Abstract
Fifty-nine Angus-cross finishing steers were used to evaluate benzoic acid, active dry yeast (Saccharomyces cerevisiae), or a combination of benzoic acid and active dry yeast when supplemented in a high-grain finishing diet on live animal performance, feeding behavior, and carcass characteristics. Steers were fed a high-grain diet for the final 106 d of finishing. Treatments were as follows: no additional supplementation (CON), 0.5% benzoic acid (ACD), 3 g per head per day active dry S. cerevisiae (YST), or both 0.5% benzoic acid and 3 g/head per day S. cerevisiae (AY). Steers were weighed every 14 d, and ultrasound was performed for rib and rump fat thickness at the beginning (day 1), middle (day 57), and end (day 99) of the experiment. Insert feeding stations were used to collect individual feeding behavior data and DMI daily throughout. Blood samples were collected on days 21 and 22 and days 99–101 to assess plane of nutrition and metabolism. Ruminal fluid samples were collected by oral gavage 4 wk prior to slaughter. Carcass characteristics were examined at a federally inspected slaughter facility. Data were analyzed using PROC GLIMMIX of SAS with initial body weight (BW) as a covariate. Benzoic acid supplementation increased (P = 0.002) overall dry matter intake (DMI) compared to YST and CON steers, which may be due to a faster eating rate (P ≤ 0.008). Animal performance parameters (BW, average daily gain, feed conversion, and ultrasound fat depth) were not different (P ≥ 0.11) among treatment groups. Aspartate aminotransferase concentration was greatest (P ≤ 0.01) for YST steers, which may have been reflected in numerically greater liver abscesses. Carcass traits did not differ (P ≥ 0.33) among treatment groups. Ruminal pH was greater (P = 0.006) for ACD steers than AY steers (pH of 6.16 vs. 5.66, respectively), which indicated that there may be an interactive effect between benzoic acid and active dry yeast. To summarize, steers fed a high-grain finishing diet supplemented with benzoic acid, active dry yeast, or both benzoic acid and active dry yeast had similar growth performance and carcass characteristics compared to those without supplementation. However, the addition of benzoic acid alone increased DMI, variation in DMI, eating rate, and ruminal pH. Future studies are warranted to further investigate the impacts of benzoic acid on the ruminal environment of feedlot cattle.
Keywords: benzoic acid, carcass characteristics, feedlot performance, feeding behavior, Saccharomyces cerevisiae
INTRODUCTION
Feed additives are commonly added to feedlot diets with the intent of reducing the prevalence and severity of coccidiosis, ruminal acidosis, and liver abscesses that adversely affect animal health and performance. Traditional feed additives, such as ionophores and antimicrobials, are typically added to the diet of finishing feedlot cattle to improve feed efficiency, energy utilization, and prevent liver abscess (Hobson and Stewart, 1997). Currently, there is a need to identify alternatives to conventional feed additives to meet consumer demands, especially antimicrobials, which can maintain animal performance and health while providing sustainable production systems for the beef industry.
Organic acids have shown antimicrobial effects by suppressing fungal activity and maintaining an acidic ruminal environment (Castillo et al., 2004). Benzoic acid is an organic acid which has been used as a feed acidifying agent and has been studied in monogastric animals. Benzoic acid supplementation has shown to improve growth performance of chickens (Amaechi and Anueyiagu, 2012; Giannenas et al., 2014) and pigs (Chen et al., 2017) and has shown improvements of markers of gut health in these studies with benzoic acid supplementation. Beef cattle are at the highest risk for gut health–related issues, such as ruminal acidosis, in the late finishing phase (Castillo-Lopez et al., 2014). However, only one other study has examined benzoic acid supplementation in beef cattle diets (Wang et al., 2020). This study examined benzoic acid as a replacement for monensin and tylosin and found no impacts on growth performance and carcass characteristics. It is not known how the impact of BA supplementation with the industry standard addition of monensin to the ration would affect performance and feed intake of finishing.
Another potential alternative to infeed antimicrobials in beef cattle diets is direct-fed microbials, such as live yeasts. Yeast as a feed additive has shown reduction of lactate accumulation in the rumen and improvements in fiber digestibility (Marden et al., 2008). Feedlot cattle are at a higher risk of developing ruminal acidosis in the finishing phase, resulting from lactate buildup in the rumen that reduces nutrient utilization by the animal (Nagaraja and Titgemeyer, 2007). Yeast supplementation in the diet of feedlot steers has the potential to improve animal performance during this period (Marden et al., 2008). Additionally, it is unknown if there is an additive effect of yeast with other potential feed additives such as BA, although the mechanisms of action for both additives are unknown.
The objectives of this experiment were to investigate the effects of supplementing benzoic acid, active dry yeast (Saccharomyces cerevisiae), or the combination of benzoic acid and active dry yeast on feedlot performance, feeding behavior, and carcass characteristics in beef steers fed high-grain diets containing monensin. It was hypothesized that adding benzoic acid, active dry yeast, or the combination of benzoic acid and active dry yeast to the diets of finishing feedlot cattle would result in improved animal performance and carcass characteristics when compared with diets containing monensin alone.
MATERIAL AND METHODS
The experiment was conducted at the Elora Beef Research Center (Elora, ON, Canada). All procedures for the experiment were approved by the Animal Care Committee (AUP# 3706) and in accordance with the Canadian Council on Animal Care (1993).
Experimental Design and Diet
Upon arrival at the University of Guelph Elora Beef Research Center, steers purchased from commercial auctions were tagged with electronic identification tags (High Performance HDX Ultra EID Tag; Allflex, Dallas, TX, USA) and assessed to be in good health by research personnel. Steers were vaccinated according to the research facility’s protocol and implanted with Synovex S hormonal implants (200 mg of progesterone, 20 mg of estradiol benzoate; Zoetis, Kalamazoo, MI, USA) 23 d before the start of the study. Steers were inspected for the presence of testicles, and non-castrated cattle were excluded from the study.
