Abstract
Obesity is now considered a “new world syndrome” because it is affecting developed and developing nations in all classes of people. Pancreatic lipase (PL) is the key enzyme for absorption of dietary lipids. Excessive consumption of cafeteria foods leads to obesity. Nutmeg is being used as a spice and it has many medicinal values. We aimed to see whether nutmeg could be used as a potential source of new lipase inhibitors and to explore its molecular mechanism in obesity. An in vitro study, which was performed to check its anti-lipase activity, showed a potent lipase inhibition (66.24%). A liquid chromatography–mass spectrometry (LC-MS) analysis was carried out to identify new compounds, and tetrahydrofuran (THF) with MW 536 at RT 15.2 min was isolated. A molecular docking study, that was carried out to check the interaction between THF and PL, found a PL inhibitory action of tetrahydrofuran. A further in vivo study was conducted to see PL inhibition. The percentage of fecal fat was higher in nutmeg treated rats. The size and number of adipose tissue fat cells were also decreased after treatment with nutmeg extract, which pointed to the inhibition of PL by the newly identified compound, THF. These four different types of studies suggested that nutmeg could potentially inhibit pancreatic lipase.
Keywords:nutmeg, tetrahydrofuran, pancreatic lipase, faecal fat percentage, adipose tissue.
INTRODUCTION
Obesity is a term applied to excess body weight with an abnormally high proportion of body fat. Thermodynamically speaking, it is an imbalance between energy intake and energy expenditure that presents a risk to health, leading to reduced life expectancy (WHO-2018). Obesity is a global health problem and its prevalence has been alarmingly increasing in developing and developed countries. Obesity is also termed the “new world syndrome” as it causes many non-communicable diseases such as diabetes, metabolic syndrome and adversely affects the body homeostasis (1). It is considered a disarray of energy balance and fundamentally a disorder of lipid metabolism (2). The sedentary lifestyle and consumption of junk food are the major reasons for the increase in the prevalence of obesity. The body mass index (BMI) is the commonly used index to assess overweight and obesity in adults (1-3). People with BMI >30 kg/m² are classified as obese. Overweight and obesity are the major risk factors for non-communicable diseases such as cardiovascular diseases, diabetes, musculoskeletal disorders and cancer. Obesity linked hyperlipidemia can increase systemic oxidative stress (4). Hence, the management of obesity is a herculean task for clinicians as it is the underlying cause of many diseases (5).
As per WHO, about 2.8 million deaths have been reported throughout the world due to complications arising from obesity and overweight (6). Globally, 650 million adults and 42 million children under the age of five are overweight or obese (WHO-2019). Interestingly, the prevalence of obesity is higher in women (about 297 million) than men (205 million) (7). As per the National Family Health Survey (NFHS-3)-2016, the prevalence of overweight and obesity in India is increasing faster than the world average. The striking feature which contributes to this progressive rise is the sedentary lifestyle (8). So, there is an urgent need to control obesity in India to prevent a further increase in the rate of obesity cases (9).
Pharmacotherapy is available and can control obesity by either decreasing the absorption of fat or increasing fat metabolism. However, the use of drugs is linked to their considerable, tolerable, and intolerable side effects, including hypertension, heart-related complications, psychiatric problem (mental illness) and constipation (10, 11). The most commonly used drug in the management of obesity is orlistat, which reduces fat absorption by inhibiting the action of lipase. However, orlistat is also associated with side effects such as gastrointestinal complications, abdominal pain, bloating, flatulence and diarrhoea, and may lead to dyspepsia and liver damage too (12). Hence, there is a need for developing safer alternatives to orlistat, which are effective and associated with minimum, or less, side effects.
