Abstract
Background
Early HIV diagnosis allows combination antiretroviral therapy (cART) initiation in the first days of life following in utero (IU) infection. The impact of early cART initiation on infant viral reservoir size in the setting of high-frequency cART nonadherence is unknown.
Methods
Peripheral blood total HIV DNA from 164 early treated (day 0–21 of life) IU HIV-infected South African infants was measured using droplet digital PCR at birth and following suppressive cART. We evaluated the impact of cART initiation timing on HIV reservoir size and decay, and on the risk of subsequent plasma viremia in cART-suppressed infants.
Results
Baseline HIV DNA (median 2.8 log10 copies/million peripheral blood mononuclear cells, range 0.7–4.8) did not correlate with age at cART initiation (0–21 days) but instead with maternal antenatal cART use. In 98 infants with plasma viral suppression on cART, HIV DNA half-life was 28 days. However, the probability of maintenance of plasma aviremia was low (0.46 at 12 months) and not influenced by HIV DNA load. Unexpectedly, longer time to viral suppression was associated with protection against subsequent viral rebound.
Conclusions
With effective prophylaxis against mother-to-child transmission, cART initiation timing in the first 3 weeks of life is not critical to reservoir size.
Keywords: early infant diagnosis, in utero HIV, HIV reservoir, reservoir decay, pediatric HIV, early treatment, digital droplet PCR, viral rebound
Peripheral blood HIV DNA levels from 164 South African in utero HIV-infected infants showed that with antiretroviral mother-to-child transmission prophylaxis, combination antiretroviral therapy initiation timing in the first 3 weeks of life does not significantly influence latent HIV reservoir size.
For children living with human immunodeficiency virus (HIV), although lifelong viral suppression with combination antiretroviral therapy (cART) is possible, rates of treatment failure and mortality on cART are higher compared to adults [1, 2], particularly for infants [3–5]. HIV DNA in resting long-lived CD4+ T cells, or the latent HIV reservoir, is established in the first days of infection [6–8] and in children and adults reactivates in the days to weeks following cART cessation [9–11], thus constitutes the ultimate barrier to HIV remission or cure. The only intervention that has been shown to reduce the latent HIV reservoir is early initiation of cART, whereby lower reservoir levels are achieved by not only restricting the initial size [12, 13] and sequence diversity [14, 15] of the viral reservoir, but also its cellular localization, skewing the reservoir to exist in shorter-lived, transitional CD4+ T-cell memory cells which decay faster than longer-lived central memory cells [16]. Because most in utero (IU) HIV infections occur in the final weeks of pregnancy [17, 18], it is possible routinely to treat HIV-infected newborns early in infection when diagnosed at birth. Early treatment along with a favorable tolerogenic neonatal immune milieu [19–21] has led to great scientific interest in the early treatment of IU HIV-infected infants as a model for HIV cure [22–24]. However, the precise age, whether it is hours or days of life, that defines early treatment to realize these benefits is unclear. In a cohort of IU HIV-infected infants in South Africa for whom cART was initiated within the first days of life, we quantified peripheral HIV DNA to determine the size and decay of the latent HIV reservoir, and the relationship with subsequent plasma virological control.
METHODS
Study Participants
Ucwaningo Lwabantwana is an observational study based in KwaZulu-Natal, South Africa, exploring early (aged <48 hours) cART for HIV-infected infants [25]. HIV-infected mothers and infants aged <21 days with confirmed positive total nucleic acid HIV polymerase chain reaction (PCR) testing at birth either via point-of-care (PoC) testing (whole blood Gene Xpert; Cepheid) or laboratory-based test result from dried blood spots (DBS; COBAS AmpliPrep/COBAS TaqMan version 2; Roche Molecular Diagnostics). Routine HIV care was provided by local Department of Health clinic staff according to South African guidelines [26]. First-line maternal cART was fixed-dose combination emtricitabine, tenofovir disoproxil fumarate, and efavirenz. Neonatal prophylaxis was commenced for all HIV-exposed infants: nevirapine (NVP) only, or NVP and zidovudine (AZT) where maternal viremia >1000 HIV RNA copies/mL was noted. Infant cART consisted of AZT/lamivudine (3TC)/NVP until 28 days for term infants or 42 weeks corrected gestational age for preterm infants, after which the regimen was switched to abacavir/3TC/ritonavir-boosted lopinavir. The study was approved by the KwaZulu-Natal and Oxfordshire Biomedical Ethics Review Committees. Written informed consent was signed by the infant’s mother or guardian. The study presented here is an analysis of all data available prior to 31 December 2019. Latent HIV reservoir measurements were undertaken at baseline then longitudinally where there was optimal cART adherence, as demonstrated by declining plasma HIV RNA.
Definitions
-
•
Acute maternal infection was defined as a mother with a negative HIV rapid antibody test at antenatal booking, followed by a positive test later in pregnancy or at delivery.
-
•
All infants received a DBS test at birth but were subgrouped into PoC and DBS according to whether or not they were first diagnosed using a PoC test.
-
•
Viral suppression was defined as 1 viral load (VL) measurement lower than the limit of detection. Where an HIV DNA measurement at suppression was not available, the measurement was made at a time point within 3 months of suppression.
-
•
Two definitions of viral rebound were used: (1) low-level rebound, as 1 VL measurement >100 HIV RNA copies/mL, and (2) high-level rebound, as 1 VL measurement >1000 HIV RNA copies/mL.
-
•
A “blip” was defined as either (1) 1 VL >100 HIV RNA copies/mL preceded and followed by an undetectable VL, or (2) a VL that increased <1 log10 HIV RNA copies/mL between consecutive monthly measurements in the context of initial VL decline.
-
•
A viral suppression (VS) subgroup (n = 98) was defined by those infants who were followed for ≥6 months postenrolment, had reached VL suppression by 6 months of age, had ≥1 HIV DNA measurement, had a VL that was less than, the same, or <1 log10 higher in consecutive monthly measurements.
