Abstract
This publication addresses the limited use of qualitative methods in neglected tropical disease (NTD) programmes. It describes a scoping literature review conducted to inform the development of a guide to inform the use of rapid qualitative assessments to strengthen NTD mass drug administration (MDA) programmes. The review assessed how qualitative methods are currently used by NTD programmes and identified qualitative approaches from other health and development programmes with the potential to strengthen the design of MDA interventions. Systematic review articles were reviewed and searched using key terms conducted on Google Scholar and PubMed. Results show that methods used by NTD programmes rely heavily on focus group discussions and in-depth interviews, often with time-consuming analysis and limited information on how results are applied. Results from other fields offered insight into a wider range of methods, including participatory approaches, and on how to increase programmatic uptake of findings. Recommendations on how to apply these findings to NTD control are made. The topic of human resources for qualitative investigations is explored and a guide to improve MDAs using qualitative methods is introduced. This guide has direct applicability across the spectrum of NTDs as well as other public health programmes.
Keywords: disease control, mass drug administration, neglected tropical diseases, participatory methods, qualitative research, programme design
Introduction
The medically and epidemiologically diverse group of 20 neglected tropical diseases (NTDs) prioritised by the WHO affect more than 1 billion people globally. More than 200 000 people die each year from snakebites, rabies and dengue, and hundreds of millions of others have experienced severe disability, disfigurement, stigmatisation and discrimination due to the full range of NTDs.1,2 Because NTDs affect people living in the most marginalised conditions, progress reducing the burden of NTDs is seen as a proxy indicator for measuring progress towards sustainable development goals and universal health coverage with equitable access to health services, leaving no one behind.2,3
The WHO's 2021–2030 road map for NTDs focuses on increased country leadership and integration, as well as achieving disease-specific impact.2 The roadmap describes intervention strategies by disease, including preventative chemotherapy (PC) as a key intervention for 10 of the 20 NTDs. PC is the large-scale administration of medicine, often delivered through mass drug administration (MDA) to treat people in endemic areas, regardless of their infection status. For the fifth consecutive year, the global NTD community has delivered more than 1 billion treatments annually since 2015.4 Delivery on this scale has resulted in lymphatic filariasis and trachoma elimination as a public health problem in 17 and 11 countries, respectively, and onchocerciasis transmission has been eliminated in four countries in the region of the Americas.2 The size of this achievement is a testament to what can be achieved when partners (including endemic country governments, the United Nations, donors, pharmaceutical company donation programmes, researchers and non-governmental organisations [NGOs]) work together.
As we turn our attention to reaching programme targets in ‘the last mile’, social sciences and qualitative methods could make a difference. For example, a programme may find ongoing low coverage at district or subdistrict level. Managers may also find that transmission continues at higher-than-expected levels, despite multiple rounds of MDA.5 As they face these issues, programme managers are increasingly grappling with challenging questions: Who are we not reaching? Why? What is their personal risk? Are they contributing to ongoing transmission? What do we need to do differently?
While a strong suite of quantitative tools has been developed in recent years to support the monitoring and evaluation of PC NTD programmes, these do not provide full answers to all the questions posed above.6 For example, a coverage survey may identify that 20% of persons surveyed have a fear of MDA drugs, but understanding what they fear and why requires a deeper contextualised understanding. The number and/or size of tablets, the result of rumours, lack of trust in the people or organisation delivering treatment, the fear of side effects experienced in the past, a mix of all these reasons or something else altogether? And what can programme managers do to help people overcome these concerns? Who should deliver the message of drug safety and how? Concern has also been voiced that quantitative surveys may be missing the same people missed during MDA, and so they remain hidden to the programme, both during MDA and during the assessments.7
Qualitative tools can be used to give a stronger voice to the communities and health workers who are often best placed to identify and solve programmatic challenges, and leads to the creation of more person-centred approaches to NTD programme delivery. The NTD 2020–2030 road map is complemented by other documents that provide frameworks for thinking more in depth about sustainability, investment, monitoring and evaluation.8,9 The monitoring and evaluation document stresses the need for increased use of qualitative methods, thus addressing the limited use of social science methods in NTDs that has been called out in several publications.4,10–14
To address this gap, we conducted a rapid scoping literature review designed to inform the development of an off the shelf ready guide on the use of qualitative methods in NTD programmes, with a focus on MDA programmes. This paper presents the results of that review and introduces the guide. While drawing mainly from the experience of PC NTDs, this guide also has direct applicability across the spectrum of NTDs, as well as other public health programmes.
Methods
We conducted this scoping literature review with the aim of gaining a better understanding of how qualitative methods are currently being used by PC NTD programmes on one hand and, on the other, to identify a range of qualitative methods being used in other public health programmes that could drive innovation within the NTD space. We therefore conducted two separate reviews: one focused on PC NTD literature and another on other health and development fields. As methods were identified we also sought to understand how their application was used to inform programme design.
The two reviews were carried out by two different researchers (AK and KB) and the results were regularly discussed with the project team (MCB, EF, AK and KB). The review on the use of qualitative methods used in PC NTDs focused on searches of specific known qualitative methods within the PC NTDs. The initial list of methods was derived from a breakout session at the 2019 Coalition of Operational Research for NTDs meeting in National Harbour. During that breakout session, researchers and programme managers identified a list of methods previously used in NTD programmatic research. This list formed the basis for searches within PubMed, combining the method with the disease name. From there, additional papers were identified through the snowball technique. To be included in the final list, papers needed to provide sufficient information on what triggered the use of the method; a summary of the data collection methods; sufficient results to comment on quality and how steps were taken to integrate findings into the programme; and (if possible) comments about the time taken for the research study, human resource needs and costs to give an idea of the feasibility of its use. Where details about resources required were not available, the researcher (AK) estimated the time and human resources for the methods based on past research experience. The final list included a selection of example papers that represent use of the different methods captured during the scoping review. The review of the broader health and development literature started with 15 systematic reviews on qualitative research from the fields of childhood vaccinology, Ebola, health emergencies, malaria, healthcare delivery, randomised controlled trials (RCTs), substance abuse and TB. From these, five rapid qualitative toolkits were identified and reviewed. In addition, searches were performed on Google Scholar and PubMed using a wide variety of search terms since the goal was to find a wide range of non-NTD literature. This included starting with broad terms such as ‘infectious disease’, ‘health’, ‘development’ AND ‘qualitative research’, ‘qualitative methods’ ‘ethnography’, ‘participatory research’ AND ‘systematic review’ and ‘review’, then narrowing the focus to those that would lend themselves to informing the design of MDA programmes for PC NTDs.
