Abstract
Pancreatic β-cells can secrete insulin via 2 pathways characterized as KATP channel -dependent and -independent. The KATP channel–independent pathway is characterized by a rise in several potential metabolic signaling molecules, including the NADPH/NADP+ ratio and α-ketoglutarate (αKG). Prolyl hydroxylases (PHDs), which belong to the αKG-dependent dioxygenase superfamily, are known to regulate the stability of hypoxia-inducible factor α. In the current study, we assess the role of PHDs in vivo using the pharmacological inhibitor dimethyloxalylglycine (DMOG) and generated β-cell-specific knockout (KO) mice for all 3 isoforms of PHD (β-PHD1 KO, β-PHD2 KO, and β-PHD3 KO mice). DMOG inhibited in vivo insulin secretion in response to glucose challenge and inhibited the first phase of insulin secretion but enhanced the second phase of insulin secretion in isolated islets. None of the β-PHD KO mice showed any significant in vivo defects associated with glucose tolerance and insulin resistance except for β-PHD2 KO mice which had significantly increased plasma insulin during a glucose challenge. Islets from both β-PHD1 KO and β-PHD3 KO had elevated β-cell apoptosis and reduced β-cell mass. Isolated islets from β-PHD1 KO and β-PHD3 KO had impaired glucose-stimulated insulin secretion and glucose-stimulated increases in the ATP/ADP and NADPH/NADP+ ratio. All 3 PHD isoforms are expressed in β-cells, with PHD3 showing the most distinct expression pattern. The lack of each PHD protein did not significantly impair in vivo glucose homeostasis. However, β-PHD1 KO and β-PHD3 KO mice had defective β-cell mass and islet insulin secretion, suggesting that these mice may be predisposed to developing diabetes.
Keywords: islet, insulin secretion, prolyl hydroxylases, cell metabolism, PHD, HIF1α, ARNT/HIF1β, hypoxia, insulin release, metabolism, pancreatic β-cell
Research in Context.
We used both the pharmacological PHD inhibitor DMOG and β-cell-specific KO mice of each isoform of PHD to assess the role of PHDs in vivo.
We show that DMOG inhibited in vivo insulin secretion in response to glucose challenge, and in perifused islets, DMOG inhibited first-phase insulin secretion but enhanced second-phase insulin secretion.
Using β-cell-specific KO mice for PHD1, PHD2, and PHD3, we show that PHD1 and PHD3 play an essential role in regulating islet insulin secretion in vitro but do not significantly impact in vivo glucose homeostasis.
We also show that β-cells from both β-PHD1 KO and β-PHD3 KO had elevated β-cell apoptosis and reduced β-cell mass. β-PHD1 KO and β-PHD3 KO islets had reduced GSIS and glucose-stimulated increases in the ATP/ADP and NADPH/NADP+ ratio.
These studies suggest that both PHD1 and PHD3 play an important role in regulating β-cell function.
Type 2 diabetes is characterized by insulin resistance and dysfunctional pancreatic β-cell insulin secretion (1). The defects associated with the impaired β-cell insulin secretion seen in type 2 diabetes are incompletely understood, but 1 key alteration is an inability to link nutrient metabolism to insulin release properly. When β-cells are functioning normally, glucose is taken up by the glucose-transporter 2 (GLUT2) and is metabolized by glycolysis to produce pyruvate (2-5). Pyruvate enters the tricarboxylic acid cycle (TCA), which leads to an increase in adenosine triphosphate (ATP) production through oxidative phosphorylation. The rise in cytosolic ATP/ADP (adenosine diphosphate) ratio leads to membrane depolarization due to closure of the plasma membrane ATP-sensitive potassium channel (KATP), which activates voltage-gated calcium channels and elevates intracellular calcium (6, 7). Calcium stimulates insulin granule exocytosis (8, 9). This pathway of regulating insulin release is called the KATP channel–dependent or triggering pathway. In addition to this pathway, there is also a KATP channel–independent pathway or the amplifying pathway for regulating insulin release that generates signals such as a rise in the NADPH/NADP+ ratio and cytosolic α-ketoglutarate (αKG) (10, 11).
We have shown that pharmacological or siRNA mediated inhibition of prolyl hydroxylases (PHDs) can regulate both the KATP channel and the KATP channel–independent pathways of insulin secretion, ATP/ADP ratio, and anaplerosis in pancreatic β-cells (12). PHDs belong to the iron- and α-ketoglutarate–dependent dioxygenase enzyme family and function as cellular oxygen sensors (13). PHDs regulate the activity of hypoxia-inducible factor-α (HIFα) through hydroxylation of proline residues in the highly conserved oxygen-dependent degradation domain of HIFα. Under normoxic conditions, hydroxylated HIFα is recognized by von Hippel–Lindau protein and undergoes proteasomal degradation. However, under hypoxic conditions, the activity of PHD is reduced, allowing HIFα to accumulate and bind to hypoxia-inducible factor-1β, triggering a hypoxic cellular response (14, 15). There are 3 PHD isoforms (PHD1, PHD2, and PHD3) each requiring oxygen and αKG as cosubstrates, and iron and ascorbate as cofactors (14-17). Previously, we have shown that the metabolism of αKG by PHDs may be the link between glucose-driven αKG generation and its role in regulating insulin secretion (10, 18).
To further explore the role of PHDs in vivo, we used the pharmacological PHD inhibitor dimethyloxalylglycine (DMOG) and generated β-cell-specific knockout (β-PHD KO) mice of each PHD isoform. We show that DMOG slightly improved glucose homeostasis but inhibited in vivo insulin secretion in response to a glucose challenge. When islets were perifused with DMOG, it inhibited the first phase of insulin secretion but enhanced the second phase of insulin secretion. We also show that DMOG inhibited oxygen consumption in isolated islets and the clonal cell line INS1 (832/13). Pharmacological inhibition of PHDs shows a complex response, suggesting there may be isoform-specific roles of PHDs in β-cells. We show that mouse pancreatic β-cells express all 3 isoforms of PHD and that the PHD isoforms express unique subcellular localization. In β-cells, PHD1 is weakly expressed in the cytosol, PHD2 is expressed primarily in the nucleus, and PHD3 is mainly expressed in the cytosol with weak nuclear expression. β-PHD1, 2, or 3 KO mice show no significant in vivo defects associated with glucose tolerance and insulin resistance, except β-PHD2 KO mice had significantly increased plasma insulin during a glucose challenge. β-Cells from both β-PHD1 KO and β-PHD3 KO had elevated β-cell apoptosis and reduced β-cell mass. Islets from β-PHD1 KO and β-PHD3 KO had impaired glucose-stimulated insulin secretion and glucose-stimulated increases in the ATP/ADP and NADPH/NADP+ ratio. Overall, none of the isoform-specific KO mice had any significant in vivo defects, except for the β-PHD1 KO and β-PHD3 KO mice having reduced β-cell mass and defective islet insulin secretion.
Materials and Methods
Reagents
All reagents were obtained from Life Technologies Inc (Burlington, ON, Canada) or SIGMA (St Louis, MO, USA) unless otherwise specified.
Generation of β-Cell-specific PHD Knockout Mice
Cre-loxP technology was used to generate β-cell-specific, single-isoform PHD1, PHD2, or PHD3 KO (β-PHD1 KO, β-PHD2 KO, and β-PHD3 KO) mice. Previously generated PHD floxed mice (19) with exon 3 in PHD1 and exon 2 in PHD2 and PHD3 flanked by loxP sites were backcrossed for 10 generations with C57Bl/6N mice (C57BL/6N mice, Charles River Laboratories, Strain code 027). Insulin-1 promoter (Ins-1)-Cre mice (C57Bl/6/129) (Jackson Laboratory, stock number 026801; Bar Harbor, ME) were bred with backcrossed PHD floxed mice, generating mice heterozygous for the floxed PHD gene and expressing Ins-1Cre transgene (PHD+/fl; Ins-1Cre+). Wild-type littermates (wt; PHD+/+; Ins-1Cre+) from each mouse model were used as controls. Data from wt animals were pooled as there was no significant difference across mouse models (data not shown). Eight- to 12-week-old β-PHD1 KO (PHD1fl/fl; Ins-1Cre+), β-PHD2 KO (PHD2fl/fl; Ins-1Cre+), and β-PHD3 KO (PHD3fl/fl; Ins-1Cre+) mice and their controls were used for experiments. Polymerase chain reaction (PCR), real-time PCR, and western blotting were used to confirm genotype (see below and Table 1 (20) for genotyping primers). Animals were maintained under controlled conditions with ad libitum access to food (Diet no. 8626; Teklad Diets, Madison, WI, USA) and water. The local ethics committee approved all animal experiments.
Intraperitoneal Glucose Tolerance Test and Insulin Tolerance Test
After a 16-hour fast, mice were administered an intraperitoneal glucose tolerance test (ipGTT) (1.5 g of glucose/kg body weight) as previously described (21-23). Blood glucose was measured from the tail vein at 0, 10, 20, 30, 60, and 120 minutes after injection using a Contour®NEXT glucometer (Bayer, Germany). Following intraperitoneal glucose injection, blood samples were collected at 0, 10, and 30 minutes into lithium heparin–coated collection tubes and separated by centrifugation. Plasma insulin levels were measured using a Rat/Mouse Insulin ELISA Kit (Millipore, Canada). After a 4-hour fast, mice were administered an insulin tolerance test (ITT) (1.2 units of insulin/kg body weight). Blood glucose was measured at 0, 10, 20, 30, 45, 60, 90, and 120 minutes post-injection.
Islet Isolation
Mouse islets were isolated and cultured as previously described (12, 21, 22). Briefly, the pancreas was perfused via the bile duct with Liberase TL and then incubated for 20 minutes at 37°C. The digested pancreas was then passed through a sieve (Bellco) and washed 3 times with ice-cold HANKS buffer followed by resuspension in islet media (RPMI 1640; Fisher Hyclone SH30027), 0.1 M HEPES, 10% fetal bovine serum, 20 mM glutamine). Islets were then hand-picked and cultured overnight before use.
Oxygen Consumption
Oxygen consumption was measured using an XF24 extracellular flux analyzer (Seahorse Bioscience, Billerica, MA) (23). Briefly, 832/13 cells were plated at 4.5 × 104 cells/well and grown to 100% confluence, or 50 islets were plated on poly-L-lysine–coated plates. On the day of the assay, cells were pretreated with 2 mM glucose for 2 hours. Oxygen consumption was then measured during the following treatments: 2 mM glucose; 8 mM glucose ± DMOG (at concentrations indicated in the figure legends); 5 µM oligomycin; 50 µM 2,4-dinitrophenol (DNP) with 20 mM pyruvate; 5 µM rotenone; and 5 µM myxothiazol. Oxygen consumption associated with ATP turnover was determined as the difference between the oxygen consumption in response to oligomycin and 8 mM glucose. Rotenone and myxothiazol were also used to assess oxygen consumption not associated with the electron transport chain.
