Abstract
Background: Concomitant atrial fibrillation (AF) in non-ST segment elevation acute coronary syndrome (NSTE-ACS) patients complicates the decision-making process regarding short- and long-term antithrombotic strategies. Patient profiles and usage rates of different antithrombotic combinations in this patient subgroup in Romania are poorly described.
Study aim: To evaluate the relationship between LA dimensions and AF recurrences (AFR) using echocardiography.
Methods: We enrolled 40 patients (56 ± 10 years; 73% males) who underwent a first RFCA for paroxysmal AF. Bi- (2DE) and three-dimensional (3DE) echocardiography was performed prior to RFCA and at 12-months follow-up. Rhythm control was monitored for up to two years after the intervention by periodic ECG Holter monitoring.
Results: Atrial fibrillation recurrences were recorded in 21 patients (52%) in the first year after RCFA. The only predictor of outcome from pre-ablation LA parameters was 3DE minimum LAVi (p = 0.042), that explained 21.4% of AF recurrences in the first year, with a cut-off value of 21.29 mL/m². The mean 3DE min LAVi was 24.29 ± 8.01 mL/ m² and patients without AFR in the first year had a lower LAVi than those with AFR (20.92 ± 6.19 mL/m² vs. 27.25 ± 8.43 mL/m², p=0.028). One year after RFCA, a decrease in medio-lateral, superior-inferior diameters and volumes were recorded in AF free patients. Eleven patients (27%) had AF recurrences after the first year and LAV 12-months after RFCA were found to be predictors of long-term outcome, with minimum LAVi as the strongest predictor of recurrences (p=0.014), explaining 36% of episodes, with a cut-off over 22.49 mL/m².
Conclusion:Radiofrequency ablation controls LA remodeling in patients with clinical success in terms of AF freedom. Left atrium 3DE volumetry is accurate in predicting RFCA outcome.
Keywords:atrial fibrillation, atrial fibrillation recurrences, left atrial dimensions, left atrial volume.
INTRODUCTION
Left atrium (LA) size assessed by echocardiography has a powerful prognostic value in multiple cardiovascular diseases, including atrial fibrillation (AF) (1). The size of LA determines the risk of AF recurrence in patients with intended rhythm control.
Echocardiography is essential in evaluating AF effect on LA size and volume, and helps identify patients at risk of AF recurrence after radiofrequency catheter ablation (RFCA) (2). Structural remodeling of LA is asymmetric and the anteroposterior size, usually evaluated in clinical practice, can underestimate LA dimensions. Left atrium volumes (LAV) are more accurate than linear dimensions (3) and, when assessed by three-dimensional echocardiography, the have the advantage of eliminating geometrical assumptions (4).
Echocardiographic predictors for AF recurrence (AFR) were evaluated in multiple studies; depending on the characteristics of the enrolled population, each parameter seems to have its role. In most studies, anteroposterior diameter and LA index volume as a marker of LA dilation predicts an unfavorable outcome after RFCA (5).
METHODS
Study design
We enrolled 40 patients who underwent a first RFCA for symptomatic drug-refractory paroxysmal AF in a low-volume ablation centre. We excluded those with non-paroxysmal AF episodes, repeated ablative procedures, ischemic or structural cardiomyopathies, significant valvular diseases, uncontrolled risk factors for AFR (thyroid disease, sleep apnoea, and chronic alcohol abuse). All patients signed an informed consent and the study protocol was approved by the Local Hospital Ethics Committee.
The ablation protocol was the same in all patients. Briefly, LA is accessed through two transseptal punctures (performed only with contrast and pressure control), where two catheters are placed: a circular duodecapolar catheter (Lasso 2151 Biosense Webster, Inc, Diamond Bar, Calif) and an ablation unidirectional catheter (Thermocool Smartouch Biosense Webster, Diamond Bar, Calif). 3D electroanatomic mapping is performed with CARTO (Biosense Webster, Inc) system, and electrograms are filtered and displayed on a commercially available electrophysiological recording system (Cardiolab, GE, Houston, Tex). The ablation lines are placed at the proximal pulmonary vein antrum. Successful isolation is defined by entrance and exit block.
