Abstract
Background
In patients with locally recurrent brain metastases (LRBMs), the role of (repeat) craniotomy is controversial. This study aimed to analyze long-term oncological outcomes in this heterogeneous population.
Methods
Craniotomies for LRBM were identified from a tertiary neuro-oncological institution. First, we assessed overall survival (OS) and intracranial control (ICC) stratified by molecular profile, prognostic indices, and multimodality treatment. Second, we compared LRBMs to propensity score-matched patients who underwent craniotomy for newly diagnosed brain metastases (NDBM).
Results
Across 180 patients, median survival after LRBM resection was 13.8 months and varied by molecular profile, with >24 months survival in ALK/EGFR+ lung adenocarcinoma and HER2+ breast cancer. Furthermore, 102 patients (56.7%) experienced intracranial recurrence; median time to recurrence was 5.6 months. Compared to NDBMs (n = 898), LRBM patients were younger, more likely to harbor a targetable mutation and less likely to receive adjuvant radiation (P < 0.05). After 1:3 propensity matching stratified by molecular profile, LRBM patients generally experienced shorter OS (hazard ratio 1.67 and 1.36 for patients with or without a mutation, P < 0.05) but similar ICC (hazard ratio 1.11 in both groups, P > 0.20) compared to NDBM patients with similar baseline. Results across specific molecular subgroups suggested comparable effect directions of varying sizes.
Conclusions
In our data, patients with LRBMs undergoing craniotomy comprised a subgroup of brain metastasis patients with relatively favorable clinical characteristics and good survival outcomes. Recurrent status predicted shorter OS but did not impact ICC. Craniotomy could be considered in selected, prognostically favorable patients.
Keywords: craniotomy, local recurrence, molecular markers, propensity score matching, recurrent brain metastasis
Key Points.
In 180 LRBMs, median survival after resection was 13.8 months; intracranial recurrence was 56.7%.
Outcomes varied by molecular profile, prognostic factors, and treatment.
LRBMs had worse OS but similar intracranial control to matched newly diagnosed BMs.
Importance of the Study.
Optimal treatment of locally recurrent brain metastases (LRBMs) after previous surgery or radiation remains an important gap in clinical knowledge. The role of (repeat) craniotomy in this context is especially controversial. Previous investigations into this question have been limited in sample size and have predated important advances in modern oncology, limiting our ability to make patient-tailored recommendations for this heterogeneous population. This study presents outcomes in a large cohort of resected LRBM patients with a special focus on variation in survival and intracranial control by molecular profile. We furthermore explore outcomes in relation to prognostic factors and different multidisciplinary treatment scenarios. Finally, we compare LRBMs against propensity-matched newly diagnosed brain metastasis patients to elucidate what role recurrent vs newly diagnosed status should play in preoperative decision-making. These results could help guide physicians’ and patients’ expectations when considering craniotomy for LRBM.
Brain metastases (BMs) are common intracranial tumors that are usually treated with a combination of surgery, stereotactic radiosurgery (SRS), whole-brain radiotherapy (WBRT), and/or systemic therapy.1 Despite advances in local treatment, BMs are reported to recur in up to 60% of patients after surgery and adjuvant SRS/WBRT.2 While surgical management of newly diagnosed brain metastases (NDBMs) is well-established by level I evidence,1 there is a lack of knowledge on optimal management strategies in the locally recurrent brain metastasis (LRBM) setting, particularly with respect to the role of (re-)resection. This has led to practice variation and a lack of resolute recommendations in current guidelines.1,3
While some studies have reported outcomes after craniotomy for recurrent BM,4–14 these have been relatively modest in sample size and predate important advances in BM treatment, such as the discovery of therapeutically targetable molecular markers in lung cancer or melanoma.15–19 The immense heterogeneity of recurrent BM patients both in terms of molecular profile and multimodal treatment approach has therefore limited meaningful interpretation thus far.
To address this gap in knowledge, the purpose of the present study was to investigate long-term oncological outcomes after craniotomy for LRBM. First, we analyze overall survival (OS) and intracranial control (ICC) stratified by molecular markers, prognostic indices, and multimodality treatment. Second, we perform propensity score-matched analysis to compare LRBM patients against NDBM patients with a similar baseline.
Materials and Methods
Data Collection and Definitions
Under Institutional Review Board approval (Partner’s IRB #2015P002352), data were gathered from a retrospectively collected departmental database at the Brigham and Women’s Hospital which contains consecutive cases of craniotomy for BM between 2007 and 2017. For the purpose of this study, patients were included if they: 1) had BMs of a primary solid tumor originating outside the central nervous system; 2) received intracranial treatment (resection, stereotactic radiosurgery (SRS), whole-brain radiotherapy (WBRT), or a combination) for NDBMs; 3) experienced local recurrence; and 4) underwent craniotomy for this recurrence. Local recurrence was defined as radiological recurrence in or adjacent to a previous resection cavity or treatment site. In case of doubt, the locality was determined by comparing magnetic resonance imaging of initial BM presentation and recurrence. Radiological recurrences that were resected and contained only necrosis or radiation-induced treatment effect on histopathological examination were not excluded, because this distinction can only be made postoperatively. In order to be representative for preoperative LRBM populations, inclusion must be agnostic of postoperative criteria. This is consistent with previous studies investigating recurrent BM resections.7,9,11,13,14 In the case of multiple intracranial recurrences over a patient’s clinical course, the different recurrences were indicated as R1, R2, R3, … Rn, with R1 being the initial craniotomy for local recurrence.