Globally, herbal products have gained an important role in the management of obesity as they are effective, affordable, and also associated with fewer side effects (13, 14). Nutmeg or mace, scientifically known as Myristica fragrans, is commonly used as a spice and it belongs to the family of Myristicaceae (15). It is used not only in culinary preparations but also as an alternative medicine for its anti-inflammatory, aphrodisiac, memory enhancer, and anticancer properties (15, 16). In Ayurvedic medicine it is used to suppress appetite, hyperlipidemia, atherosclerosis and heart ailments. It is also used to treat rheumatism, sore mouth, diarrhoea and insomnia (17, 18). Pancreatic lipase (PL) is the key enzyme in tha gastrointestinal tract (GIT) for the digestion and absorption of dietary fat (19). There are reports in the literature, but scientific evidence has not been established in obesity yet. For the treatment of metabolic syndrome, including obesity and diabetes, PL plays a key role in reducing fat percentage in the body. So, the present research was conducted to establish the lipase inhibitory effect of Myristica fragrans extract by different approaches (in vitro, in silico, in vivo, and in silico). This study may provide evidence of the antiobesity activity of nutmeg , which and may prove to be helpful in preventing and treating obesity safely, effectively, and without side effects.
MATERIAL AND METHODS
Collection of plant material and preparation of extract (20)
Fresh seeds of Myristica fragrans were purchased from an Ayurvedic medical store of Nandyal and authenticated by the Department of Botany, Rayalaseema University, Andhra Pradesh, India by Dr. K. Venkata Ratnam, PhD Assistant Professor of botany. The herbarium number of the nutmeg is 201/2016-17. Dried nutmeg was ground to fine powder, which was further used for the preparation of extract with ethyl alcohol by using a Soxhlet apparatus (20).
Pancreatic lipase inhibition assay (in vitro) (21)
The inhibitory activity of porcine pancreatic lipase (PPL) was assessed using p-nitrophenyl butyrate (p-NPB) as a substrate. The method used for assessing PL activity was modified from that previously explained by Lai et al (21).
To determine lipase inhibitory activity, extracts (final concentrations 25, 50, 100 ìg/mL) and orlistat (with the same concentrations) as a positive control were pre-incubated with PPL for one hour in a potassium phosphate buffer (0.1 mM, pH 7.2, 0.1% Tween 80) at 30°C before assaying PPL activity. After incubation at 30°C for five minutes, the amount of p-nitrophenol released in the reaction was measured at 405 nm using a UV-visible spectrophotometer. The PL inhibitory activity was calculated accor ding to the standard formula (22): Inhibition =[(B-b)/(A-a)]X100, where A is the activity of the enzyme without inhibitor, a the negative control without inhibitor, B expresses enzyme activity with inhibitor, and b the negative control with inhibitor.
The IC50 value of extracts was determined at a concentration of 25, 50, 100 ìg/mL, with orlistat being used as a positive control. The value of IC50 was calculated by the following formula:
LC-MS analysis (23)
LC-MS analysis was done to isolate and identify the phytochemicals in MFE. It was carried out by using column chromatography and purity was tested by HPLC equipped with Phenomenex Luna C18 column (150–4.6 mm, 5 m). To identify the wavelength and peak position, a PDA detector was used. The structure was confirmed by 1 H NMR and 13C NMR. The chemical structure of the isolated compound was determined by using a physicochemical and spectro- scopic data analyser (1D- and 2D-NMR and MS data) (23).
Molecular docking study (in silico) (24)
The docking simulation was carried out using Auto dock tools (24). The selected ligand molecules of tetrahydrofuran and orlistat were docked to target proteins AMPK (PDB: 4ZHX) and pancreatic lipase (PDB: 1LPB) with the molecules treated as a rigid body and the ligands being flexible. The docking experiment consisted of 10 docking runs with 150 individuals and 500,000 energy evaluations using a Lamarckian genetic algorithm (25). The docked conformation which had the least binding energy was selected to analyze the mode of binding (24). Ligand- protein interactions were analysed by a PyMol molecular viewer (The PyMOL Molecular Graphics System, Version 2.1.0 Schrödinger, LLC) and the hydrophobic effect of ligands was developed by Discovery studio 3.5 client and PoseView.
in vivo study
Fat estimation was done in cafeteria diet (CD) induced obese albino rats. The study was conducted in the Department of Pharmacology, Santhiram Medical College (SRMC), Andhra Pradesh, India. After initial acclimatization for seven days, all procedures involving laboratory animals were in accordance with the institutional animal ethical committee (Protocol approval No: (IAEC/SRMC/2017/5).