Transthoracic echocardiography was performed prior the procedure and repeated at 12-months follow-up. We used a GE Vivid 9 system (GE Vingmed, Ultrasound, AS, Horten, Norway). All patients were in sinus rhythm (SR) at the time of acquisitions and an average of three cardiac cycles were recorded. Left atrium volumetric assessment was done in a 4- and 2-chamber dedicated optimized view, using area-length method; all volumes were indexed to body surface area. 3DE acquisition of LAV was done in the 4-chamber view at end-expiratory apnoea. Maximum (max) LAV was determined at end-systole, just before mitral valve opening. The LA minimum (min) volume was determined at end-diastole, after mitral valve closure. Image analysis was performed offline using GE Echo PAC software version BT13. All images and measurements were performed by a single operator using a standard protocol, according to guidelines of the European Association of Cardiovascular Imaging guidelines and recommendations of the American Society of Echocardiography. Three-dimensional volumetric evaluation was performed using the GE Echo PAC 4D LVQ software, that is designed for the left ventricle but can also be used for LA volumetry (6).
We considered AF recurrence any episode of documented atrial tachyarrhythmia that lasted more than 30 seconds. Periodic assessments were done using 48-hour ECG monitoring and outcome was recorded up to 24 months.
The study aim was to evaluate atrial remodeling using bi- (2DE) and three-dimensional (3DE) echocardiography, and to assess LA volumes as independent predictors for AF recurrence (AFR).
Statistical analysis
Continuous variables are presented as mean ± SD (standard deviation) for uniform distribution and comparisons of the central tendency of baseline characteristics and endpoints used Student t-test, while abnormally distributed continuous variables are presented as mean (IQR) and non-parametric tests (Mann-Whitney U rank-sum test) were used for comparison. All p-values were two-sided and a p-value<0.05 was considered statistically significant. Variables with statistically significant influence over the outcome were processed with univariate linear regression. We used the following criteria to identify predictors for AFR: area under the ROC curve (AUROC) >0.650, Hosmer-Lemeshow goodness-of-fit test p >0.05 and p<0.1. The odds ratio (OR) was generated for each independent predictor. Previously identified predictors have been computed with multivariate logistic regression. The best model predicting recurrences was selected based on calibration Akaike’s Information Criterion (AIC) and Bayesian Information Criterion (BIC) as tests for Goodness-of-Fit with lowest values and Nagelkerke R² test. A threshold for AFR risk was identified in the training cohort and the sensitivity and specificity identified were reported. Validation of results was done with Hanley @ McNeill test with a p-value >0.05. We analyzed the data using SPSS (Statistical Package for the Social Sciences) version 26 software (IBM SPSS Statistics, Armonk, NY, USA; IBM Corp.).
RESULTS
We enrolled 40 patients (56 ± 10 years; 73% males) who underwent a first RFCA for paroxysmal AF. The mean duration of study monitoring was 2.1 ± 0.67 years. Baseline characteristics of the study population are summarized in Table 1.
Mean LVEF was 55.26 ± 6.40%, and diastolic dysfunction (7) was diagnosed in 25% (10/40) of patients. In our study group, mean LA anteroposterior (AP) diameter was 39.83 ± 4.45 mm, and 47.5% (19/40) of patients had a diameter > 40 mm. The mean maximum left atrium volume indexed (LAVi) at baseline was 38.15 ± 8.78 ml/m², with 72.5% (29/40) of patients having a dilated LAVi (> 34 mL/m2).
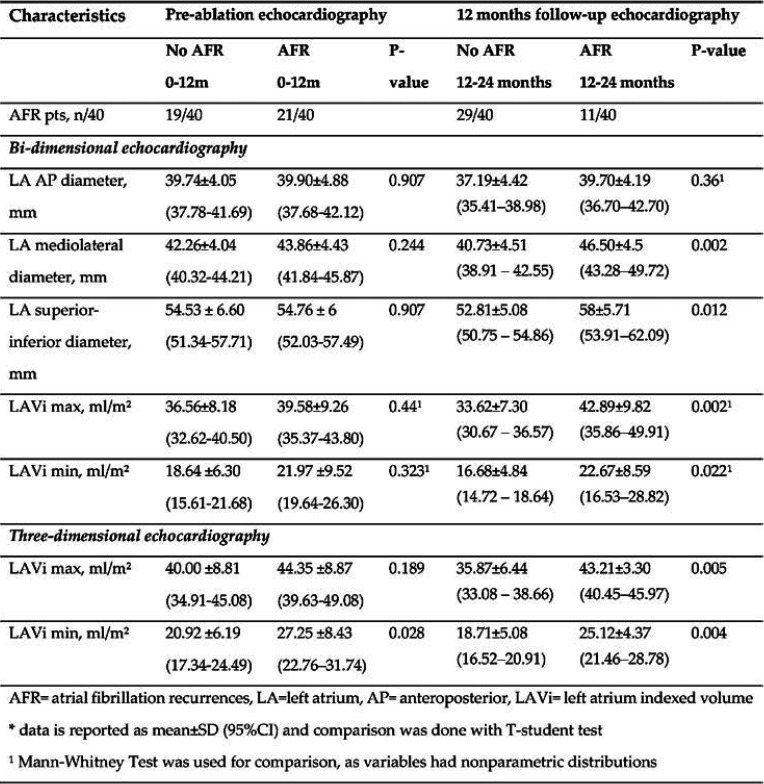
A total of 52% (21/40) patients had AFR in the first 12 months, while 27% (11/40) of subjects had AF recurrences after the first year. Prior to the RFCA procedure, no significant differences were observed regarding LA linear dimensions between patients who would develop AFR in the first years and those who would maintain SR, except for 3DE min LAVi that was significantly lower in AF-free pts (p=0.028). Twelve months after the intervention, patients who remained AF free had lower LA diameters and volumes, assessed by both 2DE and 3DE procedures, while those with AF recurrences did not show reverse remodeling of LA dimensions (Table 2).