Data were collected for the following variables: demographics, location, size and number of BMs, extracranial metastases, tumor-specific molecular markers (hormonal receptor (HR) and human epidermal growth factor receptor 2 (HER2), anaplastic lymphoma kinase (ALK), epidermal growth factor receptor (EGFR), neuroblastoma RAS (NRAS), and BRAF), local treatment modalities, administration of immunotherapy and targeted therapy, intracranial recurrence patterns and intervals, leptomeningeal recurrence, survival times, vital status, and neurologic death (i.e. death with progressive neurologic decline).20,21
Cases were grouped into distinct clinical scenarios based on the type of original treatment for NDBM, defined as follows: craniotomy after initial craniotomy (CAC), craniotomy after upfront SRS (CAS), and craniotomy after upfront WBRT (CAW). Patients treated initially with craniotomy and adjuvant SRS/WBRT were classified as CAC. No cases of neoadjuvant radiation followed by craniotomy were included; CAC and CAW were always performed after treatment failure with initial upfront radiation. Intracranial recurrence patterns were classified as local or distant; R1 is by definition a local recurrence, while R2–n could be either local or distant events.
Descriptive Statistics, Stratified Survival Analysis, and Prognostic Factors
Statistical analysis was performed on a per-patient basis unless otherwise indicated. Descriptive statistics were displayed using counts and percentages for categorical variables or medians and interquartile ranges (IQR) for continuous variables. Survival and time to recurrence in the cohort were presented from the time of craniotomy for LRBM unless otherwise indicated. Median survival times were estimated using the Kaplan-Meier method. Oncological outcomes (OS and ICC) were presented unstratified and stratified by molecular profile, clinical scenario (CAC, CAS, CAW), multimodal treatment, and expected survival as predicted by the diagnosis-specific graded prognostic assessment (ds-GPA). The ds-GPA is an established prognostic model built and validated in patients with NDBMs originating from breast cancer,22 non–small-cell lung cancer,16 melanoma,15 renal cell carcinoma,23 and other primaries.24,25
Prognostic factors for OS in LRBMs were furthermore explored using univariable and multivariable Cox proportional hazards models and the log-rank test. The multivariable model incorporated all potential prognostic factors identified in univariable regression as well as the known prognostic factors such as age, tumor origin, Karnofsky performance status (KPS), number of BMs, and presence of extracranial metastases. In a sensitivity analysis, the model was evaluated with and without the incorporation of ds-GPA score. A P-value of 0.05 was set as the boundary for statistical significance. Bonferroni correction was used to adjust for multiple hypothesis testing.
LRBM vs NDBM
We were interested in assessing how locally recurrent vs newly diagnosed BM status influenced outcomes after craniotomy for BM. We hypothesized that LRBMs had a worse prognosis than comparable NDBMs. To test this, we designed a retrospective cohort study comparing LRBMs (exposure group) with 1:3 propensity score-matched NDBMs (unexposed group). Matching was stratified by tumor type and molecular profile for subgroups with >10 LRBM patients and was carried out using the nearest neighbor method; propensity score was calculated based on age, sex, KPS, size and location of the BM, the number of BMs, the presence of extracranial metastases, and adjuvant radiation. Variable balance was assessed, and if a variable was not well balanced (P < 0.10) after matching, sensitivity analysis was performed adjusting for this variable. OS and ICC where then compared between the two groups using Cox proportional hazards models and visualized with Kaplan-Meier curves and forest plots. OS was measured until the time of death or loss to follow-up. ICC was measured until the time of intracranial recurrence, with death or loss to follow-up being censoring events. The log-rank test was used to determine statistical significance. Proportional hazards assumptions were tested using Schoenfeld residuals; assumptions were upheld (P > 0.05) unless otherwise mentioned.
Statistical Software
Data were analyzed in R version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria). The survival and survminer packages were used for survival analysis, and propensity score matching was performed using the MatchIt package.
Results
Baseline Characteristics
Out of 1069 craniotomies for BM identified in our institutional database, 197 were performed for LRBMs in 180 patients. Table 1 outlines baseline characteristics. The median age was 57 (IQR 51–66) years and 70 patients were male (38.9%). Common tumor origins were lung (36.7%), melanoma (21.7%), breast (20.0%), colorectal (3.9%), gynecological (3.9%), renal cell (2.8%) and other (11.1%) cancers. In seven (3.5%) craniotomies, histopathology revealed only necrosis or treatment effect; the remaining 190 (96.5%) resections yielded neoplastic tissue with or without treatment effect. The median interval from NDBM to LRBM was 9.9 (IQR: 5.4–18.6) months.
Table 1.
Baseline Characteristics of Locally Recurrent Brain Metastasis Patients
| Baseline characteristics | |
|---|---|
| N | 180 |
| Male (%) | 70 (38.9) |
| Age (median, IQR) | 57 (51–66) |
| Origin (%) | |
| Breast | 36 (20.0) |
| Colorectal | 7 (3.9) |
| Gynecological | 7 (3.9) |
| Lung | 66 (36.7) |
| Melanoma | 39 (21.7) |
| Other | 20 (11.1) |
| Renal | 5 (2.8) |
| Molecular target (%) | 41 (22.8) |
| Number of BMs (median, IQR) | 2 (1–3) |
| Extracranial metastases (%) | 60 (33.3) |
| Size (mean, standard deviation) | 3.00 (1.07) |
| Karnofsky Performance Status (median, IQR) | 80 (80–100) |
| Tumor locationa | |
| Frontal (%) | 58 (32.2) |
| Temporal (%) | 32 (17.8) |
| Parietal (%) | 50 (27.8) |
| Occipital (%) | 19 (10.6) |
| Cerebellar (%) | 31 (17.2) |
| Clinical scenario | |
| Craniotomy after craniotomy | 63 (35.0) |
| Craniotomy after upfront SRS | 88 (48.9) |
| Craniotomy after upfront WBRT | 29 (16.1) |
| Patients also undergoing craniotomy for second recurrence | 17 (9.4) |
Abbreviations: BMs, brain metastases; IQR, interquartile range; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
aPercentages for location may add up to >100% due to tumors extending across multiple lobes.