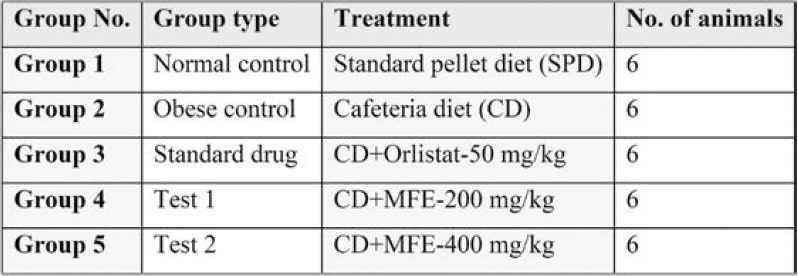
Study setting – Thirty healthy albino rats (male/female) weighing between 150-160 grams were taken from the central animal house (Reg No: 897/PO/Re/S/05/ CPCSEA), Santhiram Medical College, India, and randomly divided into five groups with six animals each (n=6) (Table 1).
Induction of obesity – Normal control ratsGroup 1) were fed with standard pellet diet containing all recommended macro- and micronutrients prepared according to AIN-93 (American Institute of Nutrition) guidelines with water ad libitum. Rats in Group 2 to Group 5 were fed with cafeteria diet for 15 weeks to induce obesity (26), and from week 16 onwards, different doses of Myristica fragrans (200, 400 mg/kg b. wt.) and standard drug (orlistat 50 mg/kg) were supplemented for 70 days (10 weeks) along with CD, as mentioned. The study had a total duration of 25 weeks.
High fat/cafeteria diet consisted of three variants along with pellet diet: 1) condensed milk + cheese bread + peanuts + pellet chow (4:1:4:1); 2) chocolate + butter biscuits + dried coconut + pellet chow (3:2:4:1); 3) cheese + boiled potatoes + butter + pellet chow (4:2:4:1). The different variants were fed on three alternate days throughout the obesity induction period of 15 weeks (26).
Estimation of faecal fat content (in vivo) (27)
Faecal matter was collected by using forceps from each rat and placed in a 50 mL conical tube that was labelled with the rat number of each group; 1 000 mg of faecal material was collected till the end of the 70th day, then completely dried and powdered. Faecal lipids were extracted with chloroform and methanol (2:1 v/v), then dissolved in 1% triton and estimated by the method of Daniel Kraus et al (27). The procedure is detailed below:
– One gram of dried faeces was homogenized in 20 mL of the chloroform-methanol mixture (2:1) and the homogenate was centrifuged at 3 000 rpm for 10 minutes.
– The supernatant was completely transferred to a fresh tube and 0.73% of sodium chloride solution was added at the rate of 0.2 mL per each mL of taken supernatant.
– After proper mixing, centrifugation at 2 400 rpm for 15 minutes was done.
– The top layer containing the non-lipids was discarded and the middle phase was gently rinsed with a chloroform-methanol mixture (2:1) without disturbing the lower phase.
– The lower organic phase containing lipids was transferred to a round bottom flask.
– The organic solvent was evaporated under vacuum using a rotary evaporator at 55–60°C with 150 rpm.
– After evaporation, the resulting residue was weighed, which indicated the total lipid content per gram of faeces (28).
Histopathological study of adipose tissue (in silico study) (28)
The adipose tissue, which was collected from five groups of rats, was kept in 10% formalin and processed to paraffin wax. Standard sections of 5 ìm were cut with a microtome, fixed on slides and stained using the haematoxylin @ eosin (H@E) staining procedures to be further observed under an optical microscope (10x) and photographed (29).
Statistical analysis
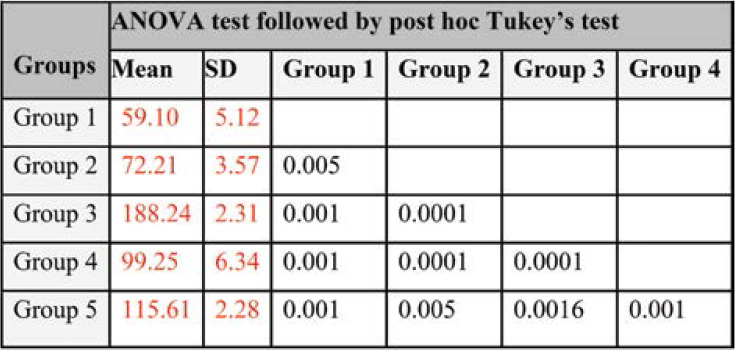
The results were expressed as mean±SD (n=6). Statistical analysis was carried out using Graph Pad Prism 8 software (version 4.03). Data was analyzed by one way ANOVA, followed by post-hoc Tukey’s multiple comparison test. Treatment groups were compared with obese group and obese group compared with normal control group. P<0.05 was considered statistically significant.