Left atrial dimensions as predictors of outcome
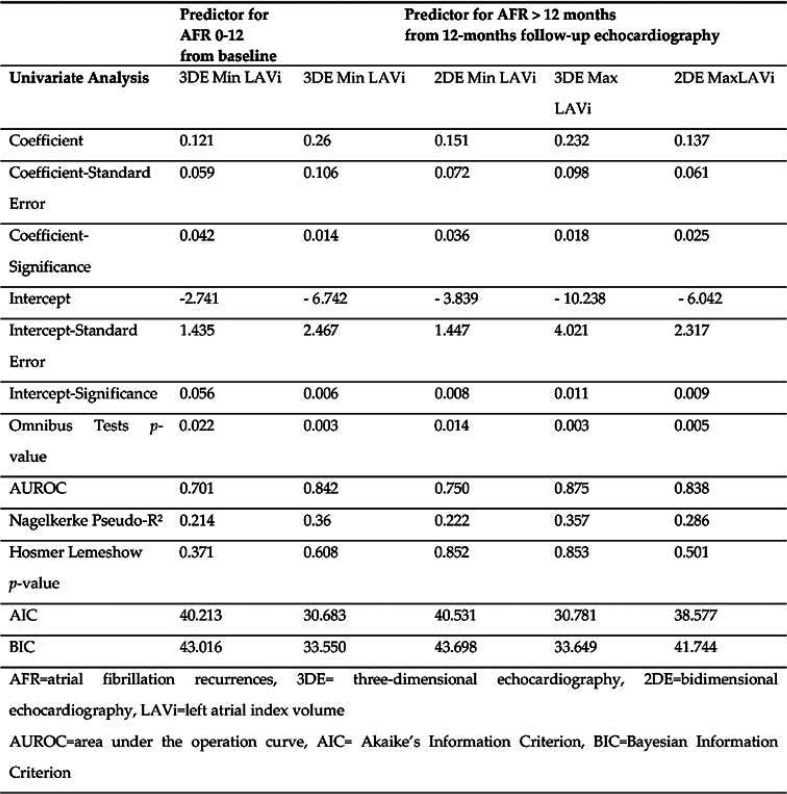
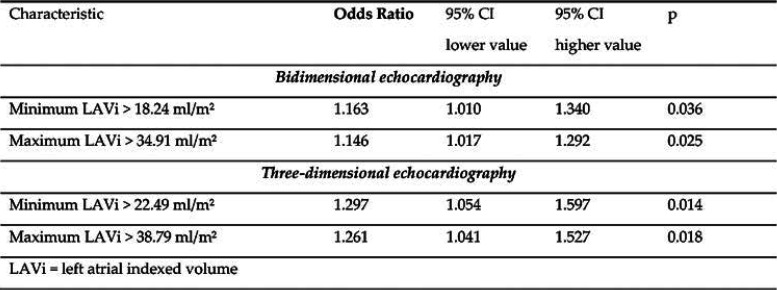
We evaluated 3D min LAVi’s prognostic role regarding ablation outcome, as its baseline value was the only echocardiographic parameter significantly different between the two groups divided by AF recurrences in the first year after RFCA. We determined that 3DE min LAVi was a predictor of AF recurrences (p=0.042), explaining 21.4% of episodes that appeared in the following 12 months after a first RFCA for paroxysmal AF. The calculated cut-off value was 21.29 mL/m², with a sensibility of 68.8% and specificity of 64.4% (AUROC=0.701, 95% CI = 0.513–0.889; p = 0.061). Patients with a min LAVi over the threshold had a 12% higher risk of AF recurrence in the first year after RFCA than those with a lower min LAVi (OR = 1.128, 95% CI = 1.004-1.268, p = 0.042).
