) is unknown.
Methods
The Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE) vaccinated 7998 asymptomatic adults with rVSVΔG-ZEBOV-GP during the 2014–2016 Ebola epidemic. In STRIVE’s immunogenicity substudy, participants provided blood samples at baseline and at 1, 6, and 9–12 months. Anti-GP binding and neutralizing antibodies were measured using validated assays. Baseline samples were tested for malaria parasites by polymerase chain reaction.
Results
Overall, 506 participants enrolled in the immunogenicity substudy and had ≥1 postvaccination antibody titer. Of 499 participants with a result, baseline malaria parasitemia was detected in 73 (14.6%). All GP enzyme-linked immunosorbent assay (ELISA) and plaque reduction neutralization test (PRNT) geometric mean titers (GMTs) at 1, 6, and 9–12 months were above baseline, and 94.1% of participants showed seroresponse by GP-ELISA (≥2-fold rise and ≥200 ELISA units/mL), while 81.5% showed seroresponse by PRNT (≥4-fold rise) at ≥1 postvaccination assessment. In participants with baseline malaria parasitemia, the PRNT seroresponse proportion was lower, while PRNT GMTs and GP-ELISA seroresponse and GMTs showed a trend toward lower responses at 6 and 9–12 months.
Conclusion
Asymptomatic adults with or without malaria parasitemia had robust immune responses to rVSVΔG-ZEBOV-GP, persisting for 9–12 months. Responses in those with malaria parasitemia were somewhat lower.
Keywords: Ebola, Ebola vaccine, immunogenicity, malaria, Sierra Leone
We assessed the immunogenicity of recombinant vesicular stomatitis virus–Zaire Ebola virus envelope glycoprotein vaccine in Sierra Leone adults with or without asymptomatic malaria parasitemia. A robust immune response persisted for 9–12 months in both study groups. Responses in participants with asymptomatic malaria parasitemia were somewhat lower.
The magnitude and complexity of the 2014–2016 Ebola outbreak led to concerns that conventional response measures would not control the outbreak. The global public health community, including affected countries recognized an urgent need for rapid development of safe and effective Ebola vaccines [1]. Several phase 1 studies of the candidate Ebola vaccine, recombinant vesicular stomatitis virus (VSV)–Zaire Ebola virus envelope glycoprotein (GP) vaccine (rVSVΔG-ZEBOV-GP; ERVEBO; Merck Sharp & Dohme, a subsidiary of Merck & Co) were conducted in Africa, Europe, and North America, and 3 phase 2/3 clinical trials were conducted in Africa, including the Centers for Disease Control and Prevention (CDC)–sponsored Sierra Leone Trial to introduce a Vaccine Against Ebola (STRIVE). These trials have provided data regarding the safety profile of this replication-competent recombinant vaccine and, in 1 trial in Guinea, demonstrated high vaccine efficacy when deployed for ring vaccination of contacts and contacts of contacts for Ebola cases [2–8]. This vaccine was deployed in subsequent Ebola outbreaks [9]. Since November 2019, it has been licensed in Europe, the United States, and several African countries and prequalified by the World Health Organization [10–13].
Although immunologic correlates of protection against Ebola have not been established, antibodies to Zaire ebolavirus GP are considered to be an indirect measure of protection [14, 15]. Challenge studies in nonhuman primates indicate that the humoral response to this GP is protective [16, 17]. Human trials have consistently documented a robust antibody response to the GP by 28 days after vaccination, with persistence for up to 2 years [2, 5–7, 18–22]. However, the titers required to prevent infection or severe outcomes remain unclear. In addition, direct comparison of human and nonhuman primate anti-GP titers is difficult, both because nonhuman primates mount higher humoral immune responses than humans and because of inherent difficulties with interpretation across studies, especially problematic for older studies that used unvalidated assays.
Understanding factors that may blunt rVSVΔG-ZEBOV-GP immunogenicity is important for widespread field implementation. Active malaria infection can inhibit the immune response to several vaccines [23–26]. Ebola outbreaks typically occur in African countries that include the countries with the highest malaria burden in the world [27], so evaluating the impact of malaria infection on rVSVΔG-ZEBOV-GP response has practical relevance. We report the results of the STRIVE immunogenicity substudy, in which validated assays were used to assess baseline seroprevalence of anti-GP and neutralizing antibodies and to document the magnitude and duration of antibody response through 9–12 months after vaccination with rVSVΔG-ZEBOV-GP. We also evaluate the association of baseline asymptomatic malaria infection with vaccine response.
MATERIALS AND METHODS
STRIVE was a clinical trial of rVSVΔG-ZEBOV-GP conducted during the 2014–2016 Ebola outbreak in Sierra Leone in which adult (≥18 years old), nonpregnant healthcare and frontline Ebola response workers were individually randomized to immediate or deferred (18–24 weeks later) vaccination and were followed up for 6 months to assess the safety and efficacy of the vaccine [8]. Detailed methods and safety results have been reported [8]. Potential participants with oral temperature >38oC or any acute illness symptoms were excluded. The rVSVΔG-ZEBOV-GP vaccine is a live attenuated replication-competent recombinant vaccine comprising an Indiana strain VSV vector in which the VSV envelope GP gene is replaced with that of the Kikwit strain of the Zaire Ebola virus. A total of 7998 participants received a single 1.0-mL nominal dose of 2 × 107 plaque-forming units/mL, given by intramuscular injection [8].
