Abstract
OBJECTIVES
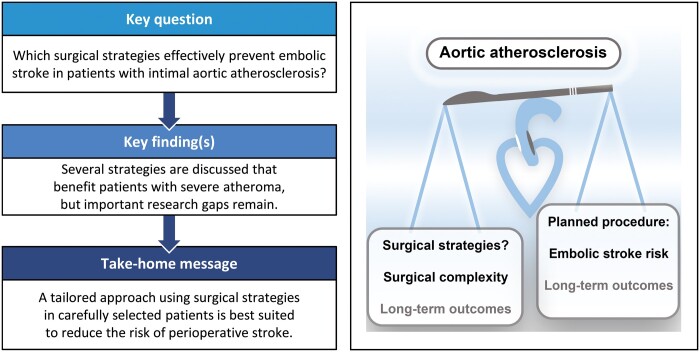
Although the incidence of perioperative stroke after cardiac surgery gradually decreased over the last decades, there is much variation between centres. This review aimed to create a concise overview of the evidence on possible surgical strategies to prevent embolic stroke in patients with intimal aortic atherosclerosis.
METHODS
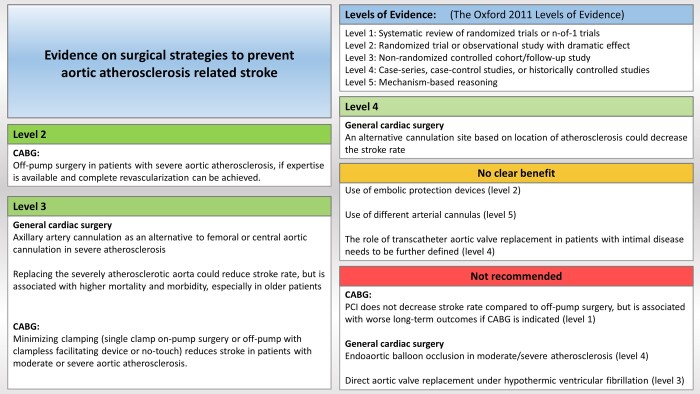
The PubMed and EMBASE databases were searched for studies on surgical management of aortic atherosclerosis and the association with perioperative stroke in cardiac surgery, including specific searches on the most common types of surgery. Articles were screened with emphasis on studies comparing multiple strategies and studies reporting on the patients’ severity of aortic atherosclerosis. The main findings were summarized in a figure, with a grade of the corresponding level of evidence.
RESULTS
Regarding embolic stroke risk, aortic atherosclerosis of the tunica intima is most relevant. Although several strategies in general cardiac surgery seem to be beneficial in severe disease, none have conclusively been proven most effective. Off-pump surgery in coronary artery bypass grafting should be preferred with severe atherosclerosis, if the required expertise is present. Although transcatheter aortic valve replacement is used as an alternative to surgery in patients with a porcelain aorta, the risk profile concerning intimal atherosclerosis remains poorly defined.
CONCLUSIONS
A tailored approach that uses the discussed alternative strategies in carefully selected patients is best suited to reduce the risk of perioperative stroke without compromising other outcomes. More research is needed, especially on the perioperative stroke risk in patients with moderate aortic atherosclerosis.
Keywords: Aortic atherosclerosis, Cardiac surgery, Perioperative stroke
In cardiac surgery, perioperative stroke is a debilitating complication that increases mortality [1].
INTRODUCTION
In cardiac surgery, perioperative stroke is a debilitating complication that increases mortality [1]. The incidence of perioperative stroke is frequently underestimated, as more cases are identified with systematic neurological screening and more cerebral damage is observed on postoperative magnetic resonance imaging (MRI) [2]. Nevertheless, over the last decades an increased understanding of mechanisms underlying perioperative stroke has led to a steady decline in the stroke rate, despite increasing complexity of the population [3]. However, there is high variation in risk-adjusted stroke rates and subsequent mortality between institutions [4]. Proven effective methods like intraoperative screening of the ascending aorta with epiaortic ultrasound (EAU) are being underused [5], potentially leading to suboptimal outcomes in patients with aortic atherosclerosis. Although many prior reviews have discussed the prevention of stroke in cardiac surgery, none have created a concise overview of the evidence on possible surgical strategies to prevent embolic stroke in patients with intimal aortic atherosclerosis.
METHODS
Search strategy
Between July and November 2020 literature searches were performed, closing on 1 November 2020 using PubMed and EMBASE (W.G.K.), focusing on studies on the association of aortic atherosclerosis and perioperative stroke, strategies in general cardiac surgical procedures and strategies in coronary artery bypass grafting (CABG) and surgical aortic valve replacement (SAVR) specifically. Articles were screened and relevant references were identified. No prior reviews with a specific focus on surgical management of intimal aortic atherosclerosis were found. The search strategies are described in the Supplementary Material. This document also contains tabulated data from the evaluated clinical studies.
Article selection
The articles were screened with regard to quality and relevance, with emphasis on studies either (i) comparing different strategies or a preventive strategy with a control group or (ii) evaluating the severity of aortic atherosclerosis. These emphases were chosen to deal with the variation in stroke risk among study populations, the confounding effect of different management policies between studies when encountering aortic atherosclerosis and the variable definitions and adjudication of stroke as an end point in studies published prior to the first standardized neurological end points consensus document [6, 7]. We focused on studies reporting the severity of atherosclerosis because the benefit of preventive strategies is very dependent on this. Clinical studies fulfilling these 2 criteria were tabulated (Supplementary Material). Studies were screened for overlapping reporting of study populations by comparing institution and enrolment period and subsequently excluded from tabulation. Right-sided valve surgery, mitral valve surgery, primary aortic surgery and reoperations were not included. The main findings are summarized in Fig. 1, grading the underlying evidence using the Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence [8].
Figure 1:
A visual summary of the strategies evaluated. CABG: coronary artery bypass grafting.
AORTIC ATHEROSCLEROSIS
Many different terms are used to describe atherosclerosis of the aorta, such as ‘shaggy aorta’, ‘hostile aorta’, ‘porcelain aorta’, aortic atheroma, aortic (soft) plaque and aortic calcification. Two types of aortic atherosclerosis should be distinguished.
