Abstract
Background
The population history of Plasmodium simium, which causes malaria in sylvatic Neotropical monkeys and humans along the Atlantic Coast of Brazil, remains disputed. Genetically diverse P vivax populations from various sources, including the lineages that founded the species P simium, are thought to have arrived in the Americas in separate migratory waves.
Methods
We use population genomic approaches to investigate the origin and evolution of P simium.
Results
We find a minimal genome-level differentiation between P simium and present-day New World P vivax isolates, consistent with their common geographic origin and subsequent divergence on this continent. The meagre genetic diversity in P simium samples from humans and monkeys implies a recent transfer from humans to non-human primates – a unique example of malaria as a reverse zoonosis of public health significance. Likely genomic signatures of P simium adaptation to new hosts include the deletion of >40% of a key erythrocyte invasion ligand, PvRBP2a, which may have favored more efficient simian host cell infection.
Conclusions
New World P vivax lineages that switched from humans to platyrrhine monkeys founded the P simium population that infects nonhuman primates and feeds sustained human malaria transmission in the outskirts of major cities.
Keywords: Plasmodium simium, Neotropical monkeys, reverse zoonosis
New World Plasmodium vivax lineages that switched from humans to platyrrhine monkeys founded the present-day Plasmodium simium population that infects nonhuman primates and feeds sustained human malaria transmission in the outskirts of major cities along the Atlantic Coast of Brazil.
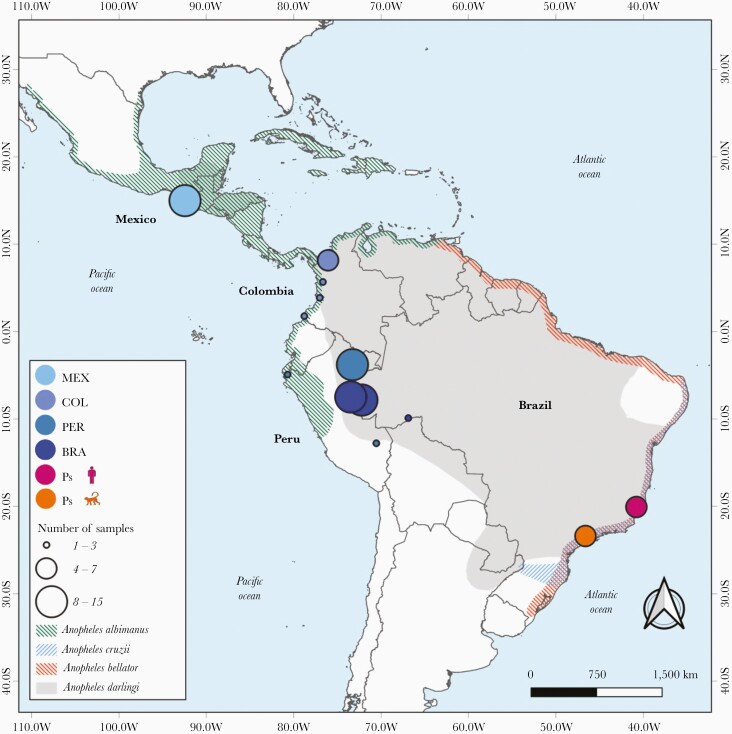
Despite the recent progress towards malaria elimination, more than 700 000 infections are reported each year in the Americas, 72% of them due to Plasmodium vivax [1]. Malaria is primarily transmitted by mosquitoes of the Nyssorhynchus subgenus, such as Anopheles darlingi in the Amazon Basin and Anopheles albimanus along the Pacific Coast of South America, Central America, and Mexico [2] (Figure 1). However, focal malaria transmission is sustained along the coast of Southeast and South Brazil by anopheline mosquitoes of the Kerteszia subgenus, mainly Anopheles cruzii and Anopheles bellator, that breed in water trapped by bromeliads in the Atlantic Forest biome [3].
Figure 1.
Map of Latin America and the Caribbean showing the distribution of Anopheles species and sites of collection of malaria parasite samples analyzed. The collection sites are shown for newly sequenced Plasmodium simium isolates (n = 11) and the New World P vivax samples from Brazil ([BRA] n = 30), Peru ([PER] n = 12), Colombia ([COL] n = 14), and Mexico ([MEX] n = 15) that were used in population genomic analysis; circle diameters are proportional to sample size. Color shading indicates the spatial distribution of Anopheles darlingi, Anopheles albimanus, Anopheles cruzii, and Anopheles bellator, the primary malaria vectors in the areas of sample collection.
Plasmodium vivax had already emerged as a human parasite in the Old World at the time the first modern humans settled in the Americas, approximately 15 000 years ago [4]. This parasite is thought to have originated from crossover infections from nonhuman primates or evolved from an ancestral parasite that infected great apes and hominids in Africa [5–7]. It was surprising to find that a closely related parasite named Plasmodium simium infects Neotropical platyrrhine monkeys [8], which diverged from African great apes and Old World catarrhine monkeys 35 million years ago [9]. Originally described in howler monkeys [10], P simium also infects wild woolly, spider, capuchin, and titi monkeys on the Atlantic Coast [8, 11, 12] but not in the Amazon [8, 13]. Parasites are genetically very similar to P vivax [14–17]. Cross-species switches are thought to be favored by the predilection of Kerteszia vectors to take bloodmeals both at the canopy of the trees, where monkeys live, and at the ground level, where humans may be found [18]. Plasmodium simium remains infectious to humans [19–21] and its zoonotic transmission challenges malaria elimination efforts in South America [22, 23].
How P simium arrived and subsequently evolved in the Americas remains disputed. Li et al [24] postulated 2 separate introductions of P vivax into the continent: one from Europe and Africa during the colonial era, founding the New World P vivax populations that circulate in the Amazon and along the Pacific Coast of northern South America, Central America, and Mexico, and another from Southeast Asia, founding the so-called Old World-type lineages, including P simium, that nowadays circulate on the Atlantic Coast of Brazil. Cormier [25] went further to speculate that P simium arrived in Rio de Janeiro with East Asian migrant workers in the early 1800s. In this study, we use population genomic approaches to reveal the close genetic affinity of P simium with present-day New World rather than Old World-type P vivax, contrary to the expectations of the Asian origin hypothesis. After their arrival in coastal Brazil, some P vivax lineages have adapted to locally abundant Kerteszia vectors and jumped from humans to sylvatic nonhuman primates, especially howler monkeys, offering an uncommon example of reverse zoonosis of public health significance.