RESULTS
Preparation of plant extract
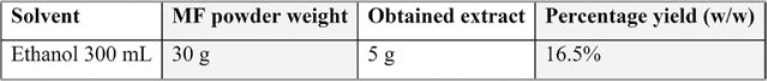
The plant extract was prepared with nutmeg powder by using a Soxhlet apparatus on continuous hot extraction process for 24 hours with ethyl alcohol. The percentage yield was higher (16.6%) with ethyl alcohol (Table 2). Percentage yield=(Obtained extract)/(Total powder) X 100.
Pancreatic lipase inhibition assay (in vitro)
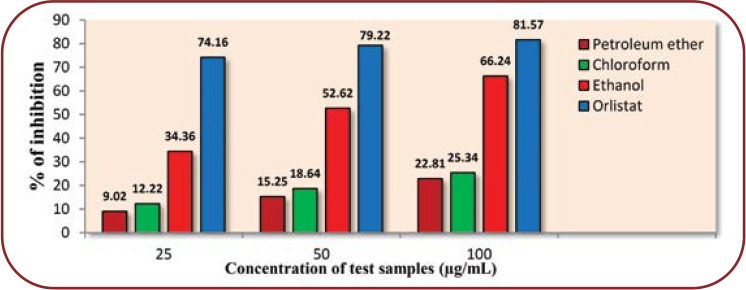
Anti-lipase activity was determined at different concentrations (25, 50, 100 μg/mL) for PPL inhibition. The inhibitory activity of MFE on pancreatic lipase ethanolic extract at a concentration of 100 μg/mL significantly inhibited (66.24%) PPL when compared to orlistat (100 ±g/mL) standard compound, a well-known anti-lipase agent, significantly inhibiting (81.57%) PPL activity (Figure 1).
The IC50 value of orlistat (6.47±0.42 ìg/mL) had a more potent anti-lipase activity than various extracts. Among the three extracts, the ethanolic one (11.35±0.11 ±g/mL) showed a better inhibitory effect on lipase than petroleum ether (16.05±0.12) ìg/mL and chloroform (18.14±0.31 ìg/mL) extracts.
LC-MS analysis
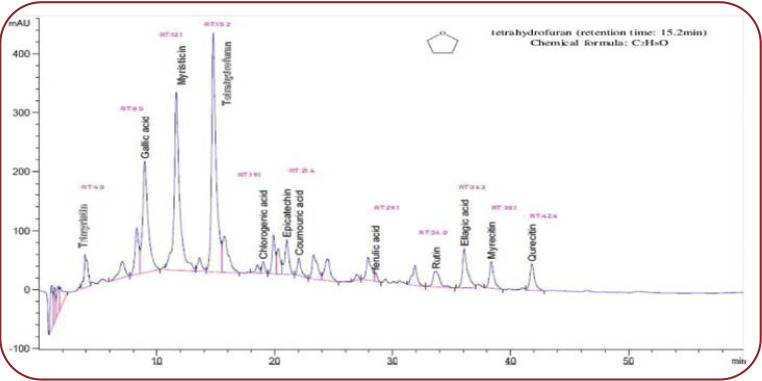
Phytochemicals were isolated by column chromatography and purity was tested by HPLC equipped with Phenomenex Luna C18 column (150–4.6 mm, 5 m). The chemical structure of the isolated compound was determined by using a physicochemical and spectroscopic data analyser (1D- and 2D-NMR and MS data). The presence of THF (MW 536) was detected (the highest peak) at RT 15.2 min along with other compounds (Figure 2).