Gene Expression
RNA was isolated using an Aurum Total RNA Mini Kit (Bio-Rad, Mississauga, ON, Canada). cDNA was synthesized using an iScript cDNA Synthesis Kit (Bio-Rad). Real-time PCR was performed using SsoFast EvaGreen Real-Time PCR Supermix (Bio-Rad) (see Table 2 for primer sequences (20)). All PHD primers were designed with 1 primer located in the floxed exon targeted for deletion. Gene expression was corrected by an internal control gene (cyclophilin) and then expressed as percent control.
Western Blotting
Islets were isolated from mice, and then 10 µg of protein was loaded on a gel. After protein transfer to a PVDF membrane, proteins were labeled using a no-stain protein labeling kit (ThermoFisher Scientific, A44449) (see Fig. 1 (20) for a representative total protein normalization blot). The PVDF membrane was then assessed for PHD1, PHD2, or PHD3 expression. Primary antibodies: rabbit anti-PHD1 (RRID:AB_10861175, Abcam), rabbit anti-PHD2 (RRID:AB_568562, ThermoFisher Scientific), rabbit anti-PHD3 (RRID:AB_2293343, ThermoFisher Scientific). They were then incubated with goat antirabbit IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor Plus 800 (RRID:AB_2633284, ThermoFisher Scientific, A32735). Blots were imaged using an iBright imaging system (ThermoFisher Scientific), and then band intensities were normalized to total protein levels using the iBright™ Analysis Software (ThermoFisher Scientific).
Comprehensive Laboratory Animal Monitoring System
Ten- to 14-week-old mice were individually placed in comprehensive laboratory animal monitoring system (CLAMS) (Columbus Instruments, Columbus, OH) metabolic chambers equipped with O2 and CO2 sensors with ad libitum access to food and water during the experiment as previously described (21, 22). The system measured the following parameters: (volume of oxygen consumed, mL/kg/hour), (volume of carbon dioxide produced, mL/kg/hour), RER (respiratory exchange ratio), heat (kcal/hour), and XY total activity (total horizontal beam breaks in counts). Mice were acclimatized for a 24-hour period, after which data were collected continuously for the next 24 hours and readings were taken every 30 minutes. An average of 2 separate trials for each mouse was used for calculations. O2 consumption and CO2 production rates were used to estimate whole-body glucose oxidation and fat oxidation rates using stoichiometric equations according to Frayn (24), with the assumption that protein oxidation was negligible. Lipid oxidation rate (g/min/kg): (1.67 × VO2) – (1.67 × VCO2). Carbohydrate oxidation rate (g/min/kg): (4.55 × VCO2) – (3.21 × VO2).
Immunohistochemistry
Immunohistochemistry was used to assess β-cell and α-cell mass as previously described (21). Briefly, the pancreas was isolated from mice and fixed in 10% aqueous buffered zinc formalin (Z-fix, Anatech Ltd, MI, USA) and then embedded in paraffin and sectioned at 3 levels separated by 100 µm. Primary antibodies: mouse anti-insulin (1:1000; RRID:AB_305690; Abcam) and rabbit antiglucagon (1:500; RRID:AB_10561971; Abcam). The EnVision™ G|2 Doublestain System, Rabbit/Mouse (DAB+/Permanent Red) (Agilent, K5361, Mississauga, ON) was used to visualize stained sections. The Aperio ScanScope was used to perform the analysis according to the manufacturer’s instructions for determining β-cell and α-cell mass.
For immunofluorescence, sections were incubated with primary antibodies: rabbit anti-PHD1 (RRID:AB_10861175, Abcam), rabbit anti-PHD2 (RRID:AB_568562, ThermoFisher Scientific), rabbit anti-PHD3 (RRID:AB_2293343, ThermoFisher Scientific), guinea pig anti-insulin (1:600; RRID:AB_10013624; Agilent), rabbit anti-Ki67 (1:50; RRID:AB_302459; Abcam), and rabbit anti-cleaved caspase-3 (1:100, RRID:AB_2070042, Cell Signalling). They were then incubated with respective conjugated secondary antibodies: Alexa Fluor 488 (RRID:AB_2340472; Abcam) and 555 (RRID:AB_10694110; Cell Signaling, Danvers, MA), and a nuclear stain: TO-PRO™-3 iodide (1:3000 in PBS, Life Technologies, T3605). Immunofluorescence was analyzed using a Nikon Eclipse Ti microscope. At least 5000 insulin-positive cells were counted for each treatment.
In Vitro Insulin Secretion
Islet glucose-stimulated insulin secretion (GSIS) was measured statically or dynamically as previously described (12, 21, 22). Briefly, static insulin secretion was measured with 10 islets per treatment and was pretreated for 1 hour in KRB with 2 mM glucose and then stimulated with 2 mM glucose (LG) or 10 mM glucose (HG) in the absence or presence of 30 mM KCl + 200 µmol/L diazoxide or 10 mM dimethylmalate (DMM) + 10 mM dimethyl α-ketoglutarate (DMαKG) for 1 hour. For dynamic islet insulin secretion, 25 islets were perifused using a BioRep Technologies perifusion system at a flow rate of 0.25 mL/min with low glucose (2 mM) KRB followed by high glucose plus or minus DMOG and then with high glucose (16.7 mM) plus 30 mM KCl. Insulin was measured using the Rat Insulin RIA kit (Millipore, Billerica, MA, USA). Islet insulin content was measured as previously described (25).
Nucleotide Measurements
Islet nucleotides were measured as previously described (12, 21, 22). Briefly, 40 islets per treatment were pretreated for 1 hour in KRB with 2 mM glucose, followed by the addition of either 2 or 10 mM glucose for 1 hour. Islets were then assessed for ATP, ADP, NADP+, and NADPH levels by high-performance liquid chromatography.
Statistical Analysis
All results are presented as mean ± SEM. Statistical significance was assessed using 1- or 2-way analysis of variance, followed by Tukey’s or Dunnett’s post hoc tests. All statistical analyses were performed using GraphPad Prism 9.
Results
The PHD Inhibitor, DMOG, Improves Glucose Tolerance but Inhibits In Vivo Insulin Secretion
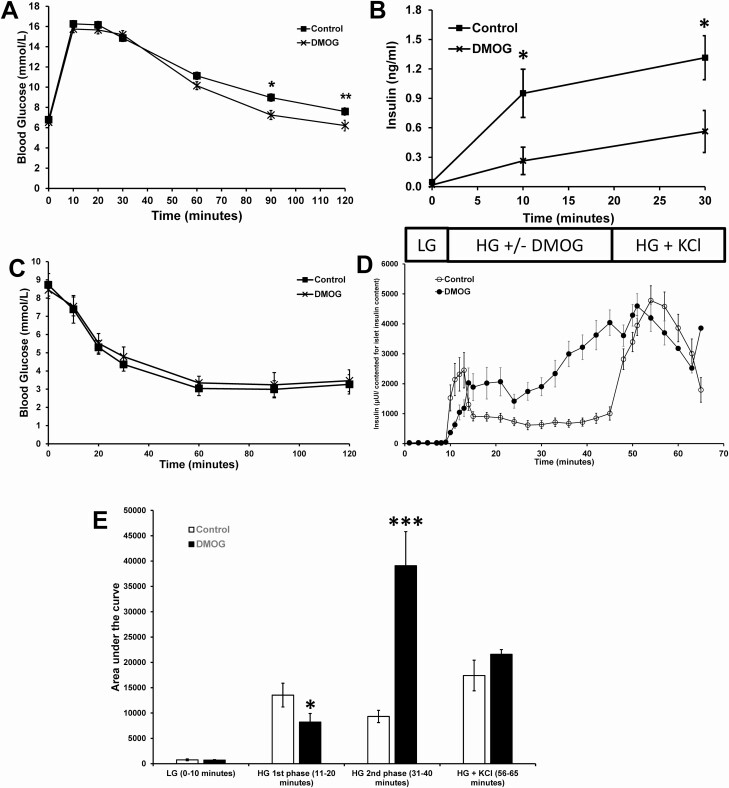
DMOG, 200 µg/g body weight, was injected intraperitoneally into C57BL/6N male mice (9-10 weeks of age), and then 10 minutes later glucose was injected followed by measuring blood glucose and plasma insulin levels for 120 minutes. Blood glucose levels during the ipGTT were significantly lower at 90 and 120 minutes in DMOG-treated mice (Fig. 1A). Blood insulin levels during the ipGTT were significantly lower at 10 and 30 minutes in DMOG-treated mice (Fig. 1B). There was no difference between control and DMOG-treated mice after an ITT (Fig. 1C).
Figure 1.
Effects of DMOG (200 µg/g body weight) on in vivo glucose homeostasis and dynamic islet insulin secretion from male C57BL/6N mice. (A) Blood glucose levels during an ipGTT (n = 12 mice per experimental group). (B) Plasma insulin levels (n = 12 mice per experimental group). (C) Insulin tolerance test (n = 12 mice per experimental group). (D) Islets were stimulated with low glucose (LG, 2 mM) for 10 minutes followed by high glucose (HG, 16.7 mM) plus or minus 5 µM DMOG for 35 minutes followed by HG + KCl (30 mM) for 20 minutes (n = 10 per experimental group; each n represents 25 islets). (E) AUC of D. Data are mean ± SEM. P < .05, **P < .01, and ***P < .001.