METHODS
Study Sites and Samples
We retain the species name P simium to refer to monkey- and human-derived malaria parasites from southeastern Brazil that morphologically resemble P vivax [20, 21]. Simian parasite deoxyribonucleic acid (DNA) for genome sequencing was obtained from blood samples drawn between 2003 and 2010 during routine veterinary care of 3 brown howler monkeys (Alouatta guariba clamitans) [26, 27] and 1 black-fronted titi monkey (Callicebus nigrifrons) [11] from Atlantic Forest fragments around São Paulo, the largest city in South America (Figure 1, Supplementary Figure 1, and Supplementary Table 1). Human P simium DNA samples were obtained between April 2001 and March 2004 from 7 febrile patients from Atlantic Forest remnants in the state of Espírito Santo [28], 740 kilometers north of São Paulo (Figure 1, Supplementary Figure 1, and Supplementary Table 1). Consistent with locally acquired infections, patients reported no recent travel to the Amazon, the main malaria-endemic area in Brazil [29].
Genome Sequencing
We enriched DNA isolated from unprocessed blood samples for target parasite DNA by selective whole-genome amplification with the primer set pvset1 [30] to prepare Nextera XT or TrueSeq Nano DNA libraries (Illumina, San Diego, CA). Paired-end short sequence reads from Illumina HiSeq 2500 or HiSeq X platforms that presented expected base call accuracy ≥99.9% were processed as described in Supplementary Methods. In brief, we mapped reads with ≥5× coverage onto the reference P vivax genome PvP01 [31] after excluding repetitive domains to which short reads are difficult to map [32]. Single-nucleotide polymorphisms (SNPs) were called, and those presenting within-sample variation in an unusually high percentage of samples were filtered out (Supplementary Methods). Heteroallelic SNP calls (0.01% to 0.05% of all SNPs per sample) were masked before downstream analyses. For comparison, we processed and analyzed raw genome sequence reads from worldwide P vivax isolates (Supplementary Table 2) in the same way.
Analysis of the Plasmodium simium pvs47, pvdpb1, and pvrbp2a Gene Orthologs
We next focused on the pvs47 (PVP01_1208000) gene encoding a gamete surface protein that may have facilitated P simium adaptation to Kerteszia mosquitoes. (Given the close similarity between P vivax and P simium orthologs, throughout this paper we use the same gene names to refer to them.) This protein controls parasite-vector compatibility in P falciparum [33] and putatively also in P vivax [34]. A maximum likelihood tree was generated with full-length translated Pvs47 sequences (434 amino acids) from P simium and worldwide P vivax isolates (Supplementary Table 3) using IQ-TREE 1.5.5 [35].
Because the efficiency of simian cell invasion may constitute a further barrier to vertebrate host switching, we also analyzed the genes that encode the Duffy Binding Protein 1 (pvdbp1; PVP01_0623800) and the Reticulocyte Binding Protein 2a (pvrbp2a; PVP01_1402400), a member of the reticulocyte binding protein (rbp) family. These are key ligands of host erythrocyte receptors [36] that display large deletions in P simium [17]. To detect deletions, we polymerase chain reaction (PCR)-amplified gene fragments and determined amplicon sizes by agarose gel electrophoresis; selected PCR products were Sanger-sequenced. The sample set analyzed and laboratory protocols are described in Supplementary Methods and Supplementary Table 3.
Data Analysis
We assessed newly obtained P simium genomes for genetic diversity and linkage disequilibrium (LD) as described in Supplementary Methods. We measured π (average number of pairwise nucleotide differences per site) and examined how it varies within 1-kb sliding windows across the genome. Tajima’s D values were calculated within 1-kb windows and their distribution was plotted. The expected value of Tajima’s D is zero under a strictly neutral model. We assessed multilocus LD by calculating the VD/VE ratio—the variance of the distribution of empirical pairwise genetic distances between samples (VD) divided by that expected under random association of alleles in a panmictic population (VE) [37]. Under panmixia, the VD/VE ratio is expected to be 1.
We used the hmmIBD software [38] to infer relatedness between P simium samples from monkeys and humans and across P simium and P vivax populations using identity-by-descent (IBD) analysis. Relatedness networks were drawn to represent sample pairs that share at least 5%, 20%, or 50% of their genomes. Principal component analysis (PCA) was carried out to explore the genetic affinities between P simium and regional populations of P vivax. We used the R package admixr [39] to compute the f4 statistic, f4 (W, X; Y, Z) [40], where (except if otherwise stated) X is P simium and Y and Z are 2 P vivax populations, to compare the proportion of shared derived alleles of P simium with every pair of P vivax populations. The outgroup (W) is P cynomolgi, the vivax-like parasite of Asian macaques [41].
Data Availability
Sequence data were deposited in the Sequence Read Archive ([SRA] SAMN17926899–SAMN17926909) and GenBank Nucleotide Database (MW560996–MW561043, MW561060–MW561072, and MW561044–MW561059) of the National Center for Biotechnology Information.
RESULTS
Genome-Wide Diversity of Plasmodium simium
We generated 11 new genome sequences of P simium (4 from monkeys and 7 from human hosts), with a mean read depth of 242× and 23.3 × 106 reads per isolate on average (Table 1 and Supplementary Table 1; Supplementary Results). Samples displayed a low complexity of infection, with FWS (within-host diversity statistic) values ranging between 0.904 and 0.988. After excluding positions with >10% missing calls in the complete set of 11 P simium sequences and 121 P vivax sequences retrieved from SRA, we ended up with a catalog of 10 738 high-confidence variable positions that were used in further analyses of P simium samples. The nonreference allele was called at 356 to 2098 genotyped sites per sample (mean, 1237), with 44.3% of the SNPs in intergenic regions, 6.8% in introns, and 48.9% in coding regions; 65.0% of coding SNPs were nonsynonymous.
Table 1.