In both reviews, once papers were identified, the full paper was read and a standardised framework in Excel (Microsoft Corporation, 2018. Microsoft Excel. Retrieved from https://office.microsoft.com/excel) was used to systematically collect information on why the method was selected, the reported feasibility of adaptation for routine programmatic use in terms of resources (human, financial and time) and the reported uptake of results.
All the authors reviewed the results and discussed how these should be applied to the design and content of the new Guide to Improving MDA Using Qualitative Methods (https://www.ntdtoolbox.org/toolbox-search/guide-improving-mda-using-qualitative-methods). In doing so, the diversity of authors’ perspectives was leveraged, including experiences as programme managers, WHO NTD staff, implementing partners, monitoring, evaluation and learning advisors, as well as researchers. The guide development was further informed by EF and MCB’s engagement with their colleagues (at country and headquarters level) on the Act to End NTDs East programme funded by United States Agency for International Development (USAID), which supports the implementation of NTD programmes in 12 countries.
Results
Qualitative methods used in NTD research
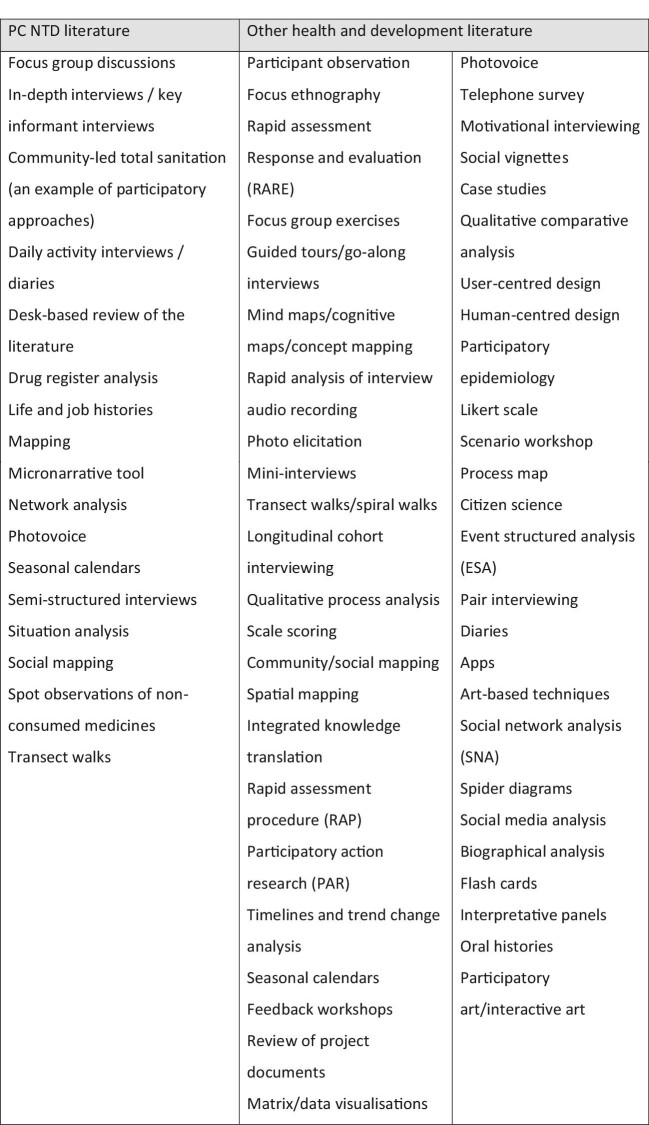
A total of 24 papers were identified for further analysis, consisting of 22 unique research papers and 2 review papers.7,15–38 Focus group discussions (FGDs) and in-depth interviews (or key informant interviews) were the most commonly used methods in the papers reviewed. There were examples of a range of other methods, including participatory approaches (Figure 1). For example, Fleming et al. used pictorial diaries, combined with in-depth interviews, to assess the opportunity costs for community drug distributors (CDDs) implementing MDAs.31 Most of the studies included in the review used more than one tool, with FGDs and in-depth interviews frequently used together, triangulating results across study types.
Figure 1.
Summary of qualitative methods found in the literature search.
Study participants commonly included the following groups: males and females (often interviewed separately), community and opinion leaders, CDDs and frontline health workers. Additionally, other important groups of interest were sometimes identified as participants in FGDs and in-depth interviews. One study from India used in-depth interviews with those individuals who did not take medicines during the last MDA.18 Another study interviewed CDDs who had resigned from carrying out MDA activities, to understand their rationale for stopping.29 Only two studies reviewed included children or adolescents as informants in their studies.15,37
Rationale for selection of qualitative method: Most of the selected papers reported using qualitative studies to better understand MDA coverage results. Some sought to increase their understanding on perspectives of the role of the deliverer (e.g. CDD) in the MDA process.14,17,19,27–29,31,35,37,38 Some of these papers were looking to build on knowledge already generated by coverage surveys.14 As Brieger writes, ‘A coverage survey is not the ideal instrument to enquire about beliefs and attitudes, and thus a smaller survey may be needed to document beliefs and test their association with coverage’.20
Team structure and logistics: Most of the papers did not describe in detail the team structure, time taken or logistical requirements to implement the studies. The reviewer (AK) used her experience to make a rough estimate of the amount of effort (low, moderate, high) needed to collect and analyse the data. For example, FGDs and in-depth interviews typically reported using recordings and producing verbatim transcripts. This can be a time-consuming process with an average 1-h FGD requiring 4–6 h to transcribe and a 1-h in-depth interview requiring 2–4 h to transcribe. Most studies were deemed to have moderate to high human resource needs, particularly when the sample size was large.