Overall Oncological Outcomes
Across 180 patients, the median follow-up was 33.6 months. Median survival measured from the time of craniotomy for LRBM was 13.8 (IQR: 6.7–27.4) months in this population; when measured from the point of initial BM diagnosis, it was 30.1 (IQR: 15.7–47.7) months. Three patients (1.7%) died within 30 days after craniotomy. One hundred two patients (56.7%) experienced some type of subsequent intracranial recurrence (R2 and further) after craniotomy; of these, 49 (27.2%) experienced initial recurrence in the resection cavity. In patients who had intracranial recurrence, the median time to recurrence was 5.6 (IQR: 3.4–10.1) months. At the end of follow-up, 125 patients (69.4%) were deceased, 74 of whom (41.1%) experienced neurologic death. Leptomeningeal disease occurred in seventeen (9.4%) patients. When excluding the seven patients who only showed treatment effect/necrosis on histopathology, median survival was 12.0 (IQR: 6.5–27.1) months, and intracranial recurrence occurred in 57.2% after a median of 5.5 (IQR: 3.4–8.8) months.
Molecular Markers
Table 2 presents outcomes related to survival and intracranial control stratified by tumor origin and molecular markers. Survival after craniotomy for R1 varied across tumor (sub)types, with the longest median survival times recorded in patients with HER2-positive breast cancer (23.1–24.2 months, n = 23) and ALK/EGFR mutated non–small-cell lung cancer (29.1 months, n = 6). Melanoma BMs had a shorter survival, even in the presence of a targetable mutation (medians of 10.7 and 10.3 months for BRAF/NRAS mutated and wildtype, respectively; n = 39). The percentage of BMs that experienced a second intracranial recurrence ranged from 40.0% (renal cell carcinoma, 2/5 cases) to 76.9% (HR-/HER2+ breast cancer, 10/13 cases). On the other hand, HER2+ breast cancer BMs had the longest time to recurrence (11.1 months for HR-/HER2+ and 17.4 months for HR+/HER2+). The worst intracranial control was seen in small-cell lung carcinoma, with 66.7% (6/9 cases) recurring after a median of only 2.5 months.
Table 2.
Postoperative Survival and Intracranial Control Stratified by Tumor Origin and Molecular Markers
| Survival by primary tumor type and molecular markers | ||||
|---|---|---|---|---|
| Tumor type | N | Median survival in months (IQR) | Intracranial recurrence; N (% of total) | Median time to recurrence in months (IQR) |
| All cases | 180 | 13.8 (6.7–27.4) | 102 (56.7) | 5.6 (3.4–10.1) |
| Lung | ||||
| Overall | 66 | 11.9 (6.4–27.1) | 40 (60.6) | 5.6 (3.3–7.9) |
| Adenocarcinoma (ALK/EGFR mutated) | 6 | 29.1 (29.1–29.1) | 3 (50.0) | 7.1 (6.9–7.2) |
| Adenocarcinoma (ALK/EGFR wildtype) | 42 | 14.7 (7.6–27.1) | 25 (59.5) | 7.1 (3.8–8.3) |
| NSCLC; non-adenocarcinoma | 9 | 7.6 (2.0–11.1) | 6 (66.7) | 4.8 (3.9–5.2) |
| SCLC | 9 | 7.0 (5.3–11.9) | 6 (66.7) | 2.5 (2.0–4.3) |
| Breast | ||||
| Overall | 36 | 23.1 (7.4–31.8) | 24 (66.7) | 8.1 (3.6–17.7) |
| Triple negative | 4 | 20.3 (7.4–31.8) | 3 (75.0) | 4.5 (3.2–18.2) |
| HR+/HER2- | 9 | 14.7 (9.5–58.7) | 6 (66.7) | 5.1 (4.1–16.2) |
| HR-/HER2+ | 13 | 24.2 (19.9–52.7) | 10 (76.9) | 11.1 (3.1–13.8) |
| HR+/HER2+ | 10 | 23.1 (7.0–27.4) | 5 (50.0) | 17.4 (3.6–18.5) |
| Melanoma | ||||
| Overall | 39 | 10.3 (5.4–27.1) | 23 (59.0) | 4.4 (3.0–7.3) |
| BRAF/NRAS mutated | 12 | 10.7 (6.9–42.3) | 7 (58.3) | 4.4 (4.3–5.8) |
| BRAF/NRAS wildtype | 27 | 10.3 (3.1–21.4) | 19 (63.3) | 4.9 (2.2–8.0) |
| Renal cell carcinoma | 5 | 7.5 (6.5–18.1) | 2 (40.0) | 8.3 (7.2–9.4) |
| Colorectal carcinoma | 7 | 10.4 (5.2–16.2) | 3 (42.9) | 3.4 (3.3–5.8) |
| Gynecological cancers | 7 | 6.7 (4.6–NR) | 3 (42.9) | 12.3 (8.5–16.8) |
| Other | 20 | 14.7 (6.9–31.4) | 7 (35.0) | 6.2 (4.2–9.3) |
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; HR, hormonal receptor; IQR, Interquartile range; NSCLC, non–small-cell lung cancer; NR, not reached; NRAS, neuroblastoma RAS; SCLC, small-cell lung cancer.