Initially, 59 Angus-crossbred steers were blocked by body weight (BW) into three groups (light: 370–416 kg, intermediate: 421–443 kg, and heavy: 445–527 kg), which determined which of the 6 pens they were assigned. Animals were housed inside the covered finishing barn in 7.16 × 14.07 m pens, bedded with wood shavings. Within each block (2 pens), steers were equally and randomly assigned to one of four treatment groups, with each pen containing two experimental treatments. Steers were adjusted to experimental diets over 33 d to transition steers from a high roughage diet to the high-concentrate finishing diet used in this study and to train steers on Insentec feeding stations. These feeding stations were part of the Insentec feeding system (Insentec B.V., Marknesse, the Netherlands) used to collect feed intake and feeding behavior data on an individual animal basis. Each pen was equipped with four feeding stations that steers were assigned to by treatment group (two feeding stations/one of two treatments assigned to a specific pen), enabling the measurement of individual feed intake. There were four dietary treatments in this study: 1) no additional supplement to the finishing diet (Table 1; CON, n = 15 steers), 2) benzoic acid (Vevovitall, DSM Nutritional Products, Parsippany, NJ, USA) fed at 0.5% on a DM basis (ACD, n =14 steers), 3) active dry yeast (S. cerevisiae, Vistacell; AB Vista, Marlborough, UK) fed at 3 grams/head per day resulting in 60 billion colony forming units (CFU) per head per day (YST, n = 15 steers), and 4) supplementation with both benzoic acid at 0.5% and active dry yeast (S. cerevisiae) at 3 g/head per day (AY, n = 15 steers). Treatment doses for benzoic acid and yeast were used according to the manufacturer’s instructions and to facilitate feeding were incorporated into the premix manufactured at a commercial feed mill, prior to addition to the total mixed ration (TMR). In each block (two pens), four or five steers were randomly assigned to one of four treatment groups, with each pen containing two different dietary treatments (two Insentec feeding stations per treatment in each pen). Steers were allowed ad libitum access to feed and at the end of the adaptation period were successfully transitioned to a highly fermentable feedlot finishing diet containing 79% high-moisture corn, 10% alfalfa haylage, 9% soybean meal, and 2% mineral premix, which included monensin on a dry matter basis (to provide a concentration of 33 mg/kg; Rumensin, Elanco Animal Health, Greenfield, IN, USA; Table 1).
Table 1.
Ingredient and chemical composition (DM basis) for experimental diets
| Item | Dietary treatment1 | |||
|---|---|---|---|---|
| CON | ACD | YST | AY | |
| Ingredient composition, % DM | ||||
| High moisture corn | 79.8 | 79.4 | 79.8 | 79.3 |
| Alfalfa haylage | 10.0 | 10.0 | 10.0 | 10.0 |
| Soybean meal + Feedlot mineral2 | 8.9 | 9.3 | 8.9 | 9.4 |
| Limestone | 1.0 | 1.0 | 1.0 | 1.0 |
| Salt | 0.3 | 0.3 | 0.3 | 0.3 |
| Chemical composition, % DM | ||||
| DM, % | 69.60 | 69.31 | 69.30 | 69.27 |
| OM | 91.03 | 91.00 | 91.10 | 91.28 |
| Crude protein | 13.20 | 13.34 | 13.48 | 13.52 |
| Crude fat | 4.87 | 4.90 | 4.83 | 4.81 |
| Starch | 52.88 | 51.61 | 52.86 | 51.83 |
| Total sugars | 4.71 | 4.68 | 4.62 | 4.69 |
| NDF | 13.48 | 14.17 | 13.19 | 14.01 |
| ADF | 6.85 | 7.54 | 6.80 | 7.47 |
| NEm, Mcal/kg | 2.04 | 2.03 | 2.05 | 2.04 |
| NEg, Mcal/kg | 1.39 | 1.38 | 1.39 | 1.38 |
| Ash | 6.25 | 6.24 | 6.17 | 6.04 |
| Ca | 0.80 | 0.80 | 0.77 | 0.80 |
| P | 0.37 | 0.37 | 0.38 | 0.37 |
| Na | 0.14 | 0.14 | 0.15 | 0.15 |
1Treatments: CON: control (not supplemented); ACD: 0.5% of benzoic acid dietary inclusion on a DM basis (DSM Nutritional Products, Parsippany, NJ, USA); YST: 3 g/head per day of Saccharomyces cerevisiae (Vistacell, AB Vista, Marlborough, UK); AY: 0.5% of benzoic acid dietary inclusion on a DM basis (DSM Nutritional Products) and 3 g/head per day of Saccharomyces cerevisiae (Vistacell, AB Vista).
2 Feedlot mineral for CON and ACD steers; 78.2% soybean meal, 13.1% limestone, 2.9% fine salt, 2.2% Biomin, 1.2% monocalcium phosphate, 0.7% potassium/magnesium, 0.6% selenium, 0.4% potassium chloride, 0.4% ruminant micro premix, 0.2% monensin (to provide a common concentration of 33 mg/kg; Rumensin, Elanco Animal Health, Greenfield, IN, USA), 0.1% magnesium oxide. Feedlot mineral for YST and AY steers; 78.0% soybean meal, 13.1% limestone, 2.9% fine salt, 2.2% Biomin, 1.2% monocalcium phosphate, 0.7% potassium/magnesium, 0.6% selenium, 0.4% potassium chloride, 0.4% ruminant micro mix, 2.3% yeast (Vistacell, AB Vista, Marlbourough, UK), 0.1% monensin (to provide a common concentration of 33 mg/kg; Rumensin, Elanco Animal Health, Greenfield, IN, USA), 0.1% magnesium oxide.
Within each pen, steers were assigned to feeding stations containing the designated dietary treatment and bunks were filled once daily (0800 h). The Insentec feeding stations enabled measurement of individual animal DMI and feeding behavior and allowed more than one treatment to be fed in each pen to designated cattle. Feeding behavior data were retrieved from Insentec feed stations and averaged for each animal over the entire feeding period (Montanholi et al., 2010). A meal was defined as a period of feed consumption with breaks no more than 7 min apart (Forbes, 2007).
Sampling and Analysis
Samples for each diet were collected weekly and stored at −20 °C until analysis. Samples were dried at 60 °C for 48 h, or until a consistent weight was achieved, and then dry matter (DM) content was determined. These DM percentages were used to calculate dry matter intake (DMI). Daily DMI for each steer was determined using the information provided from the Insentec feeding stations for individual animal as-fed intakes that were multiplied by the DM content for each diet. Individual daily variation for DMI (DMI SD) was measured for each steer against their own weekly average daily DMI.
Additional compositional analysis for the diets was conducted at a commercial laboratory (Stratford Agri Analysis, Stratford, ON, Canada) on composite samples from every four collection periods using wet chemistry methods. Crude protein was determined using a LECO FP628 nitrogen analyzer following the Association of Official Agricultural Chemists (AOAC) method 990.03 and using a protein factor of 6.25 for calculation (AOAC, 2007). Crude fat was determined using AOAC method 920.39 (AOAC, 2007). Starch was determined using a Megazyme total starch assay kit (Megazyme International Ireland, Ltd., Bray, Ireland), adopted from AOAC method 996.11 (AOAC, 2007). Total sugars were determined with AOAC method 923.09 (AOAC, 2007). Fiber fractions (NDF and ADF) were determined using an Ankom 2000 fibre analyzer (Ankom Technology, Macedon, NY, USA) utilizing Ankom Technology Method 13 and Method 12, respectively. Net energy (NEm and NEg) was calculated based on fiber fractions using prediction equations from NRC (2001). Ash was determined using AOAC method 942.05 (AOAC, 2007). Minerals were determined with aqua regia inductively coupled plasma optical emission spectrometry based on EPA 3050 and EPA 6010 methods.