Knowledge translation: Some of the study results included the need for more in-depth knowledge and perceptions of the MDA; reasons for not wanting to take the treatment offered; an understanding of which groups of people missed treatment; MDA delivery barriers and enablers; rich descriptions on the challenges to reach specific communities (e.g. geographic barriers, higher social economic status [SES]); and CDD-related challenges (e.g. poor numerical literacy and high opportunity costs).14,15,17–21,23,24,27,29,31,33–35,37,38
Evaluation of research impact: While most of the reviewed studies included a list of programme recommendations, only two papers provided information on how (or if) their findings were used by the NTD programme.32,35 These descriptions included documentation of programme changes based on the findings and future MDA coverage in areas where changes were implemented.
Related to this, a systematic review article highlighted that the types of methods used make it difficult to conclusively attribute any change in MDA coverage to changes made by the programme.26 Some authors also commented on the inability to generalise qualitative results beyond the context. Ames et al. highlighted the fact that a context description was often missing, as was any reflection on the role that the researchers may have played in influencing study results.16,28,29
Qualitative methods used in the broader health and development field
A total of 14 systematic review papers were identified for further analysis from the fields of vaccinology, Ebola, health emergencies, malaria, healthcare delivery, RCTs, substance abuse and TB.39–52 From these, 25 articles were selected for full review based on their relevance to the aims of the review.52–76 Additionally, five rapid qualitative toolkits of relevance were identified through a more general internet search.77–80 A total of 52 different qualitative methods were identified (Figure 1). Many were participatory and team-based in nature and in line with ‘implementation science’ approaches.
Rationale for selection of qualitative method: The selected articles provided a broad range of reasons for the use of qualitative methods. These included the need to generate formative insights to influence the design of programmes, such as vaccinations, childhood feeding, Ebola and malaria control,52–54,66,68,71,76 to increase community and stakeholder participation, including with racial and ethnic minority communities in HIV/AIDS prevention,70 injecting drug users73 and those undergoing aortic valve implants,56 and to understand specific operational shortcomings or failures of accepted approaches and policies, many of which were comparative studies reflecting on implementation across multiple countries.58,60,61,75
Team structure and logistics: Almost all the studies reviewed used a senior, trained social scientist, either directly involved in data collection and analysis or in training and supervision. A range of different types of personnel were employed for data collection, including nationally trained social scientists and community engagement staff (not trained in social science).58,74
Many studies were often part of longer term social science engagement performed over a few years, which served to also build capacity in research and knowledge translation.
Knowledge translation: Studies described a process of knowledge translation that started from the beginning, with a built-in process for co-design with different stakeholders and a review of existing programme materials. Stakeholder engagement continued throughout the study and resulted in adapting research questions and methods in response to preliminary results and changing programme needs. Towards the end, stakeholders analysed the relevance of the results. Methods used to facilitate this partnership, and to build the trust and respect needed for it to be effective, included member checking, peer debriefing, group analysis and stakeholder workshops (including community working groups and community advisory boards).60,67,70,73,69,75 Some studies used voting/ranking methods with end users (e.g. programme staff) to explore the feasibility of recommendations. Many papers stressed that rapid assessments can often be integrated with existing programmes to reduce costs and that they should be integrated with attention to the programme cycle.56,60,61,73
Evaluation of research impact: Surprisingly, few studies evaluated the impact of research findings on programme implementation, although some argued that integrating an evaluation component into a rapid qualitative tool is very important.69,75
Discussion
This scoping literature review showed that qualitative methods are used by PC NTD programmes for a variety of reasons, including to better understand access to treatment. However, most studies restricted their methods to in-depth or key informant interviews and FGDs, and many reported the use of time-consuming analysis methods. Few reported on how results were taken up by programmes or evaluated the impact of using results on the problem that the study was designed to address. This confirmed the authors’ observations that there is a need to better align the use of qualitative methods within NTD programmes, to provide more timely information and to tailor them towards problem solving. An increased use of qualitative methods can also be used to allow the voices of the community and beneficiaries to be heard more clearly. These perspectives—which include women, children, the rural and urban poor and people with disabilities, some of which may be caused by NTDs—are often missing in the world of NTD programming and policy, yet are vital if we are to achieve equity and reach programme goals.
Our review of the use of qualitative methods in other areas of public health and development found a much wider range of methods in use (including several rapid participatory approaches), and we found several studies that paid a lot more attention to knowledge translation and programmatic uptake of findings. However, even here, there was a surprising lack of studies that evaluated the impact of using results on programme outcomes.