Immunogenicity Substudy Enrollment, Specimen Collection, and Handling
The immunogenicity substudy enrolled participants from 1 of the 7 STRIVE study sites (Connaught Hospital, Freetown) during June–September 2015, with a goal of accruing 500 substudy participants. Malaria transmission is perennial in Sierra Leone, with peak incidence during June and July. Substudy participants provided whole-blood samples collected in serum separator tubes just before vaccination (referred to as baseline) and at 1 month (±6 days), 6 months (±16 days), and 9–12 months (±20 days) after vaccination. The specimens were transported promptly to the STRIVE immunogenicity laboratory in Connaught Hospital, where they were centrifuged within an hour of arrival at 1100–1300g for 10 minutes to further separate serum and clot. Aliquoted serum samples from baseline and follow-up and blood clots from baseline specimens were stored in Sierra Leone at −80ºC for up to 6 months, then shipped by air on dry ice to the CDC for longer-term storage. Aliquots of serum samples used in this study were sent to Sterigenics to be sterilized by treatment with gamma irradiation at the target dose of 50 kGy [28].
Malaria Testing
Malaria testing was performed at the CDC. Blood clots were homogenized in a 50-mL conical tube by passing them through a wire mesh and then centrifuging at 2000g for 5 minutes. From this homogenate, 200 µL was added directly to 20 µL of proteinase K in a 1.5-mL microcentrifuge tube, and DNA was extracted using the Qiagen DNA Mini Kit (Qiagen), according to the manufacturer’s protocol. To assay for parasite DNA, real-time photoelectron-induced transfer polymerase chain reaction (PCR) was performed, as described elsewhere [29], with species-specific nested PCR used to confirm positive results [30]. To translate between the real-time photoelectron-induced transfer PCR result and estimated parasite density, cycle threshold values were extrapolated to parasite densities (in parasites per microliter of blood) through a DNA standard curve, as described elsewhere [29].
Antibody Measurement
All samples were tested at Q2 Solutions (formerly Focus Diagnostics). Total anti-GP immunoglobulin G was measured using the validated Filovirus Animal Nonclinical Group GP indirect enzyme-linked immunosorbent assay (ELISA) [31]. Microtiter plates were coated with purified ZEBOV Kikwit recombinant GP. Serum samples were incubated in the recombinant GP–coated wells with a serial-diluted reference standard and incubated with goat anti-human immunoglobulin G horseradish peroxidase conjugate. The enzymatic reaction was stopped using a sulfuric acid solution. Optical density was measured on an ELISA plate reader and titer concentrations (in GP-ELISA units per milliliter) were calculated from the standard curve, using a 4-parameter logistic curve fit.
Neutralizing antibodies to the rVSVΔG-ZEBOV-GP vaccine strain were measured using the validated plaque reduction neutralization test (PRNT). Serum was diluted from 1:5 to 1:10 240 and mixed with an equal volume of diluted rVSVΔG-ZEBOV-GP for final dilutions of 1:10 to 1:20 480. Neutralization proceeded over an 18-hour period at 2ºC–8ºC, after which the serum-virus mixture was used to inoculate Vero cells. Viral adsorption was done at 37ºC ± 2ºC for 60 minutes, followed by a methylcellulose overlay; cells were subsequently incubated at 37ºC ± 2ºC for 2 days. Plaques were visualized with crystal violet stain and counted using the ViruSpot from Autoimmun Diagnostika. A neutralizing titer was defined as one that resulted in a 60% reduction in viral plaques in the presence of serum, compared with virus control without serum.
Data Analysis and Interpretation
Age was dichotomized as 18–50 vs >50 years of age. Baseline malaria parasitemia was analyzed as present or absent. When malaria parasites were present, parasite density was categorized as “low” (<10/μL), “moderate” (10–200/μL), or “high” (>200/μL).
For both GP-ELISA and PRNT, analyses included calculation of geometric mean titer (GMT) at baseline and at 1, 6, and 9–12 months after vaccination, as well as geometric mean fold rise (GMFR) and seroresponse at 1, 6, and 9–12 months. For GP-ELISA, seroresponse was defined 2 ways: (1) ≥2-fold increase from baseline and ≥200 ELISA units (EU)/mL, the definition that best differentiated vaccine from placebo recipients in the Partnership for Research on Ebola Vaccines in Liberia (PREVAIL) clinical trial [32]; and (2) ≥4-fold increase from baseline. These definitions were prespecified in the development plan for the vaccine, though not specifically for this study. For PRNT, seroresponse was defined as ≥4-fold increase from baseline.
The 95% confidence intervals (CIs) for GMTs and GMFRs were based on analysis of variance, and the CI for seroresponse was based on the exact binomial method. For GMTs, all serum samples obtained at baseline or during the specified postvaccination window were included, whereas a baseline result was required for calculation of GMFRs and seroresponse. Comparisons between groups were made by assessing whether or not 95% CIs overlapped. Imputation was not performed for missing data. Baseline positive results were defined as ≥200 EU/mL for GP-ELISA and as any reaction above the lower limit of quantification (LLOQ) for PRNT; the LLOQ was 36.11 EU/mL for GP-ELISA, and 35 for PRNT. For measurements below the LLOQ, samples were assigned a result of half the LLOQ (eg, 18.06 EU/mL for ELISA).