Tunica intima versus tunica media disease
Intimal atherosclerosis is a pathophysiological process that is mainly mediated by low-density-lipid cholesterol [9]. This aspect of atherosclerosis is often referred to as soft plaque. Early in this process the role of calcification is limited, while later, spotty calcification can expand, forming larger lumps or even plates. A distinct process is calcification of the medial layer of arteries, which is more likely to expand to diffuse calcified plaques, that can extend to circumferential plaques. This is associated with general, rather than local inflammation, and is often associated with type-II diabetes or chronic kidney failure [10].
Intimal atherosclerosis as a risk factor for stroke
Distinction between these 2 entities has important clinical consequences. Medial calcification and porcelain aorta can lead to mechanical difficulties (e.g. incomplete occlusion, aortic dissection) in clamping the aorta but do not seem to increase the rate of perioperative stroke as much, once intimal atherosclerosis is ruled out [11, 12]. In this review, we will focus on atherosclerosis of the intimal layer (further referred to as aortic atherosclerosis), which can be present both with and without calcification. Aortic atherosclerosis is related to the risk of perioperative stroke in patients undergoing cardiac surgery [13]. Causality of this relationship is difficult to prove but seems likely, based on several findings. Most debris captured in the aorta during cardiac surgery was found to be fibrous atheroma tissue [14]. Aortic atherosclerosis was identified by numerous studies as an independent predictor of stroke, with a higher risk in patients with more severe atherosclerosis [2, 13, 15]. Most importantly, decreasing aortic manipulation in patients with aortic atherosclerosis decreases the rate of stroke [16, 17].
Diagnosis of aortic atherosclerosis
The golden standard for the diagnosis of intimal aortic atherosclerosis is intraoperative EAU [17, 18]. Transoesophageal echocardiography is less sensitive, as the view of the distal ascending aorta is blocked by the left main bronchus [18]. Contrast-enhanced computed tomography has the advantage of preoperative screening but exposes the patient to radiation and contrast medium [12].
Prevalence of aortic atherosclerosis
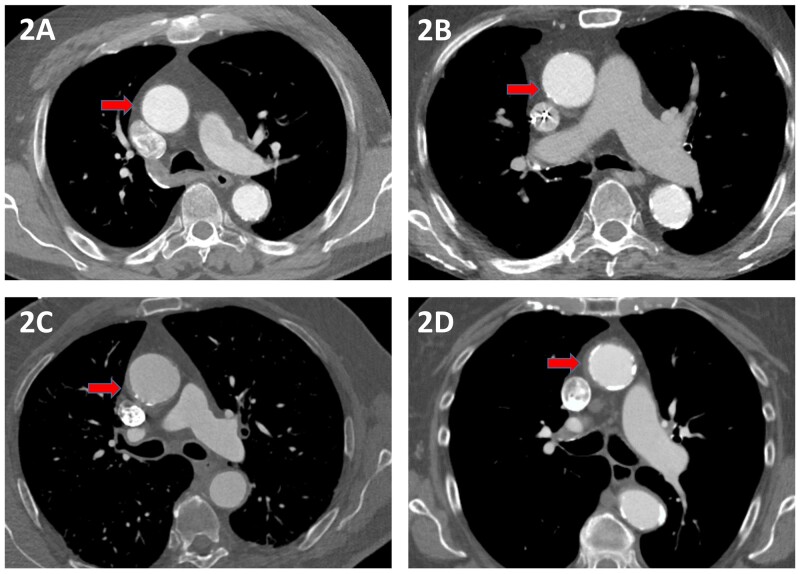
Aortic atherosclerosis is more frequent in patients undergoing cardiac surgery than in the general population, especially in patients undergoing CABG or SAVR [19, 20]. The severity is commonly categorized in 5 categories, based on the degree of intimal thickening, irregularity and mobility of the atheroma, using either of 2 classifications (Table 1) [18, 21]. Sample images of atherosclerosis severity are shown in Fig. 2. As an example, studies in patients undergoing CABG report somewhere between 27% and 68% for mild, 11% and 47% for moderate and 2% and 6% for severe atheroma [16, 22, 23]. The prevalence is influenced by variations in the classification used, population studied and type of imaging modality used.
Table 1:
Intimal atherosclerosis classifications
| Grade | Severity | Katz [20] | ASE/EACVI guideline [21] |
|---|---|---|---|
| I | Normal | Normal aorta | Intimal thickness <2 mm |
| II | Mild | Extensive intimal thickening | Focal or diffuse intimal thickness 2–3 mm |
| III | Moderate | Sessile atheroma extending <5 mm into the lumen | 3–5 mm (no mobile/ulcerated components) |
| IV | Severe | Sessile atheroma protruding >5 mm (protruding atheroma) | >5 mm (no mobile/ulcerated components) |
| V | Mobile atheroma | Complex grade 2–4 atheroma plus mobile or ulcer |
Figure 2:
Computed tomography images of the grades of aortic atherosclerosis. The red arrows indicate (A) a normal aorta; (B) mild atherosclerosis with intimal irregularity but wall thickness <3 mm; (C) moderate atherosclerosis with irregularity and wall thickness <5 mm; and (D) severe atherosclerosis with pronounced intimal thickening >5 mm.
Severity of aortic atherosclerosis
The risk of stroke increases with increasing severity of atherosclerosis [15]. In a study assessing aortic atherosclerosis with EAU before and after cannulation, new intima lesions after aortic manipulation were more frequent in moderate (11.8%) and severe (33.3%) atheroma, than in less than moderate atheroma (0.8%), implying that mainly moderate and severe atheroma are clinically relevant [23]. For severe atheroma this increased risk has been shown repeatedly [15, 21]. The risk is less well described for moderate atheroma, which is often combined with mild or severe atheroma, masking the risk for this specific category. A case–control study in 1969 patients undergoing cardiac surgery with EAU found a significantly higher prevalence of severe atheroma (10.9% vs 4.8%), but not moderate atheroma (15.2% vs 12.6%) in stroke patients [24]. Similar results were observed in another case–control study where moderate atheroma was defined as wall thickness more than 3 mm in 1 of 3 segments of the ascending aorta [15]. Another study found no association between the number of particulate emboli captured by a filter and the aortic wall thickness in patients with mild or moderate atheroma [25]. However, the risk of stroke is also related to the extent and location of the plaque within the aorta [13]. Moreover, the benefit of different surgical strategies depends also on the effectiveness and risk profiles of these strategies. Thus, no uniform cut-off value necessitating change of the surgical strategy can be provided and this should be evaluated for each alternative strategy in particular.