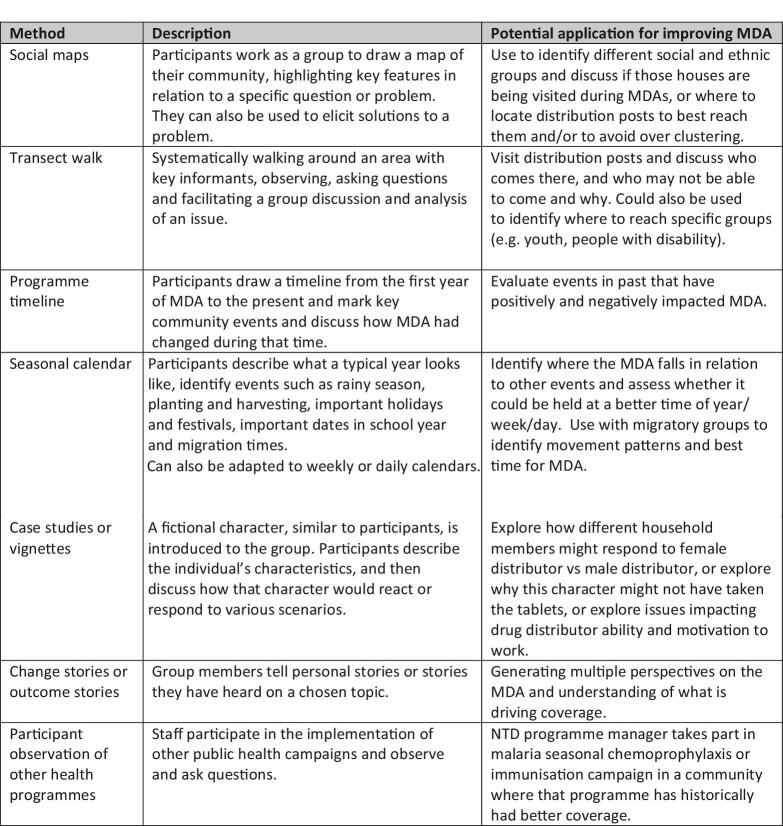
Of the methods identified in both literature reviews (Figure 1), we recommend several methods that could be adapted and used to inform and improve future design of MDAs. These are summarized in Figure 2, with examples of potential applications to NTD programmes provided. This is not intended to be an exhaustive list of possible methods (which would be potentially overwhelming), nor does it claim to be a ‘best list’, but offers a list of methods that are quick to implement, require minimal expertise in qualitative and or participatory research, and can easily be applied to solving PC NTD programmatic challenges.
Figure 2.
Summary of qualitative and participatory methods, in addition to focus groups and in-depth interviews, adopted for use in the Guide to Improving MDA using Qualitative Methods.
If results generated by qualitative studies are to be applied to specific programme challenges, knowledge translation and uptake is needed and attempts to implement changes then need to be evaluated. This requires the research team (be they researchers or programme monitoring and evaluation [M&E] staff) and the implementation team working together at every stage.11,60,67,69,70,73,75 At the beginning, they need to work together to clearly define the problem and research questions. NTD programmes have data from several sources that can be used to inform this step, including coverage surveys, routinely collected subdistrict level coverage data (broken down by age and/or gender), supervisor and other programme reports, records of post-MDA meetings, impact survey reports and tacit knowledge gained by staff from implementation experience. Collaboration needs to continue during data collection with a willingness to adapt instruments and methods. The relatively short timeframe available for evaluation between rounds of MDA further underscores the need for evaluators and implementers to collaborate closely for successful data collection and data use.
To ensure successful programme learning and adaptation, study results need to be jointly reviewed by researcher and implementer and a set of feasible recommendations co-designed. The nature of programme implementation is that it will never be perfect, especially in the settings where NTD programmes operate, the scale at which they operate and with the resources available to them. It is therefore easy to detail a long list of things that could be improved, be they training, supervision, data collection, social mobilisation, attitudes or staffing. The key is to identify the, usually considerably smaller, list of things that are most likely to affect the desired change. This is as much an art as a science. Of the methods identified in this study that facilitate that process, one that we particularly liked was the Risk, Attitude, Norms, Ability and Self-regulation (RANAS) model of behavioural change commonly used in water and sanitation. With the RANAS methodology, a framework is provided for programme implementers to use their survey results to adapt and design appropriate messages that respond to the gaps identified in the assessment.81
We found a significant lack of studies evaluating the impact of programmatic changes made based on study results. When evaluations are conducted, they usually use more quantitative methods and quasi-experimental designs like ‘before and after’ studies, with or without comparison areas. However, there are now also newer qualitative evaluation methods available, which take into consideration the complexity that ensues from the interaction of multiple programmatic changes that interact with the people and their environment in multiple, and sometimes unexpected, ways. These so-called ‘complexity aware’ methods include techniques such as most significant change, ripple effects mapping and outcome harvesting.82,83
The question of who is best suited to undertake qualitative research in the context of a programme is often hotly debated: trained researchers, M&E staff or programme managers? We have used the diversity of experience of our authors (including both programme implementers and researchers) to consider here the advantages and disadvantages of these options. NTD programmes often work with researchers trained in qualitative methods. These may be found in other units within the ministry of health, in other government sectors, in local universities and other research organisations, or with their international partners. Trained researchers have more experience with design, data collection and methods, which can lead to more efficiency and quality. They are less biased by the programme beliefs on what is happening and why, although of course they are not immune to their own biases and they lack knowledge on programme context. They usually have more time to devote to, and a preference for, more sophisticated study design: this can be a double-edged sword when time is of the essence. Engaging with outside experts requires additional funding allocated to evaluation in the programme budget and has at times produced results that are not aligned with the programme planning and budgeting cycles during which uptake of recommendations can be made. Conversely, qualitative research is often carried out by NTD programme implementers, including national or regional NTD programme managers and their teams (including M&E staff), district level health teams and international implementing partners. Programme staff have a better understanding of programme context and may have a more intuitive sense of where to probe in interviews. They tend to have more contextual knowledge that can facilitate interpretation of results, quickly filtering through all the noise. However, they may lack training in seeking out counter hypotheses, and may stop probing quickly if initial answers are in line with what they already believe. They are well positioned to apply results, although they are not immune to getting lost in lists of recommendations. As their time is already accounted for in programme budgets, they do not require additional funding, although there are opportunity costs to consider, especially when efficiencies are lost during study planning, data collection and analysis. The final decision on who performs the study will differ depending on context, funding and staff availability, and perhaps ideally will include a mixed team of both researchers and programme staff.