-
•
Adherence was documented at each clinic visit using self-reports and by measuring returned medication (if available). Nonadherence was defined as evidence or reports of missing ≥2 doses.
Laboratory Methods
VL was measured by Nuclisens EasyQ version 2.0 HIV-1 RNA PCR (bioMérieux) with a limit of detection of 20 HIV RNA copies/mL but <100 for low-volume samples. Lysed extracts from thawed peripheral blood mononuclear cells (PBMC) were used to measure total HIV DNA by droplet digital PCR (ddPCR; BioRad) with 5′ long terminal repeat or gag primers and probes, depending on the efficiency of detection in each patient [27]. The HIV DNA count was normalized using RPP30 housekeeping gene quantification to give a value per million PBMC with a 95% Poisson confidence interval, as estimated across replicates by the QuantaSoft (BioRad) software. A limit of detection for each sample was estimated according to input cell number and where HIV DNA was undetectable, the result was recorded as the limit of detection. Results were excluded if HIV DNA was undetectable and the limit of detection was >25 copies/million PBMC.
Statistics
Statistical analyses were performed using R Software version 3.6.1 [28] and GraphPad Prism version 8. Comparisons were performed using the χ 2 and Fisher exact test for categorical variables. Two-group continuous variables were compared using the t test (parametric) or the Mann-Whitney U test (nonparametric). Univariate and multivariate linear regression were calculated using the lm R function. In the baseline HIV DNA analysis, where a large number of covariates were analyzed, a penalized multivariate linear regression model was used whereby the set of relevant predicting covariates was selected using the LASSO penalty approach [29] and the optimal penalty (λ) was determined via 10-fold cross-validation using the glmnet R package [30]. The LASSO-selected covariates were then used in a (post-LASSO) multivariate linear regression to give unpenalized regression coefficient estimates. The resulting P values in a post-LASSO analysis should be interpreted with caution because variable selection via a penalized approach is a separate concept to significance. A potentially nonlinear ln-HIV DNA decay over time in the VS infants was estimated and visualized via a generalized additive mixed model (GAMM) with a random effect (intercept only) on the participant, then again in a multivariate analysis to assess for covariates influencing HIV DNA level over time, both using the mgcv R package [31]. The HIV DNA half-life (t1/2) was calculated by using the time at which the HIV DNA level was half of its value at baseline, via the predict().gam function. Pearson correlation was used to determine age of cART initiation correlation with age of VL suppression. Kaplan-Meier curves were used for time to viral suppression and rebound analyses, and Cox proportional hazard models using the coxph R function for multivariate analyses where incorporated covariates were those either significant in the baseline HIV DNA analysis or those of potential clinical relevance. All P values were 2-sided with an α of .05.
RESULTS
Baseline HIV DNA
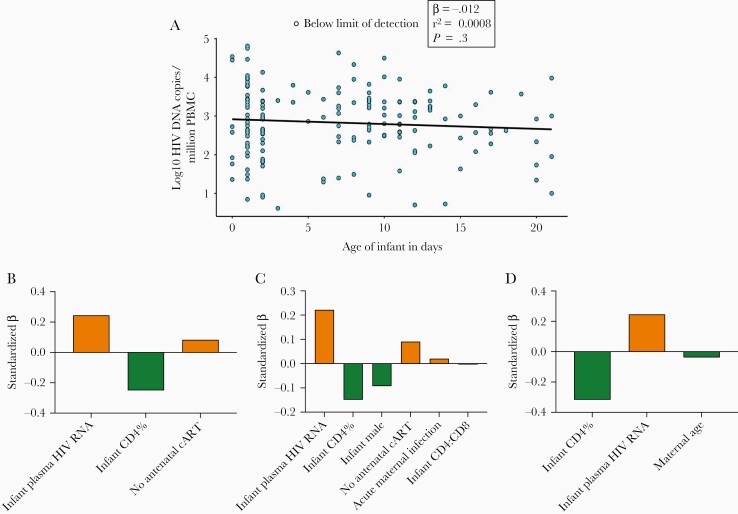
Of 191 IU HIV-infected infants enrolled, a baseline HIV DNA measurement was available for 164 infants, whose characteristics at baseline are displayed in Table 1. cART was initiated at a median age of 6.5 days (interquartile range [IQR], 1–11 days, maximum 21 days). The median infant baseline HIV DNA level was 2.8 log10 copies/million PBMC (IQR, 2.3–3.4), but levels were variable, ranging from 0.7 to 4.8 log10 copies/million PBMC. The baseline HIV DNA levels in the 77/164 (47%) PoC-tested infants did not differ from the infants diagnosed via DBS testing (P = .7, not shown). Furthermore, there was no significant relationship between baseline HIV DNA level and infant age at the time of measurement (β coefficient = −.01, P = .3; Figure 1A), even when adjusted for infant CD4 percentage and VL (P = .14, not shown) or analyzed by PoC/DBS group.
Table 1.