GENERAL CARDIAC SURGERY
Many cardiac surgical procedures are performed with cardiopulmonary bypass and under cardiac arrest. This requires arterial cannulation and cross-clamping of the aorta. Several strategies have been proposed for patients with aortic atherosclerosis, when no substantial disease-free part can be identified.
Arterial cannulas
When direct aortic cannulation in a disease-free part of the ascending aorta is still possible, aortic arch atherosclerosis could increase the risk of stroke through a ‘sandblasting effect’ [26]. The arterial cannula’s higher flow velocity can damage vulnerable plaques and dislodge debris. The extent to which this phenomenon contributes to the rate of embolic stroke is poorly defined. Several alterations on arterial cannulas have been suggested, to decrease the flow velocity and wall shear stress. No studies powered to find a clinically meaningful effect on stroke rate have been performed [27, 28]. While different types of arterial cannulas have different haemodynamic profiles, no clear recommendation can be provided on their role in preventing embolic stroke in patients with aortic atherosclerosis [29].
Embolic protection devices
Filtration or suction-based devices have been developed to capture dislodged debris before leaving the aorta. Indeed, these devices capture embolic tissue in up to 97% of patients [14]. A first randomized trial using a suction-based device observed a reduction in brain lesions on postoperative MRI [30]. A large randomized trial evaluating the filtration-based device did not observe a reduction in stroke [14]. The most recent trial randomized 383 patients undergoing SAVR between the filtration and suction-based devices or a control group. The trial was halted after the interim analysis for expected futility in observing a difference in central nervous system infarction between the 3 groups [31]. Taken together, there is some evidence that these devices can decrease the number and volume of lesions on postoperative MRI, but no clear reduction of perioperative stroke has been established.
Alternatives to clamping the diseased aorta
Instead of capturing debris after embolization, other methods aim to prevent embolization during clamping, for instance occluding the aorta with an endoaortic balloon. Instead of externally compressing and deforming the aortic walls, a balloon catheter is inflated in the ascending aorta to obstruct blood flow. In patients undergoing minimally invasive mitral valve surgery, this procedure has shown similar stroke rates compared to external clamping [32]. However, patients with significant ilio-aortic atherosclerosis are usually excluded from minimally invasive surgery. Only one study evaluated this method specifically in 52 patients with aortic atherosclerosis, reporting unfavourable outcomes [33]. A higher mortality rate was observed, both compared to a preoperative risk score and to unmatched patients with a regular approach. The stroke rate was also marginally increased (3.8% vs 0.8%, P = 0.067). Thus, currently no studies support the use of endoaortic balloon occlusion in patients with aortic atherosclerosis.
Another, more radical alternative to clamping is the use of hypothermia and ventricular fibrillation to enable intracardiac surgery. Two cohorts of 24 and 13 patients have been described performing direct SAVR under deep hypothermic circulatory arrest [34, 35]. The outcomes were not compared to a control group, but high stroke rates of 17% and 15% were observed, leading the authors of the latter study to largely abandon the strategy of direct SAVR without performing concomitant aortic surgery [36].
Alternative cannulation sites
To avoid manipulation of the ascending aorta, other sites are available for arterial cannulation. The most pragmatic alternative, especially when atherosclerosis has been diagnosed intraoperatively, is the aortic arch. Besides the increased technical complexity, a problem with this approach is that the severity of aortic atherosclerosis usually increases along the length of the aorta. Few patients with a diseased ascending aorta will have a disease-free aortic arch [19]. The same problem occurs with femoral cannulation, as the descending aorta is even more prone to atherosclerosis [19]. Despite being a safe and frequently used location in selected patients undergoing minimally invasive surgery, retrograde perfusion from the femoral artery has been associated with increased stroke rates in patients with aortic atherosclerosis [37, 38]. This association was not observed in studies on minimally invasive mitral valve surgery, when femoral cannulation was changed to aortic or axillary cannulation in patients with severe aortic atherosclerosis [39, 40]. Retrograde perfusion thus seems to be safe as long as these patients are excluded. A suitable alternative for patients with severe atheroma is the axillary artery. An experimental study showed that the altered blood flow from axillary cannulation, significantly decreased embolization of microparticles released in the ascending aorta, compared to cannulation of the ascending aorta [41]. This was supported by a recent study using computed tomography angiography screening prior to minimally invasive surgery . In 270 patients, no strokes were observed, despite rigorous postoperative neurological assessment including MRI, when switching from femoral to axillary cannulation in patients with aortoiliac wall thickness >3 mm. However, in most procedures, there remains a need for clamping, thereby decreasing, but not obviating manipulation of the diseased aorta.
Aortic replacement
A more frequently used strategy when applying deep hypothermic circulatory arrest for severe atherosclerosis is to replace the aorta altogether [34]. Circulatory arrest is usually required, as the distal ascending aorta is rarely spared, preventing the use of cross-clamping. Aortic replacement is associated with higher risks of mortality and stroke in patients with aortic atherosclerosis, when compared to other indications [43]. Supplementary Material, Table S2 shows studies that evaluated this approach in patient with severe aortic atherosclerosis. In the larger cohorts, the stroke rate ranged from 2.8% to 18%. Although these stroke rates were higher compared to control groups with less severe atherosclerosis, they were equal or lower compared to other surgical strategies, including aortic endarterectomy. However, some studies observed higher rates of other postoperative morbidity, such as postoperative bleeding and acute renal failure [35, 44, 45]. Mortality rates were also high, ranging from 3% to 25%. In a cohort where 73% of patients with severe aortic calcification underwent aortic replacement, there was a large age-related difference in mortality and stroke rates between patients aged below (3.8% and 6.4%) or above 80 years old (15.9% and 18.2%, respectively) [36]. Without larger studies and reliable control groups, mortality and stroke rates are difficult to interpret. Aortic replacement was often used as a last resort in patients with exclusively severe aortic atherosclerosis, reflecting a high surgical risk. Aortic replacement adds significant complexity to the surgery and requires surgical experience, which could influence surgical outcome in low-volume centres.