Regardless of who conducts the studies, the need for capacity strengthening on qualitative research for NTD control and elimination has been clearly stated.8–12 This need has begun to be reflected in the global NTD community through inclusion of a chapter on qualitative methods in the NTD Roadmap M&E framework and the launch of a new community of practice of researchers from different social science backgrounds focused on NTDs called iCHORDS (Improving Community Health Outcomes through Research, Dialogue and Systems Strengthening).
To respond to the need for capacity strengthening on qualitative methods, we used the results from this study, combined with a human-centred design approach84 to develop A Guide to Improving MDA using Qualitative Methods. We used rapid literature to help frame subsequent discussions with researchers, technical experts and NTD programme implementers to define the problem or issues that needed to be addressed, brainstormed how to address those problems and jointly developed a prototype guide. We also built on a previous manual developed by the WHO10 that drew on experiences from other health programmes but was reportedly difficult to use due to its length, complexity in language and requirement for more resources than are usually available.
The Guide to Improving MDA using Qualitative Methods (https://www.ntdtoolbox.org/toolbox-search/guide-improving-mda-using-qualitative-methods) provides practical guidance on topics like budgeting, includes templates for training agendas and data analysis, has modifiable questionnaires and avoids overly scientific language. It requires the study team to work with the programme team responsible for designing the MDA intervention through six steps: scoping to define the research question, methods selection, data collection, data analysis, programme adaptation and programme evaluation.
The limitations of this study include the fact that we did not scope grey literature due to time constraints, including NGO project documents, which may have highlighted the use of additional qualitative methods and provided a richer description of the uptake of results. There would be value in a future study that looks at these sources. The aim of the literature review was to provide a snapshot of some of the range of qualitative methods that have been used in NTD and public health programmatic research. As such, it does not claim to be exhaustive, however, it was useful in framing the prototype and we believe it will help drive current discussions on the role of qualitative methods in NTDs. Our focus on MDA, drawn mostly from experiences with PC NTDs, is also a limitation. Future work should address the use of qualitative research for other elements of NTDs, including health system strengthening and other types of intervention such as disease management, disability and inclusion. Finally, the guide described also needs to be tested and evaluated.
Supplementary Material
Acknowledgements
The authors thank Molly Adams for support with the formatting and design of the figures.
Contributor Information
Margaret C Baker, Global Health Division, Research Triangle Institute (RTI) International, Washington DC, WA 20005, USA.
Kevin Bardosh, Center for One Health Research, School of Public Health, University of Washington, Seattle, WA 98195, USA.
Elizabeth Fitch, Global Health Division, Research Triangle Institute (RTI) International, Washington DC, WA 20005, USA.
Pamela S Mbabazi, Department of Neglected Tropical Diseases, World Health Organization, 1221 Geneva 27, Switzerland.
Upendo Mwingira, Global Health Division, Research Triangle Institute (RTI) International, Washington DC, WA 20005, USA.
Abdel Direny, CORUS International, Washington DC, WA 20036, USA.
Laura Dean, Department of International Public Health, Liverpool School of Tropical Medicine, Pembroke Place, Liverpool, L3 5QA, UK.
Elizabeth G Sutherland, Global Health Division, Research Triangle Institute (RTI) International, Washington DC, WA 20005, USA.
Alison Krentel, School of Epidemiology and Public Health, University of Ottawa, K1G5Z3, Canada; Bruyere Research Institute, Ottawa, K1N 5C8, Canada.
Authors’ contributions
MCB and EF conceived the study; MCB, EF, KB and AK designed the study; KB and AK carried out the literature review and regularly met to discuss results with MCB and EF; all the authors contributed to the interpretation of results in the discussion; MCB drafted the manuscript with input from all authors, who also critically revised the manuscript for intellectual content. All the authors read and approved the final version of the manuscript. MCB, KB and AK are guarantors of the paper.
Funding
This study was funded with internal RTI International funds through their Strategic Investment Fund.
Competing interests
None declared.
Ethical approval
Not required.