Body weights were recorded on days 0 and 1 of the experiment, every 14 d thereafter, and on the last 2 d (days 105 and 106) before slaughter. At the beginning (day 1), middle (day 57), and end (day 99) of the experiment, ultrasound measurements for rib and rump fat depth were captured using an EXAGO ultrasound machine from Echo Control Medicinal (#L3180B, Angoulême, France) equipped with an 18 cm, 3.5 MHz transducer using anatomical landmarks as described by Wood et al. (2011). Rib and rump fat depths were measured at each time point using ImageJ software (version [1.51] Copyright 2015).
In order to monitor impacts of supplementation on metabolic status, blood metabolites were measured. Blood was collected via jugular venipuncture on days 99 to 101 at 1100 h into 10-mL lithium heparinized tubes (BD Vacutainer, Franklin Lakes, NJ) and kept on ice for at least 30 min before centrifugation for plasma samples. Concurrently, blood samples were collected into 10-mL serum separator tubes (BD Vacutainer, Franklin Lakes, NJ) and allowed to clot at room temperature for at least 30 min before storage on ice for serum samples. Plasma and serum samples were centrifuged at 3,000 × g for 20 min and stored at −20 °C until analysis. Serum analysis for blood metabolites was completed at the Animal Health Laboratory (Guelph, ON, Canada). Serum samples were analyzed using UREAL: ACN418 for urea, GLUC3: ACN717 for glucose, NEFA for non-esterified fatty acids (NEFA), RANBUT: D-3-hydroxybutyrate for ß-hydroxybutyrate (BHBA), TP2: ACN678 for total protein, CHO2I: ACN798 for total cholesterol, CAN687 for aspartate aminotransferase (AST) ALB2: ACN413 for albumin and haptoglobin was determined using the methods of Makimura and Suzuki (1982), globulin was calculated as total protein minus the albumin in each sample. Plasma samples were analyzed for serum amyloid-A (SAA) concentrations using a commercially available kit following the manufacturer’s instructions (PHASE RANGE, Tridelta Development Limited, Maynooth, Ireland). Assays were conducted using the kit supplied procedure instructions with an intra-assay CV of 5.0% and inter-assay CV of 11.4%. Microplates were read with samples in duplicate using a BioTek EPOCH 2 microplate reader at 450 nm using 630 nm as a reference (BioTek Instruments Incorporated, Winooski, VT, USA). The measuring range for bovine serum amyloid-A concentrations was 9.4–150 µg/mL.
Four weeks before slaughter, at 1100 h, ruminal fluid was collected by oral intubation using a long tube with a strainer on the end. The tube was inserted into the mouth and passed into the rumen, where fluid was drained into a flask, discarding the first portion to prevent saliva contamination. Rumen fluid was strained through three layers of cheesecloth before measuring pH using the Accumet AB150 pH meter (calibrated to pH 4 and 7 standards; >90% efficiency; Fisher Scientific, Ottawa, ON, Canada). The probe was triple rinsed with double distilled water between samples.
Steers were humanely handled and slaughtered (which included captive bolt stunning, followed by exsanguination) at a federally inspected meat processing facility following commercial industry standards and Canadian Food Inspection Agency (CFIA) inspection regulations. Liver abscess scores were recorded using the Elanco liver check system with O indicating livers were abscess free, A indicating a liver with one or two small active abscesses (less than 2.54 cm in diameter), and A+ indicating a liver with one or more large abscesses (Elanco, 2016). Hot carcass weight (HCW) was determined immediately before chilling. At approximately 3 d post-mortem, Canadian Beef Grading Agency (CBGA) graders evaluated the carcass at the 12th/13th rib interface for quality and yield grades based on the Livestock and Poultry Carcass Grading Regulations (Canadian Food Inspection Agency, 1992). Camera data were collected over the longissimus muscle on the 12th/13th rib interface for each steer using a commercial imaging system. The data were then used to determine longissimus muscle area, back fat thickness, marbling score, yield grade, and quality grade.
Statistical Methods
Data were analyzed using the GLIMMIX procedure in SAS (SAS Institute Inc. Cary, NC, USA). The data were treated as a randomized complete block design with block as the random effect, treatment as a fixed effect, and steer as the experimental unit. Initial BW was used as a covariate. Treatment means were separated using the least squares means with a Tukey–Kramer adjustment for multiple comparisons. Results were considered significant at P ≤ 0.05.
RESULTS
Four AY steers were removed from the experiment before completion due to health concerns, including severe ruminal acidosis and respiratory illness. This removal made the number of steers that completed the study for each treatment as 15 steers for CON, 14 steers for ACD, 15 steers for YST, and 11 steers for AY. These sample sizes were used for all statistical analysis after the removal of unhealthy steers from the experiment. Data from days 1–56 had sample sizes of 15, 14, 15, and 15; days 57–84 had sample sizes of 15, 14, 15, and 13; and day 85 to the end of the experiment had sample sizes of 15, 14, 15, and 11 for CON, ACD, YST, and AY, respectively.
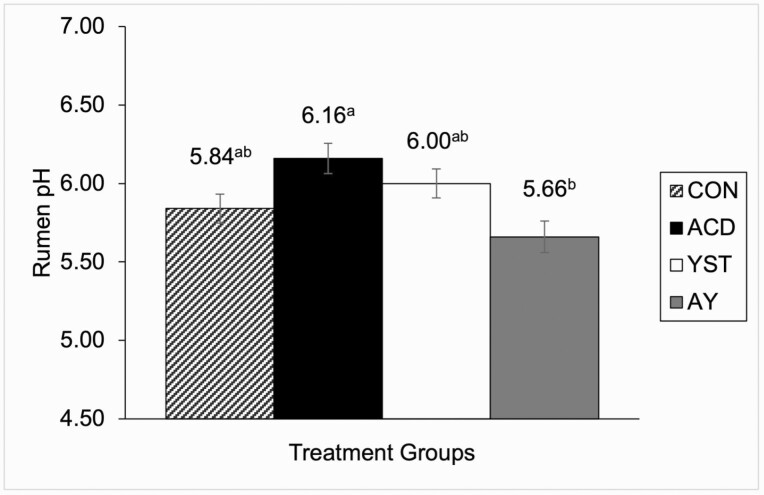
Overall DMI was greater in ACD steers than CON and YST steers having the lowest DMI (P = 0.005), whereas AY did not differ between all treatments (Table 2). Similarly, the DMI SD of steers was greater (P = 0.001) for ACD steers compared to CON, whereas YST and AY steers did not differ from the other treatments. When DMI was divided by month, ACD DMI was consistently greater than CON (P ≤ 0.02) overall timepoints. Eating rate was the fastest (P ≤ 0.008) for ACD steers over CON steers in the first half of the finishing period, second half of the finishing period, and overall experiment, whereas ACD was also greater than YST steers from days 1 to 56 and overall experiment period, but not during the second half of the finishing period. No differences between treatments were observed for all other feeding behaviour measurements. Ruminal pH was greater (P = 0.006) for ACD vs. AY steers, whereas YST and CON remained intermediate (Figure 1). Growth performance, including initial and final BW, average daily gain (ADG), feed conversion, and ultrasound fat deposition in the rib and rump did not differ (P ≥ 0.11; Table 3) among treatment groups at any time during the study.