CORONARY ARTERY BYPASS GRAFTING
Surgical strategies in CABG, mainly regarding the use of clamping and extracorporeal bypass, have extensively been studied. These strategies have not demonstrated lower stroke rates when used routinely [46, 47]. However, in selected patients, there is substantial evidence that they effectively reduce stroke rate.
On-pump coronary artery bypass grafting
A randomized trial compared a clampless versus partial clamp strategy in off-pump CABG (OPCAB) and single versus double clamp strategy in on-pump CABG patients with no or only mild atherosclerosis [48]. In these patients, the more extensive clamping strategies did not result in more microemboli, suggesting that clamping is safe in most patients undergoing CABG. In 2 propensity-matched studies comparing CABG with a single or multiple clamping strategy for the proximal anastomosis, no difference in stroke risk was observed [49, 50]. However, both propensity scores in these registry-based studies did not control for aortic atheroma. Increased use of the single clamp technique in patients with aortic atherosclerosis could have obscured the potential benefit of less clamping. No studies evaluated single or additional partial clamping specifically in high-risk patients. One study evaluating patients strictly operated on with either strategy by 1 surgeon who changed preference suggested a possible benefit of single clamping [51]. Overall, in patients with more severe atherosclerosis, the additional use of a partial clamp has not definitely been proven safe. Use of single clamping will increase clamp time by ∼15 min and measuring the graft length is performed on an empty heart, but this is not expected to have clinical consequences. The question therefore remains whether a partial clamp should be applied in patients with more severe aortic atherosclerosis.
Off-pump coronary artery bypass grafting
Similar to on-pump CABG, in OPCAB, the use of a partial clamp has not unequivocally been linked to a higher stroke rate, compared to no-touch OPCAB. In Supplementary Material, Table S3, the studies have been listed that compare these strategies and report on the severity of aortic atherosclerosis. Only one study observed an increasing stroke rate with increasing degree of aortic manipulation [52]. Interestingly, the prevalence of severe aortic atherosclerosis in the on-pump CABG group was only 0.6%, while the decrease in stroke rate compared to the no-touch group was 1.3% (2.0–0.7%). This suggests a benefit of avoiding aortic manipulation in patients with moderate aortic atherosclerosis. In 3 studies, aortic atherosclerosis was identified as an independent predictor using multivariate regression analyses [52–54]. This correlation was not observed in 2 studies evaluating the use of a clampless proximal anastomosis facilitating device [55, 56]. Apparently the use of these devices helps to prevent the increase in stroke rate associated with increasing aortic atherosclerosis severity. This is supported by the increase in benefit ratio between observed and expected stroke rate with increasing severity of aortic atherosclerosis observed when using these devices [56].
It is important to note that none of the studies included in Supplementary Material, Table S3 focused exclusively on patients with moderate or severe aortic atherosclerosis and all studies described a bias towards using the no-touch approach in patients with more severe aortic atherosclerosis [22, 52, 54]. This effect is likely to be present throughout all non-randomized studies reporting on stroke rate differences between different clamping and bypass strategies in CABG.
This and several other confounders must also be taken into account when comparing the outcomes of OPCAB to on-pump CABG. Individual randomized controlled trials in unselected study populations using either no-touch or partial clamping OPCAB have not been able to show a benefit of OPCAB over on-pump CABG [46, 47, 57]. A network meta-analysis of studies that did differentiate the degree of aortic manipulation in OPCAB showed that the rate of stroke is lowest in no-touch OPCAB and highest in on-pump CABG [58]. This finding is supported by the studies in Supplementary Material, Table S4, none of which were included in the network meta-analysis. All 3 studies included only patients with severe aortic atherosclerosis and all observed a benefit of OPCAB over on-pump CABG [59–61]. The use of OPCAB in patients with a calcified aorta has become a class I recommendation in the latest guidelines on myocardial revascularization by the European Association of Cardiothoracic Surgery [62]. Based on the studies included in Supplementary Material, Table S4, this should be extrapolated to patients with severe non-calcified aortic atherosclerosis. However, the long-term results of OPCAB are comparable to on-pump surgery only when performed by experienced surgeons and when complete revascularization is achieved [63]. Furthermore, maintaining adequate perfusion pressures and circulation during OPCAB is of special importance in patients with aortic atherosclerosis, as they are likely to suffer from cerebrovascular atherosclerosis as well. Whether this influences the risk of haemodynamic stroke in these patients remains to be investigated.
Percutaneous coronary intervention (PCI), as an alternative strategy to CABG, suffers from inherent intra-aortic manipulation. Although an analysis of pooled individual patient data from several trials randomizing patients to PCI or CABG showed a lower perioperative stroke risk after PCI, the 30-day stroke rate of no-touch OPCAB was lower when compared to PCI in a network meta-analysis, with an odds ratio of 0.92 (95% confidence interval: 0.47–1.78) [64, 65]. Moreover, PCI is associated with lower survival in patients with an indication for CABG [66]. Therefore, while PCI could be a reasonable alternative when the required surgical experience is not present, it should not be the first choice for patients with an indication for CABG.
AORTIC VALVE REPLACEMENT
In a study among 196 patients >65 years old undergoing SAVR, any aortic atherosclerosis was observed in 86% of both patients with and without perioperative stroke [6]. This highlights the high prevalence of atherosclerosis in these patients and shows that the mere presence of atherosclerosis is not enough to discriminate stroke risk. A further substudy in 129 patients who underwent postoperative cerebral MRI showed embolic type lesions in 59% of patients, accounting for 97% (77/79) of the total number of acute infarcts observed. In a multivariable model, aortic arch atheroma was correlated with embolic infarcts (odds ratio 3.4, 1.0–12.0) [2]. Another study confirmed the relevance of moderate or severe aortic atherosclerosis. A substudy of a randomized trial evaluating embolic protection devices in 326 patients undergoing SAVR observed a higher stroke rate in patients with moderate or severe atherosclerosis (8.6% vs 5.9%), although this did not reach statistical significance (P = 0.38) [67].