References
- 1. World Health Organization . Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases. World Health Organization, 2010. [Google Scholar]
- 2. World Health Organization . Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021–2030. World Health Organization, 2020. Available from: https://www.who.int/publications/i/item/9789240010352. [Google Scholar]
- 3. Fitzpatrick C, Engels D.. Leaving no one behind: a neglected tropical disease indicator and tracers for the Sustainable Development Goals. Int Health. 2016;8(suppl_1):15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mondiale de la Santé O , WHO. Weekly Epidemiological Record. 2020;95:461–76. [Google Scholar]
- 5. Burgert-Brucker CR, Zoerhoff KL, Headland Met al. Risk factors associated with failing pre-transmission assessment surveys (pre-TAS) in lymphatic filariasis elimination programs: results of a multi-country analysis. PLoS Negl Trop Dis. 2020;14(6):e0008301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Preventive chemotherapy: tools for inproving the quality of reported data and information. A field manual for implementation. World Health Organization, 2019. Available from: https://apps.who.int/iris/handle/10665/329376. [Google Scholar]
- 7. Dean L, Ozano K, Adekeye Oet al. Neglected tropical diseases as a ‘litmus test’ for universal health coverage? Understanding who is left behind and why in mass drug administration: lessons from four country contexts. PLoS Negl Trop Dis. 2019;13(11):e0007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Ending the neglect to attain the Sustainable Development Goals: a framework for monitoring and evaluating progress of the road map for neglected tropical diseases 2021−2030. World Health Organization, 2021. Available from: https://www.who.int/publications/i/item/9789240023680. [Google Scholar]
- 9. World Health Organization . Ending the neglect to attain the Sustainable Development Goals: a sustainability framework for action against neglected tropical diseases 2021–2030. World Health Organization, 2021. Available from: https://www.who.int/publications/i/item/9789240019027. [Google Scholar]
- 10. Manderson L, Aagaard-Hansen J, Allotey Pet al. Social research on neglected diseases of poverty: continuing and emerging themes. PLoS Negl Trop Dis. 2009;3(2):e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bardosh KL. Towards a science of global health delivery: a socio-anthropological framework to improve the effectiveness of neglected tropical disease interventions. PLoS Negl Trop Dis. 2018;12(7):e0006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Allotey P, Reidpath DD, Pokhrel S.. Social sciences research in neglected tropical diseases 1: the ongoing neglect in the neglected tropical diseases. Health Res Policy Syst. 2010;8(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krentel A, Gyapong M, Ogundahunsi Oet al. Ensuring no one is left behind: urgent action required to address implementation challenges for NTD control and elimination. PLoS Negl Trop Dis. 2018;12(6):e0006426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization . Towards universal coverage for preventive chemotherapy for neglected tropical diseases: guidance for assessing “who is being left behind and why”: working draft for further piloting during 2018–2019. World Health Organization, 2017. [Google Scholar]
- 15. Adeneye A, Akinwale O, Idowu Eet al. Sociocultural aspects of mass delivery of praziquantel in schistosomiasis control: the Abeokuta experience. Res Social Adm Pharm. 2007;3(2):183–98. [DOI] [PubMed] [Google Scholar]
- 16. Ames HMR, Zuske M, King JDet al. Community and drug distributor perceptions and experiences of mass drug administration for the elimination of lymphatic filariasis: a rapid review of qualitative research. Adv Parasitol. 2019;103:117–49. [DOI] [PubMed] [Google Scholar]
- 17. da-Costa Vroom FB, Aryeetey R, Boateng Ret al. Data reporting constraints for the lymphatic filariasis mass drug administration activities in two districts in Ghana: a qualitative study. SAGE Open Med. 2015;3:2050312115594083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Banerjee S, Bandyopadhyay K, Khan MFet al. Coverage of mass drug administration for elimination of lymphatic filariasis in urban Nagpur, Central India: a mixed method study. J Family Med Prim Care. 2019;8(9):3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Biritwum N-K, Garshong B, Alomatu Bet al. Improving drug delivery strategies for lymphatic filariasis elimination in urban areas in Ghana. PLoS Negl Trop Dis. 2017;11(5):e0005619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brieger WR, Otusanya SA, Oke GAet al. Factors associated with coverage in community-directed treatment with ivermectin for onchocerciasis control in Oyo State, Nigeria. Trop Med Int Health. 2002;7(1):11–8. [DOI] [PubMed] [Google Scholar]
- 21. Boyd A, Won KY, McClintock SKet al. A community-based study of factors associated with continuing transmission of lymphatic filariasis in Leogane, Haiti. PLoS Negl Trop Dis. 2010;4(3):e640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brooker SJ, Mwandawiro CS, Halliday KEet al. Interrupting transmission of soil-transmitted helminths: a study protocol for cluster randomised trials evaluating alternative treatment strategies and delivery systems in Kenya. BMJ Open. 2015;5(10):e008950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chami GF, Kontoleon AA, Bulte Eet al. Profiling nonrecipients of mass drug administration for schistosomiasis and hookworm infections: a comprehensive analysis of praziquantel and albendazole coverage in community-directed treatment in Uganda. Clin Infect Dis. 2016;62(2):200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chami GF, Kabatereine NB, Tukahebwa EM.. Profiling the best-performing community medicine distributors for mass drug administration: a comprehensive, data-driven analysis of treatment for schistosomiasis, lymphatic filariasis, and soil-transmitted helminths in Uganda. BMC Med. 2019;17(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clarke NE, Clements AC, Bryan Set al. Investigating the differential impact of school and community-based integrated control programmes for soil-transmitted helminths in Timor-Leste: the (S) WASH-D for Worms pilot study protocol. Pilot Feasibility Stud. 2016;2(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cronin T, Sheppard J, de Wildt G.. Health-seeking behaviour for schistosomiasis: a systematic review of qualitative and quantitative literature. Pan African Med J. 2013;16:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Desmond N, Solomon AW, Massae PAet al. Acceptability of azithromycin for the control of trachoma in Northern Tanzania. Trans R Soc Trop Med Hyg. 2005;99(9):656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dissak-Delon FN, Kamga G-R, Humblet PCet al. Barriers to the National Onchocerciasis Control Programme at operational level in Cameroon: a qualitative assessment of stakeholders’ views. Parasites Vectors. 