Abstract
Background
The National Tuberculosis Elimination Programme (NTEP) of India is aiming to eliminate TB by 2025. The programme has increased its services and resources to strengthen the accurate and early detection of TB. It is important to estimate the cost of TB diagnosis in India considering the advancement and implementation of new diagnostic tools under the NTEP. The objective of this study was to estimate the unit costs of providing TB diagnostic services at different levels of public health facilities with different algorithms implemented under the NTEP in Chennai, Tamil Nadu, South India.
Methods
This costing study was conducted from the perspective of the health system. This study used only secondary data and information that were available in the public domain. Data were collected with the approval of health authorities. The patient's diagnostic path from the point of registration until the final diagnosis was considered in the costing exercise. The unit costs of different diagnostic tools used in the NTEP implemented by Chennai Corporation were calculated.
Results
We estimated the unit cost of the eight laboratory tests (Ziehl–Neelsen [ZN], fluorescence microscopy [FM], x-ray, digital x-ray, gene Xpert MTB/RIF (cartridge-based nucleic acid amplification test [NAAT] that identifies rifampicin resistant Mycobacterium Tuberculosis) Mycobacterium Tuberculosis/Rifampicin [MTB/RIF], mycobacteria growth indicator tube [MGIT], line probe assay [LPA] and Lowenstein Jensen [LJ] culture) for diagnosis of drug-sensitive and drug-resistant TB. The unit costs included fixed and variable costs for smear examination by ZN microscopy (₹ [Indian Rupee] 326 [US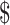 4.72], FM (₹104 [US
4.72], FM (₹104 [US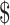 1.5]), x-ray (₹218 [US
1.5]), x-ray (₹218 [US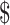 3.15]), digital X-ray (₹281 [US
3.15]), digital X-ray (₹281 [US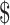 4.07]), gene Xpert MTB/RIF (₹1137 [US
4.07]), gene Xpert MTB/RIF (₹1137 [US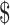 16.47]), MGIT (₹7038 [US
16.47]), MGIT (₹7038 [US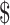 102]), LPA (₹6448 [US
102]), LPA (₹6448 [US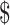 93.44]) and LJ culture (₹4850 [US
93.44]) and LJ culture (₹4850 [US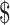 70.28]). Out of 10 diagnostic algorithms used for TB diagnosis, algorithms using only smear microscopy had the lowest cost, followed by smear microscopy with x-ray for drug-sensitive TB (₹104 [US
70.28]). Out of 10 diagnostic algorithms used for TB diagnosis, algorithms using only smear microscopy had the lowest cost, followed by smear microscopy with x-ray for drug-sensitive TB (₹104 [US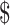 1.5] to ₹544 [US
1.5] to ₹544 [US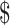 7.88]). Diagnostic algorithms for drug-resistant TB involving LPA and gene Xpert MTB/RIF were the most expensive.
7.88]). Diagnostic algorithms for drug-resistant TB involving LPA and gene Xpert MTB/RIF were the most expensive.
Conclusions
Understanding the various costs contributing to TB diagnosis in India provides crucial evidence for policymakers, programme managers and researchers to optimise programme spending and efficiently use resources.
Keywords: economic evaluation, India, provider cost, TB, TB diagnostic algorithm, TB diagnostic cost
Introduction
India has the highest TB burden in the world with 2.4 million cases reported in 2019 alone.1 The National TB Elimination Programme (NTEP) of India is aiming to eliminate TB in the country by 2025. The programme has expanded its services and increased its resources to strengthen the detection, treatment and prevention of TB. The NTEP is one of the largest public-funded programmes in the world and has ensured free treatment and diagnosis for almost 25 million patients since its inception.2 The NTEP is funded by the Government of India and global health donors to ensure free and continuous provision of diagnostic and treatment services. The provision of TB services requires considerable and sustained financial resources for delivering and maintaining the standard of TB care services. This has resulted in considerable resource investment and budgetary implications for the government.3 While there are studies that have estimated the financial and budgetary aspects of the NTEP, there is a dearth of costing-related studies on public sector TB diagnostic services.4–6 So far, costing studies conducted in TB are mostly from the patients’ perspective7–9 and there is a paucity of information on updated provider costs for TB diagnosis in India. Costing studies provide useful information to programme implementers and decision-makers for efficient use of scarce resources.10,11
It is important to estimate provider costs for TB diagnosis in India considering the advancement and implementation of new diagnostic tools under the NTEP. While there has been considerable research on the clinical aspects of advanced molecular diagnostic tools, the cost aspects of these tools in public health facilities have not been assessed to date. Estimating the unit costs for the provision of all implemented TB diagnostic services would be an important step for assessing the cost-effectiveness of TB diagnosis services in India. Costing studies are important health economic studies, which could serve as a foundation for economic evaluations of healthcare services.12 Costing studies would help to assess the budgetary implications of TB programmes and effective use of resources for delivering health services.13 The calculation of the unit cost of services is necessary and important for long-term planning of the healthcare system. With this background, in this study, we have attempted to estimate the unit costs of providing TB diagnostic services in different levels of public health facilities in Chennai, a metropolitan capital city in Tamil Nadu, South India.
Methods
Study setting
Tamil Nadu, a South Indian state with a population of 77 million represents 5.9% of India's population. Tamil Nadu is a large and economically well-developed state of India with 37 districts that has multiple urban cities and towns. The TB burden of Tamil Nadu is relatively high and a total of 110 845 cases were reported in 2019, amounting to 4.6% of the total nationwide notification. Tamil Nadu has a disproportionate burden of TB, to which the metropolitan cities of Chennai comprising the North, Central and South Chennai districts contribute 13.7% of TB cases. Chennai has an estimated TB prevalence of 203 per 100 000 population. The NTEP of the Chennai Corporation is actively engaged in providing TB prevention, diagnosis and treatment for its population of 10.9 million. In particular, NTEP Chennai Corporation has made remarkable progress in upgrading the speed and quality of mycobacteriology TB diagnostic services. Chennai has a total of 70 designated microscopy centres and 36 treatment units, along with 138 urban primary health centres and 90 family welfare centres. Every year about 63 000 to 67 000 presumptive patients are evaluated with sputum microscopy and 5600 to 6300 smear positive TB patients are diagnosed. Along with smear microscopy, new molecular diagnostic tools have been implemented in recent years for diagnosis of TB.