Unlike in CABG, no small modifications are available to avoid aortic manipulation in SAVR, as cardiopulmonary bypass, cardiac arrest and an aortotomy are imperative. Aside from the options of peripheral cannulation and aortic replacement discussed above, it is difficult to adjust the procedure upon diagnosing severe aortic atherosclerosis intraoperatively.
With the advent of transcatheter aortic valve replacement (TAVR), a new minimally invasive alternative has become available, and it has been proposed as an alternative in patients with a porcelain aorta [68]. However, despite its use in patients diagnosed with a porcelain aorta, few studies have evaluated the stroke risk of TAVR specifically in patients with intimal aortic atherosclerosis. Theoretically, extensive intra-aortic wire manipulation could dislodge atherosclerotic debris and cause embolization. Indeed, total atheroma volume was associated with stroke after TAVR in 2 case–control studies [69, 70]. Although TAVR is an alternative in some patients with a porcelain aorta, the benefit over other surgical strategies of SAVR in patients with predominantly intimal atherosclerosis remains to be investigated.
LIMITATIONS AND IMPLICATIONS FOR FUTURE RESEARCH
As this review focused on the risk of embolic stroke, the trade-off in terms of other outcomes of the surgical alternatives was not evaluated. Also, the deliberate focus on embolic stroke associated with aortic atherosclerosis prevented further discussion of other contributing aetiologies, including other embolic causes and haemodynamic stroke.
The key to successfully minimizing stroke risk in patients with aortic atherosclerosis is careful patient selection. The alternative approaches are usually technically more challenging, time-consuming or are even associated with unfavourable outcomes in the short or long term. Evidence in this field mainly consists of retrospective studies with limited sample sizes. More insight is needed in the stroke risk associated with aortic atherosclerosis in general, but mainly in patients with moderate atherosclerosis. Surgical strategies should be evaluated specifically in patients with aortic atherosclerosis. Due to low event rates and the efforts needed to evaluate aortic atherosclerosis, studies are seldom powered to evaluate individual atherosclerosis grades. In this regard, large-scale registries, such as the STS database, could be an accessible and valuable source, but only if reliable data on atherosclerosis are collected. In addition, the role of TAVR in patients with intimal atherosclerosis needs to be evaluated and compared to other alternative strategies in SAVR. As many of these patients undergo preoperative contrast-enhanced computed tomography, data should be readily available.
CONCLUSIONS
The association between aortic manipulation in patients with intimal aortic atherosclerosis and the risk of perioperative stroke have been well defined. Many strategies have been proposed to mitigate this risk, but only few have consistently been proven effective. Currently, these strategies are not consistently employed, causing variation in the perioperative stroke rates. A tailored approach, using alternative strategies in carefully selected patients, is needed to reduce the risk of perioperative stroke without compromising other outcomes. More insight in the effectiveness of alternative surgical strategies, especially in patients with moderate aortic atherosclerosis, is needed. Collecting reliable data on aortic atherosclerosis in large-scale registries is necessary.
Conflict of interest: none declared.
Author contributions
Wiebe G. Knol: Conceptualization; Data curation; Methodology; Writing—original draft. Ricardo P.J. Budde: Conceptualization; Methodology; Supervision; Writing—review & editing. Edris A.F. Mahtab: Methodology; Writing—review & editing. Jos A. Bekkers: Methodology; Writing—review & editing. Ad J.J.C. Bogers: Conceptualization; Methodology; Supervision; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Takashi Kunihara and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- CABG
Coronary artery bypass grafting
- EAU
Epiaortic ultrasound
- MRI
Magnetic resonance imaging
- OPCAB
Off-pump CABG
- PCI
Percutaneous coronary intervention
- SAVR
Surgical aortic valve replacement
- TAVR
Transcatheter aortic valve replacement
REFERENCES
- 1. Sultan I, Bianco V, Kilic A, Jovin T, Jadhav A, Jankowitz B. et al. Predictors and outcomes of ischemic stroke after cardiac surgery. Ann Thorac Surg 2020;110:448–56. [DOI] [PubMed] [Google Scholar]
- 2. Massaro A, Messe SR, Acker MA, Kasner SE, Torres J, Fanning M. et al. ; Determining Neurologic Outcomes From Valve Operations (DeNOVO) Investigators. Pathogenesis and risk factors for cerebral infarct after surgical aortic valve replacement. Stroke 2016;47:2130–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiss AJ, Svensson LG, Bakaeen FG.. Temporal improvements in perioperative stroke rates following coronary artery bypass grafting. Curr Opin Cardiol 2020;35:679–86. [DOI] [PubMed] [Google Scholar]
- 4. LaPar DJ, Quader M, Rich JB, Kron IL, Crosby IK, Kern JA. et al. Institutional variation in mortality after stroke after cardiac surgery: an opportunity for improvement. Ann Thorac Surg 2015;100:1276–82; discussion 82–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krause M, Morabito JE, Mackensen GB, Perry TE, Bartels K.. Current neurologic assessment and neuroprotective strategies in cardiac anesthesia: a survey to the membership of the society of cardiovascular anesthesiologists. Anesth Analg 2020;131:518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Messe SR, Acker MA, Kasner SE, Fanning M, Giovannetti T, Ratcliffe SJ. et al. ; Determining Neurologic Outcomes from Valve Operations (DeNOVO) Investigators. Stroke after aortic valve surgery: results from a prospective cohort. Circulation 2014;129:2253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lansky AJ, Messe SR, Brickman AM, Dwyer M, Bart van der Worp H, Lazar RM. et al. Proposed standardized neurological endpoints for cardiovascular clinical trials: an academic research consortium initiative. Eur Heart J 2018;39:1687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A. et al. OCEBM Levels of Evidence Working Group. “The Oxford Levels of Evidence 2”. 2011. Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=5653.
