See Schaffenrath et al, this issue (pp. 2095–2106).
Treatment outcomes for malignant brain tumors are poor despite extensive genome-wide molecular characterizations and decades of basic, translational, and clinical research. The complex microenvironment of brain tumors contributes to the dire prognosis for primary and secondary brain malignancies. Tumor vasculature, an essential component of tumor stroma, limits brain tumor distribution, and therefore the efficacy, of otherwise potentially efficacious drugs.1 The study by Schaffenrath et al2 offers researchers a perspective of how “better understanding of vasculature changes in human brain malignancies could lead to improved therapeutic targeting of brain tumors.” The authors employ powerful transcriptomic techniques to determine disease-related changes in the vasculature of brain tumors.
Schaffenrath et al revealed deregulation of various genes that define blood-brain barrier (BBB) function in glioblastoma (GBM) and brain metastases (BM) of non–small-cell lung cancer.2 In the normal brain, components of the BBB provide both physical and biochemical barriers to preserve homeostasis.1 Even though the role of BBB integrity for effective drug exposure and brain tumor therapy is still a matter of debate, limited and heterogeneous drug delivery into GBM and BM is a likely contributor to treatment failure.1,3 A heterogeneously disrupted BBB, whether in GBM or BM, can limit the distribution of therapies to the entirety of the tumor, which may significantly impair the efficacy of otherwise potentially curative therapies. Importantly, disruption of the BBB also may be heterogeneous with regards to physicochemical properties of drugs such that accumulation and detection of gadolinium-based contrast agents on MRI may not correspond with distribution of small or large molecule therapeutics. This delivery-efficacy hurdle points to the need for a clear understanding of the impact of tumor-induced changes in the BBB and surrounding stroma on drug delivery.
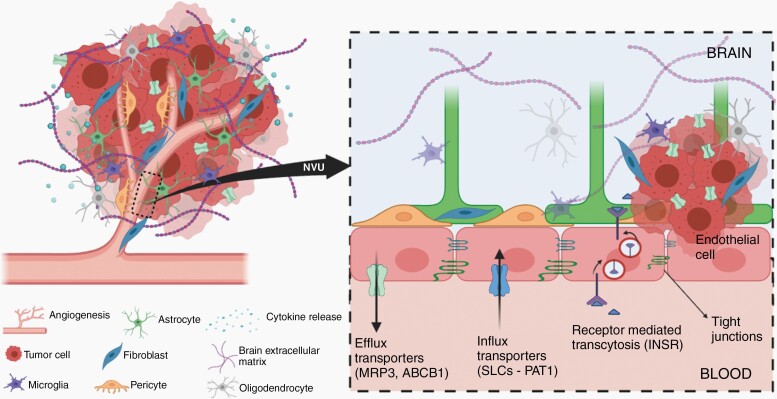
One mechanism of the BBB that restricts access of therapeutic agents to the tumor is the ATP-binding cassette (ABC) transporters expressed on endothelial cells (EC) at BBB and in tumor cells themselves. Schaffenrath et al reported profound alterations in the expression of ABC transporters in GBM and BM vasculature by performing RNA sequencing of EC isolated from primary and secondary brain tumors.2 The ABCC3 gene, encoding multidrug resistance-associated protein 3 (MRP3), is upregulated in both GBM and BM vasculature. The overexpression of MRP3 may limit the brain distribution of cisplatin, which is commonly used for lung cancer and may contribute to resistance of BM to systemic chemotherapy.4 Importantly, reduced protein level of P-glycoprotein (P-gp, encoded by ABCB1), one of the predominant efflux transporters, is observed in GBM vasculature.2 These findings suggest that changes in expression of active efflux transporters in the vasculature of brain tumor, as well as in the GBM cells,5 may influence drug delivery and hence efficacy (Figure 1).
Fig. 1.
Exploiting changes in the blood-brain barrier vasculature for the treatment of brain tumors. Abbreviation: BBB, blood-brain barrier.
In addition to the efflux transporters, various solute carriers (SLC transporters) at the BBB also have essential roles in the maintenance of brain homeostasis. The upregulated expression of genes encoding amino acid, nucleobase and nucleoside transporters indicates altered metabolic requirements of GBM EC, which implies that modified anticancer drugs such as amino acid-based prodrugs might enhance drug delivery to GBM. Schaffenrath et al found deregulation in the expression of a variety of SLC transporters in brain tumor vasculature. Proton-coupled amino acid transporter 1 (PAT1, encoded by SLC36A1) is variably overexpressed by GBM tumor cells and EC. This transporter has a low affinity and high capacity, therefore, could potentially be used as a drug delivery platform for therapeutic and diagnostic agents2 (Figure 1). 5-Aminolevulinic acid (5-ALA) is commonly used during fluorescence-guided surgery for tumor tissue visualization. 5-ALA is a substrate of PAT1 and the fluorescent signal may be influenced by changes in expression. Moreover, the discovery of the upregulation of the insulin receptor (INSR) in GBM and BM EC suggests that receptor-mediated transcytosis may also be exploited as a drug shuttle platform to deliver macromolecules to brain tumors (Figure 1).