Perspective of the study
This costing study was conducted from a health system or provider's perspective, in which direct and indirect consumption of resources for TB diagnosis using different diagnostic tools was measured. The unit costs of different diagnostic tools used in the NTEP of Chennai Corporation were calculated. The patient diagnostic path from the point of registration until final diagnosis was considered in the costing exercise (Figure A1).
Study area
This study was conducted in public sector TB diagnostic health facilities in Chennai Corporation. Data were collected from four different levels of TB diagnostic health facilities, which included (1) primary health centres (PHCs), (2) a district TB centre (DTC), (3) a government peripheral hospital (GPH) and (4) an intermediate reference laboratory (IRL). A total of 12 TB diagnostic facilities were selected for data collection (Table 1).
Table 1.
Profile of the laboratories included in the study
| Lab 1 | Lab 2 | Lab 3 | Lab 4 | Lab 5 | Lab 6 | Lab 7 | Lab 8 | Lab 9 | Lab 10 | Lab 11 | Lab 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Urban | Urban | Urban | Urban | Urban | Urban | Urban | Urban | Urban | Urban | Urban | Urban |
| Corporation zone | 4 | 4 | 4 | 4 | 5 | 4 | 5 | 4 | 4 | 8 | 8 | 9 |
| Health facility | PHC | PHC | PHC | PHC | PHC | PHC | DTC | PHC | GPH | Research centre | PHC | PHC |
| Diagnostic facility | DMC | DMC | DMC | DMC | DMC | DMC | DMC | DMC | DMC | IRL | DMC | DMC |
| Year of assessment | 2019 | 2019 | 2019 | 2019 | 2019 | 2019 | 2019 | 2019 | 2019 | 2019 | 2019 | 2019 |
| Population covered | 50 633 | 50 700 | 50 596 | 50 680 | 54 730 | 50 892 | 215 434 | 50 320 | 152 047 | NA | 229 390 | 182 362 |
| Persons requiring test for TB/month | 79 | 88 | 108 | 66 | 37 | 45 | 350 | 71 | 147 | NA | 230 | 268 |
| Diagnostic test available | ZN microscopy | ZN microscopy | ZN microscopy | ZN microscopy | ZNmicroscopy | ZN microscopy | ZN microscopyFM Gene Xpert MTB/RIFx-ray | ZN microscopy | ZN microscopyDigital x-ray | MGITDigital X-rayX-rayZN microscopyFM Gene XpertMTB/RIFLPALJ culture | ZN microscopyGene Xpert MTB/RIF | ZN microscopyGene Xpert MTB/RIF |
| Data collected | ZN microscopy | ZN microscopy | ZN microscopy | ZN microscopy | ZN microscopy | ZN microscopy | FM Gene Xpert MTB/RIFx-ray | ZN microscopy | ZN microscopyDigital x-ray | Gene Xpert MTB/RIFMGITLPALJ culture | Gene Xpert MTB/RIF | Gene Xpert MTB/RIF |
Abbreviations: DMC, designated microscopy centres; DTC, district TB centre; FM, fluorescence microscopy; Gene Xpert, cartridge-based nucleic acid amplification test (NAAT) that identifies rifampicin resistant Mycobacterium Tuberculosis; GPH, government peripheral hospital; IRL, intermediate reference laboratory; LJ, Lowenstein Jensen; LPA, line probe assay; MGIT, mycobacteria growth indicator tube; MTB/RIF, Mycobacterium Tuberculosis/Rifampicin; PHC, primary health centre; ZN, Ziehl–Neelsen microscopy.
Tools for data collection
A data extraction form was developed for data collection. This form collected information on capital costs (i.e. infrastructure, medical and non-medical equipment), human resources (HR; i.e. staff salaries), consumables and overheads. This tool also included information on costs incurred by supportive activities such as security, maintenance, administrative and water services.
Data collection
Trained field investigators visited the centres after prior approval was received from the respective authorities. The purpose of the study was explained to them in their local language. Data were collected from the secondary sources of administrative records and registries maintained at laboratories. The observational method was used to collect information wherever documentary records were not available (e.g. office space). Cost data elements, cost items and sources of data collection are provided in Table 2.
Table 2.
Cost data elements for cost analysis of TB diagnosis and data sources
| Diagnostic tool | Data element | Cost items | Source of data |
|---|---|---|---|
| ZN | Physical infrastructure | Building | Observation |
| FM | Furniture | PHC | |
| X-ray | Medical instruments | DMC | |
| Electrical instruments | DTC | ||
| IRL | |||
| GPH | |||
| Digital x-ray | Chemicals, reagents and consumables | Chemicals | Stock register |
| Gene Xpert MTB/RIF | Reagents | Lab register | |
| MGIT | Lab consumables Stationaries | Financial record | |
| LPA | Human resources | Medical officer | Indent book |
| LJ culture | Laboratory technician | Pay bill register | |
| Administrative assistant | |||
| Supporting staff |
Abbreviations: DMC, designated microscopy centres; DTC, district TB centre; FM, fluorescence microscopy; Gene Xpert, cartridge-based nucleic acid amplification test (NAAT) that identifies rifampicin resistant Mycobacterium Tuberculosis; GPH, government peripheral hospital; IRL, intermediate reference laboratory; LJ, Lowenstein Jensen; LPA, line probe assay; MGIT, mycobacteria growth indicator tube; MTB/RIF, Mycobacterium Tuberculosis/Rifampicin; PHC, primary health centre; ZN, Ziehl–Neelsen microscopy.