Comparison Against the ds-GPA
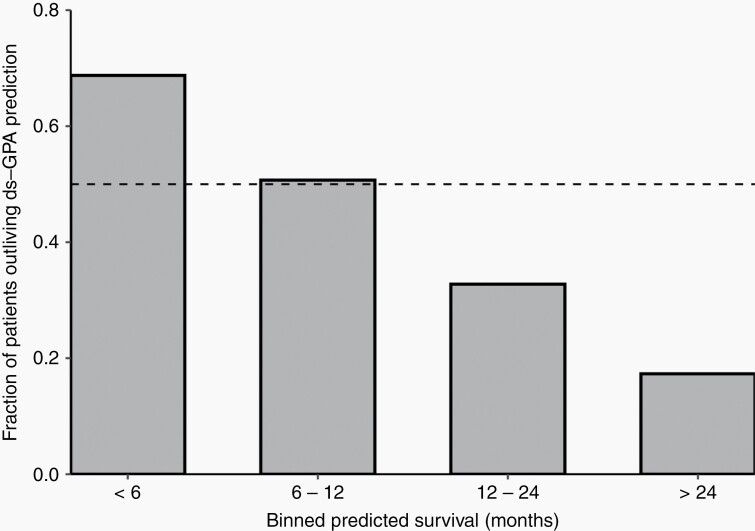
Next, we investigated how observed survival compared against predicted survival according to ds-GPA score. This comparison is outlined in Supplementary Table S1. The longest survival was observed in patients with lung adenocarcinoma or breast cancer; these patients on average lived >20 months if they had a GPA > 2). In most GPA bins, observed survival was similar to or longer than survival that would be predicted in NDBMs. Patients with shorter predicted survival tended to outlive their ds-GPA predictions, while patients with longer predicted survival did not, with a break-even point around twelve months. A visualization of this is presented in Figure 1.
Fig. 1.
Percentage of patients outliving their ds-GPA predicted survival. Legend: The diagnosis-specific graded prognostic assessment (ds-GPA) is a prognostic model constructed and validated on newly diagnosed brain metastasis patients. Despite this, a majority of locally recurrent brain metastasis patients with a predicted survival of <1 year outlived this prediction. In contrast, patients whose ds-GPA predicted longer survival generally did not live up to this prediction. The dashed line represents 50%.
Clinical Scenarios and Treatment-Stratified Outcomes
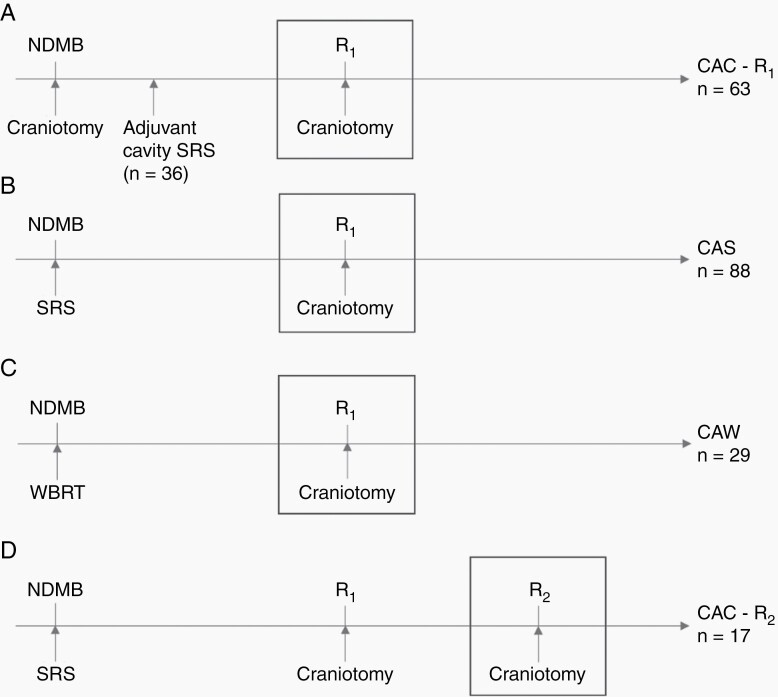
Craniotomy for recurrence comprised craniotomy after initial craniotomy (CAC; n = 63), craniotomy after upfront SRS (CAS; n = 88), or craniotomy after upfront WBRT (CAW; n = 29). In the CAC group, 36 of 63 patients had also received SRS to the cavity of their initial craniotomy. Seventeen patients received a second for R2, after initial SRS and craniotomy. These clinical scenarios are visualized in Figure 2.
Fig. 2.
Different clinical scenarios based on previous treatment at the site of local recurrence. Legend: A) Craniotomy after craniotomy for the first recurrence (CAC – R1); B) Craniotomy after upfront SRS (CAS); C) Craniotomy after upfront WBRT (CAW); D) Craniotomy after craniotomy at the second recurrence (CAC – R2). The boxes indicate the craniotomy of interest per clinical scenario, which corresponds to t = 0 in survival analysis. NDBM, newly diagnosed brain metastases; R1,2, First, second recurrence; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
Table 3 summarizes outcomes stratified by clinical scenario and multimodal treatment, including adjuvant radiation and postoperative immunotherapy and targeted therapy. Unadjusted median survival was broadly similar regardless of clinical scenario (range 11.6–14.7 months, overall log-rank P = 0.70), while longer survival was seen in patients undergoing gross total resection (14.7 vs. 11.6 months, P = 0.07), receiving adjuvant SRS as opposed to no radiation (16.1 vs. 8.2 months, P = 0.04), and receiving postoperative targeted therapy (24.2 vs. 11.7 months, P = 0.03) or immunotherapy (21.4 vs 11.6 months, P = 0.06). The incidence of subsequent intracranial recurrence varied based on clinical scenario; interestingly, this was lowest in CAC, where only 28/63 (44.4%) of patients developed a second recurrence (R2) after a median of 7.1 months. Conversely, if CAC was performed for R2, almost all (16/17; 94.1%) patients developed a third recurrence (R3), with a median time to recurrence of only 2.9 months. Supplementary Table S2 provides a detailed overview of multimodal treatment stratified by clinical scenario.