Characteristics of Newly Generated Plasmodium simium Genome Sequences From Southeastern Brazila
| Isolate | Host Species | Average Read Depth (×) | PvP01 Genome Coverage (%) | Number of Reads | Number of SNPs |
|---|---|---|---|---|---|
| 97Ps | Alouatta clamitans | 316 | 20.5 | 30 564 162 | 1042 |
| D121Ps | A clamitans | 329 | 11.7 | 31 789 162 | 863 |
| P160 | Callicebus nigrifrons | 284 | 4.8 | 27 472 914 | 610 |
| 95 | A clamitans | 117 | 12.4 | 11 349 094 | 356 |
| ALNL53 | Homo sapiens | 262 | 79.8 | 25 326 697 | 1877 |
| 143 | H sapiens | 83 | 11.2 | 8 033 616 | 1848 |
| 111 | H sapiens | 106 | 26.0 | 10 294 134 | 1337 |
| MASM | H sapiens | 365 | 19.0 | 35 279 795 | 775 |
| 761 | H sapiens | 278 | 59.3 | 26 838 145 | 721 |
| 1565MT | H sapiens | 259 | 49.0 | 25 070 079 | 2080 |
| 1272MT | H sapiens | 257 | 82.4 | 24 858 660 | 2098 |
Abbreviations: SNPs, single-nucleotide polymorphism.
aA complete version of this table is provided as Supplementary Table 1 available online.
The average genome-wide nucleotide diversity of P simium (π = 1.63 × 10–4) is approximately 3 times lower than that of P vivax populations from the Americas (Supplementary Figure 2A). Despite the small sample size, we note that P simium samples of simian origin are significantly less diverse than their counterparts of human origin (π = 1.18 × 10–4 vs 1.75 × 10–4, P = .025, Mann-Whitney U test), consistent with a bottleneck at the time parasites jumped into monkeys, with no barrier to infecting humans again following this original host switch. Domains with the top 1% π values (Supplementary Figure 3) include genes coding for the following: (1) a Plasmodium helical interspersed subtelomeric (PHIST) protein family member (PVP01_1470300) [42]; (2) 28S (PVP01_0504500) and 18S (PVP01_0202900) ribosomal RNA; and 2 proteins involved in red blood cell invasion, (3) RBP2a (PVP01_1402400) [43] and (4) cysteine-rich protective antigen (CyRPA) (PVP01_0532400) [44] (Supplementary Table 4). Plasmodium vivax genes from parasites circulating in the Amazon Basin of Brazil (BRA) are, on average, twice as diverse as their P simium orthologs; in contrast, pvrbp2a is 2.6-fold more diverse in P simium than P vivax (Supplementary Results and Supplementary Figure 4). No significant enrichment of any gene ontology (GO) term (http://geneontology.org/) was found among annotated genes within high-diversity domains (Supplementary Table 5).
Negative Tajima’s D values predominate within 1-kb windows (median D, −0.543) (Supplementary Figure 2B), with an excess of low-frequency SNPs consistent with a P simium population expansion after a recent bottleneck. Coding sequences within domains with extremely high or low Tajima’s D scores (Supplementary Figure 3), suggestive of either balancing or directional selection, are listed in Supplementary Tables 6 and 7. There is no significant enrichment of any GO term among annotated genes within domains with the top-1% or bottom-1% Tajima’s D scores (Supplementary Tables 8 and 9).
Population Structure and Shared Ancestry
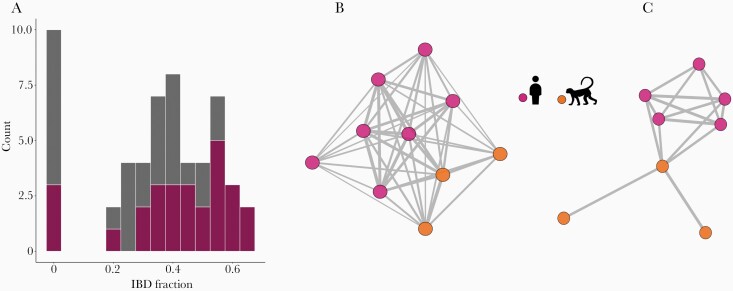
We examined LD levels to test whether simian and human P simium are part of a single panmictic population. We found significant multilocus LD at the genome level, consistent with P simium being structured into discrete, highly inbred lineages, with VD largely exceeding the variance expected under panmixia (Supplementary Figure 5). Identity-by-descent analysis indicated substantial relatedness, including between simian and human isolates from sites >700-km apart (Figures 2B and C). Eight of the 11 P. simium isolates share >50% of their genomes (equal to the average ancestry between meiotic siblings) and 10 share >20% of their genomes with at least one sample. The exception is isolate P160, derived from a titi monkey in São Paulo, which shares <10% ancestry with all other P simium isolates (Figure 2A). However, because P160 genome reads covers only 4.8% of the PvP01 reference (Table 1), its genetic affinities must be interpreted with caution (Supplementary Results). We identified a cluster of closely related parasites that includes 5 Espírito Santo human samples from 2002 ro 2003 and 3 São Paulo simian samples from 2003 and 2009. These findings suggest that the P. simium population may experience a small degree of stratification through both space and time.
Figure 2.
Shared genome ancestry in Plasmodium simium samples from monkeys and humans inferred by identity-by-descent (IBD) analysis. (A) Frequency distribution of the pairwise proportion of shared genome-wide ancestry between P simium samples. Pink bar segments represent pairwise comparisons of samples from the same vertebrate host (either humans or monkeys), whereas gray bar segments represent comparisons between different host types. (B and C) Parasite relatedness networks. Edges connect parasite pairs with shared ancestry ≥20% (B) or ≥50% (C) and unconnected samples are omitted. Edge thickness is proportional to the IBD fraction.