2019;12(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dissak-Delon FN, Kamga G-R, Humblet PCet al. Do communities really “direct” in community-directed interventions? A qualitative assessment of beneficiaries’ perceptions at 20 years of community directed treatment with ivermectin in Cameroon. Trop Med Infect Dis. 2019;4(3):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Durrans S, Last A, Boiro Het al. “Moving like birds”: a qualitative study of population mobility and health implications in the Bijagós Islands, Guinea Bissau. Soc Sci Med. 2019;230:204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fleming FM, Matovu F, Hansen KSet al. A mixed methods approach to evaluating community drug distributor performance in the control of neglected tropical diseases. Parasites Vectors. 2016;9(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Griswold E, Eigege A, Ityonzughul Cet al. Evaluation of treatment coverage and enhanced mass drug administration for onchocerciasis and lymphatic filariasis in five local government areas treating twice per year in Edo state, Nigeria. Am J Trop Med Hyg. 2018;99(2):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ipa M, Astuti EP, Ridwan Wet al. Qualitative study: leverage and Barrier Factors of Mass Drug Administration for Lymphatic Filariasis Elimination Program in Tangerang and Subang Regency. 5th Universitas Ahmad Dahlan Public Health Conference (UPHEC 2019). Amsterdam, The Netherlands: Atlantis Press;2020:116–21. [Google Scholar]
- 34. Kisoka W, Mushi D, Meyrowitsch DWet al. Dilemmas of community-directed mass drug administration for lymphatic filariasis control: a qualitative study from urban and rural Tanzania. J Biosoc Sci. 2017;49(4):447. [DOI] [PubMed] [Google Scholar]
- 35. Krentel A, Damayanti R, Titaley CRet al. Improving coverage and compliance in mass drug administration for the elimination of LF in two ‘endgame'districts in Indonesia using micronarrative surveys. PLoS Negl Trop Dis. 2016;10(11):e0005027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Legge H, Kepha S, Prochazka Met al. Implementer and recipient perspectives of community-wide mass drug administration for soil-transmitted helminths in Kwale County, Kenya. PLoS Negl Trop Dis. 2020;14(4):e0008258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muhumuza S, Olsen A, Nuwaha Fet al. Understanding low uptake of mass treatment for intestinal schistosomiasis among school children: a qualitative study in Jinja district, Uganda. J Biosoc Sci. 2015;47(4):505–20. [DOI] [PubMed] [Google Scholar]
- 38. Nujum ZT, Remadevi S, Jose Ret al. Evaluation of coverage and compliance to Mass drug administration (MDA) for lymphatic filariasis elimination–A qualitative study. Acad Med J India. 2013;1:23–27. [Google Scholar]
- 39. Abramowitz SA, Hipgrave DB, Witchard Aet al. Lessons from the West Africa Ebola epidemic: a systematic review of epidemiological and social and behavioral science research priorities. J Infect Dis. 2018;218(11):1730–8. [DOI] [PubMed] [Google Scholar]
- 40. Davis K, Minckas N, Bond Vet al. Beyond interviews and focus groups: a framework for integrating innovative qualitative methods into randomised controlled trials of complex public health interventions. Trials. 2019;20(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fitch C, Stimson GV, Rhodes Tet al. Rapid assessment: an international review of diffusion, practice and outcomes in the substance use field. Soc Sci Med. 2004;59(9):1819–30. [DOI] [PubMed] [Google Scholar]
- 42. Gagliardi AR, Berta W, Kothari Aet al. Integrated knowledge translation (IKT) in health care: a scoping review. Implement Sci. 2015;11(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McNall M, Foster-Fishman PG.. Methods of rapid evaluation, assessment, and appraisal. Am J Evaluation. 2007;28(2):151–68. [Google Scholar]
- 44. Munro SA, Lewin SA, Smith HJet al. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med. 2007;4(7):e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nofal SD, Peto TJ, Adhikari Bet al. How can interventions that target forest-goers be tailored to accelerate malaria elimination in the Greater Mekong Subregion? A systematic review of the qualitative literature. Malar J. 2019;18(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tull K. Vaccine hesitancy: guidance and interventions. K4D Helpdesk Report 672. Brighton, UK: Institute of Development Studies. 2019. Available from: https://opendocs.ids.ac.uk/opendocs/handle/20.500.12413/14747. [Google Scholar]
- 47. Pluye P, Hong QN.. Combining the power of stories and the power of numbers: mixed methods research and mixed studies reviews. Annu Rev Public Health. 2014;35:29–45. [DOI] [PubMed] [Google Scholar]
- 48. Williams HA, Jones CO.. A critical review of behavioral issues related to malaria control in sub-Saharan Africa: what contributions have social scientists made? Soc Sci Med. 2004;59(3):501–23. [DOI] [PubMed] [Google Scholar]
- 49. Larson HJ, Jarrett C, Eckersberger Eet al. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–9. [DOI] [PubMed] [Google Scholar]
- 50. Vindrola-Padros C, Vindrola-Padros B.. Quick and dirty? A systematic review of the use of rapid ethnographies in healthcare organisation and delivery. BMJ Quality Safety. 2018;27(4):321–30. [DOI] [PubMed] [Google Scholar]
- 51. Schmid P, Rauber D, Betsch Cet al. Barriers of influenza vaccination intention and behavior–a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS One. 2017;12(1):e0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dubé E, Leask J, Wolff Bet al. The WHO Tailoring Immunization Programmes (TIP) approach: review of implementation to date. Vaccine. 2018;36(11):1509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Adhikari B, Pell C, Phommasone Ket al. Elements of effective community engagement: lessons from a targeted malaria elimination study in Lao PDR (Laos). Global Health Action. 2017;10(1):1366136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Atkinson J-AM, Fitzgerald L, Toaliu Het al. Community participation for malaria elimination in Tafea Province, Vanuatu: Part I. Maintaining motivation for prevention practices in the context of disappearing disease. Malar J. 2010;9(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Balabanova D, McKee M, Koroleva Net al. Navigating the health system: diabetes care in Georgia. Health Policy Plan. 2009;24(1):46–54. [DOI] [PubMed] [Google Scholar]