Table 3.
Postoperative Survival and Intracranial Control by Different Multimodality Treatments
| Outcomes by clinical scenario | |||||
|---|---|---|---|---|---|
| Clinical scenario | N | Median survival in months (IQR) | Intracranial recurrence; N (%) | Initial recurrence in resection cavity; N (%) | Median time to intracranial recurrence in months (IQR) |
| All craniotomies | 197 | 13.8 (6.5–27.1) | 117 (59.4) | 55 (27.9) | 5.0 (3.1–8.3) |
| Craniotomies for R1 | 180 | 13.8 (6.7–27.4) | 102 (56.7) | 49 (27.2) | 5.6 (3.4–10.1) |
| Craniotomy after craniotomy | 63 | 14.7 (7.5–27.1) | 28 (44.4) | 16 (25.4) | 7.1 (3.8–12.6) |
| Craniotomy after SRS | 88 | 12.0 (6.9–29.2) | 58 (65.9) | 30 (34.1) | 5.8 (3.1–8.2) |
| Craniotomy after WBRT | 29 | 11.6 (3.0–27.4) | 15 (51.7) | 3 (10.3) | 4.2 (3.4–6.3) |
| Craniotomies for R2a | 17 | 13.8 (5.4–18.3) | 16 (94.1) | 7 (41.2) | 2.9 (0.9–4.6) |
| Outcomes by multimodal treatment for LRBM | |||||
| Treatment | N | Median survival in months (IQR) | Intracranial recurrence; N (%) | Initial recurrence in resection cavity; N (%) | Median time to intracranial recurrence in months (IQR) |
| Surgery | 180 | ||||
| Gross total resection | 102 | 14.7 (7.4–32.2) | 55 (53.9) | 24 (23.5) | 5.7 (3.5–10.7) |
| Subtotal resection | 75 | 11.6 (5.7–24.2) | 47 (62.7) | 25 (33.3) | 5.3 (3.3–8.1) |
| Undetermined | 3 | 1.9 (0.1–29.9) | 0 | 0 | - |
| Adjuvant radiation | |||||
| SRS | 40 | 16.1 (9.6–42.0) | 31 (77.5) | 12 (30.0) | 4.8 (3.5–7.8) |
| WBRT | 35 | 11.1 (6.1–24.2) | 23 (65.7) | 15 (42.9) | 5.9 (2.9–7.9) |
| Other | 14 | 16.2 (10.4–27.1) | 8 (57.1) | 4 (28.6) | 9.5 (3.4–13.4) |
| None | 74 | 8.2 (3.2–27.4) | 30 (40.5) | 14 (18.9) | 5.2 (3.3–10.3) |
| Undetermined | 17 | 14.5 (10.7–31.8) | 10 (58.8) | 4 (23.5) | 7.8 (4.8–10.2) |
| Immunotherapy after craniotomy | |||||
| Yes | 25 | 21.4 (11.7–42.3) | 17 (68.0) | 9 (36.0) | 5.3 (3.6–8.0) |
| No | 155 | 11.6 (6.5–27.1) | 85 (54.8) | 40 (25.8) | 5.7 (3.4–10.6) |
| Targeted therapy after craniotomy | |||||
| Yes | 25 | 24.2 (11.6–52.7) | 19 (76.0) | 8 (32.0) | 10.6 (3.5–18.5) |
| No | 155 | 11.7 (6.4–27.1) | 83 (53.5) | 41 (26.4) | 5.6 (3.3–8.1) |
Abbreviations: IQR, interquartile range; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
aAfter previous SRS and craniotomy.
Prognostic Factors in LRBMs
Sex, BM size, KPS, and interval between the NDBM and the LRBM were associated with OS in univariate analysis (P < 0.05), while a trend was observed for primary tumor type and targetable mutations (P < 0.10). In multivariate analysis, presence of a targetable mutation (hazard ratio (HR) 0.50; 95% Confidence Interval (CI) 0.27–0.89; P = 0.02) and higher KPS (HR 0.77 per 10 points; 95% CI 0.67–0.88; P < 0.001) predicted longer OS; tumor type, size, sex and initial diagnosis-recurrence interval were no longer significant (P > 0.05, Supplementary Table S3). After Bonferroni adjustment for fifteen degrees of freedom, KPS remained prognostic for survival (P = 0.002) but not presence of a targetable mutation (P = 0.285; Supplementary Table S3). Ds-GPA also predicted survival in univariate analysis (HR 0.70 per point increase; 95% CI = 0.56–0.89; P = 0.003). When ds-GPA was implemented into the multivariate model, however, KPS (P = 0.004) but not ds-GPA (P = 0.27) predicted survival. Kaplan-Meier curves for survival by ds-GPA, KPS and the presence of targetable mutations are presented in Supplementary Figure S1.
LRBM vs NDBM
Supplementary Tables S4 and S5 compare LRBM patient’s baseline characteristics against NDBM controls (n = 898). Before propensity matching, LRBM patients were younger, more likely to harbor a molecular target, less likely to have received adjuvant SRS, and differed in the distribution of histology and tumor location (Supplementary Table S4). After Bonferroni correction for 19 degrees of freedom, only age (median 57 vs. 61 years, post-adjustment P = 0.038) and adjuvant SRS (24.2% vs. 40.3%, post-adjustment P < 0.019) were significantly different between LRBM and NDBM patients.