The Effects of DMOG on Dynamic Insulin Secretion and Mitochondrial Respiration
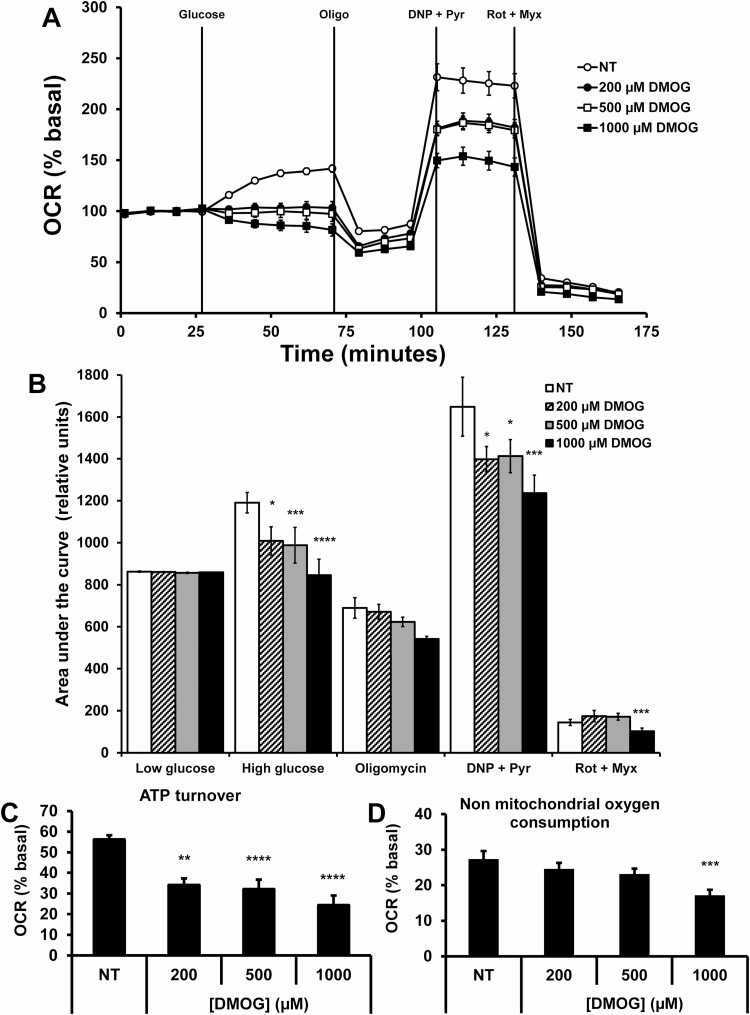
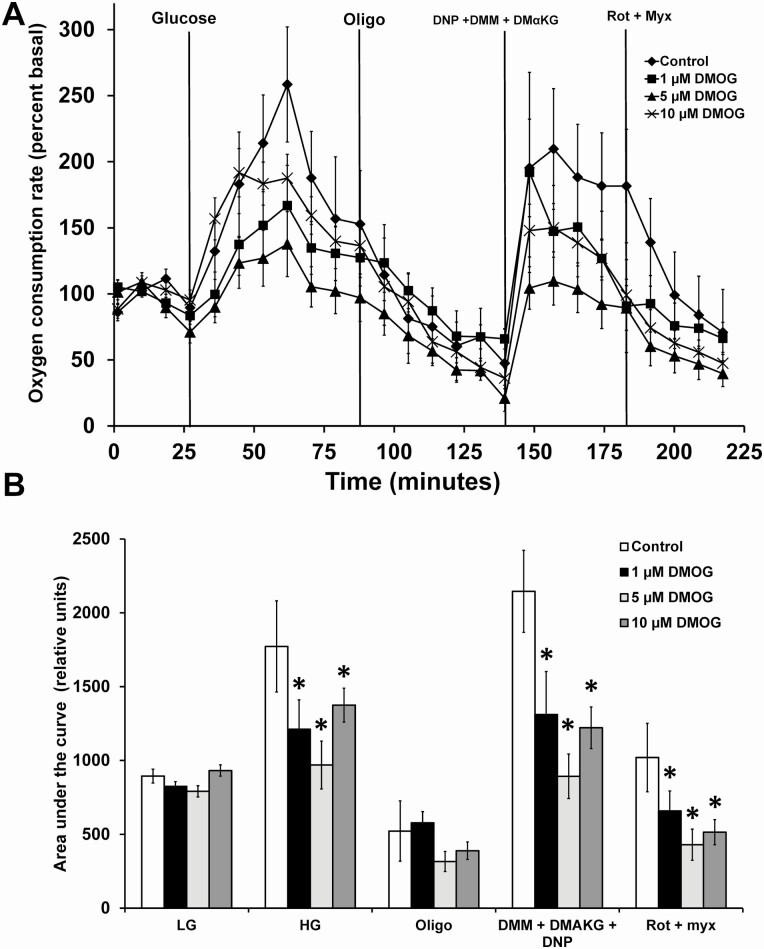
Next, we investigated the effects of DMOG on dynamic insulin secretion in vitro using C57BL/6N male mouse islets. DMOG significantly inhibited first-phase insulin secretion and significantly enhanced insulin secretion during the second phase of insulin secretion (Fig. 1). We next assessed the effects of DMOG on mitochondrial respiration in the clonal cell line 832/13 cells. Oxygen consumption rate (OCR) in response to high glucose was dose-dependently inhibited by DMOG (Fig. 2A and 2B). ATP turnover was also dose-dependently inhibited by DMOG (Fig. 2C) and nonmitochondrial respiration was only significantly inhibited at 1000 µM DMOG (Fig. 2D). OCR in response to high glucose and nonmitochondrial respiration was also inhibited by DMOG in islets from male C57BL/6N mice (Fig. 3).
Figure 2.
Mitochondrial respiration in response to DMOG in INS1 832/13 cells. (A) OCR was measured in response to low glucose (2 mM), high glucose (Glucose, 16.7 mM) plus or minus DMOG (0-1000 µM), oligomycin (Oligo, 10 µM), dinitrophenol (DNP, 50 µM) plus pyruvate (Pyr, 20 mM), and rotenone (Rot, 5 µM) plus myxothiazol (Myx, 5 µM). (B) AUC of A. (C) ATP turnover (HG OCR minus oligomycin OCR. (D) Nonmitochondrial oxygen consumption (rotenone plus myxothiazol OCR). Data are mean ± SEM (n = 10, each n represents 1 well). *P < .05, **P < .01, ***P < .001, ****P < .0001.
Figure 3.
Mitochondrial respiration in response to DMOG in male islets from C57BL/6N mice. (A) Islet OCR in response to low glucose (2 mM), high glucose (Glucose, 16.7 mM) plus or minus DMOG (0-10 µM), oligomycin (Oligo, 20 µM), DNP (100 µM) plus dimethylmalate (DMM, 10 mM) plus dimethyl αketoglutarate (DMαKG, 10 mM), and rotenone (Rot, 10 µM) plus myxothiazol (Myx,10 µM). (B) AUC of A. Data are mean ± SEM (n = 10, each n represents 1 well containing 50 islets). *P < .05.
β-Cell-specific KO of PHD1, PHD2, and PHD3
Since the effects of the PHD inhibitor, DMOG, exhibited a complex effect in vivo and in vitro, we next sought to assess the role of individual PHD proteins in β-cells using a KO mouse model. Pancreatic β-cell-specific PHD KO mice were generated by first backcrossing PHDfl/fl homozygous mice (generated previously (19)) with C57BL/6N mice for 10 generations. The PHDfl/fl homozygous mice were then crossed with the Ins-1Cre transgenic mice, which expressed the Cre recombinase under the control of the mouse Ins1 promoter. Compared with other Cre promoters such as rat insulin promoter (RIP-Cre), which has shown expression in brain regions, Ins1 is more tissue target specific and restricted to expression in β-cells (2). Exon 3 in PHD1 and exon 2 in PHD2 and PHD3 were targeted because they contain the essential His and Asp residues required for Fe2+ binding; the absence of cofactor Fe2+ renders PHD inactive. Mice were born in the expected Mendelian distribution. Genotype was confirmed using PCR.
Islets Express all 3 PHD Isoforms
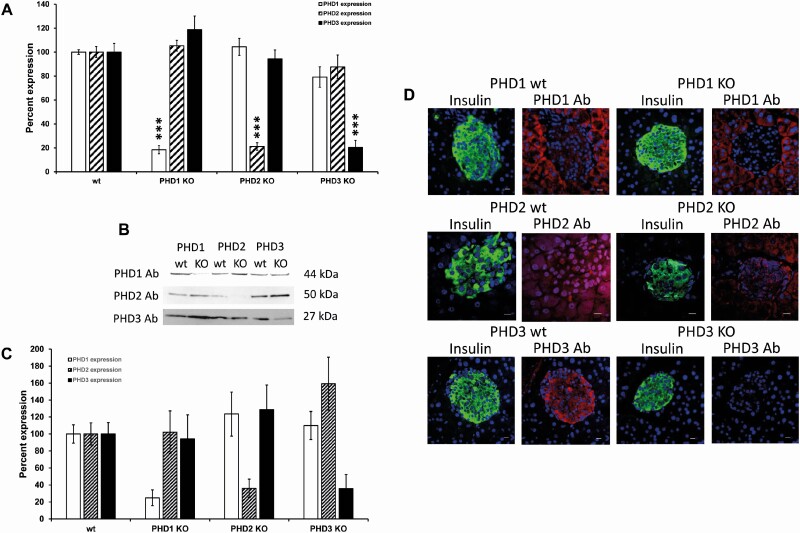
Expression of PHD1, PHD2, and PHD3 in pancreatic β-cells was assessed using real-time PCR, western blotting, and immunofluorescence. All 3 PHD isoforms were expressed at the mRNA (Fig. 4A) and protein level (Fig. 4B and 4C) and colocalized with insulin in islets as assessed by immunofluorescence (Fig. 4D). PHD1 was detected in control islets and acinar tissue with higher levels in acinar tissue (Fig. 4D). There was minimal PHD1 mRNA, protein expression, and immunofluorescent staining in islets from β-cell-specific PHD1 KO mice (Fig. 4). PHD2 was detected in both islets and acinar tissue from control mice, and β-cell-specific PHD2 KO mouse islets showed low tissue PHD2 mRNA and protein expression (Fig. 4). Unlike PHD1 and PHD2, PHD3 showed only islet expression and no acinar tissue expression (Fig. 4D). β-Cell-specific PHD3 KO mice showed reduced PHD3 mRNA and protein expression and were absent in islets when assessed by immunofluorescence (Fig. 4). The mRNA and protein expression levels of PHD1, PHD2, or PHD3 were not altered in response to knocking out individual PHD proteins (Fig. 4A-4C).
Figure 4.
PHD mRNA expression (A) in male PHD1, PHD2, and PHD3 control (wt) and β-cell-specific PHD1-3 knockout (β-PHD1-3 KO) mice (n = 6, each n represents a group of 100 islets). Gene expression was corrected by an internal control gene (cyclophilin) and then expressed as percent control. Western blotting (B and C) (expression was normalized to total protein loaded on the gel and then expressed as a percent of control) (n = 6, each n represents a group of 100 islets). Immunofluorescent staining (D) of PHD1, PHD2, and PHD3 in control (wt) and β-cell-specific PHD1-3 KO (β-PHD1-3 KO) male mice (white scale bar 10 µm). Green = insulin, red = PHD1, PHD2, or PHD3, blue = nuclei (n = 4, each n represents 1 mouse pancreas, 4 mice were used per experimental group).