Baseline Characteristics of All Infants Versus Those Virally Suppressed
| Characteristic | Baseline Infants (n = 164) | Virally Suppressed Infants (n = 98) | P Value |
|---|---|---|---|
| Infant | |||
| Sex male, No. (%) | 60 (36.6) | 36 (36.7) | 1 |
| Median birth weight, kg, median (IQR) | 2.8 (2.4–3.1) | 2.9 (2.5–3.2) | .2 |
| Median gestational age at birth, wk, median (IQR)a | 38 (36–39) | 37.5 (37–39) | .4 |
| Dual prophylactic ART, No. (%) | 111 (67.7) | 72 (73.5) | .2 |
| PoC tested, No. (%) | 77 (47) | 41 (41.8) | .1 |
| DBS PCR result indeterminate or negative, No. (%) | 15 (9) | 11 (11.2) | .4 |
| Age of cART initiation, d, median (IQR)b | 6.5 (1–11) | 7 (1–11) | .6 |
| Age of blood draw, d, median (IQR) | 7 (1–11) | 7 (1–11) | .6 |
| Neonatal admission, No. (%) | 54 (32.9) | 29 (29.6) | .4 |
| Breast feeding, No. (%) | 130 (79.3) | 77 (78.6) | .7 |
| CD4 count, cells/μL, median (IQR) | 2120 (1291–2702) | 2222 (1371–2777) | .6 |
| CD4 percentage, median (IQR) | 44.5 (35.3–52) | 45.5 (38–53) | .11 |
| CD8 count, cells/μL, median (IQR) | 1088 (685–1587) | 983 (616–1433) | .3 |
| CD8 percentage, median (IQR) | 23 (18–32) | 22 (18–28) | .3 |
| CD4:CD8, median (IQR) | 1.84 (1.17–2.77) | 2.08 (1.45–2.84) | .2 |
| Plasma viral load, copies/mL, median (IQR) | 9700 (1200–56500) | 3700 (310–30500) | .04 |
| Log10 HIV DNA copies/million PBMC, median (IQR) | 2.8 (2.3–3.4) | 2.6 (2.2–3.3) | .1 |
| Mother | |||
| Age, y, median (IQR) | 25.2 (21.9–30.3) | 24.5 (21.2–29.7) | .3 |
| Acute maternal infection, No. (%) | 43 (26.2) | 29 (29.6) | .6 |
| cART initiation postnatal, No. (%) | 15 (9.1) | 8 (8.2) | 1 |
| Duration cART in pregnancy, d, median (IQR) | 104 (13–217) | 95 (8–178) | .3 |
| Self-reported maternal nonadherence, No. (%)c | 70 (54) | 36 (48) | .5 |
| CD4 count, cells/μL, median (IQR) | 460 (317–646) | 516 (350–650) | .4 |
| CD4 percentage, median (IQR) | 24 (19–31) | 25 (19–32) | .4 |
| CD8 count, cells/μL, median (IQR) | 952 (706–1272) | 910 (660–1332) | .6 |
| CD8 percentage, median (IQR) | 52.5 (43.3–58.8) | 49 (41–58) | .4 |
| CD4:CD8. median (IQR) | 0.46 (0.32–0.7) | 0.5 (0.33–0.74) | .3 |
| Plasma viral load, HIV RNA copies/mL, median (IQR) | 3750 (488–85957) | 2250 (253–20750) | .2 |
Abbreviations: cART, combination antiretroviral therapy; DBS, dried blood spot; HIV, human immunodeficiency virus; IQR, interquartile range; PCR, polymerase chain reaction; PoC, point-of-care.
aExcluded from multivariate analysis because of missing data.
bReplaced by cART prior to blood draw in multivariate analysis.
cOf all mothers who were initiated on cART prior to labor.
Figure 1.
Baseline infant HIV DNA levels. A, Baseline HIV DNA levels as determined using droplet digital PCR on PBMC from 164 infants at a median age 7 days. Undetectable HIV DNA is represented by an open circle at the limit of detection for that measurement. Measurements are displayed according to the age of the infant at the time of measurement. Univariate linear regression model shown by the black line. B–D, Covariates selected using a penalized linear regression model (LASSO) are shown left to right along the x-axis in order of standardized β coefficient magnitude, which represents their relative influence on HIV DNA. Positive, orange bars indicate covariates associated with higher HIV DNA, while negative, green bars indicate covariates associated with lower HIV DNA. These selected covariates were used in the post-LASSO linear regression to calculate unpenalized, unstandardized β coefficients (Table 2). C, LASSO model for all 164 infants. C and D, Point-of-care tested infant and dried blood spot tested infant subgroups, respectively. Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; PBMC, peripheral blood mononuclear cell; PCR, polymerase chain reaction.
To explore the potential influence of a large number of baseline maternal and infant characteristics on baseline HIV DNA, a penalized linear regression model (LASSO) was used to select influencing covariates from a possible set of 26 variables, as listed in Table 1. In the model representing all 164 infants, relatively, the baseline covariates that were the most influential on baseline infant HIV DNA were infant VL and CD4+ T-cell percentage, followed by absence of antenatal maternal cART (Figure 1B and Supplementary Figure 1A). Infant age at blood draw did not influence HIV DNA level. The selected influential covariates were then input in a (post-LASSO) multivariate linear regression to determine their absolute influence on infant HIV DNA (Table 2). The absence of antenatal maternal cART increased infant HIV DNA by 0.6 log10 copies/million PBMC.
Table 2.
Variables Associated With Infant Baseline HIV DNA Level, a Post-LASSO Multivariate Analysis
| All Infants (n = 164) | PoC Infants (n = 77) | DBS Infants (n= 87) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | β | 95% CI | P Valuea | β | 95% CI | P Valuea | β | 95% CI | P Valuea |
| Infant plasma viral load, log10 HIV RNA copies/mL | .22 | .14 to .3 | <.0001 | .22 | .09 to .35 | .001 | .26 | .17 to .35 | <.0001 |
| Infant CD4, % | −.03 | −.04 to −.02 | <.0001 | −.01 | −.04 to .01 | .2 | −.03 | −.04 to −.02 | <.0001 |
| No antenatal cART | .61 | .24 to .98 | .002 | .76 | .13 to 1.39 | .02 | … | … | … |
| Male infant | … | … | … | −.49 | −.88 to −.09 | .02 | … | … | … |
| Acute maternal infection | … | … | … | .29 | −.08 to .66 | .1 | … | … | … |
| Infant CD4:CD8 | … | … | … | −.08 | −.28 to .11 | .4 | … | … | … |
| Maternal age, y | … | … | … | … | … | … | −.02 | −.04 to 0 | .03 |
Adjusted r2 all infants = 0.37 (P ≤ .0001); PoC infants = 0.39 (P ≤ .0001); DBS infants = 0.5 (P ≤ .0001).