These measures of vaccine immune response were calculated separately for participants in whom malaria parasitemia was present versus absent at baseline. In addition, analysis of covariance modeling of GP-ELISA and PRNT GMTs at each postvaccination time point by sex was performed, using baseline GP-ELISA and malaria parasitemia as covariates. Statistical analyses were conducted using SAS software, version 9.4.
Human Participants Protection
Trained STRIVE staff described this voluntary substudy to potential participants, and participants provided written informed consent to participate in the immunogenicity substudy; this consent was separate from and in addition to the informed consent to participate in the main STRIVE study. The immunogenicity protocol was reviewed and approved by the Sierra Leone Ethics and Review Committee, the Pharmacy Board of Sierra Leone, and the CDC Institutional Review Board. The study was conducted according to Good Clinical Laboratory Practice guidelines. STRIVE was registered with clinicaltrials.gov (NCT02378753) and the Pan African Clinical Trials Registry (PACTR201502001037220).
RESULTS
A total of 508 participants were vaccinated and enrolled in the immunogenicity substudy. For the overall GMT analysis, we excluded 2 participants who had no evaluable result from a sample obtained within the allowed postvaccination time windows, yielding 506 (99.6%) participants for analysis (Table 1). More than half of participants (293 of 506 [57.9%]) were men, and the large majority (468 of 506 [92.5%]) were 18–50 years of age. The subset of these participants who also had a baseline result were included in the overall GMFR and seroresponse analyses (503 of 506 [99.4%] for GP-ELISA and 438 of 506 [86.6%] for PRNT; Tables 1 and 2). For the malaria analyses, we included the 499 participants who had an evaluable result from the baseline malaria testing (Table 1).
Table 1.
Baseline Characteristics of Participants in the Immunogenicity Substudy of the Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE)
| Participants, No. (%) | |||||||
| Characteristic | Total (N = 506) | Any Baseline Malaria Result (n = 499) |
Malaria Negative at Baseline (n = 426) |
Malaria Positive at Baseline (n = 73) |
Parasite Density |
||
| Low (0.1 to <10/mL) (n = 31) | Moderate (10–200/mL) (n = 29) | High (>200/mL) (n = 13) | |||||
| Sex | |||||||
| Male | 293 (57.9) | 288 (57.7) | 230 (54.0) | 58 (79.4) | 29 (93.6) | 20 (69.0) | 9 (30.8) |
| Female | 213 (42.1) | 211 (42.3) | 196 (46.0) | 15 (20.6) | 2 (6.4) | 9 (31.0) | 4 (69.2) |
| Age | |||||||
| 18–50 y | 468 (92.5) | 461 (92.4) | 392 (92.0) | 69 (94.5) | 30 (96.8) | 28 (96.6) | 11 (84.6) |
| >50 y | 38 (7.5) | 38 (7.6) | 34 (8.0) | 4 (5.5) | 1 (3.2) | 1 (3.4) | 2 (15.4) |
| Positive for Ebola at baseline (before vaccination) | |||||||
| GP-ELISA | |||||||
| No | 427 (84.4) | 421 (84.4) | 366 (85.9) | 55 (75.3) | 27 (87.1) | 20 (69.0) | 8 (61.5) |
| Yes | 76 (15.0) | 75 (15.0) | 58 (13.6) | 17 (23.3) | 4 (12.9) | 8 (27.6) | 5 (38.5) |
| Missinga | 3 (0.6) | 3 (0.6) | 2 (0.5) | 1 (1.4) | 0 (0) | 1 (3.4) | 0 (0) |
| PRNT | |||||||
| No | 434 (85.8) | 427 (85.6) | 365 (85.7) | 62 (84.9) | 27 (87.1) | 24 (82.8) | 11 (84.6) |
| Yes | 4 (0.9) | 4 (0.8) | 3 (0.7) | 1 (1.4) | 0 (0) | 1 (3.5) | 0 (0) |
| Missinga | 68 (13.4) | 68 (13.6) | 58 (13.6) | 10 (13.7) | 4 (12.9) | 4 (13.8) | 2 (15.4) |
Note: Table includes participants with ≥1 evaluable result from a sample obtained within the allowed postvaccination time windows.
Abbreviations: GP-ELISA, glycoprotein enzyme-linked immunosorbent assay; PRNT, plaque reduction neutralization test.
aA baseline specimen was not available for 3 participants. An additional 65 participants had indeterminate results from baseline PRNT testing.
Table 2.