HR costs
HR costs were collected from the pay bill register. The cost related to supporting services was calculated based on the specific time used for the diagnostic facility in proportion to the service used for the whole heath facility. The unit cost for services was estimated by dividing the monthly salary by the total number of tests performed per month.
Consumable cost
Costs details of consumables were collected from the laboratory records. The unit cost was calculated by dividing the total costs of consumables by the total number of tests performed per month.
Overhead cost
The cost of water and electricity used specifically for the diagnostic facility was collected as the proportion to the water and electricity used for a whole health facility during 1 mo.
Quality control measures
The study team was supervised by the study investigators during data collection. Costs of various resources were computed from current market price value including depreciation costs. We also computed resources supplied from the NTEP based upon its standard price. Missing costs were filled with current market values adjusting for depreciation based upon life expectancy.
Cost calculation
Our primary estimate was the cost of performing a single test, i.e. the unit cost calculated. This was calculated using the ingredient approach, where the resource used was multiplied by the cost of the input. This estimate includes costs of observed resources and was calculated by a top-down method.
Top-down
In the top-down method, we used cost data and the total number of tests performed monthly at each health facility. It was calculated by dividing total monthly expenditure specifically for each test by the total numbers of tests performed monthly.14
Activity-based costing
An activity-based costing method was used for accounting activities such as registration, consultation and investigation. These costs were classified into direct (i.e. HR, consumables, instruments), indirect (i.e. building space and overhead) and fixed and variable costs. All costs were calculated in Indian Rupee (₹) and converted to US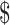 according to the 2019 exchange rate of US
according to the 2019 exchange rate of US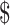 1=₹69. The study data were entered into Microsoft Excel software (Microsoft Corp., Redmond, WA, USA) and analysed using descriptive statistics methods.
1=₹69. The study data were entered into Microsoft Excel software (Microsoft Corp., Redmond, WA, USA) and analysed using descriptive statistics methods.
Depreciation costs
The depreciation costs of the equipment and building were calculated taking into consideration the useful expected life for a building and equipment.
Definitions used
Total cost: Total cost is calculated as the sum of direct and indirect costs.
Unit cost: A unit cost is a simple average or the cost per unit of outcome.
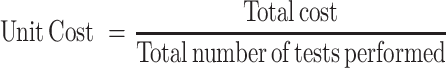 |
Data analysis
Costs incurred for all the TB diagnostic processes were calculated by accounting for all procedures and tests included. Based on the information collected, costs incurred in various diagnostic tests were estimated, such as Ziehl–Neelsen (ZN) microscopy, LED fluorescence microscopy (FM), x-ray, digital x-ray, gene Xpert MTB/RIF (cartridge-based nucleic acid amplification test (NAAT) that identifies rifampicin resistant Mycobacterium Tuberculosis) Mycobacterium Tuberculosis/Rifampicin (MTB/RIF), mycobacteria growth indicator tube (MGIT), line probe assay (LPA) and Lowenstein Jensen (LJ) culture. From these, we estimated the unit provider cost for TB diagnostic tests at different health facilities in Chennai for 2019. We also compared the unit costs of TB diagnosis with the number of tests performed at different health facilities.
Results
Profile of laboratories
In this study, we estimated the unit cost of the eight laboratory tests (ZN microscopy, FM, x-ray, digital x-ray, gene Xpert MTB/RIF, MGIT, LPA and LJ culture) used for diagnosis of TB. Eleven designated microscopy centres functioning under nine PHCs, one GPH and one DTC were selected (Table 1). All the centres are located in Chennai Corporation in different zones. For the purposes of cost calculation, one FM centre, one x-ray unit, one digital x-ray unit, three gene Xpert MTB/RIF centres and eight ZN microscopy centres were selected. Costs regarding MGIT, LPA and LJ culture were collected from one IRL. The population coverage for nine PHCs was around 50 000 each and two PHCs covered a population of >100 000. The GPH covered 152 047 and the DTC covered 215 434 people. The total number of individuals requiring TB diagnosis per centre in a month ranged from 37 to 268.
Overall unit costs for different diagnostic tests
The overall unit cost including fixed and variable costs (Table 3) for smear examination by ZN microscopy was ₹326 (US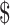 4.72) and by FM it was ₹104 (US
4.72) and by FM it was ₹104 (US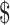 1.55). The unit costs for diagnosis by x-ray and digital X-ray were ₹218 (US
1.55). The unit costs for diagnosis by x-ray and digital X-ray were ₹218 (US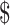 3.15) and ₹281 (US
3.15) and ₹281 (US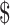 4.07), respectively. The unit cost for diagnosis by gene Xpert MTB/RIF was ₹1137 (US
4.07), respectively. The unit cost for diagnosis by gene Xpert MTB/RIF was ₹1137 (US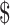 16.48), by MGIT it was ₹7038 (US
16.48), by MGIT it was ₹7038 (US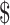 102), by LPA it was ₹6448 (US
102), by LPA it was ₹6448 (US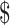 93.44) and by LJ culture it was ₹4850 (US
93.44) and by LJ culture it was ₹4850 (US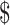 70.29). The details of unit cost for each activity (registration, consultation and investigation) for different tests by fixed and variable costs for various centres are provided in Tables A1 to A6.
70.29). The details of unit cost for each activity (registration, consultation and investigation) for different tests by fixed and variable costs for various centres are provided in Tables A1 to A6.
Table 3.