Table 2.
Feed intakes and feeding behavior for steers fed a high-grain finishing diet with no supplementation, benzoic acid, and/or live active yeast
| Item | Dietary treatment1 | SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | ACD | YST | AY | |||
| DMI, kg/d | ||||||
| Days 1 to 28 | 10.5 | 11.5 | 10.8 | 10.7 | 0.30 | 0.31 |
| Days 29 to 56 | 12.0b | 13.5a | 12.1b | 12.0b | 0.30 | 0.003 |
| Days 57 to 84 | 11.9b | 13.5a | 12.3b | 12.3ab | 0.33 | 0.008 |
| Days 85 to 106 | 11.0b | 12.3a | 11.5ab | 11.5ab | 0.26 | 0.02 |
| Overall | 11.4b | 12.6a | 11.7b | 11.8ab | 0.27 | 0.005 |
| DMI SD2 | 4.1b | 4.6a | 4.2b | 4.3ab | 0.08 | 0.001 |
| Feeding behavior3 | ||||||
| Time at feeder (min/d) | ||||||
| Days 1 to 56 | 80 | 87 | 92 | 79 | 8.9 | 0.69 |
| Days 57 to 106 | 79 | 87 | 83 | 93 | 15.4 | 0.78 |
| Days 1 to 106 | 80 | 88 | 89 | 86 | 11.7 | 0.88 |
| Visits to feeder (visits/d) | ||||||
| Days 1 to 56 | 29 | 37 | 35 | 36 | 3.3 | 0.15 |
| Days 57 to 106 | 24 | 29 | 27 | 32 | 3.2 | 0.19 |
| Days 1 to 106 | 80 | 67 | 62 | 68 | 6.3 | 0.17 |
| Time per visit (min) | ||||||
| Days 1 to 56 | 3.8 | 2.4 | 2.7 | 2.2 | 0.35 | 0.23 |
| Days 57 to 106 | 3.2 | 2.7 | 3.0 | 2.8 | 0.59 | 0.65 |
| Days 1 to 106 | 3.1 | 2.5 | 2.8 | 2.5 | 0.42 | 0.40 |
| Visit size (g DM) | ||||||
| Days 1 to 56 | 43 | 34 | 34 | 31 | 3.5 | 0.08 |
| Days 57 to 106 | 47 | 40 | 43 | 35 | 4.4 | 0.21 |
| Days 1 to 106 | 45 | 37 | 37 | 33 | 3.9 | 0.13 |
| Meals per day | ||||||
| Days 1 to 56 | 9.4 | 10.7 | 10.0 | 10.9 | 0.53 | 0.17 |
| Days 57 to 106 | 9.6 | 10.1 | 9.5 | 11.1 | 0.75 | 0.27 |
| Days 1 to 106 | 9.6 | 10.5 | 9.9 | 11.1 | 0.58 | 0.22 |
| Time per meal (min) | ||||||
| Days 1 to 56 | 8.7 | 8.2 | 9.1 | 7.5 | 0.74 | 0.39 |
| Days 57 to 106 | 8.4 | 8.2 | 8.6 | 8.4 | 1.03 | 0.92 |
| Days 1 to 106 | 8.5 | 8.2 | 8.9 | 7.9 | 0.82 | 0.79 |
| Meal size (g DM/meal) | ||||||
| Day 1 to 56 | 122 | 123 | 117 | 105 | 6.4 | 0.29 |
| Day 57 to 106 | 123 | 133 | 127 | 108 | 10.5 | 0.36 |
| Day 1 to 106 | 122 | 127 | 121 | 106 | 7.2 | 0.35 |
| Eating rate (g DM/min) | ||||||
| Days 1 to 56 | 16b | 18a | 16b | 16ab | 0.4 | 0.007 |
| Days 57 to 106 | 16b | 18a | 17ab | 17ab | 0.4 | 0.008 |
| Days 1 to 106 | 16b | 18a | 17b | 17ab | 0.4 | 0.002 |
1Treatments: CON: control (not supplemented); ACD: 0.5% of benzoic acid dietary inclusion on a DM basis (DSM Nutritional Products, Parsippany, NJ, USA); YST: 3 g/head per day of Saccharomyces cerevisiae (Vistacell, AB Vista, Marlborough, UK); AY: 0.5% of benzoic acid dietary inclusion on a DM basis (DSM Nutritional Products) and 3 g/head per day of Saccharomyces cerevisiae (Vistacell, AB Vista).
2 DMI SD = individual variation for DMI. Individual animal standard deviation measured weekly against the overall average DMI of each steer.
3 Means were calculated for each animal across weekly feeding behavior data.
a,bValues within a row with different superscripts differ significantly at P ≤ 0.05.
Figure 1.
Ruminal pH (at 1100h) for steers fed a high-grain finishing diet containing monensin without supplement (CON n = 15), with 0.5% of feed on DM basis of benzoic acid (ACD n = 14: DSM Nutritional Products), 3 g/head per day of active dry yeast (YST n = 15: Vistacell, AB Vista) or a combination of benzoic acid and yeast (AY n = 13). Ruminal pH was greater in ACD vs. AY steers (P = 0.006; SEM = 0.900).
Table 3.