Abbreviations: β, unstandardized regression coefficient estimate; cART, combination antiretroviral therapy; CI, confidence interval; DBS, dried blood spot tested; HIV, human immunodeficiency virus; PoC, point-of-care tested.
a P values for post-LASSO linear regression models should be interpreted with caution as they ignore the selection by LASSO.
The infants who were diagnosed by PoC testing were analyzed separately from the infants who were diagnosed by DBS testing to account for any selection bias between the 2 groups [25]. The median infant age at baseline blood draw in these groups was 1 day (IQR, 1–2) and 11 days (IQR, 8–14), respectively. The separate baseline HIV DNA multivariate analyses are shown in Figure 1C and 1D and Supplementary Figure 1B and 1C. In both groups, infant VL and CD4+ T-cell percentage still had the strongest relationship with HIV DNA level. Male sex was additionally associated with lower DNA load and acute maternal infection during the pregnancy with higher DNA load in the PoC group (Table 2 and Figure 1C and 1D). We observed that, after adjusting for other influencing variables, for those infants tested at birth, males had HIV DNA levels 0.5 log10 HIV copies/million PBMC lower than female infants (Table 2).
HIV DNA Decay
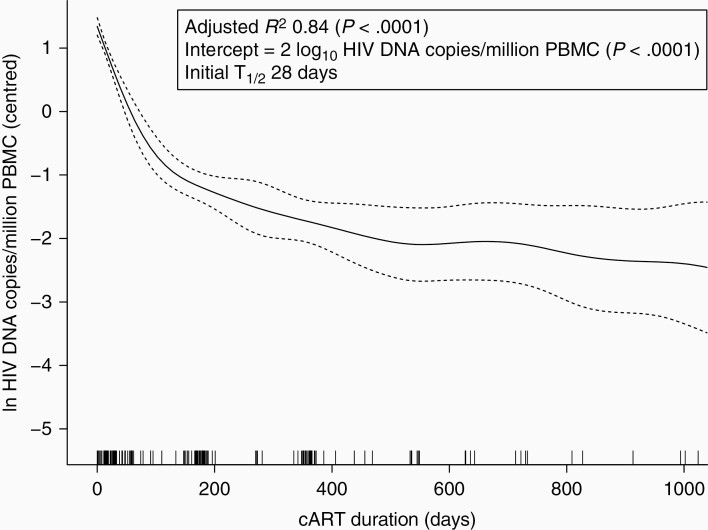
Despite early treatment, in general, virological response to cART in this cohort was suboptimal [25]. However, a subgroup of 98 infants with successful viral suppression (VS infants) were further analyzed via longitudinal measurements of HIV DNA load to examine HIV DNA decay in early treated infants (Supplementary Figure 2). To check for representativeness, the VS infants’ baseline characteristics were compared to all the infants with measurements at baseline (Table 1). The VS infants had significantly lower baseline VL (median 3700 vs 9700 HIV RNA copies/mL, P = .04) but baseline characteristics were otherwise similar. In the VS group, median age at VL suppression was 60 days (IQR, 29–90 days) and this was not significantly correlated with age at cART initiation (r2 = 0.18, P = .08, not shown), even when adjusted for baseline CD4+ T-cell percentage and VL (β = .02, P = .1, not shown). VS infant HIV DNA measurements were censored at VL rebound, resulting in 274 measurements in the first 3.5 years of life, of which 40/274 (14.5%) were below the limit of detection. The HIV DNA over time GAMM was nonlinear, well representative of the data and highly significant (adjusted R2 0.84, P < .0001; Figure 2).
Figure 2.
HIV DNA decay in the viral suppression infants. Generalized additive mixed model of log10 HIV DNA over time on cART with individual infant as a random effect (intercept only). Dashed lines, 95% confidence intervals. Vertical spikes on the x-axis denote timing of observations. Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; PBMC, peripheral blood mononuclear cell.
Rate of decay was estimated from the model, giving an initial (ie, from baseline) half-life of 28 days. At 90 days the half-life had increased to 194 days. A multivariate GAMM was used to determine the factors influencing the change in HIV DNA over time (Table 3). VL over time and age at viremia suppression significantly influenced HIV DNA decay while age of cART initiation, blips, documented nonadherence, infant sex, and CD4+ T-cell percentage over time did not (Table 3).
Table 3.
Variables Influencing HIV Decay
| Variable | Estimate (95% CI) | P Value |
|---|---|---|
| Parametric coefficients | ||
| Intercept | 2.12 (1.8 to 2.43) | <.0001 |
| Infant sex | 0.004 (−.28 to .29) | .98 |
| Blipsa | 0.12 (−.31 to .55) | .6 |
| Documented cART nonadherenceb | −0.08 (−.22 to .06) | .3 |
| PoC tested | 0.16 (−.34 to .66) | .5 |
| Approximate significance of smooth terms | EDF | P Value |
| cART duration, d | 3.19 | <.0001 |
| Infant age at cART initiation, d | 1.00 | .80 |
| Infant age suppressed, wk | 1.00 | .0003 |
| Plasma viral load, log10 HIV RNA copies/mL over time | 1.00 | <.0001 |
| CD4 percentage over time | 2.02 | .3 |
Generalized additive mixed model adjusted R2 = 0.86.
Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; EDF, effective degrees of freedom; HIV, human immunodeficiency virus; PoC, point-of-care tested.
aA blip was defined as one plasma viral load >100 HIV RNA copies/mL preceded and followed by an undetectable plasma viral load, or a plasma viral load that increased <1 log10 HIV RNA copies/mL between consecutive monthly measurements during initial viremia decline.
bEvidence or reports of missing ≥2 doses.