Overall Glycoprotein Enzyme-Linked Immunosorbent Assay and Plaque Reduction Neutralization Test Results at Baseline and 1, 6, and 9–12 Months After Vaccination with Recombinant Vesicular Stomatitis Virus–Zaire Ebola Virus Envelope Glycoprotein Vaccine, in the Immunogenicity Substudy of the Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE)
| Test and Result |
Time Point | ||||
|---|---|---|---|---|---|
| Baseline | mo 1 | mo 6 | mo 9–12 | Any Time After Vaccination | |
| GP-ELISA (N = 506)a | |||||
| Participants in GMT analysis, no. | 503 | 443 | 383 | 393 | NA |
| GMT (95% CI) | 92.7 (85.3–100.9) |
964.3 (878.7–1058.3) |
751.8 (690.6–818.4) |
760.8 (697.6–829.8) |
|
| Participants in GMFR analysis, no. | NA | 441 | 381 | 393 | NA |
| GMFR (95% CI) | 10.7 (9.6–12.0) |
8.1 (7.3–9.1) |
8.4 (7.5–9.4) |
||
| Seroresponders/participants in 1st seroresponse analysis, no. | NA | 397/441 | 341/381 | 345/393 | 446/474 |
| Seroresponse ≥2-fold increase from baseline and ≥200 EU/mL,% (95% CI) |
90.0 (86.8–92.7) |
89.5 (86.0–92.4) |
87.8 (84.1–90.9) |
94.1 (91.6–96.0) |
|
| Seroresponders/participants in 2nd seroresponse analysis, no. | NA | 352/441 | 295/381 | 292/393 | 414/474 |
| Seroresponse ≥4-fold increase from baseline, % (95% CI) | 79.8 (75.8–83.5) |
77.4 (72.9–81.5) |
74.3 (69.7–78.6) |
87.3 (84.0–92.0) |
|
| PRNT (N = 504)a | |||||
| Participants in GMT analysis, no. | 438 | 437 | 382 | 396 | NA |
| GMT (95% CI) | <35 (<35 to <35) |
116.0 (105.7–127.4) |
95.3 (86.3–105.3) |
119.9 (107.9–133.2) |
|
| Participants in GMFR analysis, no. | NA | 376 | 326 | 342 | NA |
| GMFR (95% CI) | 6.3 (5.7–7.0) |
5.4 (4.8–6.0) |
6.8 (6.1–7.6) |
||
| Seroresponders/participants in seroresponse analysis, no. | NA | 265/376 | 211/326 | 237/342 | 334/410 |
| Seroresponse ≥4-fold increase from baseline, % (95% CI) | 70.5 (65.6–75.0) |
64.7 (59.3–69.9) |
69.3 (64.1–74.1) |
81.5 (77.4–85.1) |
Abbreviations: CI, confidence interval; EU, enzyme-linked immunosorbent assay units; GMFR, geometric mean fold rise; GMT, geometric mean titer; GP-ELISA, glycoprotein enzyme-linked immunosorbent assay; NA, not applicable; PRNT, plaque reduction neutralization test.
aNumber of participants with serology data at ≥1 time point according to the study group to which they were randomized.
Malaria Testing
Among the 499 participants with a baseline malaria result, parasitemia was detected in 73 (14.6%). Parasite density was low in 31 (42.5%), moderate in 29 (39.7%), and high in 13 (17.8%). The age group distributions of those with and those without malaria parasitemia were similar, but participants with parasitemia were more likely to be men (58 of 73 [79.4%]) than women (15 of 73 [20.6%]) (Table 1). However, among those with parasitemia, women were more likely than men to have high parasite density; men were the majority of the groups with low (93.6%) or moderate (69.0%) parasite density, whereas women were the majority (69.2%) of the high-parasite-density group (Table 1).
GP-ELISA and PRNT Measurement
Positive baseline GP-ELISA (≥200 EU/mL) titers were present in 76 of 506 participants (15.0%), and positive baseline PRNT titers were present in 4 of 438 (0.9%) (Table 1). All participants with positive baseline PRNT titers had positive baseline GP-ELISA results. The distribution of GP-ELISA results for the group with missing baseline PRNT results was similar to that of the group with an evaluable result (data not shown). GP-ELISA GMTs were higher than baseline (92.7 EU/mL) at 1, 6, and 9–12 months (964.3, 751.8, and 760.8 EU/mL, respectively; Table 2 and Figure 1A).
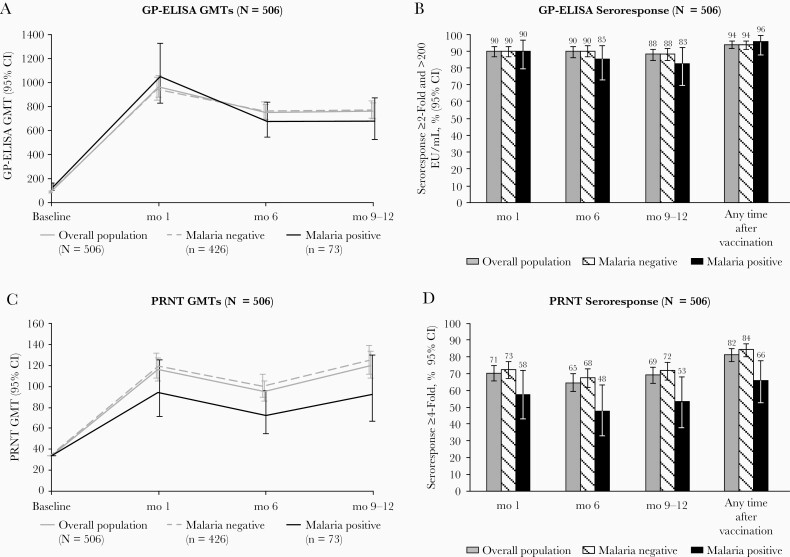
Figure 1.
Antibody responses through months 9–12 after vaccination with recombinant vesicular stomatitis virus–Zaire Ebola virus envelope glycoprotein (GP) vaccine, by baseline asymptomatic malaria infection, in the immunogenicity substudy of the Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE). A, B, GP enzyme-linked immunosorbent assay (ELISA) results. C, D, Plaque reduction neutralization test (PRNT) results, including geometric mean titers (GMTs) (A, C) and seroresponse percentages (B, D). A ≥2-fold increase from baseline along with ≥200 ELISA units (EU)/mL was the definition that best differentiated vaccine from placebo recipients in the Partnership for Research on Ebola Vaccines in Liberia (PREVAIL) clinical trial. Abbreviation: CI, confidence interval.