Geographic Origin of Plasmodium simium
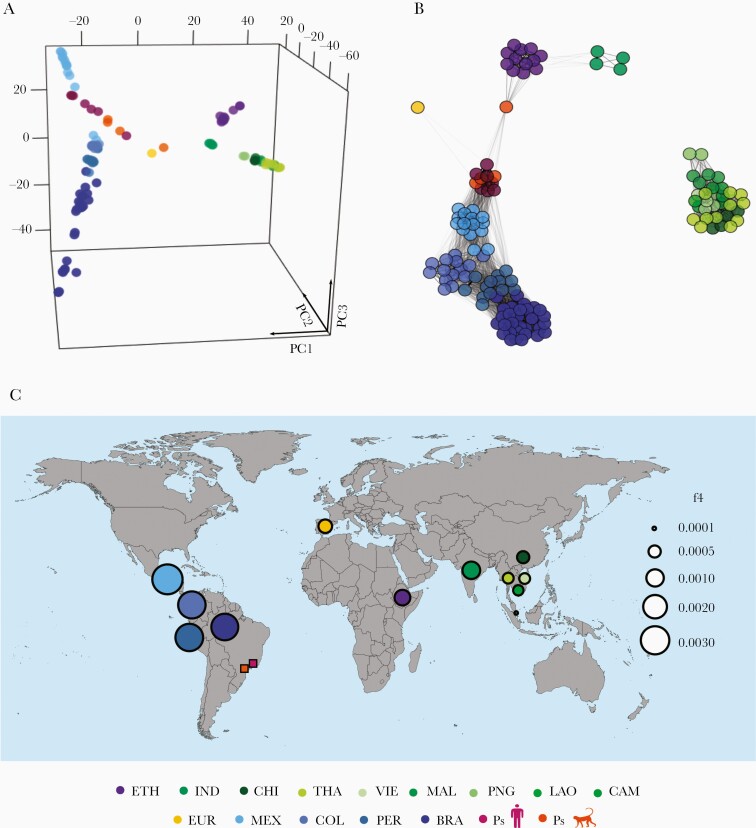
The PCA with pairwise nucleotide distances from a global data set of genomes reproduces the clear divide between New and Old World populations of P vivax found in previous studies [34]. Plasmodium simium clusters with the New World populations of P vivax—especially from Mexico (MEX) (Figure 3A), a relatively inbred population that has experienced a steady decline in recent years [34]. Identity-by-descent analysis reveals qualitatively similar clustering patterns (Figure 3B), and only parasite pairs from the same geographic region share ≥5% ancestry, which indicates limited gene flow between continents. There are 2 noteworthy exceptions: (1) the low-coverage P simium isolate P160 seems to be connected to Ethiopian (ETH) P vivax isolates, and (2) the P vivax samples from India (IND) and ETH are fairly connected to each other. The latter finding supports the hypothesis of recolonization of Africa by South Asian P vivax stocks after the local fixation of the Duffy-negative phenotype, which confers resistance to blood-stage P vivax infection [45].
Figure 3.
Genetic relatedness between Plasmodium simium and a global collection of P vivax isolates. (A) Three-eigenvector principal component analysis plot using genome-wide single-nucleotide polymorphism variation data from P simium and Plasmodium vivax isolates listed in Supplementary Table 2. Each circle represents an isolate, which is colored according to its country of origin. Populations are abbreviated as follows: BRA, Amazonian Brazil; PS, Atlantic Forest of Brazil; CAM, Cambodia; CHI, China, COL, Colombia; ETH, Ethiopia; EUR, Ebro Delta of Spain, Europe; IND, India; LAO, Laos; MAL, Malaysia, MEX, Mexico; PER, Peru; PNG, Papua New Guinea; THA, Thailand; and VIE, Vietnam. The percentage contributions of each eigenvector are as follows: PC1, 11.8%; PC2, 5.6%; and PC3, 3.9%. (B) Global parasite relatedness network inferred by identity-by-descent analysis. Edges connecting parasites are shown for pairs with ≥5% shared ancestry and unconnected isolates are omitted. Edge thickness is directly proportional to the proportion of shared ancestry, with thick edges indicating greater relatedness. (C) Values of f4 statistic, under the relationship f4 (W, X; Y, Z), where W is the macaque parasite Plasmodium cynomolgi (outgroup), X is P simium, Y is the PNG population of P vivax, and Z is each of 12 non-PNG populations of P vivax, represented as circles in the world map colored according to the country of origin. Circle sizes are directly proportional to the magnitude of the computed f4 statistics; greater circles indicate a closer relationship between Y and the P simium population from humans and monkeys (Ps, represented by squares), relative to PNG. Detailed results are shown in Supplementary Table 10.
Using f4 statistic, we show that P simium shares more derived alleles with P vivax samples from the Americas, followed by IND, ETH, and the recently characterized European isolate (EUR) [46], compared with any population from East and Southeast Asia and Papua New Guinea (PNG) (Supplementary Table 10). These results rule out an Asian origin of P simium. The greatest genetic affinity of P simium is with P vivax from MEX, followed by Colombia (COL), Peru (PER), and BRA, with the lowest affinity with PNG. Figure 3C shows the west-to-east longitudinal cline of shared ancestry between P simium and all P vivax populations represented in our global sample, in relation to PNG.
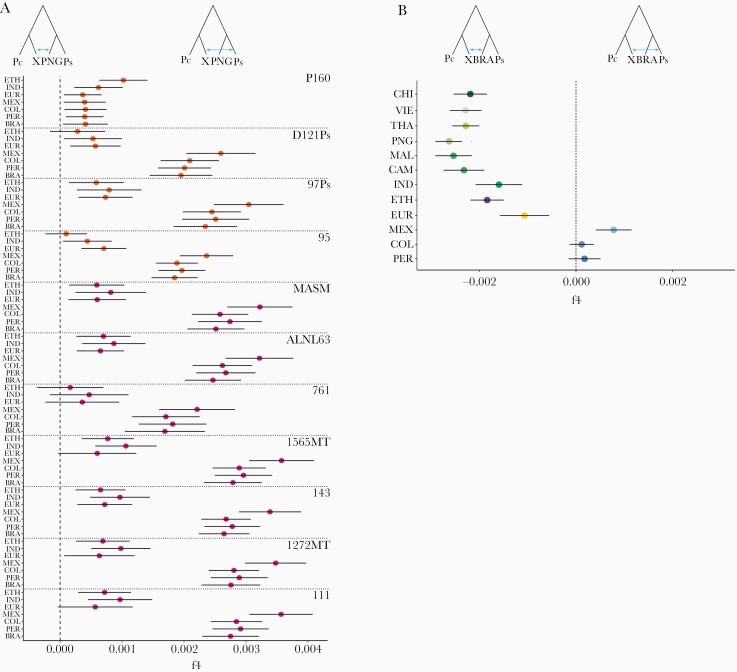
We next explored the genetic affinities of individual isolates of P simium. Each of them displays a greater genetic similarity to MEX than with the BRA, PER, COL, ETH, IND, or EUR populations of P vivax, with the exception of P160, which appears to be closer to ETH than to American populations (Figure 4A). We also compared the extent of shared ancestry of parasites from Brazil—P simium, from the Atlantic Coast, and BRA, from the Amazon—with the non-BRA P vivax populations relative to PNG. We show that MEX is significantly closer to P simium than to BRA, whereas PER and COL are nearly equidistant of P simium and BRA. Conversely, all Old World P vivax populations are significantly closer to BRA than to the P simium population (Figure 4B). This suggests that parasites from diverse geographic origins founded the highly diverse present-day BRA population [16, 47]. Conversely, with the notable exception of P160, P simium appears to have descended primarily from less admixed New World stocks of P vivax, similar to the MEX population.