HIV DNA and Plasma Viremia Control
The relationship between HIV DNA levels and plasma viremia control was explored, first by examining the time to virological suppression in all 164 infants. Baseline infant VL, CD4+ T-cell percentage and HIV DNA levels independently influenced time to suppression of plasma viremia, based on univariate Cox regression analyses and a multivariate analysis (Table 4). The addition of infant sex, age of cART initiation, and PoC testing did not individually influence time to suppression nor improve the fit of the multivariate model. In the final multivariate model, baseline log10 VL had the strongest influence on time to suppression (hazard ratio [HR], 0.55; 95% confidence interval [CI], .45–.67; P < .0001; Table 4). After this adjustment, baseline HIV DNA level still had a significant effect, where an increase of 1 log10 HIV DNA copies/million PBMC decreased the rate to suppression by 26% (P = .01; Table 4).
Table 4.
Variables Influencing Time to Viral Suppression
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Baseline infant HIV DNA, log10 copies/million PBMC | 0.59 (.49–.72) | <.0001 | 0.75 (.60–.94) | .01 |
| Baseline infant plasma viral load, log10 HIV RNA copies/mL | 0.52 (.43–.63) | <.0001 | 0.55 (.45–.66) | <.0001 |
| Baseline infant CD4, % | 1.03 (1.01–1.05) | <.0001 | 1.02 (1.01–1.04) | .008 |
| Male infant | 0.87 (.60–1.27) | .5 | … | … |
| PoC tested | 0.82 (.57–1.17) | .3 | … | … |
| Infant age at cART initiation, d | 1.01 (.98–1.04) | .7 | … | … |
Cox proportional hazard model. Wald test P ≤ .0001.
Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; PoC, point-of-care.
Next, maintenance of plasma aviremia on cART was evaluated in the VS infants with regard to (1) HIV DNA level at baseline and (2) HIV DNA at the time point VL suppression was achieved. HIV DNA load was assessed both as a continuous variable, and a categorical variable (above and below the median value). Median time to viral rebound >100 HIV RNA copies/mL was 423 days (95% CI, 320–666) and 571 days (95% CI, 280–∞) for >1000 HIV RNA copies/mL. The probability of viral rebound >1000 HIV RNA copies/mL at 12 months was 0.46 (95% CI, .34–.56). The univariate analyses did not find any significant relationship between HIV DNA load at baseline or at the time of suppression and time to viral rebound for either definition (Table 5). The best fit multivariate model for time to viral rebound >1000 HIV RNA copies/mL was statistically significant, but older infant age at viral suppression was associated with a slower rebound rate (HR, 0.93; 95% CI, .88–.98; P = .009) and not with HIV DNA load at baseline or HIV DNA load at the time point VL suppression was achieved (Table 5). The best fit multivariate model for time to low-level viral rebound was not statistically significant (Table 5).
Table 5.
Variables Influencing Time to Viral Rebound
| Viral Load >100 HIV RNA Copies/mL | Viral Load >1000 HIV RNA Copies/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Variable | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Infant HIV DNA, log10 copies/million PBMC | ||||||||
| Baseline high vs lowa | 1.12 (.67–1.87) | .7 | 1.82 (.98–3.4) | .06 | 0.89 (.5–1.59) | .7 | … | … |
| Baseline continuous | 1.05 (.79–1.4) | .8 | … | … | 0.99 (.72–1.36) | .9 | 1.37 (.91–2.06) | .1 |
| Suppression high vs lowa,b | 0.92 (.47–1.79) | .8 | … | … | 0.72 (.34–1.55) | .4 | … | … |
| Suppression continuousb | 1.05 (.67–1.64) | .8 | … | … | 0.89 (.56–1.42) | .6 | … | … |
| Male infant | 0.88 (.52–1.5) | .6 | … | … | 0.78 (.42–1.43) | .4 | … | … |
| DBS tested | 0.69 (.41–1.15) | .2 | 0.60 (.35–1.03) | .07 | 0.29 (.33–1.02) | .06 | 0.57 (.31–1.01) | .05 |
| Infant age at viral suppression, wk | 0.97 (.94–1.01) | .1 | 0.95 (.91–.99) | .02 | 0.95 (.91–1) | .047 | 0.93 (.88–.98) | .009 |
| CD4 % at suppression | 1.01 (.99–1.03) | .5 | … | … | 1.01 (.98–1.03) | .5 | … | … |
Cox proportional hazard model. Wald test viral load >100 HIV RNA copies/mL P = .06; viral load >1000 HIV RNA copies/mL P = .02.
Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; DBS, dried blood spot; HIV, human immunodeficiency virus; HR, hazard ratio.
aAbove and below the median value.
bHIV DNA measurement at the time point viral suppression is achieved or within 3 months.
Discussion
This is the largest study of HIV DNA in early treated IU HIV-infected infants. Baseline infant HIV DNA levels were low, in some cases below the limit of detection, and this was associated with maternal antenatal cART use. However, there was no evidence to suggest that for infants receiving ART as prophylaxis, which is the standard of care for HIV-exposed infants, that there was expansion of the latent HIV reservoir in the first 3 weeks of life, nor that earlier initiation of cART resulted in faster HIV DNA decay. PoC diagnosis and the subsequent blood sampling within hours of birth provided insight into clinical factors potentially influencing HIV DNA in utero. Higher HIV DNA levels were demonstrated in female infants and those born to acutely infected mothers. Lower HIV DNA was found in infants with lower VL and higher CD4+ T-cell percentages, but it is unknown whether these correlations were causal or consequential. VL at baseline and age of suppression were significantly associated with HIV DNA decay rate, but this was not further influenced by blips or documented cART nonadherence. Although lower infant HIV DNA levels were associated with a faster time to VL suppression, they were not associated with suppression maintenance, suggesting that there are immunological, virological, or behavioral factors that override the potential biological advantages of low latent HIV reservoirs in early treated IU HIV-infected infants in the first years of life.