GMFRs showed a similar pattern, peaking 1 month after vaccination and persisting at 6 months and 9–12 months after vaccination (Table 2). Seroresponse, based on the definition of ≥2-fold increase in antibody titer from baseline and antibody titer ≥200 EU/mL, occurred in ≥87.8% of participants at each postvaccination assessment and in 94.1% at ≥1 postvaccination assessment (Table 2 and Figure 1B). Seroresponse based on the definition of ≥4-fold increase in titer was lower, occurring in 87.3% at ≥1 postvaccination assessment (Table 2). For PRNT, GMTs increased to 116 at 1 month and remained elevated, at about 120, at 9–12 months (Table 2 and Figure 1C), and GMFRs and seroresponse measures also showed robust response. However, the seroresponse percentage was lower for PRNT than for GP-ELISA, with 81.5% meeting the PRNT seroresponse definition at ≥1 postvaccination assessment (Table 2 and Figure 1D).
Postvaccination GMTs were somewhat lower in men than in women, but no consistent pattern was seen by age group (Supplementary Figure 1). The baseline GMT was, by definition, higher in the baseline seropositive group. Postvaccination GMTs were also higher in this group, but the seroresponse percentages were nonetheless lower, because of the higher postvaccination GMTs required to meet either seroresponse definition from a high baseline GMT.
Association of Malaria Parasitemia With Immune Response
Baseline GP-ELISA titers were slightly higher in the group of participants in whom malaria parasitemia was present than in the group in whom it was absent (129 vs 87 EU/mL, respectively, with nonoverlapping CIs; Figure 1A and Supplementary Table 1). In the group of participants with parasitemia at baseline, postvaccination GP-ELISA GMTs and seroresponse percentages showed a trend toward lower responses at 6 and 9–12 months, although CIs overlapped widely (Figure 1B and Supplementary Table 1). PRNT analysis also showed lower point estimates for GMT titers and seroresponse percentages at each of the postvaccination time points in participants who were parasitemic at baseline, again with overlapping CIs (Figure 1C and 1D and Supplementary Table 2). However, the proportion meeting the PRNT seroresponse definition at any point after vaccination was significantly lower for parasitemic than for nonparasitemic participants (66.1% vs 84.3%, respectively; Figure 1D and Supplementary Table 2) with nonoverlapping CIs.
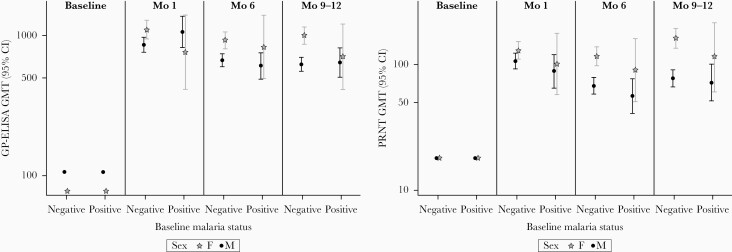
Analyses by categories of parasite density showed no consistent pattern of relationship between density and immune response (Supplementary Figure 2). Multivariate analyses of vaccine immune response including baseline malaria parasitemia (present vs absent) and baseline GP-ELISA as covariates yielded different results by sex so are presented separately (Figure 2). Among the group of participants who did not have baseline malaria parasitemia, this analysis confirmed the pattern of higher postvaccination GMTs in women. For the group of participants with malaria parasitemia, however, CIs were too broad to draw conclusions about differences by sex.
Figure 2.
Antibody responses by baseline malaria parasitemia (present vs absent) and by sex, at baseline and 1, 6 and 9–12 months after vaccination with recombinant vesicular stomatitis virus–Zaire Ebola virus envelope glycoprotein (GP) vaccine. Results from the immunogenicity substudy of the Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) and based on analysis of covariance adjusted for the GP-ELISA GMTs and baseline malaria status by visit and sex. Abbreviations: CI, confidence interval; GMT, geometric mean titer; PRNT, plaque reduction neutralization test.
DISCUSSION
The STRIVE immunogenicity data demonstrate that vaccination with rVSVΔG-ZEBOV-GP elicited robust immune responses in vaccinated persons by 1 month after vaccination, responses that persisted, albeit at slightly lower GMTs, through 9–12 months after vaccination. These results are consistent with those other studies that reported immune responses to rVSVΔG-ZEBOV-GP, using the same validated ELISA and PRNT [22, 33]. The current study demonstrates that a somewhat lower response to vaccination is achieved in participants with asymptomatic malaria parasitemia at baseline. Although most participants with asymptomatic malaria infection met seroresponse definitions at most postvaccination time points, we found significantly lower PRNT seroresponse and trends toward lower GP-ELISA seroresponse and lower PRNT and GP-ELISA GMTs.
However, although the number of participants with high malaria parasite density was small, we saw no clear dose-response relationship. Thus, the clinical and practical relevance, if any, of these findings regarding asymptomatic malaria infection are unclear. It is plausible that malaria infection, especially symptomatic infection, could reduce the immune response to vaccination; this has been reported occasionally with vaccines against other infectious diseases [26]. However, our results should be interpreted in light of the clinical trial results from Guinea [4] and the vaccine effectiveness results from the 2018–2020 outbreak in the Democratic Republic of the Congo [9]. Although neither specifically assessed the impact of malaria infection on vaccine effectiveness, both showed that rVSVΔG-ZEBOV-GP provided excellent protection against Ebola in malaria-endemic settings.