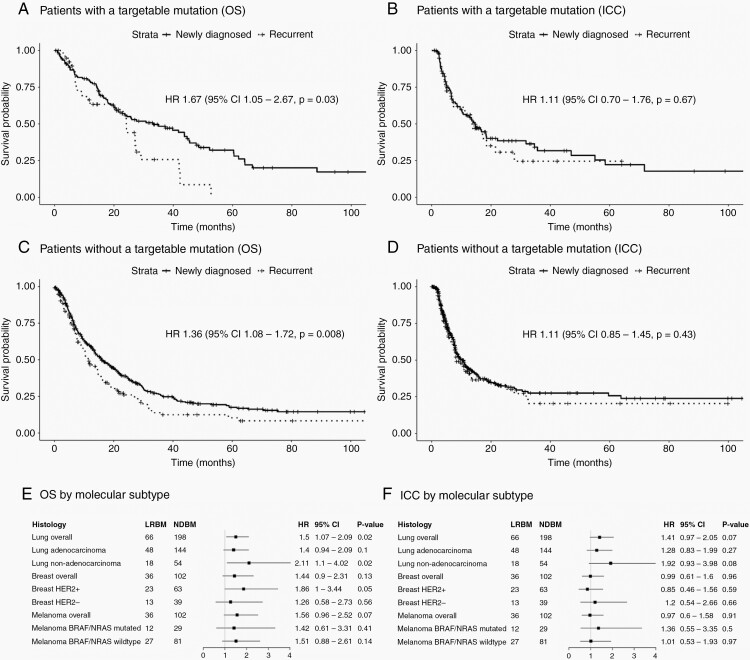
In the targetable mutation group, LRBM patients had smaller tumors (mean diameter 2.74 vs. 3.17 cm, P = 0.04) and were less likely to have undergone adjuvant SRS (24.4% vs. 42.8%, P = 0.05) compared to NDBM patients. After propensity matching, variables were well balanced. Figure 3 displays survival plots after propensity matching. LRBM patients had worse OS (HR 1.67, 95% CI 1.05–2.67, P = 0.03, Figure 3A) but similar ICC (HR 1.11, 95% CI 0.70–1.76, P = 0.67, Figure 3B) compared to NDBM patients.
Fig. 3.
Propensity score-matched comparison of newly diagnosed vs. locally recurrent brain metastases, stratified by tumor type and targetable mutations. Legend: Propensity score-matched comparison of overall survival (OS) and intracranial control (ICC) after craniotomy for newly diagnosed or locally recurrent brain metastasis (BM). Analyses are presented for subgroups of patients with (A & B) and without (C & D) a targetable mutation, as well as by molecular subtype (E & F). Subgroups were analyzed if data on > 10 LRBM cases was available.
In patients without a targetable mutation, those operated for LRBM vs. NDBM were younger (median 59 vs 62 years, P = 0.03), more likely to have undergone temporal lobe resection (20.1% vs 12.2%, P = 0.02), and less likely to have undergone adjuvant SRS (31.6 vs. 29.9%, P < 0.001). After matching variables were well balanced, LRBM patients experienced worse OS (HR 1.36, 95% CI 1.08–1.72, P = 0.008, Figure 3C) but similar ICC (HR 1.11, 95% CI 0.85–1.45, P = 0.43, Figure 3D) compared to NDBM patients.
Next, we stratified by specific molecular subgroups with > 10 LRBM patients (lung overall, adeno-, and non-adenocarcinoma, breast overall, HER+, and HER2-, melanoma overall, BRAF/NRAS+, and BRAF/NRAS-), results are presented in Figure 3E and F. The previous observation that recurrent status was associated with worse OS and similar ICC appeared to be broadly consistent across groups; for OS, effect sizes ranged from 1.26 to 2.11, although not all subgroups achieved statistical significance. There appeared to be a trend for shorter ICC after LRBM for lung cancer BMs (P = 0.07), but not for breast or melanoma subgroups (HR range 0.85–1.36, P > 0.20).
Discussion
This study aimed to investigate oncological outcomes after resection for LRBM. In the present study, median survival after LRBM resection was 13.8 months; 56.7% of patients experienced intracranial recurrence after a median of 5.6 months.
Stratified survival analysis suggested variation in outcomes based on tumor types and molecular markers. One interesting observation is that although our series contained patients with BMs from notoriously aggressive primary tumors, such as triple-negative breast cancer (n = 4) and colorectal carcinoma (n = 7), these still had relatively favorable survival outcomes (20 and 10 months, respectively). Breast cancer BMs, in general, had an excellent overall prognosis, with survival almost twice as long as lung cancer BMs (23.1 vs 11.9 months). In contrast, previous studies have found no association between primary tumor type and survival in recurrent BM6,9; moreover, tumor origin was no longer prognostic in our multivariate analysis. This could in part be due to the dominant role that KPS plays in the prognosis of BMs, in both our population and previous recurrent BM series.6,9,10,14 Additionally, the presence of targetable mutations is a factor of increasing interest in BM management17–19,26 and contributed to considerable survival variation in our data (HR 0.50, P = 0.02). While this result was no longer significant after Bonferroni adjustment, the presence of these molecular markers may still be a relevant factor which could be investigated in future studies. Because most other surgical series predate the era of molecular markers, this study is to the best of our knowledge the first to investigate the impact of targetable mutations in resected recurrent BMs.
Interestingly, LRBM patients tended to outlive their GPA-assigned prediction if predicted survival was under one year. This finding is similar to Kamp’s study8 of 32 patients with LRBMs treated with CAC that had a predicted median survival of 8.4 months by GPA but in actuality survived for a median of 12.9 months. One explanation is that if patients with LRBM and a poor prognosis were operated, additional factors not captured by the ds-GPA may have contributed to a relatively favorable fitness and surgical eligibility. On the other hand, the majority of patients with very long-predicted survival did live shorter than predicted. It could be that the divergence in survival times between NDBM and LRBM patients only becomes apparent in the long term. Thus, while the ds-GPA may predict survival in surgical LRBMs to a degree, it should be applied with caution in respect to potentially suboptimal calibration.