Molecular docking study (in silico)
The docking of phytoconstituents (THF) with pancreatic lipase (PDB ID: 1LPB) revealed pancreatic lipase inhibiting properties as compared to the standard drug, which was evident from the MolDcok score and H-bond energy (Table 3).
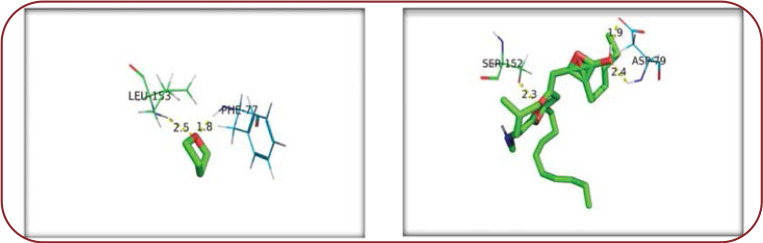
In our docking study, Phe77 and Leu153 of PL had a significant affinity by forming hydrogen bonds with different hydrogen and oxygen atoms of THF and orlistat molecules (Asp79, Ser152) (Figure 3). Tetrahydrofuran had a PL inhibitory action.
Estimation of fecal fat (in vivo)
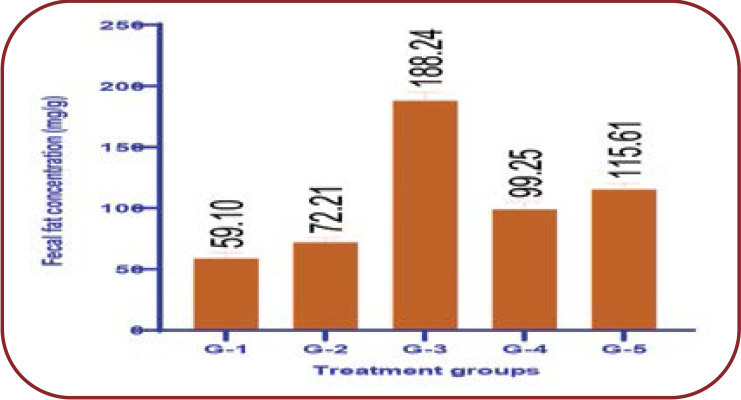
The highest excretion of lipids was observed in the faecal matter of MFE treated groups as compared to normal controls. Specifically, the groups that received orlistat and a high dose of MFE (400 mg/kg) had a high content of fat in faeces (188.24 g and 99.25 g, respectively) when compared with the obese group (P <0.0001), maybe as a result of the PL inhibitory effect in GIT (Figure 4).
Histopathological study of adipose tissue
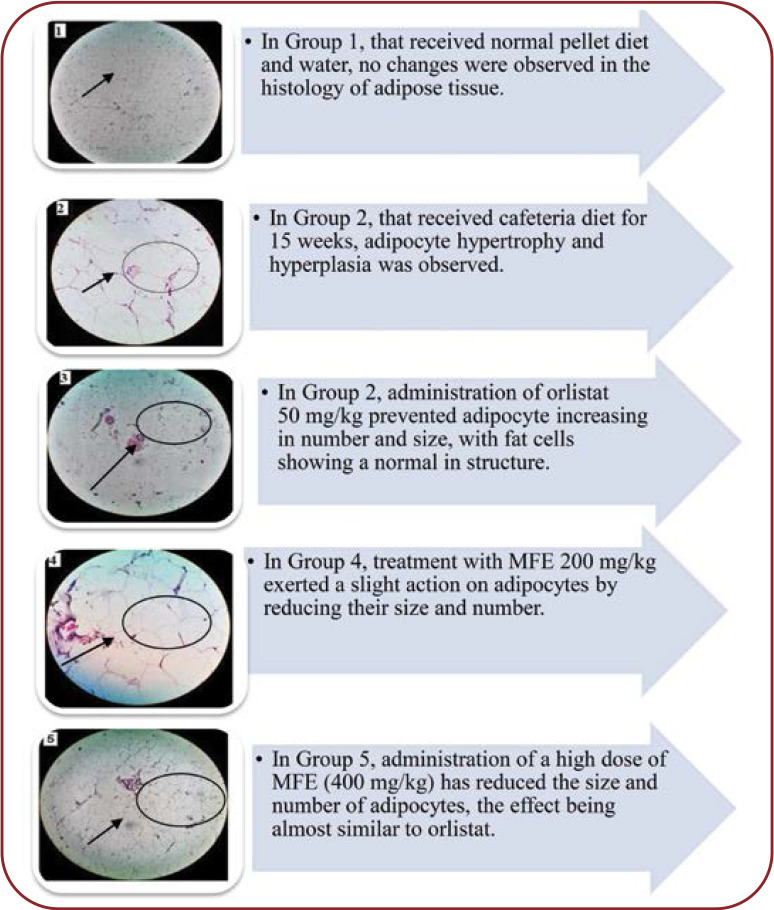
The histological study of adipose tissue has revealed the potency of anti-obesity effect of nutmeg. Histology sections of retroperitoneal adipose tissue of CD-fed rats showed hypertrophy and hyperplasia of adipocytes (picture 2) when compared to the normal control group (pic- ture 1). Supplementation of MFE decreased the size and volume of fat cells in a dose-dependent manner, with the maximum effect being observed at 400 mg/kg, as illustrated by picture 5 indicating the antiadipogenic activity of nutmeg extract (Figure 5).
DISCUSSION
Obesity is considered as metabolic syndrome and now it turns into the “new world syndrome” because people of every class and economic background are being affected all over the world (29). Current treatment strategies for obesity are not able to reach the need precisely in terms of safety and long-lasting effects. Hence, further research is required to develop newer safer alternative drugs for the management of obesity. In the present study, Myristica fragrans was selected based on the relevant literature and medicinal values of nutmeg.
The pancreatic lipase inhibitory effect of Myristica fragrans has not been explored earlier. So, the present study aimed to prove its potentiality on PL in different aspects.