Growth performance for steers fed a high-grain finishing diet with no supplementation, benzoic acid, yeast, or both benzoic acid and yeast
| Item | Treatment1 | SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | ACD | YST | AY | |||
| BW, kg | ||||||
| Initial | 488 | 502 | 493 | 488 | 21.0 | 0.38 |
| Final | 707 | 737 | 705 | 705 | 8.1 | 0.11 |
| ADG, kg/d | ||||||
| Days 1 to 28 | 2.24 | 2.28 | 2.07 | 2.25 | 0.139 | 0.65 |
| Days 29 to 56 | 2.48 | 2.80 | 2.56 | 2.55 | 0.119 | 0.20 |
| Days 57 to 84 | 2.00 | 2.29 | 1.98 | 2.22 | 0.164 | 0.21 |
| Days 85 to 106 | 1.40 | 1.27 | 1.23 | 1.34 | 0.156 | 0.89 |
| Overall | 2.07 | 2.21 | 2.00 | 2.17 | 0.077 | 0.12 |
| F:G | ||||||
| Days 1 to 28 | 4.88 | 5.31 | 5.51 | 4.88 | 0.276 | 0.50 |
| Days 29 to 56 | 5.16 | 5.21 | 4.76 | 4.99 | 0.430 | 0.87 |
| Days 57 to 84 | 6.88 | 6.08 | 6.77 | 5.69 | 0.797 | 0.67 |
| Days 85 to 106 | 12.63 | 11.29 | 10.14 | 9.01 | 3.432 | 0.64 |
| Overall | 4.92 | 5.08 | 5.18 | 4.74 | 0.226 | 0.37 |
| G:F | ||||||
| Days 1 to 28 | 0.212 | 0.203 | 0.191 | 0.209 | 0.0106 | 0.43 |
| Days 29 to 56 | 0.205 | 0.212 | 0.213 | 0.208 | 0.0094 | 0.93 |
| Days 57 to 84 | 0.169 | 0.169 | 0.159 | 0.182 | 0.0122 | 0.53 |
| Days 85 to 106 | 0.127 | 0.109 | 0.108 | 0.108 | 0.0145 | 0.62 |
| Overall | 0.208 | 0.201 | 0.197 | 0.206 | 0.0060 | 0.52 |
| Rib fat, mm | ||||||
| Initial (d 1) | 6.1 | 5.7 | 5.9 | 6.2 | 0.55 | 0.49 |
| Middle (d 57) | 9.2 | 10.2 | 9.8 | 9.7 | 0.74 | 0.78 |
| Final (d 99) | 12.2 | 12.6 | 12.6 | 12.7 | 0.87 | 0.92 |
| Rump fat, mm | ||||||
| Initial (d 1) | 7.8 | 7.6 | 7.7 | 7.8 | 0.54 | 0.54 |
| Middle (d 57) | 10.9 | 11.9 | 11.7 | 12.0 | 0.73 | 0.52 |
| Final (d 99) | 14.5 | 15.9 | 15.1 | 16.3 | 0.91 | 0.37 |
F:G = Feed to gain ratio; G:F = Gain to feed ratio.
1 Treatments: CON: control (not supplemented); ACD: 0.5% of benzoic acid dietary inclusion on a DM basis (DSM Nutritional Products, Parsippany, NJ, USA); YST: 3 g/head per day of Saccharomyces cerevisiae (Vistacell, AB Vista, Marlborough, UK); AY: 0.5% of benzoic acid dietary inclusion on a DM basis (DSM Nutritional Products) and 3 g/head per day of Saccharomyces cerevisiae (Vistacell, AB Vista).
Circulating BHBA, cholesterol, glucose, haptoglobin, NEFA, total protein, albumin, globulin, urea, and SAA concentrations along with albumin to globulin ratio (A:G) were not different (P ≥ 0.09; Table 4) among treatment groups. Circulating AST concentrations were greater (P = 0.009) for YST vs. ACD and AY steers and not different from the CON steers.
Table 4.
Circulating blood metabolite concentrations for steers fed a high-grain finishing diet with no supplementation, benzoic acid, yeast, or both benzoic acid and yeast
| Item | Treatment1 | SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | ACD | YST | AY | |||
| AST, U/L | 101ab | 84b | 126a | 100b | 9.3 | 0.009 |
| Haptoglobin, g/L | 0.09 | 0.10 | 0.11 | 0.13 | 0.02 | 0.58 |
| Serum amyloid-A, µg/mL | 37.1 | 61.0 | 53.3 | 63.5 | 13.79 | 0.41 |
| BHBA, µmol/L | 451 | 554 | 504 | 529 | 41.5 | 0.09 |
| Cholesterol, mmol/L | 2.47 | 3.00 | 2.49 | 3.17 | 0.308 | 0.17 |
| Glucose, mmol/L | 3.43 | 3.69 | 3.68 | 3.81 | 0.142 | 0.21 |
| NEFA, mmol/L | 0.10 | 0.15 | 0.11 | 0.10 | 0.021 | 0.16 |
| Total protein, g/L | 70.8 | 73.6 | 70.5 | 71.5 | 2.01 | 0.44 |
| Albumin, g/L | 35.1 | 36.6 | 34.7 | 35.6 | 0.71 | 0.10 |
| Globulin, g/L | 35.6 | 37.0 | 35.8 | 35.8 | 1.70 | 0.95 |
| A:G | 1.02 | 1.00 | 0.99 | 1.01 | 0.049 | 0.96 |
| Urea, mmol/L | 5.67 | 5.65 | 6.08 | 5.99 | 0.389 | 0.62 |
AST = Aspartate aminotransferase; BHBA = ß-hydroxybutyrate; NEFA = Non-esterifies fatty acids; A:G = Albumin to globulin ratio.
1Treatments: CON: control (not supplemented); ACD: 0.5% of benzoic acid dietary inclusion on a DM basis (DSM Nutritional Products, Parsippany, NJ, USA); YST: 3 g/head per day of Saccharomyces cerevisiae (Vistacell, AB Vista, Marlborough, UK); AY: 0.5% of benzoic acid dietary inclusion on a DM basis (DSM Nutritional Products) and 3 g/head per day of Saccharomyces cerevisiae (Vistacell, AB Vista).
a,bValues within a row with different superscripts differ significantly at P ≤ 0.05.
Hot carcass weight, dressing percentage, longissimus muscle area, fat depth, and marbling score did not differ (P ≥ 0.33; Table 5) among treatment groups. Due to the small number of animal on this experiment, statistical comparisons were not conducted on carcass grading or liver abscess scores. However, numerical frequencies were as follows: YST steers had the most carcasses grading AAA (USDA Choice equivalence) (93%), whereas AY steers had the most AA graded carcasses (USDA Select equivalence) (36%) and the only carcass grading Prime in the experiment. As for yield grade, no steers were graded as yield grade 1; however, CON steers had the most carcasses of yield grade 2 (20%), and AY steers had the highest number of yield grade 5 (27%) carcasses. The treatment group with the most abscess free (O) livers was ACD (79%), whereas YST steers had the highest number of small abscessed (A: 20%) and severely abscessed (A+: 20%) livers.
Table 5.