In this analysis, we included patients that were preoperatively thought to have LRBMs. Seven patients whose lesions postoperatively turned out to only contain necrotic tissue were not excluded; it could be argued that this leads to an impure representation of ‘true’ LRBM patients. When excluding these patients, median survival (12.0 vs 13.8 months) and intracranial control (57.2% vs 56.7%) were similar, albeit slightly less favorable. We chose this approach to keep in line with previous studies on resected recurrent BMs7,9,11,13,14; however, detailed outcomes of resection for radionecrosis after previous BM treatment are relatively understudied and could be addressed in future investigations.
Previous studies have described outcomes of CAC4–6,8,10,12,27 or CAS,7,9,11,13,14 with sample sizes ranging from 14 to 67 patients; mostly combining both local and distant recurrences into a single group. These studies report median survivals ranging from 7.5 to 15.0 months after craniotomy for recurrent BM, and survival outcomes seem to be similar after CAC or CAS.5,12 This is in line with the results of the present study, which found a median survival of 14.5 months after CAC, 12.0 months after CAS, and 11.6 months after CAW. Interestingly, these survival times are similar to survival times after craniotomy for NDBM as reported in large, recent studies.28–30 Possibly, patients who experience intracranial recurrence and are at that point still fit to undergo craniotomy comprise a select subpopulation with favorable baseline characteristics. Our data support this: when comparing unmatched cohorts, there was no difference in OS/ICC between NDBMs and LRBMs (all P-values > 0.05, data not shown). In propensity-matched comparison, LRBMs did show a tendency towards worse survival. Interestingly, ICC did not differ after craniotomy for NDBM or R1 LRBM. This suggests that the diminished survival in LRBM patients is mainly driven by systemic disease burden, and that uncontrollable intracranial disease is not the factor that determines survival after neurosurgery. Thus, for R1 scenarios, we believe recurrent status should not be a counterindication for resection in surgically eligible patients with an otherwise favorable baseline. On the other hand, craniotomy for R2 was nearly always followed by R3 (94.1%), often in the resection cavity (41.2%), after a median of only 2.9 months. This may represent a group where invasive procedures should be considered with great caution.
Limitations and Strengths
This study is limited primarily by its retrospective nature. The clinical course of resected recurrent BMs has not been studied prospectively before; future research could aim to address this knowledge gap. Our analysis of prognostic factors may have been underpowered to identify some factors with a more modest effect size. Similarly, the stratified propensity-score matching may have been underpowered for some molecular subgroups with small numbers. It should further be noted that this study was not designed to determine whether LRBMs should be resected or not in specific scenarios. Such study designs would require a comparable non-surgical control group. Rather, we aimed to charter what outcomes could be expected if craniotomy did take place. Tumor size was expressed using the largest diameter; ideally, the volumetric analysis would be a more accurate way to quantify size. This study aimed to look at those molecular markers that are currently clinically targetable and therefore did not include data on other potentially important markers, such as ROS1 for lung cancer. Last, determination of ALK/EGFR and NRAS/BRAF was not performed in BM tissue in all patients. Although it is known that there may be some discordance in these markers between primary tumors and BMs,31–33 it is not yet clinical practice to redetermine them in brain tissue. However, breast cancer subtype was always determined in the BM.
Within the context of this research question, our investigation also had several strengths. In comparison to existing literature, this cohort had a relatively large sample size, which allowed for stratified analysis. Adjustment for multiple hypothesis testing reduced the chance of type I error inflation. Finally, the use of propensity score matching enabled a meaningful comparison between LRBMs and NDBMs, controlling for prominent confounders such as performance status.
Conclusions
Patients with resected locally recurrent brain metastasis comprised a select population with favorable clinical characteristics whose outcomes were similar to those historically reported for newly diagnosed brain metastasis patients. Outcomes varied by molecular profile and other prognostic factors, in particular, KPS; however, these predictors should be validated in larger studies. In a propensity score-matched analysis, LRBM patients had a generally worse OS after craniotomy when compared to NDBM patients, but ICC was similar. These results could help guide physicians’ and patients’ expectations when considering craniotomy for LRBM.
Funding
This study did not receive any funding from internal or external sources.
Conflict of interest statement. The authors report no conflicts of interest.
Authorship statement. All authors contributed to the study conception and/or design. Data were collected by A.F.C.H., F.I., A.A., V.K. and L.D.C. Statistical analysis was performed by A.F.C.H. The first draft was written by A.F.C.H. All authors critically revised subsequent versions of the manuscript. A.F.C.H. was responsible for compiling the final draft. All authors read and approved the final draft.