Before conducting the in vivo study, an acute toxicity study was conducted to evaluate the safety of nutmeg extract in rats. After oral administration of 2 000 mg/kg of ethanolic extract of Myristica fragrans to experimental rats, they were observed at regular intervals of 1, 2, 4, 8, 12 and 24 hours, then up to 14 days for any abnormal toxic behavioural, neurological, and autonomic signs. Rats did not show any behavioural changes (reflexes, restlessness, change in sleeping pattern and locomotive disability). Also, the animals did not exhibit any physiological changes (loss of hair and redness of eyes or blindness). Mortality was not observed after Days 1, 7 and 14. So, the ethanolic extract of Myristica fragrans can be included in the category of substances with no toxicity.
Pancreatic lipase is a key enzyme for lipid absorption by hydrolysis of total dietary fats. Therefore, PL inhibition is suggested to be an effective therapy in the regulation of obesity (30). After the preparation of nutmeg ethanolic extract, an in vitro study has been initially conducted to assess its PL inhibitory property. The ethanolic extract exhibited a potent anti-lipase activity, which was very similar to that of orlistat (standard compounds). The present results support the anti-lipase property of MFE, which can manage the fat percentage in obesity.
The ethanolic extract of nutmeg was further examined for its specific chemical compounds by LC-MS analysis. Phytochemicals were isolated by column chromatography and purity was tested by HPLC. Structure was confirmed by 1 H NMR and 13C NMR. This study revealed the presence of THF (the highest peak) at RT of 15.2 min.
After detection of THF, a molecular docking study was done to observe the interaction between tetrahydrofuran and PL. The docking simulation was carried out using Auto dock tools (24). The selected ligand molecules of THF and orlistat were docked to target PL proteins (PDB: 1LPB) with the molecules treated as a rigid body and ligands being flexible. Phe77, Asp79, Ser152, and Leu153 were the key amino acid residues of the catalytic site in performing the inhibitory activity of lipase. This happens by changing the protein structure, and thereby inhibition occurs (24). In the docking study, PL had a significant affinity by forming hydrogen bonds with different hydrogen and oxygen atoms of tetrahydrofuran, showing that THF had a PL inhibitory action, which may be useful in reducing lipid absorption from GIT.