The stroma of a brain tumor is characteristically stiffer than a normal brain, due in part to changes in the extracellular matrix (ECM).2 Several angiogenesis-related genes (eg, VEGFA, ANGPT2) and genes encoding for ECM proteins (eg, LAMB1 for laminins, COL4A1 and COL4A2 for collagens, NID1 for nidogens) are upregulated in brain tumors. This upregulation of ECM components hinders diffusion, resulting in decreased exposure. This deregulation implies that the modification of tumor ECM surrounding blood vessels could be an approach to improve drug distribution throughout tumor regions. Moreover, an increased vessel permeability was shown following the reduced expression of EC junctional proteins2 (Figure 1). This heterogeneous loss of tight junctions in brain tumors, however, may not translate to an improvement of drug entry into the brain due to the presence of active efflux transporters.6,7
Another key finding by Schaffenrath et al is the identification of fibroblast-like cells in GBM stroma (Figure 1). Other than endothelial and mural cells, 2 major cell types in the neurovascular unit (NVU), there are astrocytes, oligodendrocytes, perivascular and interstitial microglia, and 2 types of fibroblast-like cells (FB1 and FB2).8 By using single-cell RNA sequencing in the mouse brain, Vanlandewijck et al discovered that fibroblast-like cells are loosely adherent to arteries and veins located between small muscle cells (SMCs) and astrocyte end-feet.8 In mouse brain, ADAM12, DPEP1, FBLN2, and CLEC3B genes are biomarkers for FB1 and FB2.2 Schaffenrath et al found upregulated ADAM12 and DPEP1 and downregulated genes FBLN2 and CLEC3B in GBM samples. How the complex regulation of these markers is related to fibroblast function is unknown. In addition, ADAM12 and CLEC3B were detected throughout blood vessels and brain parenchyma in GBM compared with localized distribution along blood vessels in the normal brain. While the specific role and function of fibroblast-like cells in GBM stroma is unclear, we might speculate that deregulation of FB1 and FB2 markers in GBM tissue may be similar to cancer-associated fibroblasts (CAFs) in other cancers. CAFs are responsible for synthesis, deposition, and remodeling of ECM in tumor stroma, and as such play an active role in regulating cancer cell proliferation, progression, and metastasis, as well as angiogenesis promotion.9 Importantly, CAFs were reported to induce resistance of cancer cells to therapy, including cytotoxic chemotherapy, radiation therapy, targeted therapy, and immunotherapy.10 Therefore, the heterogeneous expression of fibroblasts in GBM tissue needs to be further investigated to understand their role and implications in disease progression to aid in the development of novel interventions.
In summary, this study by Schaffenrath et al is a comprehensive and critical advance in understanding the response of the human brain vasculature in the setting of brain tumor growth. The observation of heterogenous dysregulation of genes defining the physical barriers (TJ proteins) and biochemical barriers (transporters and receptors) at the BBB, as well as angiogenesis pathways and ECM components, provides a deeper understanding of tumor growth and treatment opportunities. This suggests strategies focused on structural modification of drugs to evade efflux and assist the influx of drugs by utilizing receptors overexpressed in the vasculature could be used to enhance drug distribution. The identification of fibroblast-like cells associated with GBM vasculature also opens an exciting line of investigation focused on understanding the role of these specific cell types and their interactions with tumor microenvironment with regard to brain tumor biology. Progress in the development of drugs for brain tumors can be advanced when the knowledge of these alterations of the tumor microenvironment and the BBB are exploited along with a greater understanding of the inter- and intra-tumoral heterogeneity of BBB breakdown in brain tumors.
Acknowledgments
The figure was prepared using BioRender.
Conflict of interest statement. None declared.
Funding
This work was supported by the National Institutes of Health [Grants U01 CA227954, U54 CA210180, and R01 NS073610].
References
- 1. Sarkaria JN, Hu LS, Parney IF, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018;20(2):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schaffenrath J, Wyss T, He L, et al. Blood-brain barrier alterations in human brain tumors revealed by genome-wide transcriptomic profiling. Neuro Oncol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Osswald M, Blaes J, Liao Y, et al. Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin Cancer Res. 2016;22(24):6078–6087. [DOI] [PubMed] [Google Scholar]
- 4. Kishimoto S, Yasuda M, Fukushima S. Changes in the expression of various transporters as influencing factors of resistance to cisplatin. Anticancer Res. 2017;37(10):5477–5484. [DOI] [PubMed] [Google Scholar]
- 5. de Trizio I, Mariella E, d'Amati A, Francesco G, Daniela V. Expression of P-gp in glioblastoma: what we can learn from brain development. Curr Pharm Des. 2020;26(13):1428–1437. [DOI] [PubMed] [Google Scholar]
- 6. de Gooijer MC, Kemper EM, Buil LCM, et al. ATP-binding cassette transporters restrict drug delivery and efficacy against brain tumors even when blood-brain barrier integrity is lost. Cell Rep Med. 2021;2(1):100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goutal S, Gerstenmayer M, Auvity S, et al. Physical blood-brain barrier disruption induced by focused ultrasound does not overcome the transporter-mediated efflux of erlotinib. J Control Release. 2018;292:210–220. [DOI] [PubMed] [Google Scholar]
- 8. Vanlandewijck M, He L, Mäe MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554(7693):475–480. [DOI] [PubMed] [Google Scholar]
- 9. Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15(6):366–381. [DOI] [PMC free article] [PubMed] [Google Scholar]