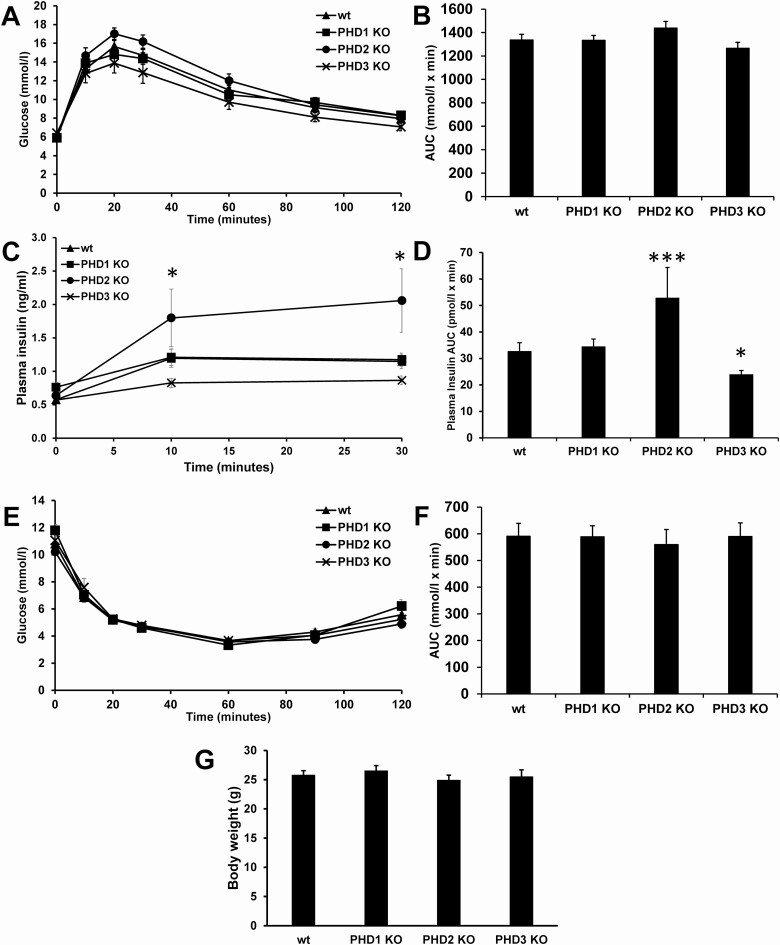
In Vivo Glucose Homeostasis
The in vivo effects on glucose homeostasis due to the loss of PHD1, PHD2, or PHD3 were assessed by ipGTT, ITTs, and CLAMS metabolic studies. The loss of PHD1, PHD2, or PHD3 did not affect the response of male mice to an ipGTT or the response to an ITT (Fig. 5A, 5B, 5E, and 5F). No significant differences in appearance or body weight (Fig. 5G) was observed at birth between control and KO littermates. Plasma insulin levels during the ipGTT were significantly higher in male β-cell-specific PHD2 KO mice and were significantly lower in β-cell-specific PHD3 KO mice compared with control mice (Fig. 5C and 5D). There were no differences seen in body weight (Fig. 5G). Similar results were found for female mice. No significant differences were seen between female control and any of the female β-cell-specific PHD KO mice in response to an ipGTT or ITT (Fig. 2 (20)). There were no significant differences seen between all 3 PHD1, 2, or 3 control mice (+/+/Cre, data not shown), or between different control mice (Fig. 3 (20)), wt mice (+/+/+ mice that have the wt allele and do not express Cre), floxed control mice (fl/fl/+ PHD1 mice that have a floxed allele but do not express Cre), and Cre control mice (+/+/Cre mice that have wild type PHD allele and express Cre).
Figure 5.
In vivo glucose homeostasis in male control (wt), β-PHD1 KO, β-PHD2 KO, and β-PHD3 KO mice. (A) Blood glucose levels during an intraperitoneal glucose tolerance test (ipGTT). (B) AUC of A. (C) plasma insulin during the ipGTT in A. (D) AUC of C. (E) Insulin tolerance test (ITT). (F) AUC of E. (G) body weight. Data are mean ± SEM (n = 10, each n represents 1 mouse, 10 mice were used per experimental group). *P < .05, ***P < .001.
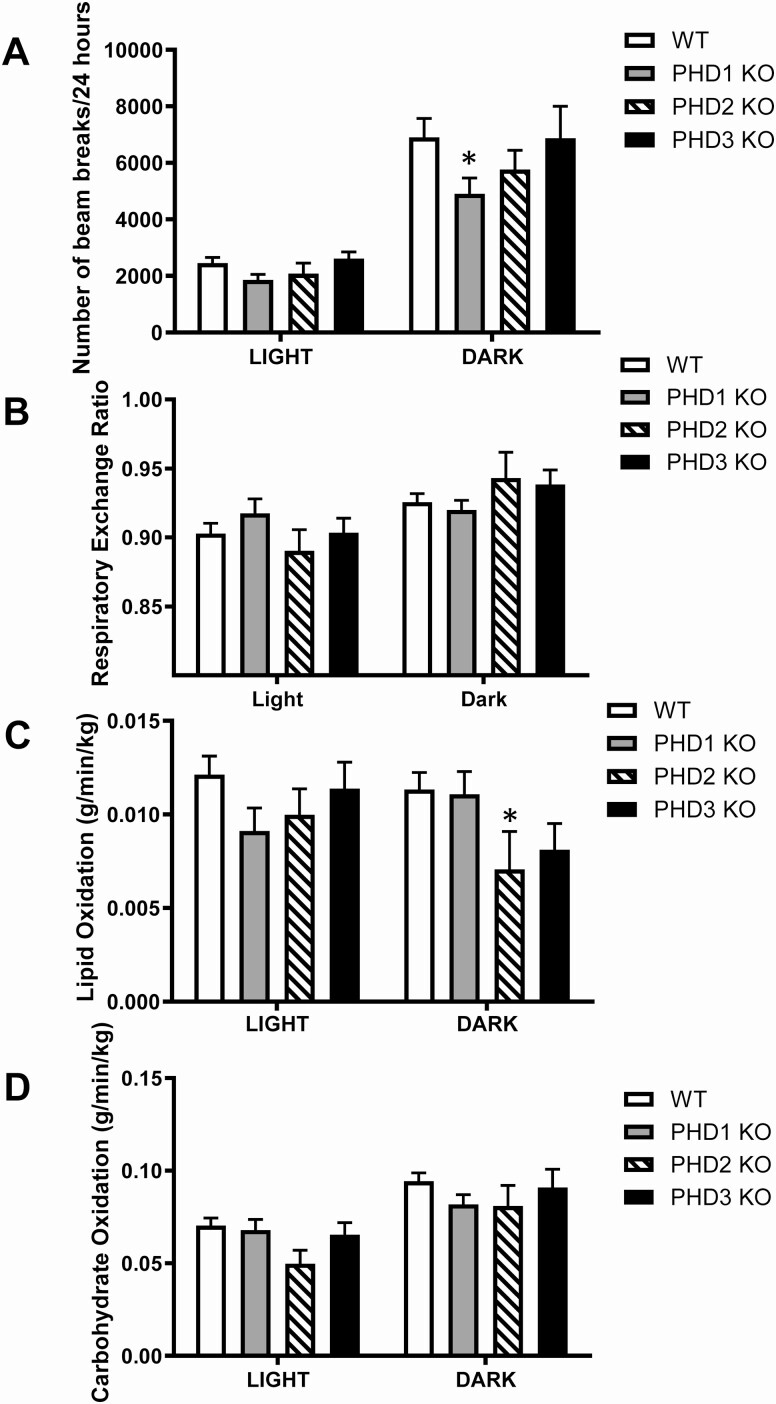
We measured whole-body energy homeostasis using indirect calorimetry to determine whether loss of PHD proteins is associated with changes in energy balance and metabolic phenotype. Mice were placed into individual metabolic cages to measure activity, RER, and in vivo lipid oxidation and carbohydrate oxidation (Fig. 6A-D). Interestingly, β-cell-specific PHD1 KO mice did not show any metabolic changes except for significantly reduced activity during the dark phase (Fig. 6A). Compared with control mice, the only difference seen in β-cell-specific PHD2 KO mice was decreased in vivo lipid oxidation (Fig. 6C). Loss of PHD3 in β-cells was not associated with any changes in metabolic phenotype (Fig. 6A-6D).
Figure 6.
Indirect calorimetry measurement of whole-body bioenergetics in male control (wt) and β-PHD1 KO, β-PHD2 KO, and β-PHD3 KO mice. (A) total activity, (B) respiratory exchange ratio (RER), (D) whole-body lipid oxidation, (E) whole-body carbohydrate oxidation. Data are mean ± SEM (n = 10, each n represents 1 mouse, 10 mice were used per experimental group). *P < .05.
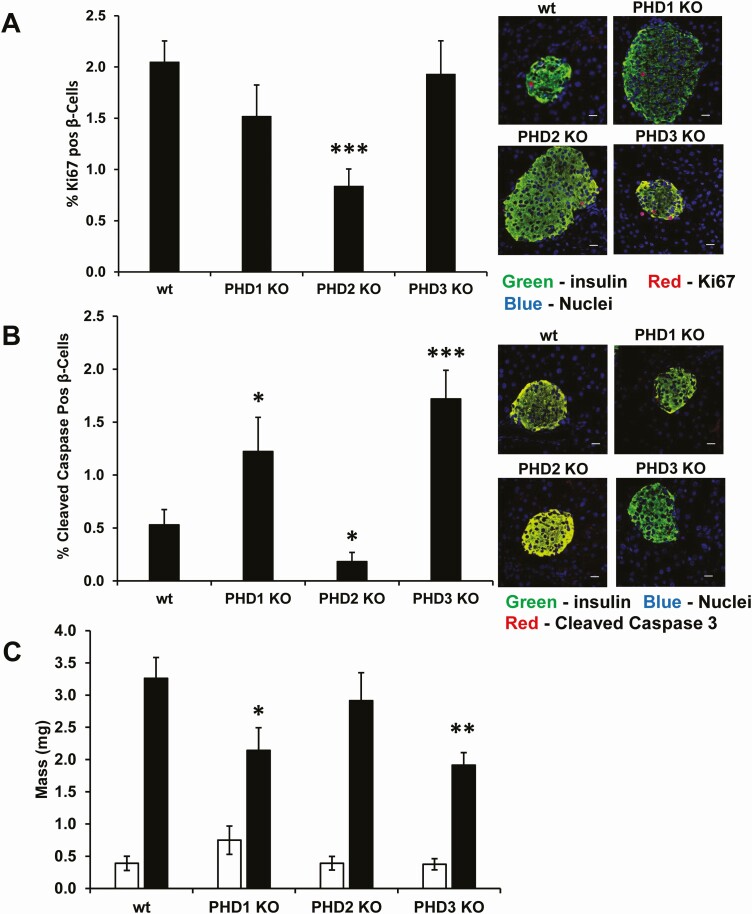
Pancreatic β-Cell Proliferation, Apoptosis, and Mass
The effects of the loss of the PHD proteins were examined next on β-cell proliferation, apoptosis, and mass. β-Cell proliferation was significantly reduced in β-cell-specific PHD2 KO β-cells compared with control mouse β-cells (Fig. 7A). β-Cell apoptosis was significantly elevated in PHD1 and PHD3 β-cell-specific KO mouse β-cells and significantly lower in β-cell-specific PHD2 KO mouse β-cells than in control mice (Fig. 7B). The net effect of the increased β-cell apoptosis and no change in β-cell proliferation resulted in significantly reduced β-cell mass in PHD1 and PHD3 β-cell-specific KO mouse β-cells compared with control β-cells (Fig. 7C). The significant changes in β-cell apoptosis and proliferation did not lead to any changes in the β-cell-specific PHD2 KO β-cell mass (Fig. 7C). There were no significant changes in α-cell mass (Fig. 7C).