CONCLUSION
Inhibition of PL activity and augmentation of lipolysis are being considered to be effective ways in the management of body weight (31). The present study revealed a limitation in the absorption of lipids at the intestinal level, which caused more excretion of fats in the feces. This is the potential mechanism by which nutmeg extract prevented weight gain in HFD-fed rats.
Histological examination of adipose tissue proved the anti-adipogenic and anti-obesity activity of nutmeg as evident from the reduced size of adipocytes in the nutmeg extract treated groups. Fat percentage decreased because dietary fat absorption inhibited. This action may be due to pancreatic lipase inhibitory action of tetrahydrofuran.
The present study results support the antiobesity activities of an ethanolic extract of Myristica fragrans by in vitro, in silico, in vivo and histopathological studies. Therefore, nutmeg may be a good alternative candidate for the treatment of obesity and metabolic syndrome.
Conflict of interests: none declared
Financial support: none declared.
TABLE 1.
Research design and grouping of animals
TABLE 2.
Myristica fragrans extract yielded with ethyl alcohol
FIGURE 1.
Anti-lipase activity of MFE and orlistat
FIGURE 2.
LC-MS analysis showing tetrahydrofuran on the highest peak with RT 15.2 min
TABLE 3.
in silico study of tetrahydrofuran effect on pancreatic lipase
FIGURE 3.
Docking results of tetrahydrofuran and orlistat with pancreatic lipase
TABLE 4.
Results of one-way ANOVA followed by post hoc Tukey’s test. Values were represented as mean±SD (n=6) and statistically analysed by one-way ANOVA, followed by a post hoc multiple comparison test, with p <0.001 extremely significant, p <0.01 very significant, p <0.05 significant, and p >0.05 not significant
FIGURE 4.
Effect of nutmeg extract on intestinal PL (inhibitory effect on fat absorption)
FIGURE 5.
Effect of MFE on adipose tissue after 70 days of treatment
Contributor Information
Vangoori YAKAIAH, Santhiram Medical College, Nandyal (AP), India.
Anusha DAKSHINAMOORTHI, Sri Ramachandra Institute of Higher Education and Research, Chennai, India.
Sree SUDHA TY, Department of Pharmacology, AIIMS-Raipur, Chattisgarh, India.
References
- 1.WHO | World Health Organization. Fact Sheets. www.who.int.news.facts sheets. 2018.
- 2.Birari RB, Gupta S, Mohan CG. Anti-obesity and lipid-lowering effects of Glycyrrhiza chalcones. Experimental and computational studies. Phytomedicine. 2011;18:795–801. doi: 10.1016/j.phymed.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Shiva Kumar A, et al. Antiobesity, antioxidant and hepatoprotective effect of Diallyl trisulphide (DATS) alone or in combination with Orlistat on HFD induced obese rats. Biomedicine & Pharmacotherapy. 2017;93:81–87. doi: 10.1016/j.biopha.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 4.Brahma Naidu P, Nemani H, Meriga B. Mitigating efficacy of piperine in the physiological derangements of high fat diet-induced obesity in Sprague Dawley rats. Chem Biol Interact. 2014;221:42–51. doi: 10.1016/j.cbi.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Cooke D, Bloom S. The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat Rev Drug Discovery. 2006;5:919–931. doi: 10.1038/nrd2136. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Fact Sheets: Obesity and overweight [ http://www.who.int/mediacentre/ factsheets/en/index. html. 2013.
- 7.World Health Organization, Global health observatory (GHO) data. Obesity Risk Factors. http://www.who.int/gho/ncd/risk_ factors/obesity_text/en/index.html. 2017.