Some complexities in our data that bear on the finding of lower vaccine response in participants with baseline malaria parasitemia merit further discussion. First, our observation that baseline GP-ELISA titers were higher in malaria-positive participants was unexpected. The lack of increased baseline PRNT seropositivity in malaria-positive participants argues against their having been exposed to Ebola, and no STRIVE participants had a clinical history of Ebola disease. Rather, we believe this finding is most likely attributable to nonspecific cross-reactivity due to the immune activation caused by malaria or to higher rates of previous non-Ebola infections that yielded cross-reactive antibodies. Perhaps baseline malaria infection helps explain the observation of higher baseline titers in other studies conducted in African populations, compared with non-African populations, although gamma irradiation of the STRIVE specimens certainly also contributed to the higher baseline titers we observed [22, 33]. STRIVE participants are likely to have had frequent malaria exposure throughout their lives; responses may differ in populations in which malaria is less common.
Second, seroresponse and GMFR, by definition, relate postvaccination titers to baseline titers. The lower seroresponse we observed in participants with baseline malaria parasitemia is thus to some extent attributable to higher baseline titers. However, this is not the whole story, as absolute postvaccination GMTs were also lower in participants with baseline malaria parasitemia. Third, further complicating the interpretation of the malaria data, is that women in our study were substantially less likely than men to have baseline malaria parasitemia—present in 7% of women versus 20% of men. Women appeared to have more vigorous responses to rVSVΔG-ZEBOV-GP than men, even in developed country settings where malaria is not endemic [20]. As our study included only 15 women with baseline malaria parasitemia, 4 of whom had a baseline positive GP-ELISA, it was not large enough to disentangle the effects of baseline GP-ELISA seropositivity, malaria parasitemia, and sex on vaccine response. It is also important to emphasize that this study was not designed or powered for hypothesis testing, so we have not conducted formal statistical testing.
Beyond our novel findings concerning the impact of asymptomatic baseline malaria infection, our results generally accord with the existing scientific literature on the immunogenicity of rVSVΔG-ZEBOV-GP. Robust immune responses have been demonstrated in separate studies in both regions considered to be at risk for Ebola Zaire outbreaks (African countries with previous outbreaks) and regions not at risk (North America and Europe) [2, 3, 5–7, 18, 19, 22]. Similarly to several previous studies, we found that a fraction of vaccinated persons—on the order of 5%–15%, depending on the postvaccination time point—did not produce anti-GP antibodies according to our criteria, though the implications for protection are unclear.
The impact of human immunodeficiency virus (HIV) infection on vaccine response is a concern, especially in high-prevalence areas. The trial in Liberia reported modest reductions in immune response with HIV infection [6]. STRIVE did not test participants for HIV infection, which is uncommon in adults in Sierra Leone, with an estimated prevalence of <2%, and no participants with self-reported HIV infection were included in the immunogenicity evaluation [34].
Our findings have several limitations beyond those already discussed. Regarding our malaria-related results, it is important to emphasize that the STRIVE participants were asymptomatic; our results cannot be generalized to persons with symptomatic malaria. Moreover, neither children nor pregnant women were included in STRIVE, yet both groups commonly experience malaria infection with high parasite density. Our results may not generalize to these groups, either. The differences we observed were generally small, with overlapping confidence limits, and their clinical implications for protection are unclear. It would not be surprising if any effects on vaccine immunogenicity were larger with symptomatic malaria, which is usually associated with higher parasite density.
We did not assess malaria infection after vaccination, but given the intensity of malaria transmission in Sierra Leone, it is likely that additional malaria infections occurred in many participants during follow-up. Our study provides no information about the possible impact of malaria infection during the postvaccination period on antibody durability. The use of validated assays is a strength of our study, but our results must be interpreted knowing that serum samples were gamma-irradiated to eliminate any theoretical risk of Ebola infection to laboratory workers. Gamma irradiation causes an approximate 20% elevation in prevaccination antibodies measured by the GP-ELISA, though not in those measured by the PRNT [28]. Irradiation also produces an approximate 20% reduction in postvaccination antibodies measured by both GP-ELISA and PRNT [35]. These changes are unlikely to change our general conclusions, since they are expected to affect all serum samples in the study similarly.
