Abstract
Background: Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders affecting women of reproductive age. The goal of this study was to present the suitable diet recommended by the international literature for women with polycystic ovary syndrome to alleviate their symptoms.
Methods: A systematic search of electronic databases containing medical topics was conducted.
Results: A total number of 123 articles were retrieved and seven of them were relevant to our chosen topic concerning the diet-related polycystic ovary syndrome. According to research, it seems that diet plays a very important role on the clinical picture and laboratory findings of PCOS. According to the included studies, the change in the diet of women brought positive results in terms of clinical appearance of the syndrome. Ôhis review presents the type of diet that is deemed helpful in the clinical and laboratory picture of the syndrome.
Conclusion: In the future, more research should be conducted on a larger population with PCOS and for a longer period of time, during which subjects would be given a specific diet. It would also be important to compare diet to mild exercise and dietary supplementation.
Keywords:polycystic ovary syndrome, PCOS, polycystic ovaries, amenorrhea, PCOS and nutrition, diet and PCOS, diet in women with PCOS, nutrition in women with PCOS, dietary changes in women with PCOS, eating habits in PCOS.
BACKGROUND
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders affecting 6-10% of reproductive age women worldwide (1). There have been many attempts to define PCOS. Nevertheless, even today this abnormality is not fully understood due to the complexity and heterogeneity of the clinical picture. After a thorough study, diagnostic criteria include: a) hyperandrogenism, including hypertrichosis and/or hyperandrogenemia; b) oligomenorrhea and/or polycystic ovary disease; and c) exclusion of other causes of androgen hypersecretion or associated disorder (2).
Polycystic ovary syndrome is characterized by a phenotypic heterogeneity. However, some clinical symptoms and disorders, particularly menstrual disorders, are observed in the majority of patients. Oligo- or anovulation is among the most important disorders, which leads to oligomenorrhea or amenorrhea. Clinical hyperandrogenism (including hirsutism, alopecia, acne, and acanthosis nigricans) and biochemical hyperandrogenemia, usually assessed by detecting laboratory markers of androgen growth (3), are also identified. In addition, obesity and infertility are observed in 70-80% of women with PCOS (4). Finally, according to guidelines of the International PCOS Network, the image of the ovaries is as follows: ovarian volume >10 cm3 and/or at least 20 follicles 2-9 mm in size in at least one ovary is required (5). The etiology of PCOS appears to be multifactorial. Causes include hypothalamic-pituitary disorders, ovarian or adrenal disorders, genetic factors, and hyperinsulinemia due to insulin resistance.
Often, women with PCOS have insulin resistance – their bodies can make insulin but cannot use it effectively, increasing their risk for type 2 diabetes. Women with PCOS, especially if they are overweight, have a greater risk of developing other serious health problems such as diabetes (more than half of women with PCOS develop type 2 diabetes by the age of 40), gestational diabetes (which puts the pregnancy and the baby at risk and can lead to type 2 diabetes later in life for both the mother and child), heart disease, hypertension, high LDL-cholesterol and low HDL-cholesterol, sleep apnea (6). Polycystic ovary syndrome is also linked to depression and anxiety, though the connection between the two conditions is not fully understood (6).
Polycystic ovary syndrome has a variety of therapeutic approaches due to heterogeneity in its clinical manifestation. Initially, lifestyle changes are suggested, including more exercise and proper nutrition. These changes not only lead to weight loss, reduced insulin resistance, lower incidence of type 2 diabetes and reduced hyperandrogenism, but also enhance women's fertility as menstruation is restored. When pregnancy is not sought, the main treatments include birth control pills, progesterone, and metformin. If pregnancy is sought and weight loss does not help ovulation, menstruation can be induced with clomiphene citrate with or without metformin, aromatase inhibitors, gonadotropins, and ovarian drilling. In case none of the above ways leads to pregnancy then, the patient is encouraged to use in vitro fertilization (5).
It should also be noted that the microbiome may have an important role in PCOS. The hypothesis that alterations in the microbiome are involved in the genesis of PCOS has been postulated. Most of the surveys conducted so far have focused on the connection between intestinal bacteria with sex hormones and insulin-resistance – a relationship with hyperandrogenism has been described in the first case, and chronic low-grade inflammation by activating the immune system, with increased production of proinflammatory cytokines (which interferes with insulin receptor function, causing insulin resistance/hyperinsulinemia), in the second case; also, the role of gastrointestinal hormones such as Ghrelin and peptide YY (PYY), bile acids, interleukin-22 and Bacteroides vulgatus has been highlighted. The lower genital tract microbiome would be affected by changes in PCOS patients too (7).
Aim
The goal of the present study is to find which is the suitable currently recommended diet in the literature for women with PCOS to alleviate their symptoms.