Estimated number of tests per month and estimated unit cost
| Estimated unit costs | ||||
|---|---|---|---|---|
| Diagnostic tools | Estimated number of tests | Fixed | Variable | Total |
| ZN | 641 | ₹291 (US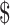 4.21) 4.21) |
₹35 (US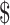 0.51) 0.51) |
₹326 (US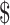 4.72) 4.72) |
| FM | 34 | ₹66 (US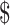 0.95) 0.95) |
₹38 (US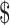 0.55) 0.55) |
₹104 (US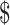 1.50) 1.50) |
| X-ray | 32 | ₹154 (US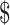 2.23) 2.23) |
₹64 (US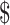 0.93) 0.93) |
₹218 (US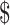 3.16) 3.16) |
| Digital x-ray | 57 | ₹269 (US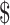 3.89) 3.89) |
₹12 (US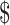 0.18) 0.18) |
₹281 (US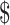 4.07) 4.07) |
| Gene Xpert MTB/RIF | 29 | ₹1082 (US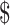 15.68) 15.68) |
₹55 (US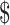 0.80) 0.80) |
₹1137 (US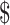 16.48) 16.48) |
| MGIT | 56 | ₹6048 (US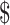 87.65) 87.65) |
₹990 (US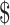 14.35) 14.35) |
₹7038 (US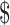 102) 102) |
| LPA | 16 | ₹4819 (US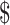 69.84) 69.84) |
₹1629 (US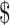 23.60) 23.60) |
₹6448 (US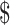 93.44) 93.44) |
| LJ | 44 | ₹4723 (US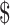 68.44) 68.44) |
₹127 (US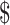 1.84) 1.84) |
₹4850 (US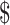 70.29) 70.29) |
Abbreviations: FM, fluorescence microscopy; Gene Xpert, cartridge-based nucleic acid amplification test (NAAT) that identifies rifampicin resistant Mycobacterium Tuberculosis; LJ, Lowenstein Jensen; LPA, line probe assay; MGIT, mycobacteria growth indicator tube; MTB/RIF, Mycobacterium Tuberculosis/Rifampicin; ZN, Ziehl–Neelsen microscopy.
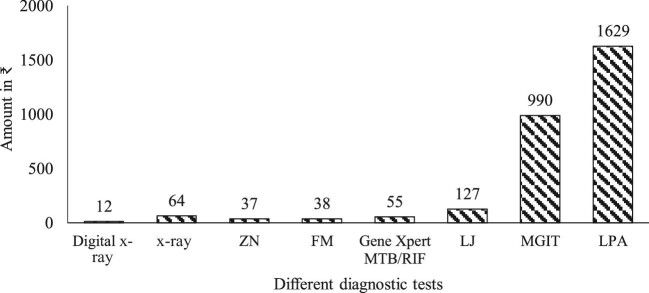
Variable costs
Variable costs for ZN smear microscopy tests were lower than those for FM tests (₹35 vs ₹38; US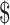 0.52 vs US
0.52 vs US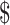 0.55); costs for digital x-ray tests were lower than for manual x-ray tests (₹12 vs ₹64; US
0.55); costs for digital x-ray tests were lower than for manual x-ray tests (₹12 vs ₹64; US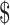 0.17 vs US
0.17 vs US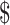 0.92). The variable cost was less for LJ culture compared with gene Xpert MTB/RIF, MGIT and LPA (₹127 [US
0.92). The variable cost was less for LJ culture compared with gene Xpert MTB/RIF, MGIT and LPA (₹127 [US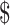 1.84] vs ₹55 [US
1.84] vs ₹55 [US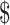 0.79], ₹990 [US
0.79], ₹990 [US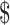 14.34] and ₹1629 [US
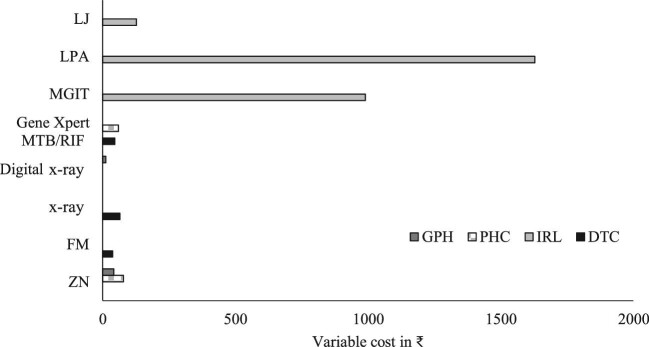
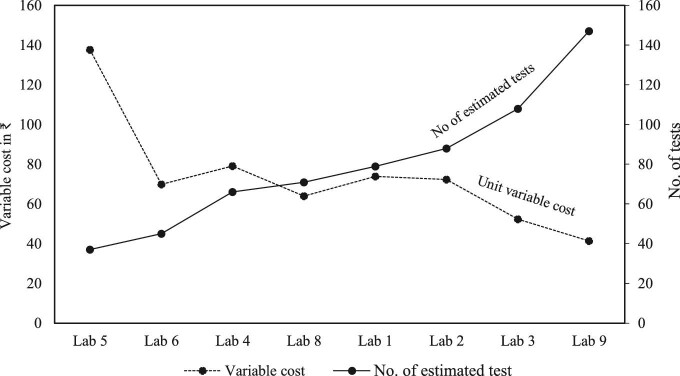
14.34] and ₹1629 [US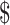 23.6]), respectively (Figure 1). ZN microscopy tests at PHCs incurred higher variable costs compared with the costs incurred at the level of GPH and DTC (Figure 2). For all the diagnostic tests, fixed costs were found to be higher than variable costs. There was an inverse relationship between the total number of tests and unit variable costs (Figure 3).
23.6]), respectively (Figure 1). ZN microscopy tests at PHCs incurred higher variable costs compared with the costs incurred at the level of GPH and DTC (Figure 2). For all the diagnostic tests, fixed costs were found to be higher than variable costs. There was an inverse relationship between the total number of tests and unit variable costs (Figure 3).
Figure 1.
Unit variable costs for different TB diagnostic tests.
Figure 2.
Unit variable costs for TB diagnosis at different levels of health facilities.
Figure 3.
The association between the number of tests done and unit variable costs.