We found higher infant HIV DNA than the only 2 other studies of HIV DNA in IU HIV-infected prevention of mother-to-child transmission (PMTCT)-exposed infants in the first days of life (median 2.8 vs 2.1 and 2.2 log10 HIV DNA copies/million PBMC) [12, 13], although this may be related to small sample sizes in the other studies (n = 9 and 7 vs n = 164 here). HIV DNA levels in the first days of life from all 3 studies were approximately 1 log10 copies/million PBMC lower than in IU HIV-infected infants not exposed to PMTCT [32]. Maternal cART use in pregnancy in our study was common (approximately 75% [25]). The ratio of umbilical cord blood to maternal blood ART levels is close to 1 [33], and in South Africa, serum drug levels of NVP and AZT when administered at prophylaxis dosing are often indistinguishable from those observed following treatment dosing [26, 34, 35]. Thus, this substantial infant exposure to ART transplacentally and postnatally as prophylaxis prior to cART initiation is lowering or restricting infant latent HIV reservoir size.
From these low levels, HIV DNA decayed quickly with an initial HIV DNA half-life of 28 days. This was shorter than the 2.7 months observed by Veldsman et al in their analysis of infants with similar ages of cART initiation and VL suppression [13], although in their analysis the half-life was taken at 6 months, in comparison to the initial decline we have reported. IU HIV transmission is estimated to occur at a median of 14 days prior to delivery [17], and our infants were on average 1 week postdelivery, thus 3 weeks postinfection, but the HIV DNA decay rate in the infants was faster than the approximate half-life of decay of 12 weeks for adults treated in the first days of infection [7], demonstrating benefits of the neonatal immune system. It was intriguing to note that although HIV DNA decay was significantly associated with VL, it was not influenced by blips or documented nonadherence (Table 3). This raises the possibility that the initial degree and length of plasma viremia is more crucial in terms of reservoir size compared with short periods of viremia later, also shown in analytic treatment interruption studies in adults [36, 37]. However, it may have been that 3-monthly VL measurements and self-reports of adherence were simply insensitive markers for intermittent viremia.
Similar to our multivariate model of HIV DNA decay, most pediatric studies have found lower levels of HIV DNA associated with younger infant age at suppression [10, 12, 13, 16, 38–45]. The key difference for our cohort was there was no association between age of cART initiation and age of suppression, probably because previous studies analyzed age of cART initiation in the order of months, compared to our narrow window of age of initiation (21 days). Interestingly, we found that time to rebound was faster in those infants who suppressed plasma viremia earlier. This may have been due to more effective HIV-specific immune responses in those infants who were exposed to plasma viremia for longer, delaying rebound [9]. Alternatively, this finding may instead be due to behavioral factors, where mothers of viremic infants in the first months receive the most attentive adherence support, addressing central issues such as disclosure, and developed robust cART administration skills.
Studies have shown correlations between lower peripheral total HIV DNA levels and superior maintenance of plasma aviremia in children [43, 46], but compared to these, our participants had a much higher probability of VL rebound (0.46 at 12 months compared with 0.01 in Kuhn et al [43]), but were also much younger, so less likely to be cART adherent ([3, 4]). It is possible that lower HIV DNA levels are useful in slowing viral rebound in the context of occasional missed doses, but not for the higher levels of nonadherence seen in younger children.
The findings of our study are relevant to settings like South Africa, with well implemented PMTCT programs, including antenatal cART, neonatal prophylaxis, and HIV testing at birth. Although we did not find any benefit in terms of latent HIV reservoir size for initiating cART in the first hours versus days of life, it is important to make the point that this should not be used as an argument against PoC testing for early infant diagnosis, which has been shown to decrease test turnaround time and increase rates of cART initiation [47, 48], crucial for KwaZulu-Natal, where only 25% of infants return for confirmatory HIV testing following an initial positive DBS test [49]. Furthermore, the innate immunological benefits of cART initiation in the first days of life are enabled by PoC testing [12].
We used total PBMC HIV DNA as a surrogate marker for latent HIV reservoir which may have overestimated HIV DNA able to contribute to plasma viremia. However, measurement of total HIV DNA still has clinical relevance, as defective and unintegrated HIV DNA triggers inflammation contributing to disease progression [50]. There was an inverse relationship between CD4+ T-cell percentage and HIV DNA, therefore in the case of low CD4+ T-cell count there would have been an even higher concentration of HIV DNA per CD4+ T cell than measured.
This HIV DNA decay analysis benefited from a large sample size, but its generalizability may be limited by excluding those infants with prolonged time to suppression and higher baseline plasma viral load who may have had intrinsically different viral or immune characteristics. However, inadequate plasma viral suppression for those infants was more likely secondary to cART nonadherence, a scenario for which HIV DNA decay is less relevant.
In conclusion, where PMTCT programs are implemented well, and the standard of care can achieve HIV diagnosis at birth and cART initiation within the first 3 weeks of life, the priority for IU HIV-infected infant cART initiation should be timed to maximize caregiver adherence to achieve and sustain viral suppression, rather than aiming for lower HIV DNA as a hallmark of HIV remission.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge the study participants and their families, along with the Ucwaningo Lwabantwana study and HIV Pathogenesis Programme laboratory staff.
Author contributions. Study management: J. R. M., N. B., I. F., R. F., J. R., K. S., V. N., I. F., M. A., N. I, T. N., and P. G. Laboratory assays: J. R. M., V. A. V., E. A., M. C. G. G., M. C. P., and N. I. Data analysis: J. R. M. and A. G. Drafting of manuscript: J. R. M. and P. G. Editing: all authors. Study conceptualization T. N. and P. G. Study leads: J. M. P. and P. G.