METHODS
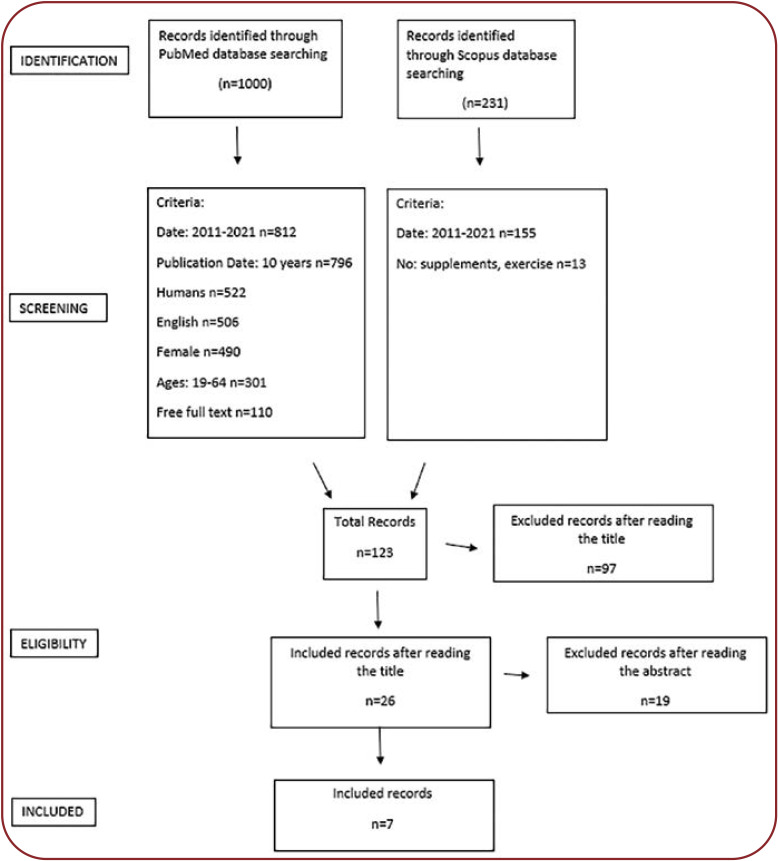
A systematic search of electronic databases containing medical literature was conducted (PubMed and Scopus). This review aimed to detect articles containing quantitative studies on PCOS in relation to nutrition, which were written in English between 2011 and 2021. The selection criteria included prospective quantitative studies that used samples of women aged between 19 to 64. The terms used to select the articles were “polycystic ovary syndrome”, “PCOS”, “nutrition”, “diet” “no supplements”, “no exercise”. The resulting articles were then evaluated by their title. Articles with a title that was not compatible to the purpose of the systematic review were excluded. Afterwards, abstracts of the remaining studies were appraised, and those that did not meet the inclusion criteria and purpose of the systematic review have been also excluded. Titles and abstracts were reviewed by two authors. For the remaining studies, a search for their full text version was done, and finally, those that did not meet the purpose of the review were rejected.
RESULTS
A total number of 123 articles were retrieved, but only seven of them were relevant on diet-related polycystic ovary syndrome.
In 2014, Pohlmeier and his colleagues (8) studied the effect of diet on metabolic disorders of PCOS. Ten obese women with this syndrome were selected and they followed a diet for eight weeks. Research has been done to determine if this low-starch and dairy diet would improve oxidation of fasting fat and postprandial fat after a liquid dinner of saturated fat. The diet contained pure animal protein, non-starchy vegetables and fruits, nuts, seeds, and oils, red wine and full-fat cheese. The results of the study showed a decrease in fasting insulin and the two-hour insulin curve. A beneficial change in waist-buttocks ratio, free fat, body mass, fasting glucose or two-hour glucose curve, HbA1c and free testosterone has been also found. However, there was a correlation between weight and free testosterone. Researchers concluded that this diet led to an increased oxidation of fasting fat after a meal in overweight and obese women with PCOS, which was not significantly correlated with changes in BMI, fasting insulin, or testosterone.
In 2015, Phy and his colleagues (9) conducted a prospective study to observe whether a low starch diet and dairy products would lead to weight loss, improvement of insulin resistance and reduction of testosterone. Their study involved 24 obese women with PCOS who followed a low starch and dairy diet for eight weeks. Their daily diet contained pure animal protein, fish and shellfish, eggs, non-starchy vegetables, low-sugar fruits, avocados, olives, nuts, seeds, fats, red wine, fresh high fat cheese. Grains, beans, other dairy products, and sugar (concentrated fruit juices, raw sugar, honey, agave nectar, etc) were excluded from the diet because of their potential to raise blood sugar. Regularly eating foods with high sugar content can overload the body’s ability to produce enough insulin. After the eight-week test, there was a significant reduction in all body measurements. Diet had also improved insulin sensitivity and total and free testosterone. This intervention seemed auspicious as weight loss in patients with PCOS was difficult.