Supplementary Material
References
- 1. Nahed BV, Alvarez-Breckenridge C, Brastianos PK, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of surgery in the management of adults with metastatic brain tumors. Neurosurgery. 2019;84(3):E152–E155. [DOI] [PubMed] [Google Scholar]
- 2. Lamba N, Muskens IS, DiRisio AC, et al. Stereotactic radiosurgery versus whole-brain radiotherapy after intracranial metastasis resection: a systematic review and meta-analysis. Radiat Oncol. 2017;12(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ammirati M, Cobbs CS, Linskey ME, et al. The role of retreatment in the management of recurrent/progressive brain metastases: a systematic review and evidence-based clinical practice guideline. J Neuro-Oncol. 2010;96(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Zabin M, Ullrich WO, Brawanski A, Proescholdt MA. Recurrent brain metastases from lung cancer: the impact of reoperation. Acta Neurochir. 2010;152(11):1887–1892. [DOI] [PubMed] [Google Scholar]
- 5. Arbit E, Wroński M, Burt M, Galicich JH. The treatment of patients with recurrent brain metastases. A retrospective analysis of 109 patients with nonsmall cell lung cancer. Cancer. 1995;76(5):765–773. [DOI] [PubMed] [Google Scholar]
- 6. Bindal RK, Sawaya R, Leavens ME, Hess KR, Taylor SH. Reoperation for recurrent metastatic brain tumors. J Neurosurg. 1995;83(4):600–604. [DOI] [PubMed] [Google Scholar]
- 7. Jeon YS, Koh YC, Song SW, Cho J, Lim SD. Palliative resection of metastatic brain tumors previously treated by stereotactic radiosurgery. Brain Tumor Res Treat. 2016;4(2):116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamp MA, Fischer I, Dibue-Adjei M, et al. Predictors for a further local in-brain progression after re-craniotomy of locally recurrent cerebral metastases. Neurosurg Rev. 2018;41(3):813–823. [DOI] [PubMed] [Google Scholar]
- 9. Kano H, Kondziolka D, Zorro O, Lobato-Polo J, Flickinger JC, Lunsford LD. The results of resection after stereotactic radiosurgery for brain metastases. J Neurosurg. 2009;111(4):825–831. [DOI] [PubMed] [Google Scholar]
- 10. Kennion O, Holliman D. Outcome after craniotomy for recurrent cranial metastases. Br J Neurosurg. 2017;31(3):369–373. [DOI] [PubMed] [Google Scholar]
- 11. Mu F, LucasJT, Jr., Watts JM, et al. Tumor resection with carmustine wafer placement as salvage therapy after local failure of radiosurgery for brain metastasis. J Clini Neuro. 2015;22(3):561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schackert G, Schmiedel K, Lindner C, Leimert M, Kirsch M. Surgery of recurrent brain metastases: retrospective analysis of 67 patients. Acta Neurochir. 2013;155(10):1823–1832. [DOI] [PubMed] [Google Scholar]
- 13. Truong MT, St Clair EG, Donahue BR, et al. Results of surgical resection for progression of brain metastases previously treated by gamma knife radiosurgery. Neurosurgery. 2006;59(1):86–97; discussion 86. [DOI] [PubMed] [Google Scholar]
- 14. Vecil GG, Suki D, Maldaun MV, Lang FF, Sawaya R. Resection of brain metastases previously treated with stereotactic radiosurgery. J Neurosurg. 2005;102(2):209–215. [DOI] [PubMed] [Google Scholar]
- 15. Sperduto PW, Jiang W, Brown PD, et al. Estimating survival in melanoma patients with brain metastases: an update of the graded prognostic assessment for melanoma using molecular markers (Melanoma-molGPA). Int J Radiation Oncol Biol Phys. 2017;99(4):812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sperduto PW, Yang TJ, Beal K, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol. 2017;3(6):827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang T, Min W, Li Y, Yue Z, Wu C, Zhou C. Radiotherapy plus EGFR TKIs in non-small cell lung cancer patients with brain metastases: an update meta-analysis. Cancer Med. 2016;5(6):1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petrelli F, Lazzari C, Ardito R, et al. Efficacy of ALK inhibitors on NSCLC brain metastases: a systematic review and pooled analysis of 21 studies. PLoS One. 2018;13(7):e0201425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rulli E, Legramandi L, Salvati L, Mandala M. The impact of targeted therapies and immunotherapy in melanoma brain metastases: a systematic review and meta-analysis. Cancer. 2019;125(21):3776–3789. [DOI] [PubMed] [Google Scholar]
- 20. McTyre ER, Johnson AG, Ruiz J, et al. Predictors of neurologic and nonneurologic death in patients with brain metastasis initially treated with upfront stereotactic radiosurgery without whole-brain radiation therapy. Neuro Oncol. 2017;19(4):558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New Eng J Med. 1990;322(8):494–500. [DOI] [PubMed] [Google Scholar]
- 22. Subbiah IM, Lei X, Weinberg JS, et al. Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast cancer and brain metastases. J Clin Oncol. 2015;33(20):2239–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sperduto PW, Deegan BJ, Li J, et al. Estimating survival for renal cell carcinoma patients with brain metastases: an update of the Renal Graded Prognostic Assessment tool. Neuro Oncol. 2018;20(12):1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4259 patients. Int J Radi Oncol Biol Phys. 2010;77(3):655–661. [DOI] [PubMed] [Google Scholar]
- 25. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clini Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leone JP, Lin NU. Systemic therapy of central nervous system metastases of breast cancer. Curr Oncol Rep. 2019;21(6):49. [DOI] [PubMed] [Google Scholar]
- 27. Sundaresan N, Sachdev VP, DiGiacinto GV, Hughes JE. Reoperation for brain metastases. J Clini Oncol. 1988;6(10):1625–1629. [DOI] [PubMed] [Google Scholar]
- 28. Lee CH, Kim DG, Kim JW, et al. The role of surgical resection in the management of brain metastasis: a 17-year longitudinal study. Acta Neurochir. 2013;155(3):389–397. [DOI] [PubMed] [Google Scholar]
- 29. Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kavouridis VK, Harary M, Hulsbergen AFC, et al. Survival and prognostic factors in surgically treated brain metastases. J Neuro-Oncol. 2019;143(2):359–367. [DOI] [PubMed] [Google Scholar]
- 31. Hulsbergen AFC, Claes A, Kavouridis VK, et al. Subtype switching in breast cancer brain metastases: a multicenter analysis. Neuro Oncol. 2020;22(8):1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shih DJH, Nayyar N, Bihun I, et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat Genet. 2020;52(4):371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dono A, Takayasu T, Yan Y, et al. Differences in genomic alterations between brain metastases and primary tumors. Neurosurgery. 2021;88(3):592–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.