Disclaimer . The views expressed in this publication are those of the authors and not necessarily those of African Academy of Sciences (AAS), New Partnership for Africa’s Development Planning and Coordinating (NEPAD) Agency, Wellcome Trust, or the UK Government.
Financial support. This work was supported by the Wellcome Trust (grant numbers WT104748MA to P. G. and 110110/Z/15/Z to P. C. M.); the National Institutes of Health (grant number RO1-AI133673 to P. G.); and Grifols (to J. M. P.). T. N. was supported in part by the South African Department of Science and Innovation through the National Research Foundation (South African Research Chairs Initiative, grant number 64809); the Victor Daitz Foundation; and the Sub-Saharan African Network for TB/HIV Research Excellence, a Developing Excellence in Leadership, Training, and Science (DELTAS) Africa Initiative (grant number DEL-15-006). The DELTAS Africa Initiative is an independent funding scheme of the AAS Alliance for Accelerating Excellence in Science in Africa and supported by the NEPAD Agency with funding from the Wellcome Trust (grant number 107752/Z/15/Z) and the UK Government.
Potential conflicts of interest. J. R. M. received travel costs for an HIV conference in 2018 from Cepheid. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. UNAIDS. Global AIDS update 2019. http://rstesa.unaids.org/documents/publications/79-2019-global-aids-update-en/file. Accessed 2 January 2020.
- 2. Boerma RS, Boender TS, Bussink AP, et al. . Suboptimal viral suppression rates among HIV-infected children in low- and middle-income countries: a meta-analysis. Clin Infect Dis 2016; 63:1645–54. [DOI] [PubMed] [Google Scholar]
- 3. Meyers TM, Yotebieng M, Kuhn L, Moultrie H. Antiretroviral therapy responses among children attending a large public clinic in Soweto, South Africa. Pediatr Infect Dis J 2011; 30:974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teasdale CA, Sogaula N, Yuengling KA, et al. . HIV viral suppression and longevity among a cohort of children initiating antiretroviral therapy in Eastern Cape, South Africa. J Int AIDS Soc 2018; 21:e25168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuhn L, Strehlau R, Shiau S, et al. ; LEOPARD Study Team . Early antiretroviral treatment of infants to attain HIV remission. EClinicalMedicine 2020; 18:100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leyre L, Kroon E, Vandergeeten C, et al. . Abundant HIV-infected cells in blood and tissues are rapidly cleared upon ART initiation during acute HIV infection. Sci Transl Med. 2020; 12:eaav3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ananworanich J, Chomont N, Eller LA, et al. ; RV217 and RV254/SEARCH010 Study Groups . HIV DNA set point is rapidly established in acute HIV infection and dramatically reduced by early ART. EBioMedicine 2016; 11:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whitney JB, Hill AL, Sanisetty S, et al. . Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 2014; 512:74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zanchetta M, Anselmi A, Vendrame D, et al. . Early therapy in HIV-1-infected children: effect on HIV-1 dynamics and HIV-1-specific immune response. Antivir Ther 2008; 13:47–55. [PubMed] [Google Scholar]
- 10. Martínez-Bonet M, Puertas MC, Fortuny C, et al. . Establishment and replenishment of the viral reservoir in perinatally HIV-1-infected children initiating very early antiretroviral therapy. Clin Infect Dis 2015; 61:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pankau MD, Wamalwa D, Benki-Nugent S, et al. . Decay of HIV DNA in the reservoir and the impact of short treatment interruption in kenyan infants. Open Forum Infect Dis 2018; 5:13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia-Broncano P, Maddali S, Einkauf KB, et al. . Early antiretroviral therapy in neonates with HIV-1 infection restricts viral reservoir size and induces a distinct innate immune profile. Sci Transl Med 2019; 11:eaax7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veldsman KA, Janse van Rensburg A, Isaacs S, et al. . HIV-1 DNA decay is faster in children who initiate ART shortly after birth than later. J Int AIDS Soc 2019; 22:e25368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palma P, Zangari P, Alteri C, et al. . Early antiretroviral treatment (eART) limits viral diversity over time in a long-term HIV viral suppressed perinatally infected child. BMC Infect Dis 2016; 16:742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Zyl GU, Katusiime MG, Wiegand A, et al. . No evidence of HIV replication in children on antiretroviral therapy. J Clin Invest. 2017; 127:3827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luzuriaga K, Tabak B, Garber M, et al. . HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis 2014; 210:1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rouzioux C, Costagliola D, Burgard M, et al. . Estimated timing of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission by use of a Markov model. The HIV Infection in Newborns French Collaborative Study Group. Am J Epidemiol 1995; 142:1330–7. [DOI] [PubMed] [Google Scholar]
- 18. Lallemant M, Jourdain G, Le Coeur S, et al. . A trial of shortened zidovudine regimens to prevent mother-to-child transmission of human immunodeficiency virus type 1. Perinatal HIV Prevention Trial (Thailand) Investigators. N Engl J Med 2000; 343:982–91. [DOI] [PubMed] [Google Scholar]
- 19. Kollmann TR, Crabtree J, Rein-Weston A, et al. . Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 2009; 183:7150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nguyen M, Leuridan E, Zhang T, et al. . Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS One 2010; 5:e10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Wit D, Olislagers V, Goriely S, et al. . Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood 2004; 103:1030–2. [DOI] [PubMed] [Google Scholar]
- 22. Goulder PJ, Lewin SR, Leitman EM. Paediatric HIV infection: the potential for cure. Nat Rev Immunol 2016; 16:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shiau S, Abrams EJ, Arpadi SM, Kuhn L. Early antiretroviral therapy in HIV-infected infants: can it lead to HIV remission? Lancet HIV 2018; 5:e250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Persaud D, Gay H, Ziemniak C, et al. . Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 2013; 369:1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Millar JR, Bengu N, Fillis R, et al. . High-frequency failure of combination antiretroviral therapy in paediatric HIV infection is associated with unmet maternal non-adherence. EClinicalMedicine 2020; 22:100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Republic of South Africa National Department of Health. Guideline for the prevention of mother to child transmission of communicable infections, 2019. https://www.knowledgehub.org.za/system/files/elibdownloads/2019-10/PMTCTGuideline28Octobersigned.pdf. Accessed 28 December 2019.