Figure 4.
Genetic affinities of Plasmodium simium with New World isolates of Plasmodium vivax. (A) To explore the genetic affinities of individual isolates of P simium, we computed the f4 statistic for W corresponding to Plasmodium cynomolgi (Pc, outgroup), X to each of the 11 individual isolates of P simium (Ps), Y to the Papua New Guinean (PNG) population of P vivax, and Z to one of the American (Amazonian Brazil [BRA], Mexico [MEX], Peru [PER], and Colombia [COL]), Indian (IND), Ethiopian (ETH), or European (EUR) populations of P vivax, that are indicated on the y-axis legend. Sample codes are shown to the right. Note that P160 is the only P simium isolate that is genetically closer to non-American (ETH and to some extent IND) than American populations of P vivax (also see Supplementary Results). (B) To compare the genetic affinities of the P simium and BRA populations (from the Atlantic Coast and Amazon Basin of Brazil, respectively) with each of the non-BRA P vivax populations, we used the f4 statistic with W corresponding to Plasmodium cynomolgi (Pc), X to the P simium (Ps) population, Y to BRA, and Z to each of 12 non-BRA populations of P vivax, which are indicated on the y-axis legend.
There is minimal genetic differentiation between P simium and P vivax populations from the Americas (Supplementary Figure 6; Supplementary Results). The average divergence between BRA and P simium (between-species π = 4.92 × 10–4) is identical to the pairwise polymorphism within the BRA population (within-species π = 4.93 × 10–4; P = .8504, Mann-Whitney U test). Moreover, the differentiation between BRA and MEX (within-species π = 6.10 × 10–4) is significantly greater than the average divergence between MEX and P simium (between-species π = 4.74 × 10–4; P = 4.1 × 10–15, Mann-Whitney U test) and that between BRA and P simium (P = 2.0 × 10–16, Mann-Whitney U test).
Pathway to a Reverse Zoonosis
Plasmodium simium experimentally infects anophelines from North America, Africa, South Asia, Southeast Asia, and the South Pacific [48], but no study has compared its infectiousness to Nyssorhinchus and Kerteszia anophelines or investigated adaptative changes in key proteins, such as Pvs47, that may affect vector compatibility. Not surprisingly, we show that Pvs47 sequences of P simium cluster together with those of P vivax from the Pacific Coast of Colombia and Amazonian Brazil and are clearly divergent from Old World sequences (Figure 5A), most likely as a result of selection imposed by adaptation to local vectors [34]. In addition, we found 2 amino acid changes present in P simium Pvs47 but absent in global P vivax populations (Figure 5B and Supplementary Table 11) that merit further investigation.
Figure 5.
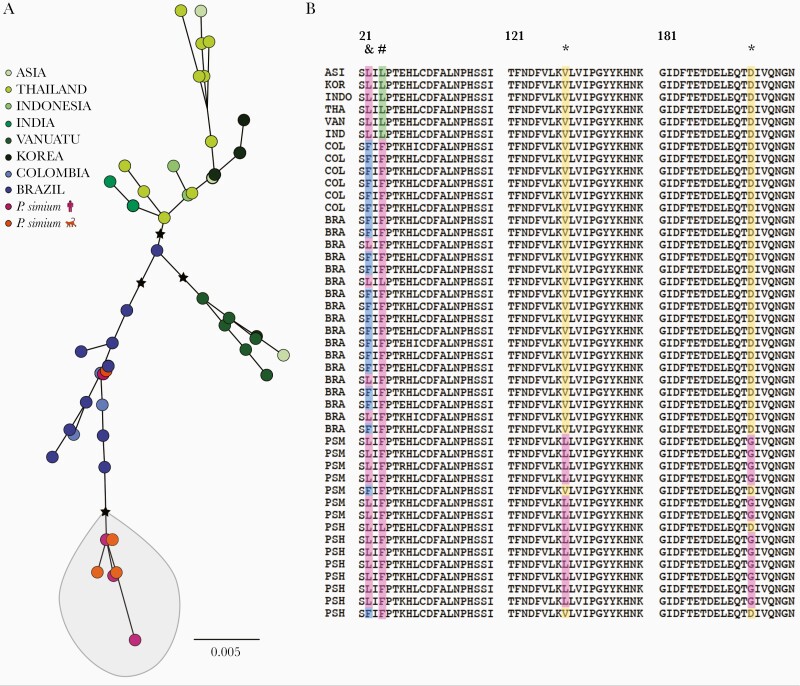
Global diversity in the Plasmodium vivax gamete surface protein Pvs47, a key determinant of parasite-vector compatibility. (A) Maximum likelihood tree of Pvs47 protein sequences from P vivax and Plasmodium simium generated with the best-fit protein evolution model (FLU + I). Black stars indicate basal nodes with bootstrap support >85% (1000 pseudoreplicates). Note that P simium clusters with New World P vivax isolates. (B) Amino acid alignment of the variable domain of Pvs47 (residues 21–200); the full-length protein has 434 amino acids. * indicates amino acid changes private of P simium; # indicates an amino acid change shared between P simium and New World P vivax lineages; and & indicates an amino acid change private of New World P vivax lineages. A single Pvs47 sequence represents all Asian lineages of P vivax because they are >99% identical [34]. Sample origins are abbreviated as follows: ASI, Asia (country not specified); BRA, Amazonian Brazil; COL, Colombia; IND, India; INDO, Indonesia; KOR, South Korea; PSH, P simium of human origin; PSM, P simium of simian origin; THA, Thailand; and VAN, Vanuatu. Sample origins are given in Supplementary Table 3 and Pvs47 polymorphisms are listed in Supplementary Table 11.