Different components of costs
The different components of unit costs included direct costs (HR, consumables, instruments) and indirect costs (building, overhead), as given in Table 4. The direct and indirect costs for different diagnostic tests, respectively, are ZN microscopy (₹321 [US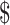 4.65] and ₹5 [US
4.65] and ₹5 [US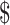 0.07]); FM (₹101 [US
0.07]); FM (₹101 [US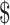 1.46] and ₹3 [US
1.46] and ₹3 [US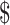 0.04]); x-ray (₹205 [US
0.04]); x-ray (₹205 [US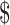 2.97] and ₹13 [US
2.97] and ₹13 [US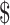 0.18]); digital x-ray (₹271 [US
0.18]); digital x-ray (₹271 [US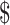 3.92] and ₹10 [US
3.92] and ₹10 [US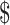 0.14]); gene Xpert MTB/RIF (₹879 [US
0.14]); gene Xpert MTB/RIF (₹879 [US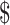 12.73] and ₹258 [US
12.73] and ₹258 [US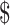 3.73]); MGIT (₹6993 [US
3.73]); MGIT (₹6993 [US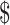 101.34] and ₹45 [US
101.34] and ₹45 [US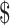 0.65]); LPA (₹6412 [US
0.65]); LPA (₹6412 [US 92.92] and ₹36 [US
92.92] and ₹36 [US 0.52]); and Lowenstein Jensen (LJ) culture (₹4820 [US
0.52]); and Lowenstein Jensen (LJ) culture (₹4820 [US 69.85] and ₹30 [US
69.85] and ₹30 [US 0.43]). It was observed that indirect costs were higher for ZN, FM, x-ray, digital x-ray and gene Xpert MTB/RIF tests. Direct costs were higher for MGIT, LPA and LJ culture tests. Within the direct costs, HR component costs were proportionally higher for almost all the tests. Instrument costs were less compared with HR costs and for indirect costs component infrastructure cost (i.e. buildings) had the largest share.
0.43]). It was observed that indirect costs were higher for ZN, FM, x-ray, digital x-ray and gene Xpert MTB/RIF tests. Direct costs were higher for MGIT, LPA and LJ culture tests. Within the direct costs, HR component costs were proportionally higher for almost all the tests. Instrument costs were less compared with HR costs and for indirect costs component infrastructure cost (i.e. buildings) had the largest share.
Table 4.
Different components of unit cost
| Direct | Indirect | |||||
|---|---|---|---|---|---|---|
| HR | Consumables | Instruments | Building | Overhead | Total | |
| ZN | ₹273 (US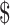 3.95) 3.95) |
₹34 (US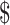 0.49) 0.49) |
₹14 (US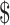 4 0.20) 4 0.20) |
₹4 (US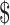 0.05) 0.05) |
₹1 (US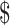 0.01) 0.01) |
₹326 (US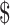 4.72) 4.72) |
| FM | ₹48 (US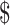 0.69) 0.69) |
₹37 (US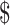 0.53) 0.53) |
₹17 (US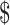 0.24) 0.24) |
₹2 (US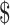 0.02) 0.02) |
₹1 (US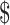 0.01) 0.01) |
₹104 (US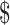 1.50) 1.50) |
| X-ray | ₹134 (US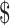 1.94) 1.94) |
₹57 (US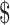 0.82) 0.82) |
₹14 (US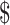 0.20) 0.20) |
₹6 (US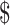 0.08) 0.08) |
₹7 (US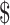 0.10) 0.10) |
₹218 (US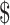 3.16) 3.16) |
| Digital x-ray | ₹258 (US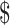 3.73) 3.73) |
₹9 (US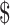 0.13) 0.13) |
₹4 (US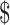 0.05) 0.05) |
₹7 (US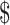 0.10) 0.10) |
₹3 (US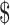 0.04) 0.04) |
₹281 (US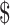 4.07) 4.07) |
| Gene Xpert MTB/RIF | ₹183 (US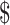 2.65) 2.65) |
₹51 (US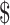 0.73) 0.73) |
₹645 (US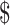 9.36) 9.36) |
₹254 (US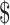 3.69) 3.69) |
₹4 (US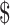 0.05) 0.05) |
₹1137 (US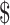 16.48) 16.48) |
| MGIT | ₹4875 (US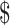 70.65) 70.65) |
₹946 (US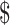 5.01) 5.01) |
₹1172 (US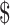 16.98) 16.98) |
₹1 (US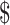 0.01) 0.01) |
₹44 (US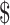 0.63) 0.63) |
₹7038 (US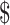 102) 102) |
| LPA | ₹4795 (US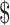 69.49) 69.49) |
₹1594 (US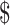 23.10) 23.10) |
₹23 (US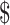 0.33) 0.33) |
₹1 (US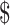 0.01) 0.01) |
₹35 (US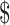 0.50) 0.50) |
₹6448 (US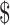 93.44) 93.44) |
| LJ | ₹4689 (US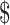 67.95) 67.95) |
₹98 (US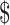 4 1.42) 4 1.42) |
₹33 (US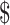 0.47) 0.47) |
₹1 (US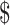 0.01) 0.01) |
₹29 (US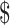 0.33) 0.33) |
₹4850 (US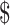 61.59) 61.59) |
Abbreviations: FM, fluorescence microscopy; Gene xpert, cartridge-based nucleic acid amplification test (NAAT) that identifies rifampicin resistant Mycobacterium Tuberculosis; LJ, Lowenstein Jensen; LPA, line probe assay; MGIT, mycobacteria growth indicator tube; MTB/RIF, Mycobacterium Tuberculosis/Rifampicin; ZN, Ziehl–Neelsen microscopy.