In conclusion, the STRIVE immunogenicity substudy reaffirms previous studies demonstrating robust immune response to rVSVΔG-ZEBOV-GP. Vaccination yields binding and functional antibody titers that remain elevated through 9–12 months after vaccination. Asymptomatic malaria infection may lower this response to some extent, highlighting knowledge gaps about the possible effects of malaria infection on vaccine-induced immune response and the importance of evaluating new vaccines in African populations, whose distinct exposure history to malaria and other infectious agents may have implications for vaccine response. More than 300 000 persons were vaccinated with rVSVΔG-ZEBOV-GP in the public health response to the second-largest Ebola outbreak in history, with 3470 cases and 2287 deaths, in 2018–2020 [36]. Many observers have credited the vaccine with helping prevent an even larger catastrophe [9]. Since then, rVSVΔG-ZEBOV-GP was again deployed in outbreaks in the Democratic Republic of the Congo and Guinea [37, 38]. Noting that the vaccine is being used for responses involving a wide range of participants with differing health status living in malaria-endemic areas, it is encouraging to know that most vaccinated persons, with or without asymptomatic malaria parasitemia, can be expected to have a strong immune response.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful to the staff members of all the organizations in Sierra Leone and the United States that made the Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE) and its immunogenicity substudy possible. We acknowledge the College of Medicine and Allied Health Sciences, University of Sierra Leone, and Ministry of Health and Sanitation staff in Sierra Leone who worked so hard and effectively to implement the trial and the substudy, including Zainab Bangura, Mohamed Kamara, Sallieu Kamara, Juliet Sevallie, Jacob Tengbeh, and Josephine Kai Tongie. We also acknowledge Stephanie Sincock (US Biomedical Advanced Research and Development Authority), Natalie Lukovsky-Akhsanov (US National Institutes of Health), and Peter Browning, Julie Chatt-Soroka, Ebenezer David, Maureen Diaz, Susan Hiers, Curtis Huber, Wendi McDonald, Mark Mandelbaum, Panagiotis Maniatis, Deborah Moore, Laura Rose, and David Wang (US Centers for Disease Control and Prevention [CDC]), whose efforts in Atlanta and Sierra Leone were essential. Mary E. Hanson, PhD, of Merck Sharp & Dohme, a subsidiary of Merck & Co, provided medical writing assistance, and Karyn Davis, also of Merck Sharp & Dohme, provided editorial assistance.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. The STRIVE study was supported by the Centers for Disease Control and Prevention, the Biomedical Advanced Research and Development Authority, and the National Institutes of Health, with additional support from the CDC Foundation. The STRIVE immunogenicity substudy was supported by the US Department of Health and Human Services, the Office of the Assistant Secretary of Preparedness and Response, and the Biomedical Advanced Research and Development Authority (contracts HHSO10201500002C, HHSO10201600031C, and HHSO10201700012C) and by the US Department of Defense, Defense Threat Reduction Agency (contract HDTRA1-15-C-0058).
Potential conflicts of interest. J. S. and K. L. are employees of Merck Sharp & Dohme, a subsidiary of Merck & Co, and may own stock or hold stock options in the company. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Ebola strategy—Ebola and Marburg virus disease epidemics: preparedness, alert, control, and evaluation. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
- 2. Agnandji ST, Huttner A, Zinser ME, et al. . Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med 2016; 374:1647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halperin SA, Arribas JR, Rupp R, et al. ; V920-012 Study Team . Six-month safety data of recombinant vesicular stomatitis virus–Zaire Ebola virus envelope glycoprotein vaccine in a phase 3 double-blind, placebo-controlled randomized study in healthy adults. J Infect Dis 2017; 215:1789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henao-Restrepo AM, Camacho A, Longini IM, et al. . Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 2017; 389:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huttner A, Dayer JA, Yerly S, et al. ; VSV-Ebola Consortium . The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 2015; 15:1156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kennedy SB, Bolay F, Kieh M, et al. ; PREVAIL I Study Group . Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med 2017; 377:1438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Regules JA, Beigel JH, Paolino KM, et al. ; rVSVΔG-ZEBOV-GP Study Group . A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med 2017; 376:330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samai M, Seward JF, Goldstein ST, et al. ; STRIVE Study Team . The Sierra Leone Trial to Introduce a Vaccine against Ebola: an evaluation of rVSV∆G-ZEBOV-GP vaccine tolerability and safety during the West Africa Ebola outbreak. J Infect Dis 2018; 217:S6–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Preliminary results on the efficacy of rVSV-ZEBOV-GP Ebola vaccine using the ring vaccination strategy in the control of an Ebola outbreak in the Democratic Republic of the Congo: an example of integration of research into epidemic response. https://www.who.int/csr/resources/publications/ebola/ebola-ring-vaccination-results-12-april-2019.pdf. Accessed 10 April 2020.
- 10. Eisele P, Irvine S.. ERVEBO® (Ebola Zaire vaccine, live) now registered in four African countries, within 90 days of reference country approval and WHO prequalification. Democratic Republic of the Congo one of the first African countries to register ERVEBO. 2020. https://www.merck.com/news/ervebo-ebola-zaire-vaccine-live-now-registered-in-four-african-countries-within-90-days-of-reference-country-approval-and-who-prequalification/. Accessed 4 November 2020. [Google Scholar]
- 11. European Medicine Agency. Ervebo: Ebola Zaire vaccine (rVSV∆G-ZEBOV-GP, live). European Medicine Agency, 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/ervebo. Accessed 20 June 2020. [Google Scholar]