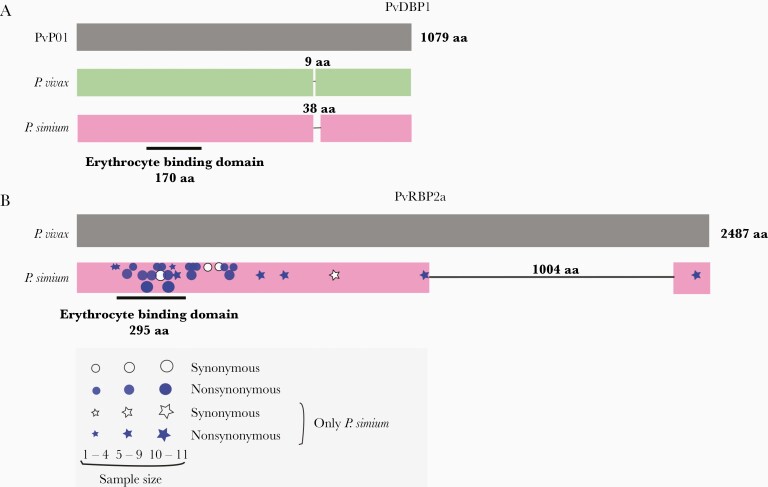
The red blood cell ligands PvDBP1 and PvRBP2a display marked divergence between P vivax and P simium. We identified the previously described 38-amino acid deletion in PvDBP1 in 7 of 16 P simium samples from humans and 5 of 9 samples from monkeys (Figure 6A; Supplementary Figures 7 and 8). However, the deletion is unlikely to modify the erythrocyte binding affinity of P simium PvDBP1, because its cysteine-rich 170-amino acid binding motif remains intact. Worldwide P vivax isolates present a much smaller deletion (9 amino acids) in the same PvDBP1 domain, relative to the reference PvP01 sequence. All P simium samples analyzed present the same private E→K amino acid substitution (Supplementary Figure 8).
Figure 6.
Deletions in the Plasmodium simium PvDBP1 and PvRBP2a ligands of red blood cells. (A) Schematic representation of PvDBP1 showing the 9-amino acid (aa) deletion found in the vast majority of worldwide Plasmodium vivax isolates, relative to the PvP01 reference sequence from Papua New Guinea, and the 38-aa deletion that may occur in P simium isolates, but not in P vivax. The location of the 170-aa erythrocyte binding motif of PvDBP is indicated. (B) Schematic representation of PvRBP2a showing the 1004-aa deletion found in all P simium isolates but not in worldwide P vivax populations. The location of the 295-aa erythrocyte binding motif of PvRBP2a is indicated [43]. The approximate location of synonymous and nonsynonymous nucleotide replacements at the pvrbp2a locus observed in newly sequenced P simium genome sequences is indicated by white and blue symbols, respectively; symbol sizes are directly proportional to the number of sequences showing the mutation. Stars and circles indicate nucleotide replacements found only in P simium and those also found in P vivax populations, respectively. See Supplementary Figure 8 and Supplementary Table 11 for further details.
More strikingly, all P simium samples from humans (n = 16) and monkeys (n = 7) display the same 1004-amino acid deletion in exon 2 of PvRBP2a [17] (Figure 6B and Supplementary Figures 6 and 7). This deletion preserves the reading frame but removes 44% of the coding sequence—therefore, it is very likely to destabilize the protein structure [43] and affect erythrocyte binding affinity. No deletion occurs in P vivax PvRBP2a (Supplementary Figures 8 and 9). Eight private amino acid changes and 1 private synonymous nucleotide replacement are present throughout P simium PvRBP2a (Figure 6B and Supplementary Table 11), which ranks among the most diverse coding sequences of this parasite (Supplementary Table 4; Supplementary Figure 4).
DISCUSSION
We have characterized, at the genome level, New World malaria parasites that infect humans and platyrrhine monkeys and sustain zoonotic malaria transmission on the Atlantic Coast of Brazil [20, 21]. The paucity of genomic diversity in P simium is consistent with its recent jump from humans to howler monkeys and a few other Neotropical nonhuman primates [14, 15]. Although natural and experimental human infections with nonhuman primate malaria parasites are well known [49], in this study we address the much less common case of a host switch from humans to sylvatic monkeys, which characterizes a reverse zoonosis. The new hosts constitute a vast infectious reservoir that remains unaddressed by malaria elimination efforts [22, 23, 26, 29].
The P simium isolates from platyrrhine monkeys and humans have close genome-wide affinity with P vivax from the New World. We hypothesize that P vivax stocks from southern Europe and Africa that arrived in the Atlantic Coast during the colonial era [18] evolved to infect Kerteszia anophelines and local nonhuman primate species, in addition to humans. Similar species of platyrrhine monkeys are rarely found to harbor P simium across the Amazon Basin, although these same hosts comprise a significant sylvatic reservoir of the zoonotic malariae-like parasite Plasmodium brasilianum in the region [8, 13, 15]. More importantly, the meagre genome-wide divergence between P simium and New World P vivax, compared with similar or even higher levels of intraspecific polymorphism in P vivax populations across the continent [34, 47] (Supplementary Figure 6), argues against the current placement of P simium and P vivax in separate species.
Signatures of adaptation to new vectors are expected to occur in the P simium genome. As in P falciparum [33], the substantial genetic differentiation at the pvs47 locus between Old World and New World P vivax is thought to have allowed parasites to evade the Jun N-terminal kinase (JNK) immune response of Neotropical Nyssorhynchus vectors [34], which diverged from the lineage leading to Old World anophelines approximately 100 million years ago. Our analysis of Pvs47 sequences in P vivax and P simium suggests that local Kerteszia vectors may have similarly imposed a selective pressure on parasites that, once introduced in South America, remained on the Atlantic Coast, instead of spreading to the Nyssorhynchus-dominated interior of the continent.