Costs for different diagnostic algorithms used under the NTEP
Of the 10 diagnostic algorithms used for TB diagnosis, the algorithm using only smear microscopy was lowest followed by the algorithm using smear microscopy with x-ray for detecting drug-sensitive TB (₹104 to ₹544; US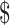 1.50 to US
1.50 to US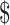 7.8). Diagnostic algorithms involving smear microscopy with digital x-ray and gene Xpert MTB/RIF ranged from ₹1459 (US
7.8). Diagnostic algorithms involving smear microscopy with digital x-ray and gene Xpert MTB/RIF ranged from ₹1459 (US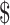 21.14) to ₹1681 (US
21.14) to ₹1681 (US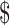 21.36). Algorithms involving LPA were more expensive, ranging from ₹6770 (US
21.36). Algorithms involving LPA were more expensive, ranging from ₹6770 (US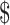 98.11) to ₹8129 (US
98.11) to ₹8129 (US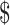 117.8). Diagnostic algorithms for drug-resistant TB were the most expensive involving LPA and gene Xpert MTB/RIF (Table 5). With respect to the fixed and variable costs for all diagnostic algorithms, fixed costs were higher in comparison with variable costs.
117.8). Diagnostic algorithms for drug-resistant TB were the most expensive involving LPA and gene Xpert MTB/RIF (Table 5). With respect to the fixed and variable costs for all diagnostic algorithms, fixed costs were higher in comparison with variable costs.
Table 5.
Costs for different diagnostic algorithms used under the NTEP
| S. no. | Algorithm | Fixed | Variable | Total |
|---|---|---|---|---|
| 1 | ZN | ₹291 (US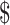 4.21) 4.21) |
₹35 (US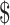 0.51) 0.51) |
₹326 (US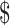 4.72) 4.72) |
| 2 | ZN and x-ray | ₹445 (US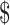 6.45) 6.45) |
₹99 (US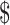 1.43) 1.43) |
₹544 (US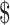 7.88) 7.88) |
| 3 | ZN, x-ray and Gene Xpert MTB/RIF | ₹1527 (US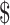 22.13) 22.13) |
₹154 (US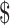 2.23) 2.23) |
₹1681 (US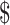 24.36) 24.36) |
| 4 | ZN, x-ray and LPA | ₹5264 (US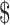 76.29) 76.29) |
₹1728 (US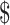 25.04) 25.04) |
₹6992 (US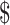 101.33) 101.33) |
| 5 | ZN, x-ray Gene Xpert MTB/RIF and LPA | ₹6346 (US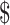 91.97) 91.97) |
₹1783 (US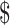 25.84) 25.84) |
₹8129 (US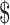 117.81) 117.81) |
| 6 | FM | ₹66 (US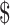 0.96) 0.96) |
₹38 (US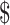 0.55) 0.55) |
₹104 (US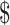 1.55) 1.55) |
| 7 | FM and x-ray | ₹220 (US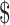 3.19) 3.19) |
₹102 (US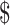 1.47) 1.47) |
₹322 (US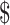 4.66) 4.66) |
| 8 | FM, x-ray and Gene Xpert MTB/RIF | ₹1302 (US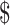 18.87) 18.87) |
₹157 (US 2.27) 2.27) |
₹1459 (US 21.14) 21.14) |
| 9 | FM, x-ray and LPA | ₹5039 (US 73.03) 73.03) |
₹1731 (US 25.08) 25.08) |
₹6770 (US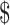 98.11) 98.11) |
| 10 | FM, x-ray, Gene Xpert MTB/RIF and LPA | ₹6121 (US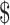 88.71) 88.71) |
₹1786 (US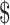 25.88) 25.88) |
₹7907 (US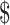 114.59) 114.59) |
Abbreviations: FM, fluorescence microscopy; Gene Xpert, cartridge-based nucleic acid amplification test (NAAT) that identifies rifampicin resistant Mycobacterium Tuberculosis; LJ, Lowenstein Jensen; LPA, line probe assay; MGIT, mycobacteria growth indicator tube; MTB/RIF, Mycobacterium Tuberculosis/Rifampicin; ZN, Ziehl–Neelsen microscopy.
Discussion
The Government of India has committed to eliminate TB by 2025, 5 y ahead of the global elimination target set under the Sustainable Development Goals.15 As part of the ‘End TB strategy’, the Ministry of Health and Family Welfare (MoHFW), Government of India has developed the National Strategic Plan (NSP) for TB elimination. The NSP was implemented in 2017 with additional financial resources to rapidly reduce TB. The NTEP aims to bring significant reductions to the TB burden by implementing new diagnostic tools and early TB diagnosis of active TB.16 The current costing study provides comprehensive evidence of the different costs incurred for TB diagnosis under the NTEP in India. While other studies have provided cost estimates for TB, our study updates and reflects the latest changes brought about under the ambit of the NTEP, which has expanded with new diagnostic TB services. Assessing the utilisation of healthcare resources remains critical for long-term planning and for evaluating the programme’s outcomes and effectiveness.17 This study’s findings could provide inputs to policymakers in developing appropriate scale-up plans and helps with decisions on the efficient provision of new diagnostic tools for diagnosis of pulmonary TB.
Our study estimated the costs for all eight diagnostic tools used in drug-sensitive and drug-resistant TB diagnosis used in different levels of health facilities. The unit total costs (i.e. fixed and variable costs) for diagnosing drug-sensitive TB ranged from ₹104 (US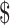 1.5) to ₹326 (US
1.5) to ₹326 (US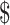 4.72). Diagnosis of drug-resistant TB was found to be many times (>15-fold) higher than the unit cost for diagnosing drug-sensitive TB. This agrees with the finding from a similar setting, in which 89.9% of diagnostic costs were for drug-resistant TB.18 For both drug-sensitive and drug-resistant TB, the fixed costs were significantly higher than the variable costs. The costing method we used in this study accounted for the entire process that the patient goes through during their diagnostic visit to a facility, from registration to completion of diagnosis. This method helped to estimate all the possible resources and services incurred for TB diagnosis. However, our cost estimates need to be interpreted within the context of a metropolitan city with optimal resources and better diagnostic facilities, thus may underestimate the costs associated with places with less optimal facilities.