- 9. Boren J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2020;41:2313–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T. et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J 2014;35:1515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nishi H, Mitsuno M, Ryomoto M, Miyamoto Y.. Comprehensive approach for clamping severely calcified ascending aorta using computed tomography. Interact CardioVasc Thorac Surg 2010;10:18–20. [DOI] [PubMed] [Google Scholar]
- 12. Asenbaum U, Nolz R, Puchner SB, Schoster T, Baumann L, Furtner J. et al. Coronary artery bypass grafting and perioperative stroke: imaging of atherosclerotic plaques in the ascending aorta with ungated high-pitch CT-angiography. Sci Rep 2020;10:13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van der Linden J, Hadjinikolaou L, Bergman P, Lindblom D.. Postoperative stroke in cardiac surgery is related to the location and extent of atherosclerotic disease in the ascending aorta. J Am Coll Cardiol 2001;38:131–5. [DOI] [PubMed] [Google Scholar]
- 14. Banbury MK, Kouchoukos NT, Allen KB, Slaughter MS, Weissman NJ, Berry GJ. et al. ; ICEM 2000 Investigators. Emboli capture using the Embol-X intraaortic filter in cardiac surgery: a multicentered randomized trial of 1,289 patients. Ann Thorac Surg 2003;76:508–15; discussion 15. [DOI] [PubMed] [Google Scholar]
- 15. Goto T, Baba T, Matsuyama K, Honma K, Ura M, Koshiji T.. Aortic atherosclerosis and postoperative neurological dysfunction in elderly coronary surgical patients. Ann Thorac Surg 2003;75:1912–18. [DOI] [PubMed] [Google Scholar]
- 16. Lyons JM, Thourani VH, Puskas JD, Kilgo PD, Baio KT, Guyton RA. et al. Intraoperative epiaortic ultrasound scanning guides operative strategies and identifies patients at high risk during coronary artery bypass grafting. Innovations (Phila) 2009;4:99–105. [DOI] [PubMed] [Google Scholar]
- 17. Rosenberger P, Shernan SK, Loffler M, Shekar PS, Fox JA, Tuli JK. et al. The influence of epiaortic ultrasonography on intraoperative surgical management in 6051 cardiac surgical patients. Ann Thorac Surg 2008;85:548–53. [DOI] [PubMed] [Google Scholar]
- 18. Goldstein SA, Evangelista A, Abbara S, Arai A, Asch FM, Badano LP. et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2015;28:119–82. [DOI] [PubMed] [Google Scholar]
- 19. Khoury Z, Gottlieb S, Stern S, Keren A.. Frequency and distribution of atherosclerotic plaques in the thoracic aorta as determined by transesophageal echocardiography in patients with coronary artery disease. Am J Cardiol 1997;79:23–7. [DOI] [PubMed] [Google Scholar]
- 20. Goland S, Trento A, Czer LS, Eshaghian S, Tolstrup K, Naqvi TZ. et al. Thoracic aortic arteriosclerosis in patients with degenerative aortic stenosis with and without coexisting coronary artery disease. Ann Thorac Surg 2008;85:113–19. [DOI] [PubMed] [Google Scholar]
- 21. Katz ES, Tunick PA, Rusinek H, Ribakove G, Spencer FC, Kronzon I.. Protruding aortic atheromas predict stroke in elderly patients undergoing cardiopulmonary bypass: experience with intraoperative transesophageal echocardiography. J Am Coll Cardiol 1992;20:70–7. [DOI] [PubMed] [Google Scholar]
- 22. Manabe S, Fukui T, Miyajima K, Watanabe Y, Matsuyama S, Shimokawa T. et al. Impact of proximal anastomosis procedures on stroke in off-pump coronary artery bypass grafting. J Card Surg 2009;24:644–9. [DOI] [PubMed] [Google Scholar]
- 23. Ura M, Sakata R, Nakayama Y, Goto T.. Ultrasonographic demonstration of manipulation-related aortic injuries after cardiac surgery. J Am Coll Cardiol 2000;35:1303–10. [DOI] [PubMed] [Google Scholar]
- 24. Davila-Roman VG, Murphy SF, Nickerson NJ, Kouchoukos NT, Schechtman KB, Barzilai B.. Atherosclerosis of the ascending aorta is an independent predictor of long-term neurologic events and mortality. J Am Coll Cardiol 1999;33:1308–16. [DOI] [PubMed] [Google Scholar]
- 25. Bonatti J, van Boven WJ, Nagele G, Shahin G, Schachner T, Laufer G. et al. ; Assessment Of the Risk of Emboli Transmission In Coronary Surgery Study Group. Do particulate emboli from the ascending aorta in coronary bypass grafting correlate with aortic wall thickness? Interact CardioVasc Thorac Surg 2006;5:716–20. [DOI] [PubMed] [Google Scholar]
- 26. Swaminathan M, Grocott HP, Mackensen GB, Podgoreanu MV, Glower DD, Mathew JP.. The “sandblasting” effect of aortic cannula on arch atheroma during cardiopulmonary bypass. Anesth Analg 2007;104:1350–1. [DOI] [PubMed] [Google Scholar]
- 27. Grooters RK, Thieman KC, Schneider RF, Nelson MG.. Assessment of perfusion toward the aortic valve—using the new dispersion aortic cannula during coronary artery bypass surgery. Tex Heart Inst J 2000;27:361–5. [PMC free article] [PubMed] [Google Scholar]
- 28. Lata AL, Hammon JW, Deal DD, Stump DA, Kincaid EH, Kon ND.. Cannula design reduces particulate and gaseous emboli during cardiopulmonary bypass for coronary revascularization. Perfusion 2011;26:239–44. [DOI] [PubMed] [Google Scholar]
- 29. McDonald CI, Bolle E, Lang HF, Ribolzi C, Thomson B, Tansley GD. et al. Hydrodynamic evaluation of aortic cardiopulmonary bypass cannulae using particle image velocimetry. Perfusion 2016;31:78–86. [DOI] [PubMed] [Google Scholar]