The deletion of >40% of the coding sequence of PvRBP2a, combined with numerous private amino acid changes, is the most likely signature of adaptation to nonhuman primate hosts identified in the P simium genome. We hypothesize that PvRBP2a-mediated erythrocyte binding has been drastically reduced or abolished in P simium, favoring alternative ligands for more efficient monkey infection. This is reminiscent of the loss of EBA165, the red blood cell ligand of Laverania parasites that recognizes ape-specific sialic acids on erythrocytes, allowing P falciparum to infect humans [50]. Whether P simium binds to and invades human and simian erythrocytes with similar efficiency is unknown. Typically, P simium parasitemias are low in natural human and simian infections, with mild or absent clinical manifestations [19, 21, 22, 28]. We note, however, that we obtained human P simium samples from febrile hosts with patent parasitemias, which may have biased our analysis towards parasites with increased virulence. How P simium PvRBP2a interacts with its (undetermined) receptor on human and simian red blood cells remains unexplored [36], and experimental approaches are severely limited by the lack of a continuous culture in vitro for this parasite.
CONCLUSIONS
We conclude that P simium parasites from humans and monkeys comprise New World-type P vivax lineages that have very recently adapted to Neotropical nonhuman primates in the Atlantic Forest ecosystem. Likely genomic signatures of adaptation to these new vertebrate hosts include a massive deletion and extensive amino acid polymorphism in a key erythrocyte ligand, which may have favored more efficient simian host cell infection. Addressing the zoonotic reservoir is critical for preventing human malaria in the outskirts of major urban hubs, such as São Paulo and Rio de Janeiro, along the Atlantic Coast of Brazil [21, 22, 27].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful to Maria José Menezes and Rebecca Kuzma for expert laboratory support, Rodrigo M. Corder for help in data analysis, Igor C. Johansen for preparing maps, and Seth Redmond for the useful insights.
Financial support. This work was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil (2016/18740-9 [to M. U. F.], 2014/10919-4 [to A. M. R. C. D.], and 2009/51466-4 [to J. L. C.-D.]), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil (301011/2019-2; to M. U. F.), and the Wellcome Trust, United Kingdom (207486/Z/17/Z; to L. D. P. R.). This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Grant Number U19AI110818 to the Broad Institute (to D. E. N.). T. C. d. O., L. C.-D., and M. U. F. acknowledge scholarships from CNPq, Brazil. P. T. R. receives a postdoctoral scholarship from FAPESP. T. C. d. O. received a travel scholarship from the Fulbright Foreign Student Program for Brazil, USA.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Presented in part: 67th Annual Meeting of the American Society of Tropical Medicine and Hygiene, October 30, 2018, New Orleans, LA; 7th International Conference on Plasmodium vivax Research, June 24–28, 2019, Paris, France.
References
- 1. Ferreira MU, Castro MC. Malaria situation in Latin America and the Caribbean: residual and resurgent transmission and challenges for control and elimination. Methods Mol Biol 2019; 2013:57–70. [DOI] [PubMed] [Google Scholar]
- 2. Sinka ME, Rubio-Palis Y, Manguin S, et al. . The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic précis. Parasit Vectors 2010; 3:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marrelli MT, Malafronte RS, Sallum MA, Natal D. Kerteszia subgenus of Anopheles associated with the Brazilian Atlantic rainforest: current knowledge and future challenges. Malar J 2007; 6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harcourt AH. Human phylogeography and diversity. Proc Natl Acad Sci U S A 2016; 113:8072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prugnolle F, Rougeron V, Becquart P, et al. . Diversity, host switching and evolution of Plasmodium vivax infecting African great apes. Proc Natl Acad Sci U S A 2013; 110:8123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu W, Li Y, Shaw KS, et al. . African origin of the malaria parasite Plasmodium vivax. Nat Commun 2014; 5:3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loy DE, Plenderleith LJ, Sundararaman SA, et al. . Evolutionary history of human Plasmodium vivax revealed by genome-wide analyses of related ape parasites. Proc Natl Acad Sci U S A 2018; 115:E8450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deane LM. Simian malaria in Brazil. Mem Inst Oswaldo Cruz 1992; 87:1–20. [DOI] [PubMed] [Google Scholar]
- 9. Schrago CG, Russo CA. Timing the origin of New World monkeys. Mol Biol Evol 2003; 20:1620–5. [DOI] [PubMed] [Google Scholar]
- 10. da Fonseca F. Plasmodium of a primate of Brazil. Mem Inst Oswaldo Cruz 1951; 49:543–53. [DOI] [PubMed] [Google Scholar]
- 11. Bueno MG.[doctoral dissertation]. São Paulo: Faculty of Veterinary Medicine of the University of São Paulo; 2012. [Google Scholar]
- 12. de Alvarenga DA, de Pina-Costa A, de Sousa TN, et al. . Simian malaria in the Brazilian Atlantic forest: first description of natural infection of capuchin monkeys (Cebinae subfamily) by Plasmodium simium. Malar J 2015; 14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Araújo MS, Messias MR, Figueiró MR, et al. . Natural Plasmodium infection in monkeys in the state of Rondônia (Brazilian Western Amazon). Malar J 2013; 12:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim CS, Tazi L, Ayala FJ. Plasmodium vivax: recent world expansion and genetic identity to Plasmodium simium. Proc Natl Acad Sci U S A 2005; 102:15523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tazi L, Ayala FJ. Unresolved direction of host transfer of Plasmodium vivax v. P. simium and P. malariae v. P. brasilianum. Infect Genet Evol 2011; 11:209–21. [DOI] [PubMed] [Google Scholar]
- 16. Rodrigues PT, Valdivia HO, de Oliveira TC, et al. . Human migration and the spread of malaria parasites to the New World. Sci Rep 2018; 8:1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mourier T, de Alvarenga DAM, Kaushik A, et al. . The genome of the zoonotic malaria parasite Plasmodium simium reveals adaptions to host-switching [preprint]. BioRxiv 841171. [DOI] [PMC free article] [PubMed]
- 18. Deane LM, Ferreira Neto JA, Lima MM. The vertical dispersion of Anopheles (Kerteszia) cruzi in a forest in southern Brazil suggests that human cases of malaria of simian origin might be expected. Mem Inst Oswaldo Cruz 1984; 79:461–3. [DOI] [PubMed] [Google Scholar]
- 19. Deane LM, Deane MP, Ferreira Neto J. Studies on transmission of simian malaria and on a natural infection of man with Plasmodium simium in Brazil. Bull World Health Organ 1966; 35:805–8. [PMC free article] [PubMed] [Google Scholar]