4.72). Diagnosis of drug-resistant TB was found to be many times (>15-fold) higher than the unit cost for diagnosing drug-sensitive TB. This agrees with the finding from a similar setting, in which 89.9% of diagnostic costs were for drug-resistant TB.18 For both drug-sensitive and drug-resistant TB, the fixed costs were significantly higher than the variable costs. The costing method we used in this study accounted for the entire process that the patient goes through during their diagnostic visit to a facility, from registration to completion of diagnosis. This method helped to estimate all the possible resources and services incurred for TB diagnosis. However, our cost estimates need to be interpreted within the context of a metropolitan city with optimal resources and better diagnostic facilities, thus may underestimate the costs associated with places with less optimal facilities.
We also assessed the unit cost for TB diagnosis with various diagnostic algorithms used under the NTEP. This was undertaken to assess the cost differences of implementing specific diagnostic algorithms, which would involve either single or multiple diagnostic tools. Estimating the cost for individual algorithms is useful for comparing cost inputs and diagnostic outcomes. We found that the algorithm used for diagnosing drug-resistant TB was much more expensive than the algorithm used for diagnosing drug-sensitive TB. A similar study performed in an Indian state reported that the estimated programme cost was higher for all the strategies using gene Xpert MTB/RIF for drug-resistant TB.19 It was also suggested that the cost of the gene Xpert MTB/RIF test is cheaper when used in higher TB prevalence settings where more presumptive TB cases access diagnostic facilities.20 The study also identified an inverse relationship between the total number of tests and unit variable costs, similar to earlier research.20
Diagnosis involving smear microscopy alone was the cheapest of all the algorithms for drug-sensitive TB. Similar findings regarding the lower cost for smear microscopy were reported in an earlier study conducted in India.19 The diagnostic sensitivity of an algorithm should be considered when interpreting the cost consequences. To achieve the 95% case TB detection set by the NTEP and accelerate early diagnosis of TB cases, multiple diagnostic algorithms would be required based on field feasibility, programme requirements and resource constrains. The cost estimates from our study regarding different diagnostic algorithms serve as an evidence base for making informed decisions regarding the implementation of diagnostic strategies. This could help to achieve TB diagnostic goals set by the NSP under the NTEP.1
In terms of the resources required, funding for the provision of TB prevention, diagnostic and treatment services have doubled since 2006, but still fall far short of what is needed. Globally, the amount required for TB control in 2019 was US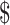 3.3 billion, which was less than the US
3.3 billion, which was less than the US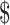 10.1 billion estimated by the Stop TB Partnership. Among the BRICS group of countries (i.e. Brazil, the Russian Federation, India, China and South Africa), 57% (US
10.1 billion estimated by the Stop TB Partnership. Among the BRICS group of countries (i.e. Brazil, the Russian Federation, India, China and South Africa), 57% (US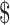 3.7 billion) of available funding for TB was reported in 2020, of which 97% was from domestic sources. With respect to India, 92% of funding is from domestic sources.
3.7 billion) of available funding for TB was reported in 2020, of which 97% was from domestic sources. With respect to India, 92% of funding is from domestic sources.
In India, domestic funding quadrupled from 2016 to 2019.21 For the NTEP in India, a total of US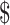 2.4 billion has been estimated for diagnosis, treatment, administration and patient support services for 2017–2020. Of this, US
2.4 billion has been estimated for diagnosis, treatment, administration and patient support services for 2017–2020. Of this, US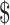 134 million (9%) has been estimated for additional diagnostic alone.22 In this context, understanding the various costs contributing to TB diagnosis are essential to optimise programme spending and efficiently use resources.
134 million (9%) has been estimated for additional diagnostic alone.22 In this context, understanding the various costs contributing to TB diagnosis are essential to optimise programme spending and efficiently use resources.
Our study has limitations. First, in this study, cost calculations were only from the perspective of the government programme and do not include costs incurred by the private sector. We conducted our study in a public sector hospital where TB patients are treated free of charge, hence our cost estimates could be substantially different from those for patients diagnosed in the private healthcare sector. Further, the costs estimates made using observational methods may not reflect actual costs and may thus affect diagnostic costs. The unit cost estimation for diagnosis by each diagnostic tool and algorithm may not be same as in other situations, as the values will differ depending upon the total number of tests performed or the total number of presumptive TB patients.
Conclusions
Unit costs for TB diagnosis in India provide essential evidence for policymakers, programme managers and researchers to understand the budgetary and financial implications of diagnostic services implemented under the NTEP. Understanding the various costs contributing to TB diagnosis is essential to optimise programme spending and efficient use of resources. The current updated TB diagnostic cost estimates will help to conduct economic evaluations of different TB diagnostic interventions and assess their cost effectiveness.
Supplementary Material
Contributor Information
Malaisamy Muniyandi, Department of Health Economics, ICMR-National Institute for Research in Tuberculosis, Chennai-600031, India.
Jayabal Lavanya, District TB Office, National TB Elimination Programme, Chennai.
Nagarajan Karikalan, Department of Health Economics, ICMR-National Institute for Research in Tuberculosis, Chennai-600031, India.
Balakrishnan Saravanan, Department of Health Economics, ICMR-National Institute for Research in Tuberculosis, Chennai-600031, India.
Sellappan Senthil, Department of Health Economics, ICMR-National Institute for Research in Tuberculosis, Chennai-600031, India.
Sriram Selvaraju, Department of Epidemiology, ICMR-National Institute for Research in Tuberculosis, Chennai.
Rajesh Mondal, Department of Bacteriology, ICMR-National Institute for Research in Tuberculosis, Chennai.
Supplementary data
Supplementary data are available at International Health online.