Carcass characteristics for steers fed a high-grain finishing diet with no supplementation, benzoic acid, yeast, or both benzoic acid and yeast
| Item1 | Treatment2 | SEM | P-value | |||
|---|---|---|---|---|---|---|
| CON | ACD | YST | AY | |||
| HCW, kg | 389 | 396 | 385 | 383 | 6.8 | 0.75 |
| Dressing percentage, % | 58.3 | 55.8 | 56.0 | 56.5 | 1.51 | 0.33 |
| LM area, cm2 | 94.8 | 94.0 | 93.1 | 89.4 | 3.53 | 0.68 |
| Backfat thickness, mm | 21.9 | 22.6 | 21.1 | 22.2 | 2.86 | 0.97 |
| Marbling score | 475 | 475 | 424 | 436 | 53.5 | 0.72 |
| Yield grade | 3.90 | 3.99 | 3.50 | 4.13 | 0.507 | 0.69 |
| Quality grade | ||||||
| A | 0 | 0 | 0 | 0 | – | – |
| AA | 5 | 3 | 1 | 4 | – | – |
| AAA | 10 | 11 | 14 | 6 | – | – |
| Prime | 0 | 0 | 0 | 1 | – | – |
| Liver score | ||||||
| O | 11 | 11 | 9 | 8 | – | – |
| A | 2 | 1 | 3 | 2 | – | – |
| A+ | 2 | 2 | 3 | 1 | – | – |
HCW = Hot carcass weight; LM = Longissimus dorsi muscle.
1 Treatments: CON: control (not supplemented); ACD: 0.5% of benzoic acid dietary inclusion on a DM basis (DSM Nutritional Products, Parsippany, NJ, USA); YST: 3 g/head per day of Saccharomyces cerevisiae (Vistacell, AB Vista, Marlborough, UK); AY: 0.5% of benzoic acid dietary inclusion on a DM basis (DSM Nutritional Products) and 3 g/head per day of Saccharomyces cerevisiae (Vistacell, AB Vista).
2 Dressing percentage was calculated with a 4% pencil shrink for ending live weight. LM = longissimus muscle. Marbling score (devoid = 200 to 299, traces = 300 to 399, slight = 400 to 499, small = 500 to 599, modest = 600 to 699). Canadian Beef Grading Agency (CBGA) standards were used to calculate yield grade (YG1 = 1, YG2 = 2, YG3 = 3, YG4 = 4, YG5 = 5) and quality grade (A = 1, AA = 2, AAA = 3, Prime = 4). Liver score according to the Elanco scoring system; O as abscess free, A as a liver with one or two small active abscesses (less than one inch in diameter), and A+ as a liver with one or more large abscesses.
DISCUSSION
This study investigated the effects of supplementing benzoic acid, active dry yeast, or a combination of benzoic acid and active dry yeast on feedlot performance, feeding behavior, and carcass characteristics of beef steers finished on a high-grain diet.
Dry matter intake was consistently numerically greater for ACD steers, whereas DMI for AY steers was not different compared to all treatment groups, suggesting that benzoic acid did not negatively impact feed intake. While a similar response was observed for day to day variation in DMI, this did not affect overall feed conversion among treatment groups. There is limited research into the impact of benzoic acid supplementation on beef cattle performance. While monensin is known to suppress DMI for feedlot cattle (Wood et al., 2016), Wang et al. (2020) reported no differences in DMI between steers fed a negative control (no monensin or tylosin supplementation), positive control (monensin and tylosin supplementation), or benzoic acid diet (no monensin or tylosin supplementation) at the same benzoic acid supplementation level as the present study. While similar DMI among treatment groups was expected in the present study, the addition of benzoic acid to the basal diet containing monensin increased DMI versus cattle fed a control diet containing monensin. These results suggest that other mechanisms may be involved in the regulation of feed intake when benzoic acid is included in a monensin-supplemented finishing diet. Eating rate (g DM consumed/min) was elevated in ACD steers when compared with CON steers. This, combined with no observed differences for all other feeding behavior parameters when compared with CON steers, could explain the increased overall DMI and greater variation in day to day DMI. When diet composition and structure are the same, eating rate has proven to be an indirect measure of preference in finding feed that can be used for motivation of cattle (Spörndly and Asberg, 2006). Although the steers in the present study only had access to one diet, the increased eating rate for ACD steers may be attributed to improved diet palatability. Previous work with active dry yeast indicated a decrease in DMI, feeder visits, and eating rate, which may suggest lower palatability with active dry yeast (Williams, 2019). This may partially explain the increased DMI for ACD steers when compared with YST steers, and the numerically lower DMI for AY steers.
Ruminal acidosis generally occurs when there is erratic feeding behavior. More specifically, there is an increased risk for ruminal acidosis challenges with larger meal sizes and daily variation in feeding time or meals (Owens et al., 1998). Meal size was not different in the present study, and therefore not likely a contributing factor to the observed difference in ruminal pH. However, variation in DMI was greater for ACD steers compared with CON and YST steers. Ruminal pH was lowest for AY steers (5.66), with numerically intermediate pH values for CON and YST steers (5.84 and 6.00, respectively), and ACD steers (6.16) had the greatest ruminal pH. Monensin was provided in all diets to help minimize the risk of poor rumen health caused by a diet containing readily fermentable high moisture corn approaching 80% of total DM with a large number of fine particles. Since ruminal pH was only measured on a single spot sample, it would be beneficial to more thoroughly investigate the impact on ruminal pH over a longer duration in order to verify these results. Similarly, further investigations on the impact on rumen fermentation, volatile fatty acid concentration, and microbiome changes are also warranted.
Although there is limited research on ruminants, monogastric research on benzoic acid supplementation indicates varied responses for intake and performance when benzoic acid is included in the diet. Previous research has found that benzoic acid supplementation improved BW gains in weaned pigs and broiler chickens, along with positively impacting feed conversion (Giannenas et al., 2014; Chen et al., 2017). The majority of monogastric research on benzoic acid supplementation has found no impact on performance traits, as was similarly observed in the present study for FCR and fat deposition (Hassan, 2016). One experiment in broiler chickens found that a 0.2% inclusion of benzoic acid depressed growth (Józefiak et al., 2010). Given limited literature evaluating benzoic acid supplementation for ruminants, further research is warranted to understand these observed differences in feed intake and feeding rate.
The addition of yeast alone did not impact any feed intake, feeding behavior, or animal performance measurements. This agrees with Carrasco et al. (2016) but does not align with Geng et al. (2016). There has been extensive variation in animal response to yeast supplementation in previous feedlot cattle research. This is primarily due to the extensive study-to-study variation in experimental design, dose level, yeast varieties, and time on feed (Jiao et al., 2018). Since monensin was included in all diets in this present study, perhaps no additional performance or carcass benefits can be observed when adding yeast to diets containing monensin. Further research evaluating the mechanistic action and ruminal impacts for cattle fed yeast supplemented diets with/without monensin is warranted.