Figure 7.
β-Cell proliferation (A), apoptosis (B), and α- and β-cell mass (C) in male control (wt), β-PHD1 KO, β-PHD2 KO, and β-PHD3 KO mice. (C) α-Cell mass white bars and β-cell mass black bars. Data are mean ± SEM (n = 6-9 mice, each n represents 1 mouse pancreas, each experimental group had an n between 6 and 9, from these a minimum 3000 insulin-positive cells were counted per genotype). *P < .05, **P < .01, ***P < .001. White scale bar 10 µm. Green = insulin, red = Ki67 or cleaved caspase 3, blue = nuclei.
In Vitro Islet Insulin Secretion
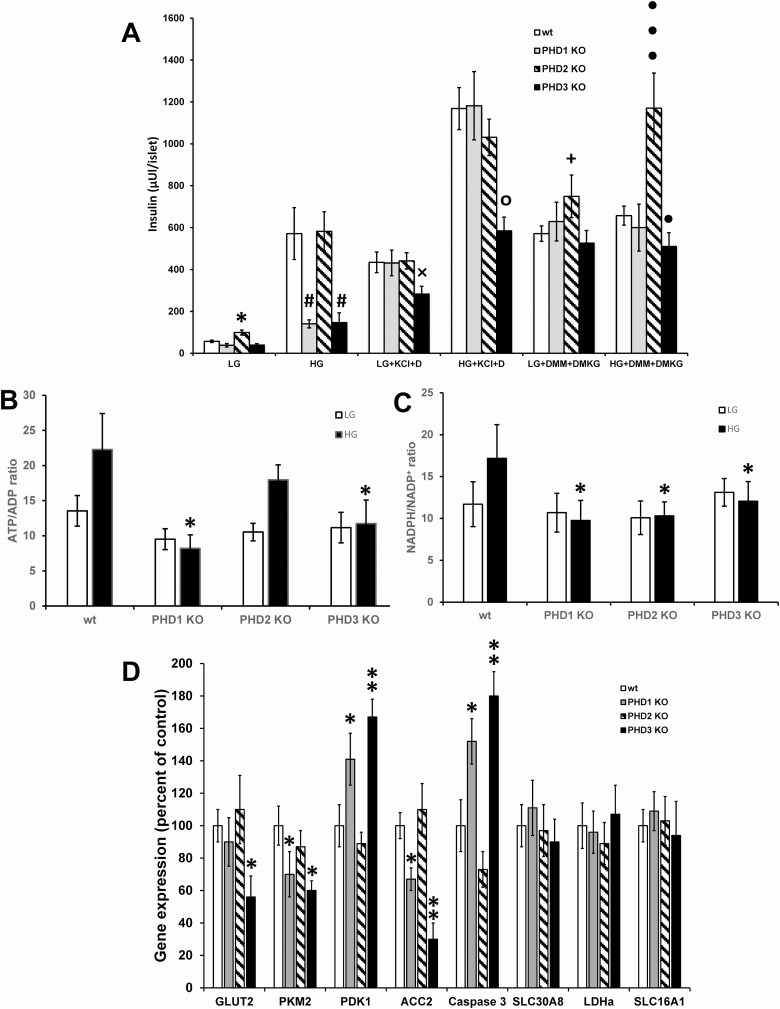
In vitro insulin secretion from isolated male control and PHD KO islets was measured in response to low glucose, high glucose, KCl plus diazoxide, and TCA cycle metabolites. β-Cell-specific PHD2 KO islets had significantly higher insulin release in response to low glucose compared with control islets (Fig. 8A). In addition, β-cell-specific PHD2 KO islets had significantly enhanced insulin release in response to TCA cycle intermediates dimethylmalate (DMM) and DMαKG compared with control islets (Fig. 8A). Stimulation of islets with high glucose led to significantly lower insulin secretion from PHD1 and PHD3 KO islets than from control islets (Fig. 8A). β-Cell-specific PHD3 KO islets showed a significantly lower insulin secretion in response to both low glucose plus KCl plus diazoxide and high glucose plus KCl plus diazoxide than control islets (Fig. 8A). The impaired insulin secretion seen in β-cell-specific PHD3 KO islets in response to high glucose, low glucose plus KCl plus diazoxide, and high glucose plus KCl plus diazoxide could be rescued by stimulating islets with TCA cycle intermediates DMM and DMαKG (Fig. 8A). The impaired insulin secretion seen in male islets was similar in female islets except for only β-cell-specific PHD3 KO islets and not β-cell-specific PHD1 KO had defective insulin secretion in response to high glucose (Fig. 2e (20)). Islet insulin content was not significantly different between any of the KO mouse models (data not shown). This data suggest that the β-cell-specific PHD3 KO islets have defective KATP channel–dependent and –independent pathways for insulin secretion.
Figure 8.
Islet insulin secretion and nucleotides from male control (wt), β-PHD1 KO, β-PHD2 KO, and β-PHD3 KO islets. (A) Islet insulin secretion in response to low glucose (2 mM; LG), high glucose (10 mM; HG), LG or HG plus 10 mM dimethylmalate (DMM) and dimethyl α-ketoglutarate (DMαKG), and LG or HG plus 30 mM potassium chloride (KCl) and 200 µM diazoxide (D). Mean ± SEM (n = 24-43 groups of 10 islets per treatment per genotype, islets were obtained from 5 to 7 mice per genotype). *P < .05 LG control vs LG PHD KO, #P < .05 HG control vs HG PHD KO, xP < .05 LG plus KCl plus diazoxide control vs LG plus KCl plus diazoxide PHD KO, °P < .05 HG plus KCl plus diazoxide control vs HG plus KCl plus diazoxide PHD KO, +P < .05 LG plus DMM plus DMαKG control vs LG plus DMM plus DMαKG PHD KO, •P < .05, •••P < .001 HG plus DMM plus DMαKG control vs HG plus DMM plus DMαKG PHD KO. (B) Islet ATP/ADP ratio (C) and NADPH/NADP+ ratio from male control (wt), β-PHD1 KO, β-PHD2 KO, and β-PHD3 KO islets. Low glucose (LG, 2 mM); high glucose (HG, 10 mM)(n = 10-14 groups of 25 islets per treatment per genotype, islets were obtained from 4-6 mice per genotype). *P < .05 HG wt vs HG PHD KO islets. (D) Gene expression from male islets (n = 8 groups of 100 islets per treatment per genotype, islets were obtained from 6 or 7 mice per genotype). Gene expression was corrected by an internal control (cyclophilin) and then expressed as percent control. Data are mean ± SEM.
ATP/ADP Ratio and NADPH/NADP+ Ratio
Nutrient-mediated changes in the ATP/ADP ratio and NADPH/NADP+ ratio are 2 key signaling molecules involved in regulating insulin secretion in response to glucose. We next assessed the islet ATP/ADP ratio and NADPH/NADP+ responses to low and high glucose. Both β-cell-specific PHD1 and PHD3 KO islets had significantly impaired ATP/ADP ratio response to high glucose compared with control islets (Fig. 8B). All 3 β-cell-specific PHD1, PHD2, and PHD3 KO islets had significantly impaired NADPH/NADP+ ratio response to high glucose compared with control islets (Fig. 8C).
Gene Expression
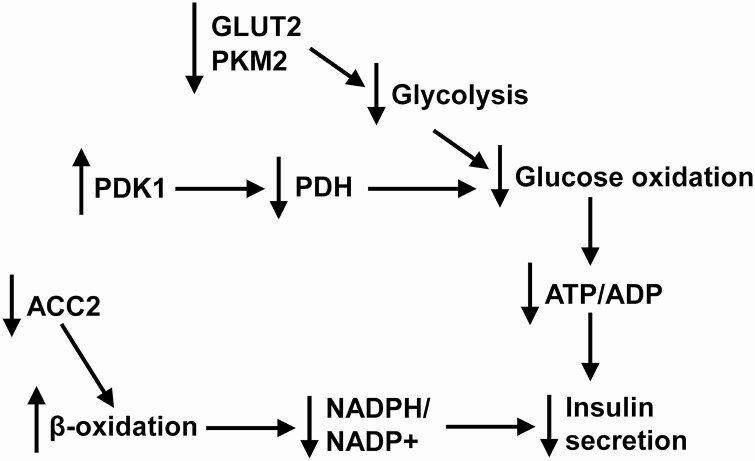
The effects of the loss of the individual PHD proteins on the expression of critical genes involved in regulating β-cell function were assessed in isolated islets. Pyruvate kinase (muscle type 2, PKM2) and acetyl-CoA carboxylase-2 (ACC2) gene expression were significantly lower in PHD1 and PHD3 KO islets than in control islets, whereas glucose-transporter-2 (GLUT2) was only significantly lower in PHD3 KO islets (Fig. 8D). Pyruvate dehydrogenase kinase-1 (PDK1) and caspase 3 were significantly elevated in PHD1 and PHD3 KO islets compared with control islets (Fig. 8D). The expression of SLC30A8 (ZnT8, involved in Zn2+ transport into insulin granules), lactate dehydrogenase (LDHa, a disallowed gene in β-cells), and monocarboxylate transporter-1 (SLC16A1 [MCT1], a disallowed gene in β-cells) was not altered by the loss of any of the PHD proteins (Fig. 8D). The decreased expression of the glycolytic proteins GLUT2 and PKM2 and elevated mRNA levels of PDK1, which inhibits pyruvate dehydrogenase and glucose oxidation, can partially explain the decreased ATP/ADP ratio and GSIS seen in the PHD1 and PHD3 KO islets. The loss of ACC2, which negatively controls lipid entry into mitochondria by producing malonyl CoA that inhibits carnitine palmitoyltransferase I (CPT1), increases lipid transport into mitochondria and shunts anaplerotic substrates away from the generation of NADPH/NADP+ and can also inhibit insulin secretion. The higher caspase 3 mRNA levels may partly explain the increased β-cell apoptosis seen in the PHD1 and PHD3 KO mice.