- 56. Baumbusch J, Wu S, Lauck SBet al. Exploring the synergies between focused ethnography and integrated knowledge translation. Health Res Policy Systems. 2018;16(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Burgess-Allen J, Owen-Smith V. Using mind mapping techniques for rapid qualitative data analysis in public participation processes. Health Expect. 2010;13(4):406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Closser S, Rosenthal A, Maes Ket al. The global context of vaccine refusal: insights from a systematic comparative ethnography of the global polio eradication initiative. Med Anthropol Q. 2016;30(3):321–41. [DOI] [PubMed] [Google Scholar]
- 59. Dubé E, Vivion M, Sauvageau Cet al. “Nature does things well, why should we interfere?” Vaccine hesitancy among mothers. Qual Health Res. 2016;26(3):411–25. [DOI] [PubMed] [Google Scholar]
- 60. Ezard N, Oppenheimer E, Burton Aet al. Six rapid assessments of alcohol and other substance use in populations displaced by conflict. Conflict Health. 2011;5(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Flores W, Chang J, Barillas E.. Rapid assessment of the performance of malaria control strategies implemented by countries in the Amazon subregion using adequacy criteria: case study. Malar J. 2011;10(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Godoy-Ramirez K, Byström E, Lindstrand Aet al. Exploring childhood immunization among undocumented migrants in Sweden-following qualitative study and the World Health Organization's guide to tailoring immunization Programmes (TIP). Public Health. 2019;171:97–105. [DOI] [PubMed] [Google Scholar]
- 63. Grietens KP, Gryseels C, Dierickx Set al. Characterizing types of human mobility to inform differential and targeted malaria elimination strategies in Northeast Cambodia. Sci Rep. 2015;5(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grietens KP, Xuan XN, Ribera JMet al. Social determinants of long lasting insecticidal hammock-use among the Ra-Glai ethnic minority in Vietnam: implications for forest malaria control. PLoS One. 2012;7(1):e29991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hagan JE, Smith W, Pillai SKet al. Implementation of Ebola case-finding using a village chieftaincy taskforce in a remote outbreak—Liberia, 2014. Morb Mortal Wkly Rep. 2015;64(7):183. [PMC free article] [PubMed] [Google Scholar]
- 66. Heyerdahl LW, Pugliese-Garcia M, Nkwemu Set al. “It depends how one understands it:” a qualitative study on differential uptake of oral cholera vaccine in three compounds in Lusaka, Zambia. BMC Infect Dis. 2019;19(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kamineni VV, Turk T, Wilson Net al. A rapid assessment and response approach to review and enhance advocacy, communication and social mobilisation for tuberculosis control in Odisha state, India. BMC Public Health. 2011;11(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mushi AK, Schellenberg J, Mrisho Met al. Development of behaviour change communication strategy for a vaccination-linked malaria control tool in southern Tanzania. Malar J. 2008;7(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mohammed S, Sajun SZ, Khan FS.. Harnessing Photovoice for tuberculosis advocacy in Karachi, Pakistan. Health Promot Int. 2015;30(2):262–9. [DOI] [PubMed] [Google Scholar]
- 70. Needle RH, Trotter RT, Singer Met al. Rapid assessment of the HIV/AIDS crisis in racial and ethnic minority communities: an approach for timely community interventions. Am J Public Health. 2003;93(6):970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pelto GH, Armar-Klemesu M, Siekmann Jet al. The focused ethnographic study ‘assessing the behavioral and local market environment for improving the diets of infants and young children 6 to 23 months old’ and its use in three countries. Maternal Child Nutrition. 2013;9:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pell CL, Adhikari B, Myo Thwin Met al. Community engagement, social context and coverage of mass anti-malarial administration: comparative findings from multi-site research in the Greater Mekong sub-Region. PLoS One. 2019;14(3):e0214280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stimson GV, Fitch C, Jarlais DDet al. Rapid assessment and response studies of injection drug use: knowledge gain, capacity building, and intervention development in a multisite study. Am J Public Health. 2006;96(2):288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tangseefa D, Monthathip K, Tuenpakdee Net al. “Nine Dimensions”: a multidisciplinary approach for community engagement in a complex postwar border region as part of the targeted malaria elimination in Karen/Kayin State, Myanmar. Wellcome Open Res. 2018;3:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Theiss-Nyland K, Ejersa W, Karema Cet al. Operational challenges to continuous LLIN distribution: a qualitative rapid assessment in four countries. Malar J. 2016;15(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pathmanathan I, O'Connor KA, Adams MLet al. Rapid assessment of Ebola infection prevention and control needs—six districts, Sierra Leone, October 2014. Morb Mortal Wkly Rep. 2014;63(49):1172. [PMC free article] [PubMed] [Google Scholar]
- 77. Atun R, Lennox-Chhugani N, Drobniewski Fet al. A framework and toolkit for capturing the communicable disease programmes within health systems: tuberculosis control as an illustrative example. Euro J Public Health. 2004;14(3):267–73. [DOI] [PubMed] [Google Scholar]
- 78. Chandler CI, Reynolds J, Palmer JJet al. ACT consortium guidance: qualitative methods for international health intervention research. London, UK: LSHTM, 2013. [Google Scholar]
- 79. Griffiths UK, Hanvoravongchai P, Oliveira-Cruz Vet al. A toolkit for assessing the impacts of measles eradication activities on immunization services and health systems at country level. London, UK: LSHTM, 2010. [Google Scholar]
- 80. World Health Organization . Rapid assessment of alcohol and other substance use in conflict-affected and displaced populations: a field guide. Rapid assessment of alcohol and other substance use in conflict-affected and displaced populations: a field guide. UN High Commissioner for Refugees (UNHCR), 2008. [Google Scholar]
- 81. Mosler H-J. A systematic approach to behavior change interventions for the water and sanitation sector in developing countries: a conceptual model, a review, and a guideline. Int J Environ Health Res. 2012;22(5):431–49. [DOI] [PubMed] [Google Scholar]
- 82. Lee B, Sommerville P, Farley Set al. SPACES MERL: systems and Complexity White Paper. Washington, DC, USA:US Agency for International Development, 2016. [Google Scholar]
- 83. Peterson C, Skolits G.. Evaluating unintended program outcomes through Ripple Effects Mapping (REM): application of REM using grounded theory. Eval Program Plann. 2019;76:101677. [DOI] [PubMed] [Google Scholar]
- 84. Brown T, Wyatt J.. Design thinking for social innovation. 2010;8:30–35. Available from: https://www.researchgate.net/publication/273354145_Design_Thinking_for_Social_Innovation_By. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.