- 27. Morón-López S, Puertas MC, Gálvez C, et al. . Sensitive quantification of the HIV-1 reservoir in gut-associated lymphoid tissue. PLoS One 2017; 12:e0175899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. https://www.r-project.org/. [Google Scholar]
- 29. Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med 1997; 16:385–95. [DOI] [PubMed] [Google Scholar]
- 30. Simon N, Friedman J, Hastie T, Tibshirani R. Regularization paths for Cox’s proportional hazards model via coordinate descent. J Stat Softw 2011; 39:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wood SN. Generalized additive models: an introduction with R. 2nd ed. Boca Raton - London - New York: Chapman and Hall/CRC, 2017. [Google Scholar]
- 32. Scott-Algara D, Rouzioux C, Blanche S, et al. . In untreated HIV-1-infected children, PBMC-associated HIV DNA levels and cell-free HIV RNA levels are correlated to distinct T-lymphocyte populations. J Acquir Immune Defic Syndr 2010; 53:553–63. [DOI] [PubMed] [Google Scholar]
- 33. McCormack SA, Best BM. Protecting the fetus against HIV infection: a systematic review of placental transfer of antiretrovirals. Clin Pharmacokinet 2014; 53:989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cressey TR, Punyawudho B, Le Coeur S, et al. ; PHPT-5 Study Team . Assessment of nevirapine prophylactic and therapeutic dosing regimens for neonates. J Acquir Immune Defic Syndr 2017; 75:554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Republic of South Africa National Department of Health. 2019 ART clinical guidelines for the management of HIV in adults, pregnancy, adolescents, children, infants and neonates,2019. https://www.knowledgehub.org.za/elibrary/2019-art-clinical-guidelines-management-hiv-adults-pregnancy-adolescents-children-infants. Accessed 10 January 2020.
- 36. Steingrover R, Pogány K, Fernandez Garcia E, et al. . HIV-1 viral rebound dynamics after a single treatment interruption depends on time of initiation of highly active antiretroviral therapy. AIDS 2008; 22:1583–8. [DOI] [PubMed] [Google Scholar]
- 37. Salantes DB, Zheng Y, Mampe F, et al. . HIV-1 latent reservoir size and diversity are stable following brief treatment interruption. J Clin Invest 2018; 128:3102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Foster C, Pace M, Kaye S, et al. ; CHERUB Investigators . Early antiretroviral therapy reduces HIV DNA following perinatal HIV infection. AIDS 2017; 31:1847–51. [DOI] [PubMed] [Google Scholar]
- 39. Uprety P, Patel K, Karalius B, et al. ; Pediatric HIV/AIDS Cohort Study (PHACS) . Human immunodeficiency virus type 1 DNA decay dynamics with early, long-term virologic control of perinatal infection. Clin Infect Dis 2017; 64:1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Persaud D, Palumbo PE, Ziemniak C, et al. . Dynamics of the resting CD4+ T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS 2012; 26:1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bitnun A, Ransy DG, Brophy J, et al. . Clinical correlates of human immunodeficiency virus-1 (HIV-1) DNA and inducible HIV-1 RNA reservoirs in peripheral blood in children with perinatally acquired HIV-1 infection with sustained virologic suppression for at least 5 years. Clin Infect Dis. 2019; 70:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McManus M, Mick E, Hudson R, et al. ; PACTG 356 Investigators . Early combination antiretroviral therapy limits exposure to HIV-1 replication and cell-associated HIV-1 DNA levels in infants. PLoS One 2016; 11:e0154391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kuhn L, Paximadis M, Da Costa Dias B, et al. . Age at antiretroviral therapy initiation and cell-associated HIV-1 DNA levels in HIV-1 infected children. PLoS One 2018; 13:e0195514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van Zyl GU, Bedison MA, Van Rensburg AJ, Laughton B, Cotton MF, Mellors JW. Early antiretroviral therapy in South African children reduces HIV-1-infected cells and cell-associated HIV-1 RNA in blood mononuclear cells. J Infect Dis 2015; 212:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moragas M, Distefano M, Mecikovsky D, et al. . Impact of the time to achieve viral control on the dynamics of circulating HIV-1 reservoir in vertically infected children with long-term sustained virological suppression: a longitudinal study. PLoS One 2018; 13:e0205579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tagarro A, Chan M, Zangari P, et al. . Early and highly suppressive antiretroviral therapy are main factors associated with low viral reservoir in European perinatally HIV-infected children. J Acquir Immune Defic Syndr 2018; 79:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bianchi F, Cohn J, Sacks E, Bailey R, Lemaire JF, Machekano R; EGPAF POC EID Study Team . Evaluation of a routine point-of-care intervention for early infant diagnosis of HIV: an observational study in eight African countries. Lancet HIV 2019; 6:e373–81. [DOI] [PubMed] [Google Scholar]
- 48. Technau KG, Kuhn L, Coovadia A, Murnane PM, Sherman G. Xpert HIV-1 point-of-care test for neonatal diagnosis of HIV in the birth testing programme of a maternity hospital: a field evaluation study. Lancet HIV 2017; 4:e442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith S, Govender K, Moodley P, La Russa P, Kuhn L, Archary M. Impact of shifts to birth testing on early infant diagnosis program outcomes in KwaZulu-Natal, South Africa. Pediatr Infect Dis J 2019; 38:e138–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Imamichi H, Dewar RL, Adelsberger JW, et al. . Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc Natl Acad Sci U S A 2016; 113:8783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.