- 20. Buery JC, Rodrigues PT, Natal L, et al. . Mitochondrial genome of Plasmodium vivax/simium detected in an endemic region for malaria in the Atlantic Forest of Espírito Santo state, Brazil: do mosquitoes, simians and humans harbour the same parasite? Malar J 2017; 16:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brasil P, Zalis MG, de Pina-Costa A, et al. . Outbreak of human malaria caused by Plasmodium simium in the Atlantic Forest in Rio de Janeiro: a molecular epidemiological investigation. Lancet Glob Health 2017; 5:e1038–46. [DOI] [PubMed] [Google Scholar]
- 22. Abreu FVS, Santos ED, Mello ARL, et al. . Howler monkeys are the reservoir of malarial parasites causing zoonotic infections in the Atlantic forest of Rio de Janeiro. PLoS Negl Trop Dis 2019; 13:e0007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Multini LC, Marrelli MT, Beier JC, Wilke ABB. Increasing complexity threatens the elimination of extra-Amazonian malaria in Brazil. Trends Parasitol 2019; 35:383–7. [DOI] [PubMed] [Google Scholar]
- 24. Li J, Collins WE, Wirtz RA, Rathore D, Lal A, McCutchan TF. Geographic subdivision of the range of the malaria parasite Plasmodium vivax. Emerg Infect Dis 2001; 7:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cormier LA. The historical ecology of human and wild primate malarias in the New World. Diversity 2010; 2:256–80. [Google Scholar]
- 26. Duarte AM, Malafronte Rdos S, Cerutti C Jr, et al. . Natural Plasmodium infections in Brazilian wild monkeys: reservoirs for human infections? Acta Trop 2008; 107:179–85. [DOI] [PubMed] [Google Scholar]
- 27. Yamasaki T, Duarte AM, Curado I, et al. . Detection of etiological agents of malaria in howler monkeys from Atlantic Forests, rescued in regions of São Paulo city, Brazil. J Med Primatol 2011; 40:392–400. [DOI] [PubMed] [Google Scholar]
- 28. Cerutti C Jr, Boulos M, Coutinho AF, et al. . Epidemiologic aspects of the malaria transmission cycle in an area of very low incidence in Brazil. Malar J 2007; 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferreira MU, Castro MC. Challenges for malaria elimination in Brazil. Malar J 2016; 15:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cowell AN, Loy DE, Sundararaman SA, et al. . Selective whole-genome amplification is a robust method that enables scalable whole-genome sequencing of Plasmodium vivax from unprocessed clinical samples. mBio 2017;8:e02257-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Auburn S, Böhme U, Steinbiss S, et al. . A new Plasmodium vivax reference sequence with improved assembly of the subtelomeres reveals an abundance of pir genes. Wellcome Open Res 2016; 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pearson RD, Amato R, Auburn S, et al. . Genomic analysis of local variation and recent evolution in Plasmodium vivax. Nat Genet 2016; 48:959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Molina-Cruz A, Canepa GE, Alves E Silva TL, et al. . Plasmodium falciparum evades immunity of anopheline mosquitoes by interacting with a Pfs47 midgut receptor. Proc Natl Acad Sci U S A 2020; 117:2597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hupalo DN, Luo Z, Melnikov A, et al. . Population genomics studies identify signatures of global dispersal and drug resistance in Plasmodium vivax. Nat Genet 2016; 48:953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 2015; 32:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kanjee U, Rangel GW, Clark MA, Duraisingh MT. Molecular and cellular interactions defining the tropism of Plasmodium vivax for reticulocytes. Curr Opin Microbiol 2018; 46:109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haubold B, Travisano M, Rainey PB, Hudson RR. Detecting linkage disequilibrium in bacterial populations. Genetics 1998; 150:1341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schaffner SF, Taylor AR, Wong W, Wirth DF, Neafsey DE. hmmIBD: software to infer pairwise identity by descent between haploid genotypes. Malar J 2018; 17:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Petr M, Vernot B, Kelso J. admixr-R package for reproducible analyses using ADMIXTOOLS. Bioinformatics 2019; 35:3194–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patterson N, Moorjani P, Luo Y, et al. . Ancient admixture in human history. Genetics 2012; 192:1065–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tachibana S, Sullivan SA, Kawai S, et al. . Plasmodium cynomolgi genome sequences provide insight into Plasmodium vivax and the monkey malaria clade. Nat Genet 2012; 44:1051–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Warncke JD, Vakonakis I, Beck HP. Plasmodium helical interspersed subtelomeric (PHIST) proteins, at the center of host cell remodeling. Microbiol Mol Biol Rev 2016; 80:905–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gruszczyk J, Lim NT, Arnott A, et al. . Structurally conserved erythrocyte-binding domain in Plasmodium provides a versatile scaffold for alternate receptor engagement. Proc Natl Acad Sci U S A 2016; 113:E191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knuepfer E, Wright KE, Kumar Prajapati S, et al. . Divergent roles for the RH5 complex components, CyRPA and RIPR in human-infective malaria parasites. PLoS Pathog 2019; 15:e1007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Culleton R, Carter R. African Plasmodium vivax: distribution and origins. Int J Parasitol 2012; 42:1091–7. [DOI] [PubMed] [Google Scholar]
- 46. van Dorp L, Gelabert P, Rieux A, et al. . Plasmodium vivax malaria viewed through the lens of an eradicated European strain. Mol Biol Evol 2020; 37:773–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Oliveira TC, Rodrigues PT, Menezes MJ, et al. . Genome-wide diversity and differentiation in New World populations of the human malaria parasite Plasmodium vivax. PLoS Negl Trop Dis 2017; 11:e0005824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Collins WE, Sullivan JS, Galland GG, et al. . Plasmodium simium and Saimiri boliviensis as a model system for testing candidate vaccines against Plasmodium vivax. Am J Trop Med Hyg 2005; 73:644–8. [PubMed] [Google Scholar]
- 49. Martinelli A, Culleton R. Non-human primate malaria parasites: out of the forest and into the laboratory. Parasitology 2018; 145:41–54. [DOI] [PubMed] [Google Scholar]
- 50. Proto WR, Siegel SV, Dankwa S, et al. . Adaptation of Plasmodium falciparum to humans involved the loss of an ape-specific erythrocyte invasion ligand. Nat Commun 2019; 10:4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data were deposited in the Sequence Read Archive ([SRA] SAMN17926899–SAMN17926909) and GenBank Nucleotide Database (MW560996–MW561043, MW561060–MW561072, and MW561044–MW561059) of the National Center for Biotechnology Information.