- 12. Jasarevic T. WHO prequalifies Ebola vaccine, paving the way for its use in high-risk countries. World Health Organization, 2019. https://www.who.int/news/item/12-11-2019-who-prequalifies-ebola-vaccine-paving-the-way-for-its-use-in-high-risk-countries. Accessed 12 November 2019. [Google Scholar]
- 13. US Food and Drug Administration. ERVEBO, Ebola Zaire vaccine, live. US Food and Drug Administration, 2020. https://www.fda.gov/vaccines-blood-biologics/ervebo. accessed 15 April 2020. [Google Scholar]
- 14. Medaglini D, Santoro F, Siegrist CA. Correlates of vaccine-induced protective immunity against Ebola virus disease. Semin Immunol 2018; 39:65–72. [DOI] [PubMed] [Google Scholar]
- 15. Grais RF, Kennedy SB, Mahon BE, et al. . Estimation of the correlates of protection of the rVSVΔG-ZEBOV-GP Zaire ebolavirus vaccine: a post-hoc analysis of data from phase 2/3 clinical trials. Lancet Microbe 2021; 2:e70–e8. [DOI] [PubMed] [Google Scholar]
- 16. Jones SM, Feldmann H, Ströher U, et al. . Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med 2005; 11:786–90. [DOI] [PubMed] [Google Scholar]
- 17. Qiu X, Fernando L, Alimonti JB, et al. . Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong Ebola GP-specific immune responses. PLoS One 2009; 4:e5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agnandji ST, Fernandes JF, Bache EB, et al. ; VEBCON Consortium . Safety and immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine in adults and children in Lambaréné, Gabon: a phase I randomised trial. PLoS Med 2017; 14:e1002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ElSherif MS, Brown C, MacKinnon-Cameron D, et al. ; Canadian Immunization Research Network . Assessing the safety and immunogenicity of recombinant vesicular stomatitis virus Ebola vaccine in healthy adults: a randomized clinical trial. CMAJ 2017; 189:E819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huttner A, Agnandji ST, Combescure C, et al. ; VEBCON; VSV-EBOVAC; VSV-EBOPLUS Consortia . Determinants of antibody persistence across doses and continents after single-dose rVSV-ZEBOV vaccination for Ebola virus disease: an observational cohort study. Lancet Infect Dis 2018; 18:738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huttner A, Siegrist CA. Durability of single-dose rVSV-ZEBOV vaccine responses: what do we know? Expert Rev Vaccines 2018; 17:1105–10. [DOI] [PubMed] [Google Scholar]
- 22. Halperin SA, Das R, Onorato MT, et al. ; V920-012 Study Team . Immunogenicity, lot consistency, and extended safety of rVSVΔG-ZEBOV-GP vaccine: a phase 3 randomized, double-blind, placebo-controlled study in healthy adults. J Infect Dis 2019; 220:1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fowkes FJ, Boeuf P, Beeson JG. Immunity to malaria in an era of declining malaria transmission. Parasitology 2016; 143:139–53. [DOI] [PubMed] [Google Scholar]
- 24. Scholzen A, Sauerwein RW. Immune activation and induction of memory: lessons learned from controlled human malaria infection with plasmodium falciparum. Parasitology 2016; 143:224–35. [DOI] [PubMed] [Google Scholar]
- 25. Williamson WA, Greenwood BM. Impairment of the immune response to vaccination after acute malaria. Lancet 1978; 1:1328–9. [DOI] [PubMed] [Google Scholar]
- 26. Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev 2019; 32:e00084–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization World malaria report 2018. Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- 28. Grant-Klein RJ, Antonello J, Nichols R, et al. . Effects of gamma-irradiated human serum samples from rVSVΔG-ZEBOV-GP (V920) vaccines using a validated plaque-reduction neutralization test. Am J Trop Med Hyg 2021; 104:1751–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lucchi NW, Narayanan J, Karell MA, et al. . Molecular diagnosis of malaria by photo-induced electron transfer fluorogenic primers: PET-PCR. PLoS One 2013; 8:e56677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Snounou G. Detection and identification of the four malaria parasite species infecting humans by PCR amplification. Methods Mol Biol 1996; 50:263–91. [DOI] [PubMed] [Google Scholar]
- 31. Rudge TL Jr, Sankovich KA, Niemuth NA, et al. . Development, qualification, and validation of the filovirus animal nonclinical group anti-Ebola virus glycoprotein immunoglobulin G enzyme-linked immunosorbent assay for human serum samples. PLoS One 2019; 14:e0215457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Antonello J, Grant-Klein RJ, Nichols R, Kennedy SB, Dubey S, Simon JK. Serostatus cutoff levels and fold increase to define seroresponse to recombinant vesicular stomatitis virus—Zaire Ebola virus envelope glycoprotein vaccine: an evidence-based analysis. Vaccine 2020; 38:4885–91. [DOI] [PubMed] [Google Scholar]
- 33. Boum Y, Juan-Giner A, Hitchings M, et al. . Humoral and cellular immune response induced by rVSVDeltaG-ZEBOV-GP vaccine among frontline workers during the 2013–2016 West Africa Ebola outbreak in Guinea. Vaccine 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dwyer-Lindgren L, Cork MA, Sligar A, et al. . Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature 2019; 570:189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grant-Klein RJ, Antonello J, Nichols R, Dubey S, Simon J. Effect of gamma irradiation on the antibody response measured in human serum from subjects vaccinated with recombinant vesicular stomatitis virus-Zaire Ebola virus envelope glycoprotein vaccine. Am J Trop Med Hyg 2019; 101:207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. World Health Organization. 10th Ebola outbreak in the Democratic Republic of the Congo declared over; vigilance against flare-ups and support for survivors must continue. https://www.who.int/news-room/detail/25-06-2020-10th-ebola-outbreak-in-the-democratic-republic-of-the-congo-declared-over-vigilance-against-flare-ups-and-support-for-survivors-must-continue. Accessed 6 October 2020.
- 37. World Health Organization. 11th Ebola outbreak in the Democratic Republic of the Congo declared over. https://www.afro.who.int/news/11th-ebola-outbreak-democratic-republic-congo-declared-over. Accessed 14 December 2020.
- 38. Hackett KM. Ebola vaccinations launch in guinea. 2021. https://www.vaxbeforetravel.com/2021/02/23/ebola-vaccinations-launch-guinea#:~:text=The%20vaccination%20began%20just%2024%20hours%20after%20Guinea,from%20Merck%20in%20the%20initial%20phase%20of%20immunization. Accessed19 May 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.