Discussion
In the current study, we assessed the role of PHD proteins in pancreatic β-cells in vivo. Using both the pharmacological PHD inhibitor DMOG and β-cell-specific KO mice of each isoform of PHD, we show that PHD1 and PHD3 play an important role in regulating islet insulin secretion but do not have a significant impact on in vivo glucose homeostasis. We show that DMOG inhibited in vivo insulin secretion in response to glucose challenge, and in perifused islets, and DMOG inhibited first-phase insulin secretion but enhanced second-phase insulin secretion. We also show that β-cells from both β-PHD1 KO and β-PHD3 KO had elevated β-cell apoptosis and reduced β-cell mass. β-PHD1 KO and β-PHD3 KO islets had reduced GSIS and glucose-stimulated increases in the ATP/ADP and NADPH/NADP+ ratio. The defective insulin secretion seen in the β-PHD1 KO and β-PHD3 KO islets was associated with reduced mRNA levels of PKM2, ACC2, and elevated PDK1. We also saw an increase in caspase 3 mRNA levels in the β-PHD1 KO and β-PHD3 KO islets. β-PHD2 KO mice had increased plasma insulin during the ipGTT in vivo but had no defect in in vitro islet insulin secretion. These studies suggest that both PHD1 and PHD3 play an essential role in regulating β-cell function.
PHD proteins belong to the αKG-dependent dioxygenase superfamily of enzymes that use 1 oxygen atom in the oxidative decarboxylation of αKG producing succinate and CO2, while the second oxygen atom is used in the hydroxylation of proline residues on target proteins (13, 16). The 3 mammalian isoforms of PHDs have distinct tissue expression profiles and subcellular localization (14, 16, 26). There are several variants of each of the PHD isoforms that are produced via alternative splicing of exons 4 and 5, but it is unlikely that they have any sufficient enzymatic activity or stability (14, 17). In some cell types, it has been shown that PHD1 is expressed in the nucleus, and PHD2 is mainly localized in the cytosol, whereas PHD3 is found in both the cytosol and the nucleus (14, 16, 26). We are the first to assess the tissue distribution in the pancreas and show that β-cells express all 3 isoforms of PHD, with PHD3 showing the highest level of β-cell expression compared with PHD1 and PHD2. We also show that PHD3 was expressed only in islets, whereas PHD1 and PHD2 are expressed in both acinar and islet cells. We found that PHD1 and PHD3 were expressed primarily in the cytosol of islets, whereas PHD2 was expressed in both the cytosol and nucleus of islets.
There are a few inconsistencies in the DMOG experiments. The strong inhibition of plasma insulin without any significant changes in ipGTT was not due to changes in insulin sensitivity since there were no changes in insulin sensitivity. We are uncertain about why these differences exist, but it may be related to the unique effects of inhibiting all 3 isoforms of PHD at the same time. We are currently attempting to generate a triple KO mouse line of PHD mice to address this possibility. Another possible explanation for the different responses to DMOG vs the KO mice is that DMOG looked more at the acute inhibition of the PHD proteins, whereas the KO mouse studies looked more at the effects of the chronic loss of the PHD proteins. In addition, DMOG inhibits all 3 PHD isoforms. Given these differences, it is not surprising that there would be a few unique responses with these 2 different experimental approaches. For the most part, the results are consistent between mice treated with DMOG and the PHD3 KO mice, with inhibition of in vivo insulin secretion in response to an ipGTT and no major defects in glucose responses during the ipGTT. There may also be DMOG mediated off-target and on-target effects independent of its impact on β-cell function and may also affect liver, adipose, or muscle cells. To address these issues it would be interesting to generate liver-, adipose-, and muscle-specific PHD KO mice in combination with β-cell-specific PHD KO mice to address the response to DMOG but this is beyond the scope of the current set of studies.
Several PHD KO mouse studies have been published to date, but none of them looked at the role of PHDs in pancreatic β-cells (19, 27-46). Mice with whole-body KO of PHD1 and PHD3 are viable, whereas mice completely lacking PHD2 are embryonically lethal and die due to placental defects (19, 37, 38). Mice with whole-body PHD1 KO have reduced basal oxygen consumption and were protected from skeletal muscle ischemia, and this was partly due to reduced mitochondrial respiration and increased glycolytic ATP production that was mediated by HIF2α induction of pyruvate dehydrogenase kinase isoforms 1 and 4 (37). Mice with whole-body KO of PHD3 have a hypofunctional sympathoadrenal system and hypotension (19, 38).
There have also been a few tissue-specific PHD KO studies. Adipocyte-specific PHD2 KO mice were resistant to high-fat diet–induced obesity and glucose intolerance (27), whereas liver-specific ablation of PHD3 improved insulin sensitivity and prevented the development of diabetes in response to a high-fat diet (45, 46). Liver-specific KO of all 3 PHD isoforms led to severe erythrocytosis, vascular malformation, and massive lipid accumulation in the liver (42). In our β-cell-specific PHD KO mice, we show no major defects in vivo, but in vitro islets from PHD1 and PHD3 β-cell-specific KO mice had defective insulin secretion associated with impaired KATP channel–dependent and –independent pathways. It is surprising that there was a lack of an in vivo ipGTT defect in any of the PHD KO models and this may be due to some compensatory changes in the PHD proteins. However, although there were some changes in PHD2 protein expression in PHD3 KO mice the analysis of multiple blots trended up for PHD2, but the data did not reach a significant difference. We also did not see any differences in PHD mRNA expression. In combination with the lower β-cell mass, defective insulin secretion in vivo and in vitro, the PHD3 KO mice may be prone to developing diabetes if they are stressed with an HFD.
Caveats to the current set of studies are that we did not use wt mice (wt PHD allele with no Cre) or floxed control mice (floxed PHD allele with no Cre) in all experiments. However, in response to ipGTT none of the 3 controls showed a significant difference in glucose homeostasis (Fig. 3 (20)). The addition of these controls would provide important information about whether or not the floxed allele or Cre affects our mouse models. Of the 3 possible mouse controls (wt, floxed mice, or Cre controls) the most important is the Cre control. The 2 other controls were not included in all studies in part because there are no published studies showing that floxing an allele can affect mouse glucose homeostasis; however, there are a few papers showing that Cre expression can negatively affect glucose homeostasis in some Cre mouse deleter strains. The original paper published on the PHD floxed mice published by our group showed no effect of floxing the PHD alleles in any of the transgenic mice (19). We also used the Thorens Ins1Cre deleter mouse line (47). At the start of these studies, this was the best β-specific Cre deleter mouse line available. These mice showed only expression of Cre in β-cells and not in any other tissues. In addition, the authors showed no effect of Cre compared with a no Cre mouse control. More recently, these Cre deleter mice were shown to be susceptible to DNA hypermethylation silencing, which may affect Cre expression and gene KO efficiency (48). This was addressed by assessing PHD mRNA expression in our KO mice with at least 1 primer designed to the targeted floxed exon for all 3 PHD KO mice. If the Cre allele was methylated, there would be no change in PHD mRNA levels which was not the case in the current set of studies.
High glucose stimulates transient hypoxia in pancreatic β-cells, likely due to increased nutrient-stimulated oxygen consumption. The hypoxia that occurs after glucose stimulation of β-cells becomes more pronounced when islet oxygen availability is limited. This transient hypoxia, however, does not lead to the activation of HIF1α (49). Exposing β-cells to reduced oxygen levels for long periods leads to a decrease in insulin biosynthesis, insulin content and inhibits insulin secretion, which may be due to the induction of HIF1α, which causes a shift to anaerobic metabolism (49-52). Hypoxia also decreases the mRNA for the diabetes risk gene SLC30A8 and Zn2+ concentrations in β-cells (53). The high-fat diet–fed mice and diabetic ob/ob mice have poor islet microcirculation leading to impaired islet oxygen delivery and hypoxia (49). Islets from patients with type 2 diabetes have also been shown to have poor microcirculation, and increased nutrient-stimulated oxygen consumption, limiting the ability of β-cells to produce ATP in response to glucose stimulation (54). The long-term hypoxia leads to PHD inhibition which allows for HIF1α stabilization and activates target genes involved in the switch to anaerobic metabolism and angiogenesis to increase oxygen supply (14-17, 26, 29, 54-56). Although the adaption to hypoxia by inducing HIF1α may protect islets, the elevated HIF1α also blocks nutrient-stimulated insulin secretion (49, 57). These studies suggest that diabetes-induced hypoxia leads to an inhibition of PHDs, which elevates HIFα and promotes islet survival during hypoxia but this protection comes at a cost of reduced β-cell function. Our studies support the idea that the lack of PHD1 and PHD3 can have a negative impact on β-cell function. Surprisingly, while it did not lead to the development of diabetes, it is possible that the mice are more prone to the development of diabetes.
In addition to the role of oxygen and Fe2+ in regulating PHDs, TCA cycle intermediates succinate and fumarate have also been reported to inhibit PHD activity (16, 17, 54, 55, 58, 59). All 3 isoforms of PHDs are inhibited by fumarate and succinate, with fumarate having a Ki value between 50 and 80 μM and succinate between 350 and 460 μM (55). We have shown that nutrient metabolism in β-cells leads to the generation of αKG, and this αKG can act as an insulin secretagogue (60-63). We have also shown, as well as others, that inhibition of PHDs impairs nutrient-stimulated insulin secretion (12, 57, 64). These studies suggest that αKG may be essential in defining the extramitochondrial role of αKG in insulin secretion. We have previously shown that EDHB (ethyl-3,4-dihydroxybenzoate), a potent inhibitor of PHDs (65, 66), suppressed insulin secretion and blunted the glucose-stimulated increase in the ATP/ADP ratio and the increase in TCA intermediates (12). These effects were primarily mediated through PHD1 and PHD3 inhibition as targeted siRNA-mediated knockdown of PHD3 resulted in a similar inhibition of insulin secretion (12). These studies are supported by the current work using β-cell-specific PHD1 and PHD3 KO mice.
The levels of TCA cycle intermediates are rapidly elevated by treating β-cells with high glucose because they express high levels of the anaplerotic mitochondrial enzyme pyruvate carboxylase (1, 22, 61, 62, 67). One key TCA cycle intermediate that is increased by glucose in β-cells is αKG. We have shown that the transport of αKG from mitochondria to the cytosol is facilitated by the αKG carrier (2-oxoglutarate carrier) (68). Using either pharmacological or siRNA-mediated inhibition of 2-oxoglutarate carrier in 832/13 β-cells and primary rat islets leads to a significant reduction in insulin secretion in response to nutrients, suggesting that αKG needs to be transported to the cytosol to affect insulin secretion (68). We have shown that anaplerotically derived αKG plays a crucial role in regulating nutrient-stimulated insulin secretion (61, 68-70) and that αKG may regulate insulin secretion through its metabolism by PHDs (12, 63, 71). In addition to αKG, it has been shown that succinate and fumarate can also regulate PHD activity and HIFα stabilization (72, 73). When succinate levels are elevated by inhibiting succinate dehydrogenase, this reduces PHD activity (73). Also, fumarate, pyruvate, and oxaloacetate can lead to the stabilization of HIFα in vitro (72, 74). Some TCA cycle intermediates can have unique effects depending on the PHD isoforms; for example, citrate has been shown to inhibit PHD3 more effectively than PHD1 or PHD2 (55).