- 30. Bolotin G, Huber CH, Shani L, Mohr FW, Carrel TP, Borger MA. et al. Novel emboli protection system during cardiac surgery: a multi-center, randomized, clinical trial. Ann Thorac Surg 2014;98:1627–33; discussion 33–4. [DOI] [PubMed] [Google Scholar]
- 31. Mack MJ, Acker MA, Gelijns AC, Overbey JR, Parides MK, Browndyke JN. et al. ; Cardiothoracic Surgical Trials Network (CTSN). Effect of cerebral embolic protection devices on CNS infarction in surgical aortic valve replacement: a randomized clinical trial. JAMA 2017;318:536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kowalewski M, Malvindi PG, Suwalski P, Raffa GM, Pawliszak W, Perlinski D. et al. Clinical safety and effectiveness of endoaortic as compared to transthoracic clamp for small thoracotomy mitral valve surgery: meta-analysis of observational studies. Ann Thorac Surg 2017;103:676–86. [DOI] [PubMed] [Google Scholar]
- 33. Zingone B, Gatti G, Rauber E, Pappalardo A, Benussi B, Dreas L.. Surgical management of the atherosclerotic ascending aorta: is endoaortic balloon occlusion safe? Ann Thorac Surg 2006;82:1709–14. [DOI] [PubMed] [Google Scholar]
- 34. Gillinov AM, Lytle BW, Hoang V, Cosgrove DM, Banbury MK, McCarthy PM. et al. The atherosclerotic aorta at aortic valve replacement: surgical strategies and results. J Thorac Cardiovasc Surg 2000;120:957–63. [DOI] [PubMed] [Google Scholar]
- 35. Aranki SF, Nathan M, Shekar P, Couper G, Rizzo R, Cohn LH.. Hypothermic circulatory arrest enables aortic valve replacement in patients with unclampable aorta. Ann Thorac Surg 2005;80:1679–86; discussion 86–7. [DOI] [PubMed] [Google Scholar]
- 36. Kaneko T, Neely RC, Shekar P, Javed Q, Asghar A, McGurk S. et al. The safety of deep hypothermic circulatory arrest in aortic valve replacement with unclampable aorta in non-octogenarians. Interact CardioVasc Thorac Surg 2015;20:79–84. [DOI] [PubMed] [Google Scholar]
- 37. Murzi M, Cerillo AG, Gasbarri T, Margaryan R, Kallushi E, Farneti P. et al. Antegrade and retrograde perfusion in minimally invasive mitral valve surgery with transthoracic aortic clamping: a single-institution experience with 1632 patients over 12 years. Interact CardioVasc Thorac Surg 2017;24:363–8. [DOI] [PubMed] [Google Scholar]
- 38. Grossi EA, Loulmet DF, Schwartz CF, Ursomanno P, Zias EA, Dellis SL. et al. Evolution of operative techniques and perfusion strategies for minimally invasive mitral valve repair. J Thorac Cardiovasc Surg 2012;143:S68–70. [DOI] [PubMed] [Google Scholar]
- 39. Williams RD, Foley NM, Vyas R, Huang S, Kertai MD, Balsara KR. et al. Predictors of stroke after minimally invasive mitral valve surgery without the cross-clamp. Semin Thorac Cardiovasc Surg 2020;32:47–56. [DOI] [PubMed] [Google Scholar]
- 40. Barbero C, Marchetto G, Ricci D, El Qarra S, Attisani M, Filippini C. et al. Right minithoracotomy for mitral valve surgery: impact of tailored strategies on early outcome. Ann Thorac Surg 2016;102:1989–95. [DOI] [PubMed] [Google Scholar]
- 41. Hedayati N, Sherwood JT, Schomisch SJ, Carino JL, Markowitz AH.. Axillary artery cannulation for cardiopulmonary bypass reduces cerebral microemboli. J Thorac Cardiovasc Surg 2004;128:386–90. [DOI] [PubMed] [Google Scholar]
- 42. Etz CD, Plestis KA, Kari FA, Silovitz D, Bodian CA, Spielvogel D. et al. Axillary cannulation significantly improves survival and neurologic outcome after atherosclerotic aneurysm repair of the aortic root and ascending aorta. Ann Thorac Surg 2008;86:441–7. [DOI] [PubMed] [Google Scholar]
- 43. Hagl C, Galla JD, Spielvogel D, Bodian C, Lansman SL, Squitieri R. et al. Diabetes and evidence of atherosclerosis are major risk factors for adverse outcome after elective thoracic aortic surgery. J Thorac Cardiovasc Surg 2003;126:1005–12. [DOI] [PubMed] [Google Scholar]
- 44. Zingone B, Gatti G, Spina A, Rauber E, Dreas L, Forti G. et al. Current role and outcomes of ascending aortic replacement for severe nonaneurysmal aortic atherosclerosis. Ann Thorac Surg 2010;89:429–34. [DOI] [PubMed] [Google Scholar]
- 45. Rokkas CK, Kouchoukos NT.. Surgical management of the severely atherosclerotic ascending aorta during cardiac operations. Semin Thorac Cardiovasc Surg 1998;10:240–6. [DOI] [PubMed] [Google Scholar]
- 46. Diegeler A, Borgermann J, Kappert U, Breuer M, Boning A, Ursulescu A. et al. ; GOPCABE Study Group. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med 2013;368:1189–98. [DOI] [PubMed] [Google Scholar]
- 47. Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu SS, Paolasso E. et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012;366:1489–97. [DOI] [PubMed] [Google Scholar]
- 48. Halkos ME, Anderson A, Binongo JNG, Stringer A, Lasanajak Y, Thourani VH. et al. Operative strategies to reduce cerebral embolic events during on- and off-pump coronary artery bypass surgery: a stratified, prospective randomized trial. J Thorac Cardiovasc Surg 2017;154:1278–85.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Araque JC, Greason KL, Li Z, Heins CN, Stulak JM, Daly RC. et al. On-pump coronary artery bypass graft operation: is one crossclamp application better than two? J Thorac Cardiovasc Surg 2015;150:145–9. [DOI] [PubMed] [Google Scholar]