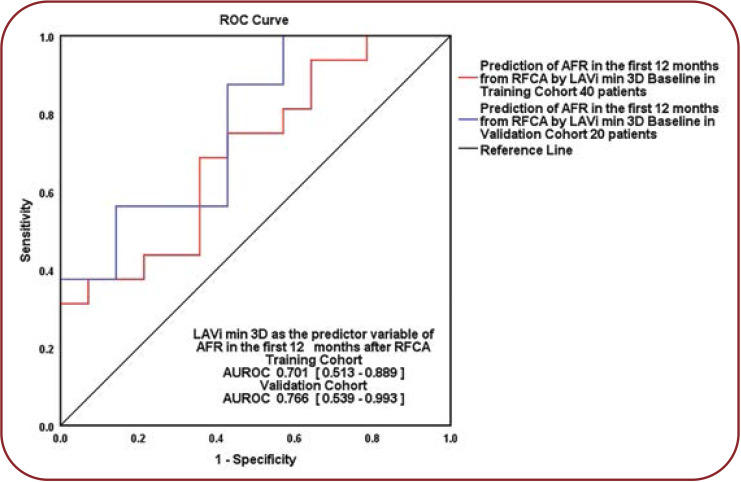
The equation of prediction was computed as follows: Ln (OR for AFR) = - 2.741 + 0.121 x [baseline 3DE min LAVi]. We validated our result on a 20-patient internal cohort (randomly chosen, respecting the percentages of 52%: nine AF-free patients and 11 with AF recurrences: AUROC = 0.766, 95% CI = 0.539-0.993, p = 0.063) (Figure 1).
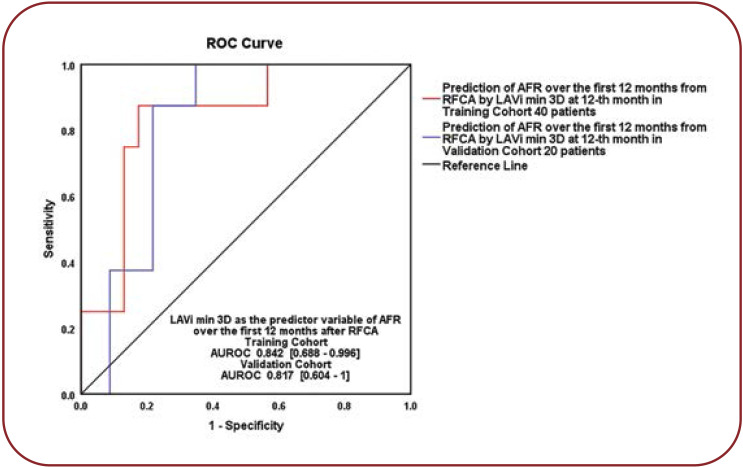
We compared 3DE LAVi values from the 12-month assessment and determined that is the strongest predictor of outcome (p=0.014), explaining 36% of latter AFR. We calculated the Patients with 3DE min LAVi over the cut-off value >22.49 mL/m² (AUROC = 0.842, 95% CI = 0.688-0.996, p = 0.004, 87.5% sensibility and 82.6% specificity), had a 29% higher risk of AFR after the first year (OR = 1.297, 95% CI = 1.054-1.597, p = 0.014). The prediction equation is the following: Ln (OR for AFR >12 months) = - 6.742 + 0.26 x [3DE min LAVi]. We validated the result on a randomly assigned subgroup from our study population (AUROC = 0.817, 95% CI = 0.604-1; p = 0.045), with a good superposition of the two ROC curves (Figure 2).
3DE min LAVi shows a significant reverse remodeling after RFCA, with a decrease in mean volume by 5.24 mL/m² (p = 0.02) 12-months after RFCA. Reverse remodeling is significant in AF-free patients (p = 0.047), but not in patients with AFR (p=0.327). Patients who will no longer develop AFR after the first year also show a significant reverse remodeling of min 3DE LAVi (p=0.034) compared to pre-ablation values.