Authors’ contributions:
MM, NK and SRS were responsible for conceptualisation of the study, data collection, supervision and project administration. MM, NK, SS and RM were responsible for the methodology. MM, SS and RM were responsible for the formal analysis. BS, SS, JL, MM and RM were responsible for data collection. MM, NK and SS were responsible for the original manuscript draft preparation. JL, BS, SRS and RM were responsible for reviewing and editing the manuscript. All the authors have read and agreed to the final version of the manuscript. MM, NK, SS and BS revised the complete manuscript following the reviewers’ comments. JL, SS, SRS and RM agreed to submission of the revised manuscript.
Funding:
None.
Competing interests:
None declared.
Ethical approval:
Not required.
Data availability:
The authors have the research data, which are available upon reasonable request.
References
- 1. Central TB Division, Ministry of Health and Family Welfare (MoHFW), Government of India. India TB Report 2020. National TB Elimination Programme Annual Report. Central TB Division, MoHFW, Government of India; 2020. Available at www.tbcindia.gov.in [accessed April 15, 2020]. [Google Scholar]
- 2. Central TB Division, Ministry of Health and Family Welfare (MoHFW), Government of India. India TB Report 2016 to 2020. National TB Elimination Programme Annual Report. Central TB Division, MoHFW, Government of India; 2016 - 2020. Available at www.tbcindia.gov.in [accessed April 15, 2020]. [Google Scholar]
- 3. Mouseli A, Barouni M, Amiresmaili Met al. Cost-price estimation of clinical laboratory services based on activity-based costing: a case study from a developing country. Electron Phys . 2017;9:4077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babu R, Sagili K, Jacob A, Chadha AA. Resource optimisation for tuberculosis elimination in India. Economic Political Weekly. 2016;L1:26–7. [Google Scholar]
- 5. Centre for Budget and Policy Studies . Public Expenditure under Revised National Tuberculosis Eradication of Tuberculosis Control Programme (RNTCP). Karnataka, India: Centre for Budget and Policy Studies; 2015. [Google Scholar]
- 6. Dholakia Ravindra, Almeida Joel, WHO Global Tuberculosis Programme. The Potential Economic Benefits of the DOTS Strategy Against TB in India. World Health Organization; 1997. [Google Scholar]
- 7. Chandra A, Kumar R, Kant S, Parthasarathy R, Krishnan A. Direct and indirect patient costs of tuberculosis care in India. Trop Med Int Health. 2020;25(7):803–12. [DOI] [PubMed] [Google Scholar]
- 8. Sarin R, Vohra V, Singla N, Thomas B, Krishnan R, Muniyandi M. Identifying costs contributing to catastrophic expenditure among TB patients registered under RNTCP in Delhi metro city in India. Indian J Tuberc. 2019;66(1):150–7. [DOI] [PubMed] [Google Scholar]
- 9. Muniyandi M, Thomas BE, Karikalan Net al. Association of tuberculosis with household catastrophic expenditure in South India. JAMA Netw Open. 2020;3(2):e1920973. [DOI] [PubMed] [Google Scholar]
- 10. Dang A, Likhar N, Alok U. Importance of economic evaluation in health care: an Indian perspective. Value Health Reg Issues. 2016;9:78–83. [DOI] [PubMed] [Google Scholar]
- 11. Ahmadkiadaliri A, Haghparast-Bidgoli H, Zarei A. Measuring efficiency of general hospitals in the south of Iran. World Appl Sci. J 2011;13:1310–6. [Google Scholar]
- 12. Xu X, Grossetta Nardini HK, Ruger JP. Micro-costing studies in the health and medical literature: protocol for a systematic review. Syst Rev 2014;3:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hendriks ME, Kundu P, Boers ACet al. Step-by-step guideline for disease-specific costing studies in low-and middle-income countries: a mixed methodology. Glob Health Action. 2014;7:23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rupert S, Vassall A, Raizada Net al. Bottom-up or top-down: unit cost estimation of tuberculosis diagnostic tests in India. Int J Tuberc Lung Dis. 21(4):375–80. [DOI] [PubMed] [Google Scholar]
- 15. Uplekar M, Weil D, Lonnroth Ket al. WHO's new End TB Strategy. Lancet. 2015;385:1799–801. [DOI] [PubMed] [Google Scholar]
- 16. Pai M. TB control requires new tools, policies, and delivery models. Indian J Tuberc. 2015;62(1):1–3. [DOI] [PubMed] [Google Scholar]
- 17. Wurtz R, White WD. The cost of tuberculosis: utilization and estimated charges for the diagnosis and treatment of tuberculosis in a public health system. Int J Tuberc Lung Dis. 1999;3(5):382–7. [PubMed] [Google Scholar]
- 18. Atif M, Syed ASS, Asrul AS, Muhammad A, Zaheer-Ud-Din B. Resource utilization pattern and cost of tuberculosis treatment from the provider and patient perspectives in the state of Penang, Malaysia BMC Health Serv Res. 2014;14:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chadha VK, George Sebastian, Kumar P. Cost analysis of different diagnostic algorithms for pulmonary tuberculosis varying in placement of Xpert MTB/RIF. Ind J Tub. 2016;63:19–27. [DOI] [PubMed] [Google Scholar]
- 20. Debes AK, Gilman RH, Onyango-Makumbi C, Ruff A, Oberhelman R, Dowdy DW. Cost-effectiveness of diagnostic algorithms for tuberculosis in children less than 5 years of age. Pediatr Infect Dis J. 2017;36(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organisation . Global Tuberculosis Report 2019. Licence: CC BY-NC-SA 3.0 IGO. WHO/CDS/TB/2019.15. Geneva, Switzerland: World Health Organization, 2019. [Google Scholar]
- 22. Central TB Division, Ministry of Health and Family Welfare (MoHFW), Government of India. National strategic plan for TB Elimination 2017-2025. Central TB Division, MoHFW, Government of India; 2017. Available at https://tbcindia.gov.in/WriteReadData/NSP%20Draft%2020.02.2017%201.pdf [accessed May 25, 2020]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors have the research data, which are available upon reasonable request.