- 50. Alaeddine M, Badhwar V, Grau-Sepulveda MV, Wei LM, Cook CC, Halkos ME. et al. Aortic clamping strategy and postoperative stroke. J Thorac Cardiovasc Surg 2018;156:1451–57.e4. [DOI] [PubMed] [Google Scholar]
- 51. Grega MA, Borowicz LM, Baumgartner WA.. Impact of single clamp versus double clamp technique on neurologic outcome. Ann Thorac Surg 2003;75:1387–91. [DOI] [PubMed] [Google Scholar]
- 52. Moss E, Puskas JD, Thourani VH, Kilgo P, Chen EP, Leshnower BG. et al. Avoiding aortic clamping during coronary artery bypass grafting reduces postoperative stroke. J Thorac Cardiovasc Surg 2015;149:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kinnunen EM, Juvonen T, Biancari F.. Use of blood products and diseased ascending aorta are determinants of stroke after off-pump coronary artery bypass grafting. J Cardiothorac Vasc Anesth 2015;29:1180–6. [DOI] [PubMed] [Google Scholar]
- 54. Kapetanakis EI, Stamou SC, Dullum MK, Hill PC, Haile E, Boyce SW. et al. The impact of aortic manipulation on neurologic outcomes after coronary artery bypass surgery: a risk-adjusted study. Ann Thorac Surg 2004;78:1564–71. [DOI] [PubMed] [Google Scholar]
- 55. Kim DJ, Lee SH, Joo HC, Yoo KJ, Youn YN.. Effects of a proximal seal system on neurologic outcomes of off-pump coronary artery bypass grafting. Int Heart J 2019;60:593–600. [DOI] [PubMed] [Google Scholar]
- 56. Thourani VH, Razavi SA, Nguyen TC, Kilgo PD, Puskas JD, Guyton RA. et al. Incidence of postoperative stroke using the heartstring device in 1,380 coronary artery bypass graft patients with mild to severe atherosclerosis of the ascending aorta. Ann Thorac Surg 2014;97:2066–72. [DOI] [PubMed] [Google Scholar]
- 57. Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E. et al. On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med 2009;361:1827–37. [DOI] [PubMed] [Google Scholar]
- 58. Zhao DF, Edelman JJ, Seco M, Bannon PG, Wilson MK, Byrom MJ. et al. Coronary artery bypass grafting with and without manipulation of the ascending aorta a network meta-analysis. J Am Coll Cardiol 2017;69:924–36. [DOI] [PubMed] [Google Scholar]
- 59. Cavallaro P, Itagaki S, Seigerman M, Chikwe J.. Operative mortality and stroke after on-pump vs off-pump surgery in high-risk patients: an analysis of 83 914 coronary bypass operations. Eur J Cardiothorac Surg 2014;45:159–64. [DOI] [PubMed] [Google Scholar]
- 60. Mishra M, Malhotra R, Karlekar A, Mishra Y, Trehan N.. Propensity case-matched analysis of off-pump versus on-pump coronary artery bypass grafting in patients with atheromatous aorta. Ann Thorac Surg 2006;82:608–14. [DOI] [PubMed] [Google Scholar]
- 61. Sharony R, Grossi EA, Saunders PC, Galloway AC, Applebaum R, Ribakove GH. et al. Propensity case-matched analysis of off-pump coronary artery bypass grafting in patients with atheromatous aortic disease. J Thorac Cardiovasc Surg 2004;127:406–13. [DOI] [PubMed] [Google Scholar]
- 62. Sousa-Uva M, Neumann FJ, Ahlsson A, Alfonso F, Banning AP, Benedetto U. et al. ; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019;55:4–90. [DOI] [PubMed] [Google Scholar]
- 63. Gaudino M, Benedetto U, Bakaeen F, Rahouma M, Tam DY, Abouarab A. et al. Off-versus on-pump coronary surgery and the effect of follow-up length and surgeons’ experience: a meta-analysis. J Am Heart Assoc 2018;7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Head SJ, Milojevic M, Daemen J, Ahn JM, Boersma E, Christiansen EH. et al. Stroke rates following surgical versus percutaneous coronary revascularization. J Am Coll Cardiol 2018;72:386–98. [DOI] [PubMed] [Google Scholar]
- 65. Zhao DF, Edelman JJ, Seco M, Bannon PG, Vallely MP.. Stroke risk following anaortic off-pump CABG versus PCI. J Am Coll Cardiol 2018;72:2679–80. [DOI] [PubMed] [Google Scholar]
- 66. Thuijs D, Kappetein AP, Serruys PW, Mohr FW, Morice MC, Mack MJ. et al. ; SYNTAX Extended Survival Investigators. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019;394:1325–34. [DOI] [PubMed] [Google Scholar]
- 67. Iribarne A, Pan S, McCullough JN, Mathew JP, Hung J, Zeng X. et al. Impact of aortic atherosclerosis burden on outcomes of surgical aortic valve replacement. Ann Thorac Surg 2020;109:465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Idrees J, Roselli EE, Raza S, Krishnaswamy A, Mick S, Kapadia S. et al. Aborted sternotomy due to unexpected porcelain aorta: does transcatheter aortic valve replacement offer an alternative choice? J Thorac Cardiovasc Surg 2015;149:131–4. [DOI] [PubMed] [Google Scholar]
- 69. Yu K, Rishi P, Anthony DP, Muhammad H, Mohammed Q, Kiyoko U. et al. Aortic atheroma burden predicts acute cerebrovascular events after transcatheter aortic valve implantation: insights from volumetric multislice computed tomography analysis. EuroIntervention 2016;12:783–9. [DOI] [PubMed] [Google Scholar]
- 70. Miyasaka M, Sharma RP, Maeno Y, Taguri M, Yoon SH, Kawamori H. et al. Investigation of computed-tomography based predictors of acute stroke related to transcatheter aortic valve replacement: aortic wall plaque thickness might be a predictive parameter of stroke. J Invasive Cardiol 2020;32:E18–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.