We have also found significant differences between patients who would remain AF-free and those who would have AFR after the first year: medio-lateral and superior-inferior LA diameters, LA min and max volumes assessed by 2DE and 3DE; while all LAVi were found to be independent predictors of AFR 2DE min LAVi (p=0.036), 3DE max LAVi (p=0.025) and 3DE max LAVi (p=0.018) (Table 3 and Table 4).
DISCUSSION
The main findings of our study are as follows: LA anteroposterior (AP) diameter is not a prognostic marker for AF recurrences after RFCA, while LA remodeling evaluated by volumetric assessment adds important prognostic information.
Left atrium AP diameter as marker of LA dilation was intensely studied in relation to AFR risk after ablation. An increased diameter was associated with a higher risk of AFR (8). We did not find any differences in LA AP diameter with respect to AF recurrence at 12-month echocardiographic LA evaluation: superior-inferior, mediolateral LA diameters and LA volumes were significantly lower in patients with no AFR.
Left atrial volume reflects LA remodeling better than linear diameters and is more precise in assessing LA enlargement, a risk factor of AFR after ablation (9). Badano et al show that, by using only LA diameters and area measurements, more than 50% of patients may be misclassified regarding the degree of LA dilation, while 3D LAVi is more accurate than 2DE LAVi (10). The literature threshold of 2D LAVi over 34 mL/m² has a sensitivity of 70% and specificity of 91% to predict AF recurrences (11), while there is no cut-offs generally accepted for 3DE. Volumes assessed by 3D are larger compared with 2D, normality ranges for 3DE versus 2DE: max LAVi 43 vs 35 mL/m² and min LAVi 18 vs 14 mL/m² (12).
With respect to the above discussed reference values, prior to intervention mean LAVi was higher in both study groups (arrhythmia-free and AFR groups). At 12-month follow-up, arrhythmia- free patients will demonstrate a significant decrease in LAV compared to those with AFR. Conventional 2DE found no differences between groups, while 3DE enabled us to notice that min LAVi was lower in patients without AFR and also qualified for being a predictor of first year outcome after RFCA.
Most studies evaluated max LAVi as predictor of outcome after RF ablation. To our knowledge, limited data is available regarding LA min volume and its prognostic role in patients with AF. According to Fatema et al (13), min LA volume is associated with a first AF episode, while Schaaf et al proved (14) it to be the best predictor of paroxysmal AF. Motoc et al have also found that a 3DE min LAVi over 23.69 mL/m² was a predictor of AFR after paroxysmal AF ablation (15). We determined a cut-off over 21.29 mL/m² to predict first year AFR and explain our lower threshold as we did not exclude patients with AFR only in the blanking period.
Study limitations
This is a single center study with a small number of patients from a low-volume RFCA; therefore, larger clinical studies are warranted to confirm our findings. 3DE LAV was assessed with a non-dedicated software package and could not be compared to the gold standard method for chamber quantification: cardiac magnetic resonance. Nevertheless, the same software was used in other clinical trials and we enrolled a homogenous population, without any baseline clinical or biological parameters that may have influenced prognosis. Internal validation of our results showed a good superposition of the ROC curves.
Future perspectives
3DE can provide more accurate LA measurements and prognostic information about the outcome after AF ablation interventions. Routine measurement of minimum LAVi can impact clinical practice not only by selecting patients with a better chance to be “cured” by RFCA, but also by monitoring those who are at higher risk for AF recurrences.
CONCLUSION
Atrial remodeling is more accurately assessed using volumetric measurement by 3DE. 3DE min LAVi was the only independent predictor of outcome in the first year after RFCA, suggesting that subtle structural remodeling had an important role in triggering AF episodes. LA remodeling assessed by 3DE shows that minimum LAVi evaluated at 12 months after the intervention was the best predictor of long-term AF recurrence.
Conflict of interests: none declared
Financial support: none declared.
Acknowledgement: The authors of this paper would like to thank Prof. Dr. Serban Balanescu, Department of Cardiology, Elias Emergency University Hospital, for his support.
TABLE 1.
Patient characteristics (n = 40)
FIGURE 1.
Baseline LAVi over 21.29 mL/m² as predictor of AFR in the first year and internal validation. Training cohort versus validation cohort, Hanley@McNeil p = 0.58. LAVi = left atrial index volume, 3D = three-dimensional echocardiography, AFR = atrial fibrillation recurrences
TABLE 2.
Echocardiographic characteristics
FIGURE 2.