Our studies on the role of PHD proteins support the idea that the link between glucose metabolism and PHD-regulated insulin release may be anaplerotically derived αKG. The metabolism of αKG by PHDs could lead to proline hydroxylation of critical proteins regulating insulin release. In addition to HIFs, PHDs can also modulate the activities of Pax2 (75), mTORC1 (17), PKM2, ATF4, β2AR, NFkB, p27, wnt/β-catenin, acetyl CoA carboxylase 2 (ACC2), and caspase-3 (33, 44, 76-80). Interestingly, PHD3 has the widest range of suggested targets (77), and our current paper supports a strong role for PHD3 in nutrient-regulated insulin secretion. We also show that the mRNA levels of PHD targets PKM2, ACC2 and caspase 3 are altered in the β-PHD1 KO and β-PHD3 KO islets (Fig. 9). Our current studies support the concept that long-term changes in PHD activity can elevate HIFα and have a negative impact on β-cell function leading to the development of type 2 diabetes (10). In contrast, short-term changes in PHD activity may have a positive effect on β-cell function in an αKG-dependent and HIFα-independent fashion to promote GSIS. In conclusion, we show that each of the PHD proteins plays a unique role in pancreatic β-cells, with PHD1 and PHD3 playing a more prominent role in regulating β-cell function. It is also possible that the lack of these 2 proteins may predispose metabolically stressed mice to the development of type 2 diabetes.
Figure 9.
PHD1 and PHD3 regulated insulin secretion. The loss of PHD1 and PHD3 is associated with reduced GLUT2, PKM2, and ACC2 and the elevation of PDK1. The loss of GLUT2 and PKM2 lowers glycolysis, and the increased PDK1 inhibits pyruvate dehydrogenase (PDH), which reduces glucose oxidation, ATP/ADP, and insulin secretion. The loss of ACC2 can reduce malonyl CoA levels allowing CPT1 mediated shunting of anaplerotic substrates to β-oxidation. This would lower the β-cells ability to generate NADPH/NADP+ leading to an inhibition of insulin secretion.
Acknowledgments
Financial Support : This work was supported by Canadian Institute of Health Research (CIHR) grants to J.W.J. (PJT-159552). D.J.H. was supported by MRC (MR/N00275X/1 and MR/S025618/1) and Diabetes UK (17/0005681) Project Grants. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Starting Grant 715884 to D.J.H.).
Author Contributions : G.H.F. provided the floxed mice. M.H., S.J., and J.W.J. performed the mouse islet studies and wrote the paper. E.J. helped with some of the in vivo and mouse islet studies. Evaluation of whole-body bioenergetics in mice using CLAMS was carried out in collaboration with ART. M.H., S.J., E.J., D.N., F.C., D.J.H., A.R.T., G.H.F., and J.W.J. helped with revising the paper. J.W.J. is responsible for the integrity of the work as a whole. All authors approved the final version of the paper.
Disclosure Statement : The authors declare that there is no duality of interest associated with this manuscript.
Glossary
Abbreviations
- αKG
α-ketoglutarate
- ADP
adenosine diphosphate
- ARNT/HIF1β
aryl hydrocarbon receptor nuclear translocator/hypoxia-inducible factor-1β
- ATP
adenosine triphosphate
- AUC
area under the curve
- DMαKG
dimethyl α-ketoglutarate
- DMOG
dimethyloxalylglycine
- DMM
dimethylmalate
- DNP
2,4-dinitrophenol
- GLUT2
glucose-transporter 2
- GSIS
glucose-stimulated insulin secretion
- GTT
glucose tolerance test
- HIF
hypoxia-inducible factor
- ipGTT
intraperitoneal glucose tolerance test
- ITT
insulin tolerance test
- KO
knockout
- OCR
oxygen consumption rate
- PCR
polymerase chain reaction
- PHD
prolyl-4-hydroxylase
- RER
respiratory exchange ratio
- TCA
tricarboxylic acid
- V̇CO2
volume of carbon dioxide produced
- V̇O2
volume of oxygen consumed; wt, wild type
Data Availability
All data discussed are presented in the article or available at DataDryad.org (20).
References
- 1. Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(3):193-205. [DOI] [PubMed] [Google Scholar]
- 2. Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987;67(4):1185-1248. [DOI] [PubMed] [Google Scholar]
- 3. Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54(2):87-143. [DOI] [PubMed] [Google Scholar]
- 4. Newgard CB, Matchinsky FM. Substrate control of insulin release. In: Maurice Goodman H, Jefferson LS, Cherrington AD, eds. Handbook of Physiology Section 7: The Endocrine System Volume II: The Endocrine Pancreas and Regulation of Metabolism. Oxford University Press; 2001:125-151. [Google Scholar]
- 5. Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem. 1995;64:689-719. [DOI] [PubMed] [Google Scholar]
- 6. MacDonald PE, Rorsman P. The ins and outs of secretion from pancreatic beta-cells: control of single-vesicle exo- and endocytosis. Physiology (Bethesda). 2007;22:113-121. [DOI] [PubMed] [Google Scholar]
- 7. Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P. Hierarchy of the beta-cell signals controlling insulin secretion. Eur J Clin Invest. 2003;33(9):742-750. [DOI] [PubMed] [Google Scholar]
- 8. Mariot P, Gilon P, Nenquin M, Henquin JC. Tolbutamide and diazoxide influence insulin secretion by changing the concentration but not the action of cytoplasmic Ca2+ in beta-cells. Diabetes. 1998;47(3):365-373. [DOI] [PubMed] [Google Scholar]
- 9. Gilon P, Henquin JC. Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic B-cell. J Biol Chem. 1992;267(29):20713-20720. [PubMed] [Google Scholar]
- 10. Hoang M, Joseph JW. The role of α-ketoglutarate and the hypoxia sensing pathway in the regulation of pancreatic β-cell function. Islets. 2020;12(5):108-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huypens PR, Huang M, Joseph JW. Overcoming the spatial barriers of the stimulus secretion cascade in pancreatic β-cells. Islets. 2012;4(1):1-116. [DOI] [PubMed] [Google Scholar]
- 12. Huang M, Paglialunga S, Wong JM, Hoang M, Pillai R, Joseph JW. Role of prolyl hydroxylase domain proteins in the regulation of insulin secretion. Physiol Rep. 2016;4(5):e12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hausinger RP. FeII/alpha-ketoglutarate-dependent hydroxylases and related enzymes. Crit Rev Biochem Mol Biol. 2004;39(1):21-68. [DOI] [PubMed] [Google Scholar]
- 14. Myllyharju J. Prolyl 4-hydroxylases, master regulators of the hypoxia response. Acta Physiol (Oxf). 2013;208(2):148-165. [DOI] [PubMed] [Google Scholar]
- 15. Appelhoff RJ, Tian YM, Raval RR, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem. 2004;279(37):38458-38465. [DOI] [PubMed] [Google Scholar]
- 16. Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393-402. [DOI] [PubMed] [Google Scholar]
- 17. Boulahbel H, Durán RV, Gottlieb E. Prolyl hydroxylases as regulators of cell metabolism. Biochem Soc Trans. 2009;37(Pt 1):291-294. [DOI] [PubMed] [Google Scholar]
- 18. Sadiku P, Willson JA, Dickinson RS, et al. Prolyl hydroxylase 2 inactivation enhances glycogen storage and promotes excessive neutrophilic responses. J Clin Invest. 2017;127(9):3407-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takeda K, Ho VC, Takeda H, Duan LJ, Nagy A, Fong GH. Placental but not heart defects are associated with elevated hypoxia-inducible factor alpha levels in mice lacking prolyl hydroxylase domain protein 2. Mol Cell Biol. 2006;26(22):8336-8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joseph JW. Supplemental figures. Dryad. 2021. Deposited October 20, 2021 https://datadryad.org/stash/share/zDX6r9nBMXLrLSbpdIxvlM_gGwAL4dO_YiTFxkd-FoA.