- 8.Behla S, Misra A. Management of obesity in adult Asian Indians. Indian Heart Journal. 2017;69:539–544. doi: 10.1016/j.ihj.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg C, Khan SA, Ansari SH, Garg M. Prevalence of obesity in Indian women. Obes Rev. 2010;11:105–108. doi: 10.1111/j.1467-789X.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers R.J, Tschop M.H. Anti-obesity drugs: past, present and future. Dis Model Mech. 2012;5:621–625. doi: 10.1242/dmm.009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooke D, Bloom S. The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat Rev Drug Discov. 2006;5:919–993. doi: 10.1038/nrd2136. [DOI] [PubMed] [Google Scholar]
- 12.Ballinger A, Peikin S.R. Orlistat: its current status as an anti-obesity drug. Eur J Pharmacol. 2002;440:109–117. doi: 10.1016/s0014-2999(02)01422-x. [DOI] [PubMed] [Google Scholar]
- 13.Kasbia, Gursevak S. Functional foods and nutraceuticals in the management of obesity. Nutrition & Food Science. 2005;35:344–352. [Google Scholar]
- 14.Mukherjee M. Human digestive and metabolic lipases – a brief review. J Mol Catal B Enzyme. 2003;22:369–376. [Google Scholar]
- 15.Preetee Jaiswal. Biological Effects of Myristica fragrans. ARBS Annual Review of Biomedical Sciences. 2009;11:21–29. [Google Scholar]
- 16.Rosengarten F Jr. The book of spices, 1st ed, Livingston Publishing Company. 1969. p. 489.
- 17.Olajide OA, Ajayi FF, Ekhelar AL, Awe SO. Medicinal uses of Nutmeg. Phytother Res. 1999;13:344–345. doi: 10.1002/(SICI)1099-1573(199906)13:4<344::AID-PTR436>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A, Mathur R, Dixit VP. Prevention of hypercholesterolemia and atherosclerosis in rabbits after supplementation of Myristica fragrans seed extract. Indian J Physiol Pharmacol. 1995;39:407–410. [PubMed] [Google Scholar]
- 19.Sang-M Jeon. Regulation and functions of AMPK in physiology and diseases. Experimental and Molecular Medicine. 2016;48:2092–2098. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qing-Wen Zhang. Techniques for extraction and isolation of natural products: a comprehensive review. Chinese Medicine. 2018;13:20. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai HY, Ong SL. In vitro lipase inhibitory effect of thirty-two selected plants in Malaysia. Asian J Pharm Clin R. 2014;7:3. [Google Scholar]
- 22.Urmi Chedda. In vitro pancreatic lipase inhibition potential of commonly used Indian spices. IOSR Journal of Pharmacy. 2016;6:10–13. [Google Scholar]
- 23.Rao VR, Raju SS, Sarma VU, et al. Simultaneous determination of bioactive compounds in Piper nigrum L. and a species comparision study using HPLC-PDA. Nat Prod Res. 2011;25:1288–1294. doi: 10.1080/14786419.2010.535158. [DOI] [PubMed] [Google Scholar]
- 24.Morris GM, Huey R, Lindstrom W, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. Journal of Computational Chemistry. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huey R, Morris GM, Olson AJ, Goodsell DS. A Semiempirical Free Energy Force Field with Charge-Based Desolvation J. Computational Chemistry. 2007;28:1145–1152. doi: 10.1002/jcc.20634. [DOI] [PubMed] [Google Scholar]
- 26.Hebbani Nagarajappa, Shivaprasad. Effect of Coleus forskohlii extract on cafeteria diet-induced obesity in rats. Pharmacognosy Research. 2014;6:42–45. doi: 10.4103/0974-8490.122916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniel Kraus, Qin Yang. Lipid extraction from Mouse Feces. Bio Protoc. 2015;5:e1375. doi: 10.21769/bioprotoc.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemani Harishankar, Putcha Uday Kumar. Obesity-associated pathophysiological & Histological changes in WNIN obese mutant rats. Indian J Med Res. 2011;134:330–340. [PMC free article] [PubMed] [Google Scholar]
- 29.Terence J Wilkin. Metabolic syndrome: maladaptation to a modern world. Journal of the Royal Society of Medicine. 2004;97:511–519. doi: 10.1258/jrsm.97.11.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siew-Ling Ong. In vitro lipase inhibitory effect of thirty two selected plants in Malaysia. AJPCR. 2017;2:12. [Google Scholar]
- 31.Yoshikawa M, Shimoda H, Nishida N, et al. Salacia reticulata and its polyphenolic constituents with lipase inhibitory and lypophilic activities have mild antiobesity effects in rats. J Nutr. 2002;132:1819–1824. doi: 10.1093/jn/132.7.1819. [DOI] [PubMed] [Google Scholar]