12-month min LAVi over 22.49 mL/m² as predictor of AFR after the first year. Training cohort versus validation cohort, Hanley@McNeil p=0.79. LAVi = left atrial index volume, 3D = three-dimensional echocardiography, AFR = atrial fibrillation recurrences
TABLE 3.
Independent predictors of AFR
TABLE 4.
Quantification of importance (OR) for 12-month LA volumes
Contributor Information
Lavinia-Lucia MATEI, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; Department of Cardiology, Elias Emergency University Hospital, Bucharest, Romania.
Liviu-Nicolae GHILENCEA, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; Department of Cardiology, Elias Emergency University Hospital, Bucharest, Romania.
Gabriel-Cristian BEJAN, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Sebastian STOICA, Department of Cardiology and Cardiovascular Surgery, Emergency University Hospital, Bucharest, Romania.
Ruxandra DRAGOI-GALRINHO, Department of Cardiology and Cardiovascular Surgery, Emergency University Hospital, Bucharest, Romania.
Calin SILISTE, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; Department of Cardiology and Cardiovascular Surgery, Emergency University Hospital, Bucharest, Romania.
Dragos VINEREANU, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; Department of Cardiology, Elias Emergency University Hospital, Bucharest, Romania.
References
- 1.Blume GG, Mcleod CJ, Barnes ME, et al. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr. 2011;12:421–430. doi: 10.1093/ejechocard/jeq175. [DOI] [PubMed] [Google Scholar]
- 2.Jeevanantham V, Ntim W, Navaneethan SD, et al. Meta-Analysis of the Effect of Radiofrequency Catheter Ablation on Left Atrial Size, Volumes and Function in Patients With Atrial Fibrillation. Am J Cardiol. 2010;105:1317–1326. doi: 10.1016/j.amjcard.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 3.Lester SJ, Ryan EW, Schiller NB, Foster E. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–832. doi: 10.1016/s0002-9149(99)00446-4. [DOI] [PubMed] [Google Scholar]
- 4.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 5.Liżewska-Springer A, Dąbrowska-Kugacka A, Lewicka E, et al. Echocardiographic predictors of atrial fibrillation recurrence after catheter ablation: A literature review. Cardiol J. 2020;27:848–856. doi: 10.5603/CJ.a2018.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuusisto JK, Järvinen VM, Sinisalo JP. Validation of 3D echocardiographic volume detection of left atrium by human cadaveric casts. BMC Med Imaging. 2018;18:43. doi: 10.1186/s12880-018-0282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang J, Wang Y, Tang K, et al. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: a systematic review and meta-analysis of observational studies. Europace. 2012;14:638–645. doi: 10.1093/europace/eur364. [DOI] [PubMed] [Google Scholar]
- 9.Njoku A, Kannabhiran M, Arora R, et al. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. EP Europace. 1;2018:20. doi: 10.1093/europace/eux013. [DOI] [PubMed] [Google Scholar]
- 10.Badano LP, Pezzutto N, Marinigh R, et al. How many patients would be misclassified using M-mode and two-dimensional estimates of left atrial size instead of left atrial volume? A three-dimensional echocardiographic study: J Cardiovasc Med. 2008;9:476–484. doi: 10.2459/JCM.0b013e3282f194f0. [DOI] [PubMed] [Google Scholar]
- 11.Shin S-H, Park M-Y, Oh W-J, et al. Left Atrial Volume Is a Predictor of Atrial Fibrillation Recurrence After Catheter Ablation. J Am Soc Echocardiogr. 2008;21:697–702. doi: 10.1016/j.echo.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Badano LP, Miglioranza MH, Mihaila S, et al. Left Atrial Volumes and Function by Three-Dimensional Echocardiography: Reference Values, Accuracy, Reproducibility, and Comparison With Two-Dimensional Echocardiographic Measurements. Circ Cardiovasc Imaging. 2016. [DOI] [PubMed]
- 13.Fatema K, Barnes ME, Bailey KR, et al. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr . 2016. [DOI] [PubMed]
- 14.Schaaf M, Andre P, Altman M, et al. Left atrial remodelling assessed by 2D and 3D echocardiography identifies paroxysmal atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2017;18:46–53. doi: 10.1093/ehjci/jew028. [DOI] [PubMed] [Google Scholar]
- 15.Motoc A, Abugattas J-P, Roosens B, et al. Left atrium remodeling predicts late recurrence of paroxysmal atrial fibrillation after second generation cryoballoon ablation. Cardiovasc Ultrasound. 2018;16:19. doi: 10.1186/s12947-018-0137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]