Limited treatment differences in animal performance in this study and the lack of blood metabolite impacts seen in previous research (Bühler et al., 2010; Carrasco et al., 2016) resulted in the expectation that blood metabolite concentrations would be similar among treatment groups in the present study. However, circulating aspartate aminotransferase (AST) concentrations were elevated for the YST group. In the past, the addition of active dry S. cerevisiae to diets reduced the occurrence of abscessed livers in steers (Ran et al., 2018; Ran et al., 2018); however, this was not observed in the present study. Additionally, liver enzyme profiles are often regarded as a poor indicator for the occurrence of liver abscesses in a clinical setting (Abdelaal et al., 2014). The number of animals in the present study was too small to conclude the efficacy of benzoic acid in reducing liver abscesses, and further research is warranted.
Since there were no treatment differences in ADG, feed conversion, or fat deposition throughout the study, similarities among treatment groups for carcass traits were expected. Research using benzoic acid as a supplement in diets for broiler chickens found an increase in final body weights when benzoic acid was supplemented at 0.6% and 1.2% of the diet, whereas lower final body weights were found in birds supplemented at 1.8% and 2.4% when compared with the control group (Amaechi and Anueyiagu, 2012). These authors attributed the effects of benzoic acid on final body weights to differences in animal feed intake and feed conversion among treatment groups. In contrast, Hassan (2016) found that benzoic acid supplementation at 0.4% and 0.8% in broiler diets had no impact on carcass weight or dressing percentage. A major difference in these two experiments is that the energy density of the diet in the Hassan (2016) experiment was much greater (~3200 kcal/kg) than (~2800 kcal/kg) in Amaechi and Anueyiagu (2012). Perhaps the mode of action for benzoic acid in the host is dependent on energy availability.
As for live yeast supplementation, similar results to the present experiment have been reported in the past (Magrin et al., 2018), where yeast in the diet did not impact carcass parameters. Magrin et al. (2018) reported that although DMI was increased for yeast supplemented steers, this did not affect feed conversion ratio or ADG, with no impact on carcass characteristics. Others have found that yeast supplementation increased HCW when feeding active dry yeast (Geng et al., 2016) or decreased HCW when a yeast fermentation product was fed (Swyers et al., 2014). These differences in HCW outcomes could be attributed to differences in yeast additive type, where each type may have different efficacy levels and mechanistic actions. Without proven mechanisms of action for yeast supplementation, it is difficult to pinpoint the exact differences between yeast products and their differences in animal response. In the present study, neither active dry yeast, benzoic acid, nor the combination of benzoic acid and active dry yeast had any impacts on carcass traits.
To conclude—results from this experiment suggest that steers fed a high-grain finishing diet supplemented with benzoic acid, active dry yeast (Saccharomyces cerevisiae), or both benzoic acid and active dry yeast performed the same as the control group (no benzoic acid or active dry yeast supplementation) and had similar carcass characteristics. Steers fed a diet with benzoic acid had greater DMI and a faster eating rate when compared with CON and YST steers. Providing yeast in the diet did not impact feeding behaviour and significantly increased circulating serum aminotransferase concentrations. This suggests that supplementation did not impact steer performance, carcass characteristics, or have any negative impacts on feed intake. Additionally, further research is needed to better assess impacts on ruminal pH and gut health in beef finishing steers, as “spot sample” rumen pH measurements in the present study may suggest positive improvement in rumen pH with BA supplementation.
IMPLICATIONS
This preliminary research on benzoic acid in high-grain finishing diets may indicate potential as an antibiotic alternative for feedlot cattle. Results also show that the supplementation of benzoic acid, active live Saccharomyces cerevisiae, or in a combination in finisher diets with monensin did not impact carcass characteristics or performance, but BA supplementation increased DMI relative to control and yeast, suggesting no negative impacts on feed intake.
ACKNOWLEDGMENTS
This work was funded with full or partial financial support from the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA), Beef Farmers of Ontario, and the Weston Seeding Food Innovation Program (George Weston Limited). In-kind support was provided from DSM Nutritional Products and AB Vista. The authors would like to thank Timothy Caldwell, Amber Zupan, Tysson Amidon, and staff at the Elora Beef Research Center and the Meat Lab staff for assistance during the experiment.
LITERATURE CITED
- Abdelaal, A. M., Gouda S. M., and Tharwat M., . 2014. Clinico-biochemical, ultrasonographic and pathological findings of hepatic abscess in feedlot cattle and buffaloes. Vet. World, 7:5. [Google Scholar]
- Amaechi, N., and Anueyiagu C. F., . 2012. The effect of dietary benzoic acid supplementation on growth performance and intestinal wall morphology of broilers. Online J. Anim. Feed Res., 2:401–404. [Google Scholar]
- AOAC International. 2007. Official methods of analysis of AOAC international, 18th edition. AOAC Int., Gaithersburg, MD. [Google Scholar]
- Bühler, K., Liesegang A., Bucher B., Wenk C., and Broz J.. . 2010. Influence of benzoic acid and phytase in low-phosphorus diets on bone characteristics in growing-finishing pigs. J. Anim. Sci. 88:3363–3371. doi: 10.2527/jas.2009-1940. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care. 1993. Guide to the use of experimental animals. CCAC, Ottawa, ON. [Google Scholar]
- Carrasco, C., Medel P., Fuentetaja A., Ranilla M. J., and Carro M. D.. . 2016. Effect of disodium/calcium malate or supplementation on growth performance, carcass quality, ruminal fermentation products, and blood metabolites of heifers. J. Anim. Sci. 94:4315–4325. doi: 10.2527/jas.2016-0616. [DOI] [PubMed] [Google Scholar]
- Castillo, C., Benedito J. L., Mendez J., Pereira V., Lopez-Alonso M., Miranda M., and Hernandez J.. . 2004. Organic acids as a substitute for monensin in diets for beef cattle. Animal Feed Science and Technology, 115:101–116. doi: 10.1016/j.anifeedsci.2004.02.001 [DOI] [Google Scholar]