- 21. Hoang M, Paglialunga S, Bombardier E, Tupling AR, Joseph JW. The loss of ARNT/HIF1β in male pancreatic β-cells is protective against high-fat diet-induced diabetes. Endocrinology. 2019;160(12):2825-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pillai R, Paglialunga S, Hoang M, et al. Deletion of ARNT/HIF1β in pancreatic beta cells does not impair glucose homeostasis in mice, but is associated with defective glucose sensing ex vivo. Diabetologia. 2015;58(12):2832-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patterson JN, Cousteils K, Lou JW, Manning Fox JE, MacDonald PE, Joseph JW. Mitochondrial metabolism of pyruvate is essential for regulating glucose-stimulated insulin secretion. J Biol Chem. 2014;289(19):13335-13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(2):628-634. [DOI] [PubMed] [Google Scholar]
- 25. Joseph JW, Jensen MV, Ilkayeva O, et al. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem. 2006;281(47):35624-35632. [DOI] [PubMed] [Google Scholar]
- 26. Fong GH, Takeda K. Role and regulation of prolyl hydroxylase domain proteins. Cell Death Differ. 2008;15(4):635-641. [DOI] [PubMed] [Google Scholar]
- 27. Matsuura H, Ichiki T, Inoue E, et al. Prolyl hydroxylase domain protein 2 plays a critical role in diet-induced obesity and glucose intolerance. Circulation. 2013;127(21):2078-2087. [DOI] [PubMed] [Google Scholar]
- 28. Franke K, Kalucka J, Mamlouk S, et al. HIF-1α is a protective factor in conditional PHD2-deficient mice suffering from severe HIF-2α-induced excessive erythropoiesis. Blood. 2013;121(8):1436-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rishi MT, Selvaraju V, Thirunavukkarasu M, et al. Deletion of prolyl hydroxylase domain proteins (PHD1, PHD3) stabilizes hypoxia inducible factor-1 alpha, promotes neovascularization, and improves perfusion in a murine model of hind-limb ischemia. Microvasc Res. 2015;97:181-188. [DOI] [PubMed] [Google Scholar]
- 30. Oriowo B, Thirunavukkarasu M, Selvaraju V, et al. Targeted gene deletion of prolyl hydroxylase domain protein 3 triggers angiogenesis and preserves cardiac function by stabilizing hypoxia inducible factor 1 alpha following myocardial infarction. Curr Pharm Des. 2014;20(9):1305-1310. [DOI] [PubMed] [Google Scholar]
- 31. Ikeda J, Ichiki T, Matsuura H, et al. Deletion of phd2 in myeloid lineage attenuates hypertensive cardiovascular remodeling. J Am Heart Assoc. 2013;2(3):e000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duan LJ, Takeda K, Fong GH. Prolyl hydroxylase domain protein 2 (PHD2) mediates oxygen-induced retinopathy in neonatal mice. Am J Pathol. 2011;178(4):1881-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adluri RS, Thirunavukkarasu M, Dunna NR, et al. Disruption of hypoxia-inducible transcription factor-prolyl hydroxylase domain-1 (PHD-1-/-) attenuates ex vivo myocardial ischemia/reperfusion injury through hypoxia-inducible factor-1alpha transcription factor and its target genes in mice. Antioxid Redox Signal. 2011;15(7):1789-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li X, Sutherland S, Takeda K, Fong GH, Lee FS. Integrity of the prolyl hydroxylase domain protein 2: erythropoietin pathway in aging mice. Blood Cells Mol Dis. 2010;45(1):9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takeda K, Fong GH. Prolyl hydroxylase domain 2 protein suppresses hypoxia-induced endothelial cell proliferation. Hypertension. 2007;49(1):178-184. [DOI] [PubMed] [Google Scholar]
- 36. Zhang Q, Gu J, Li L, et al. Control of cyclin D1 and breast tumorigenesis by the EglN2 prolyl hydroxylase. Cancer Cell. 2009;16(5):413-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aragonés J, Schneider M, Van Geyte K, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40(2):170-180. [DOI] [PubMed] [Google Scholar]
- 38. Bishop T, Gallagher D, Pascual A, et al. Abnormal sympathoadrenal development and systemic hypotension in PHD3-/- mice. Mol Cell Biol. 2008;28(10):3386-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen RL, Nagel S, Papadakis M, et al. Roles of individual prolyl-4-hydroxylase isoforms in the first 24 hours following transient focal cerebral ischaemia: insights from genetically modified mice. J Physiol. 2012;590(16):4079-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bishop T, Talbot NP, Turner PJ, et al. Carotid body hyperplasia and enhanced ventilatory responses to hypoxia in mice with heterozygous deficiency of PHD2. J Physiol. 2013;591(14):3565-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hodson EJ, Nicholls LG, Turner PJ, et al. Regulation of ventilatory sensitivity and carotid body proliferation in hypoxia by the PHD2/HIF-2 pathway. J Physiol. 2016;594(5):1179-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Duan LJ, Takeda K, Fong GH. Hematological, hepatic, and retinal phenotypes in mice deficient for prolyl hydroxylase domain proteins in the liver. Am J Pathol. 2014;184(4):1240-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rahtu-Korpela L, Karsikas S, Hörkkö S, et al. HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes. 2014;63(10):3324-3333. [DOI] [PubMed] [Google Scholar]
- 44. Zeng H, Chen JX. Conditional knockout of prolyl hydroxylase domain protein 2 attenuates high fat-diet-induced cardiac dysfunction in mice. PLoS One. 2014;9(12):e115974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taniguchi CM, Finger EC, Krieg AJ, et al. Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nat Med. 2013;19(10):1325-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yano H, Sakai M, Matsukawa T, et al. PHD3 regulates glucose metabolism by suppressing stress-induced signalling and optimising gluconeogenesis and insulin signalling in hepatocytes. Sci Rep. 2018;8(1):14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thorens B, Tarussio D, Maestro MA, Rovira M, Heikkilä E, Ferrer J. Ins1(Cre) knock-in mice for beta cell-specific gene recombination. Diabetologia. 2015;58(3):558-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mosleh E, Ou K, Haemmerle MW, et al. Ins1-Cre and Ins1-CreER gene replacement alleles are susceptible to silencing By DNA hypermethylation. Endocrinology. 2020;161(8):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sato Y, Endo H, Okuyama H, et al. Cellular hypoxia of pancreatic beta-cells due to high levels of oxygen consumption for insulin secretion in vitro. J Biol Chem. 2011;286(14):12524-12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Spegel P, Malmgren S, Sharoyko VV, Newsholme P, Koeck T, Mulder H. Metabolomic analyses reveal profound differences in glycolytic and tricarboxylic acid cycle metabolism in glucose-responsive and -unresponsive clonal beta-cell lines. Biochem J. 2011;435(1):277-284. [DOI] [PubMed] [Google Scholar]
- 51. Ma Z, Moruzzi N, Catrina SB, Grill V, Björklund A. Hyperoxia inhibits glucose-induced insulin secretion and mitochondrial metabolism in rat pancreatic islets. Biochem Biophys Res Commun. 2014;443(1):223-228. [DOI] [PubMed] [Google Scholar]
- 52. Ma Z, Moruzzi N, Catrina SB, Hals I, Oberholzer J, Grill V, Bjorklund A. Preconditioning with associated blocking of Ca2+ inflow alleviates hypoxia-induced damage to pancreatic beta-cells. PLoS ONE. 2013;8(7):e67498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gerber PA, Bellomo EA, Hodson DJ, et al. Hypoxia lowers SLC30A8/ZnT8 expression and free cytosolic Zn2+ in pancreatic beta cells. Diabetologia. 2014;57(8):1635-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bento CF, Pereira P. Regulation of hypoxia-inducible factor 1 and the loss of the cellular response to hypoxia in diabetes. Diabetologia. 2011;54(8):1946-1956. [DOI] [PubMed] [Google Scholar]
- 55. Koivunen P, Hirsilä M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem. 2007;282(7):4524-4532. [DOI] [PubMed] [Google Scholar]
- 56. Puri S, Cano DA, Hebrok M. A role for von Hippel-Lindau protein in pancreatic beta-cell function. Diabetes. 2009;58(2):433-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cheng K, Ho K, Stokes R, et al. Hypoxia-inducible factor-1alpha regulates beta cell function in mouse and human islets. J Clin Invest. 2010;120(6):2171-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294(5545):1337-1340. [DOI] [PubMed] [Google Scholar]
- 59. Tennant DA, Gottlieb E. HIF prolyl hydroxylase-3 mediates alpha-ketoglutarate-induced apoptosis and tumor suppression. J Mol Med (Berl). 2010;88(8):839-849. [DOI] [PubMed] [Google Scholar]
- 60. Pillai R, Huypens P, Huang M, et al. Aryl hydrocarbon receptor nuclear translocator/hypoxia-inducible factor-1{beta} plays a critical role in maintaining glucose-stimulated anaplerosis and insulin release from pancreatic {beta}-cells. J Biol Chem. 2011;286(2):1014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huang M, Joseph JW. Metabolomic analysis of pancreatic β-cell insulin release in response to glucose. Islets. 2012;4(3):210-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Huang M, Joseph JW. Assessment of the metabolic pathways associated with glucose-stimulated biphasic insulin secretion. Endocrinology. 2014;155(5):1653-1666. [DOI] [PubMed] [Google Scholar]
- 63. Rabaglia ME, Gray-Keller MP, Frey BL, Shortreed MR, Smith LM, Attie AD. Alpha-Ketoisocaproate-induced hypersecretion of insulin by islets from diabetes-susceptible mice. Am J Physiol Endocrinol Metab. 2005;289(2):E218-E224. [DOI] [PubMed] [Google Scholar]
- 64. Fallon MJ, MacDonald MJ. Beta-cell alpha-ketoglutarate hydroxylases may acutely participate in insulin secretion. Metabolism. 2008;57(8):1148-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li B, Takeda K, Yokoyama S, Shibahara S. A prolyl-hydroxylase inhibitor, ethyl-3,4-dihydroxybenzoate, induces haem oxygenase-1 expression in human cells through a mechanism independent of hypoxia-inducible factor-1alpha. J Biochem. 2008;144(5):643-654. [DOI] [PubMed] [Google Scholar]
- 66. Wang J, Buss JL, Chen G, Ponka P, Pantopoulos K. The prolyl 4-hydroxylase inhibitor ethyl-3,4-dihydroxybenzoate generates effective iron deficiency in cultured cells. FEBS Lett. 2002;529(2-3):309-312. [DOI] [PubMed] [Google Scholar]
- 67. Maechler P, Carobbio S, Rubi B. In beta-cells, mitochondria integrate and generate metabolic signals controlling insulin secretion. Int J Biochem Cell Biol. 2006;38(5-6):696-709. [DOI] [PubMed] [Google Scholar]
- 68. Odegaard ML, Joseph JW, Jensen MV, et al. The mitochondrial 2-oxoglutarate carrier is part of a metabolic pathway that mediates glucose- and glutamine-stimulated insulin secretion. J Biol Chem. 2010;285(22):16530-16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huypens P, Pillai R, Sheinin T, et al. The dicarboxylate carrier plays a role in mitochondrial malate transport and in the regulation of glucose-stimulated insulin secretion from rat pancreatic beta cells. Diabetologia. 2011;54(1):135-145. [DOI] [PubMed] [Google Scholar]
- 70. Ronnebaum SM, Ilkayeva O, Burgess SC, et al. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006;281(41):30593-30602. [DOI] [PubMed] [Google Scholar]
- 71. Lorenz MA, El Azzouny MA, Kennedy RT, Burant CF. Metabolome response to glucose in the beta-cell line INS-1 832/13. J Biol Chem. 2013;288(15):10923-10935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Isaacs JS, Jung YJ, Mole DR, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8(2):143-153. [DOI] [PubMed] [Google Scholar]
- 73. MacKenzie ED, Selak MA, Tennant DA, et al. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27(9):3282-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dalgard CL, Lu H, Mohyeldin A, Verma A. Endogenous 2-oxoacids differentially regulate expression of oxygen sensors. Biochem J. 2004;380(Pt 2):419-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yan B, Jiao S, Zhang HS, et al. Prolyl hydroxylase domain protein 3 targets Pax2 for destruction. Biochem Biophys Res Commun. 2011;409(2):315-320. [DOI] [PubMed] [Google Scholar]
- 76. German NJ, Yoon H, Yusuf RZ, et al. PHD3 loss in cancer enables metabolic reliance on fatty acid oxidation via deactivation of ACC2. Mol Cell. 2016;63(6):1006-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jaakkola PM, Rantanen K. The regulation, localization, and functions of oxygen-sensing prolyl hydroxylase PHD3. Biol Chem. 2013;394(4):449-457. [DOI] [PubMed] [Google Scholar]
- 78. Cummins EP, Berra E, Comerford KM, et al. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci U S A. 2006;103(48):18154-18159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xue J, Li X, Jiao S, Wei Y, Wu G, Fang J. Prolyl hydroxylase-3 is down-regulated in colorectal cancer cells and inhibits IKKbeta independent of hydroxylase activity. Gastroenterology. 2010;138(2):606-615. [DOI] [PubMed] [Google Scholar]
- 80. Van Welden S, Laukens D, Ferdinande L, De Vos M, Hindryckx P. Differential expression of prolyl hydroxylase 1 in patients with ulcerative colitis versus patients with Crohn’s disease/infectious colitis and healthy controls. J Inflamm (Lond). 2013;10(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data discussed are presented in the article or available at DataDryad.org (20).