- Castillo-Lopez, E., Wiese B. I., Hendrick S., McKinnon J. J., McAllister T. A., Beauchemin K. A., and Penner G. B.. . 2014. Incidence, prevalence, severity, and risk factors for ruminal acidosis in feedlot steers during backgrounding, diet transition, and finishing. J. Anim. Sci. 92:3053–3063. doi: 10.2527/jas.2014-7599. [DOI] [PubMed] [Google Scholar]
- Chen, J. L., Zheng P., Zhang C., Yu B., He J., Yu J., Luo J. Q., Mao X. B., Huang Z. Q., and Chen D. W.. . 2017. Benzoic acid beneficially affects growth performance of weaned pigs which was associated with changes in gut bacterial populations, morphology indices and growth factor gene expression. J. Anim. Physiol. Anim. Nutr. (Berl). 101:1137–1146. doi: 10.1111/jpn.12627. [DOI] [PubMed] [Google Scholar]
- Elanco. 2016. Elanco’s liver check service. Available from: https://assets.ctfassets.net/fistk1blxig0/2XZ3TB1cEMGsMuUGAIOAU2/2b87e28193d1e7f7ca6634537265ed24/USBBUTPX0081_1__-_Liver_Abscess_Service_Detailer.pdf
- Forbes, J. M., 2007. Voluntary food intake and diet selection in farm animals, 2nd edition. CABI, Wallingford, UK; Cambridge, MA. [Google Scholar]
- Geng, C. Y., Ren L. P., Zhou Z. M., Chang Y., and Meng Q.-X.. . 2016. Comparison of active dry yeast (Saccharomyces cerevisiae) and yeast culture for growth performance, carcass traits, meat quality and blood indexes in finishing bulls: Comparison of yeast preparations. Anim. Sci. J. 87:982–988. doi: 10.1111/asj.12522 [DOI] [PubMed] [Google Scholar]
- Giannenas, I., Papaneophytou C., Tsalie E., Triantafillou E., Tontis D., and Kontopidis G., . 2014. The effects of benzoic acid and essential oil compounds in combination with protease on the performance of chickens. J. Anim. Feed Sci. 23:73–81. doi: 10.22358/jafs/65719/2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan, R. I. M. 2016. Effect of feeding benzoic acid on performance of broiler chickens. J. Adv. Vet. Res. 6:118–122. [Google Scholar]
- Hobson, P. N., and Stewart C. S., . 1997. The rumen microbial ecosystem, 2nd edition. Blackie Acedemic & Professional. Boundary Row, London. [Google Scholar]
- Jiao, P. X., He Z. X., Ding S., Walker N. D., Cong Y. Y., Liu F. Z., Beauchemin K. A., and Yang W. Z., . 2018. Impact of strain and dose of live yeast and yeast derivatives on in vitro ruminal fermentation of a high-grain diet at two pH levels. J. Plaizier, editor. Can. J. Anim. Sci. 98:477–487. doi: 10.1139/cjas-2017-0079 [DOI] [Google Scholar]
- Józefiak, D., Kaczmarek S., and Rutkowski A.. . 2010. The effects of benzoic acid supplementation on the performance of broiler chickens. J. Anim. Physiol. Anim. Nutr. (Berl). 94:29–34. doi: 10.1111/j.1439-0396.2008.00875.x. [DOI] [PubMed] [Google Scholar]
- Magrin, L., Gottardo F., Fiore E., Gianesella M., Martin B., Chevaux E., and Cozzi G., . 2018. Use of a live yeast strain of Saccharomyces cerevisiae in a high-concentrate diet fed to finishing Charolais bulls: effects on growth, slaughter performance, behavior, and rumen environment. Anim. Feed Sci. Tech. 241:84–93. doi: 10.1016/j.anifeedsci.2018.04.021 [DOI] [Google Scholar]
- Marden, J. P., Julien C., Monteils V., Auclair E., Moncoulon R., and Bayourthe C.. . 2008. How does live yeast differ from sodium bicarbonate to stabilize ruminal pH in high-yielding dairy cows? J. Dairy Sci. 91:3528–3535. doi: 10.3168/jds.2007-0889. [DOI] [PubMed] [Google Scholar]
- Montanholi, Y. R., Swanson K. C., Palme R., Schenkel F. S., McBride B. W., Lu D., and Miller S. P.. . 2010. Assessing feed efficiency in beef steers through feeding behavior, infrared thermography and glucocorticoids. Animal 4:692–701. doi: 10.1017/S1751731109991522. [DOI] [PubMed] [Google Scholar]
- Nagaraja, T. G., and Titgemeyer E. C.. . 2007. Ruminal acidosis in beef cattle: the current microbiological and nutritional outlook. J. Dairy Sci. 90(Suppl 1):E17–E38. doi: 10.3168/jds.2006-478. [DOI] [PubMed] [Google Scholar]
- NRC. 2001. Nutrient requirements of dairy cattle. National Academies Press, 2001. [PubMed] [Google Scholar]
- Owens, F. N., Secrist D. S., Hill W. J., and Gill D. R.. . 1998. Acidosis in cattle: a review. J. Anim. Sci. 76:275–286. doi: 10.2527/1998.761275x. [DOI] [PubMed] [Google Scholar]
- Ran, T., Shen Y. Z., Saleem A. M., AlZahal O., Beauchemin K. A., and Yang W. Z.. . 2018. Using ruminally protected and nonprotected active dried yeast as alternatives to antibiotics in finishing beef steers: growth performance, carcass traits, blood metabolites, and fecal Escherichia coli. J. Anim. Sci. 96:4385–4397. doi: 10.1093/jas/sky272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spörndly, E., and Asberg T.. . 2006. Eating rate and preference of different concentrate components for cattle. J. Dairy Sci. 89:2188–2199. doi: 10.3168/jds.S0022-0302(06)72289-5. [DOI] [PubMed] [Google Scholar]
- Swyers, K. L., Wagner J. J., Dorton K. L., and Archibeque S. L.. . 2014. Evaluation of Saccharomyces cerevisiae fermentation product as an alternative to monensin on growth performance, cost of gain, and carcass characteristics of heavy-weight yearling beef steers. J. Anim. Sci. 92:2538–2545. doi: 10.2527/jas.2013-7559. [DOI] [PubMed] [Google Scholar]
- Wang, L. M., Mandell I. B., and Bohrer B. M., . 2020. Effects of feeding essential oils and benzoic acid to replace antibiotics on finishing beef cattle growth, carcass characteristics, and sensory attributes. Appl. Anim. Sci. 36:145–156. doi: 10.15232/aas.2019-01908 [DOI] [Google Scholar]
- Williams, M. S., 2019. Natural feed additives for feedlot beef cattle: Impact of a fibrolytic enzyme additive on digestibility and performance in the grower phase, and Saccharomyces cerevisiae impacts on rumen health and performance in the finishing phase. MSc thesis, University of Guelph, Guelph, Canada. [Google Scholar]
- Wood, K. M., Salim H., McEwen P. L., Mandell I. B., Miller S. P., and Swanson K. C., . 2011. The effect of corn or sorghum dried distillers grains plus solubles on growth performance and carcass characteristics of crossbred beef steers. Anim. Feed Sci. Tech. 165:23–30. doi: 10.1016/j.anifeedsci.2011.02.011 [DOI] [Google Scholar]
- Wood, K. M., Pinto A. C., Millen D. D., Kanafany Guzman R., and Penner G. B.. . 2016. The effect of monensin concentration on dry matter intake, ruminal fermentation, short-chain fatty acid absorption, total tract digestibility, and total gastrointestinal barrier function in beef heifers. J. Anim. Sci. 94:2471–2478. doi: 10.2527/jas.2016-0356. [DOI] [PubMed] [Google Scholar]