In 2017, a survey was conducted by Szczuko and his associates in Poland (10), in an attempt to find whether a particular diet affected the inflammatory factors. Thus, a three-month study was performed on 24 women with PCOS. The researchers intervened in their diet that contained five meals a day. The products used as sources of carbohydrates (five portions per day) were oatmeal, whole grain rye or graham bread, brown rice, groats (wheat, millet, and buckwheat), sporadically potatoes, and whole meal pasta. The carbohydrate products selected for the diets were characterized by lowered glycemic index (GI). The products recommended in diets as source of proteins (one portion of meat and two portions of dairy products per day) included eggs, lean meat without skin (turkey, chicken), fish, mainly sea fish (sole, salmon, tuna), semi-skimmed pasteurized milk and dairy products (2% fat quark, natural yoghurt, and buttermilk), nuts and seeds (almonds, pumpkin seeds, sunflower seeds, sesame seeds, and poppy seeds), and legumes (soy, red lentils, beans, and peas). The products chosen as sources of fat (two portions per day on average) were raw oils (rapeseed oil, and olive oil), oily fruits (e.g., avocado) as well as nuts, fish, meat, and dairy products. Fruits and vegetables with low GI were also included in diets. They were present in every meal to supplement the diet with vitamins and minerals. Patients were recommended to use braising, roasting, cooking in water, and steaming as heat treatment techniques to prepare their food. The results of this research showed that there was no significant difference between the two phenotypes of PCOS. The three-month reduction diet facilitated the synthesis of inflammatory mediators but was probably too short to assure the suppression of inflammatory reactions.
Two years later, in 2019, Barrea and his colleagues conducted a cross sectional/case control study in Italy (11). They examined whether the Mediterranean diet was related to PCOS and whether these women followed this regime. The study involved 112 women with PCOS and 112 women as a control group who followed the Mediterranean diet between 2014 and 2019. According to the results, women with PCOS consumed less virgin olive oil, legumes, fish, and nuts than the control group did. In addition, they consumed fewer complex carbohydrates, fiber, unsaturated fatty acids, monounsaturated fatty acids, n-3 polyunsaturated fatty acids and more simple carbohydrates, total fat, saturated fatty acids, polyunsaturated fatty acids, and n-6. In conclusion, there seems to be a direct correlation between the adherence to the Mediterranean diet and women with PCOS and a different body composition characterized by low polyhydroxyalkanoates (PHAs) and no fat. These data could support a therapeutic role of individual foods and nutrients of the Mediterranean diet in PCOS, helping to reduce the inflammatory condition that paves the way for insulin resistance and hyperandrogenemia. In addition, PHAs are an indicator of the clinical severity of PCOS.
In 2019, in Iran, Shishehgar et al (12) conducted a study to determine the effect of low-calorie hypocaloric diet on human variables and insulin resistance in women with or without PCOS as well as the effect of this diet on clinical and hormonal characteristics of women with PCOS. All participants were urged to eat lean meat, whole grains, low-fat dairy, non-starchy vegetables, and vegetable oils, and were prohibited from consuming fast food or high-salt foods. The results of the mentioned study showed that this diet led to similar benefits in the anthropometric and metabolic characteristics of obese women with or without PCOS. In addition, in women with PCOS, menstrual cycles and biochemical and clinical features improved after six months. Thus, low testosterone levels and an increase in sex hormone binding globulin (SHBG) were observed in women with PCOS. Decreased insulin due to weight loss and reduced hyperandrogenemia were also seen.
In 2020, Kazemi et al (13) conducted a cross-sectional study to determine which dietary plan affected ovarian morphology. A survey was conducted on 111 women with PCOS. Four different diet plans were used: 1) healthy eating index (HEI-2015); 2) alternative healthy eating index (HEI-2010); 3) alternative Mediterranean diet (aMED); and 4) dietary approaches to stop hypertension. The study concluded that the aMED and DASH diets improved the appearance of the ovaries. These diets are characterized by favorable combinations of dietary ingredients, which include high dietary fiber, processed composition and ratio of macronutrients, vitamins and minerals and other dietary agents with antioxidant properties. These diets were shown to reduce insulin resistance, dysglycemia, hyperandrogenism and obesity, also causing satiety and weight loss in women. These interventions appear to improve ovarian steroidogenesis and affect ovarian development by regulating follicle stimulation hormone (FSH). The aMED and DASH diets indicate a decrease in red meat intake and an increase in protein intake from fish and dairy. This leads to a reduction in the number of follicles and blastocysts. Reduced beverage and sodium intake is recommended at DASH, which increases glucose regulation, reduces insulin resistance, and improves abdominal fat deposition.
Finally, in 2020, in Italy, Paoli et al (14) conducted a study that studied the effect of a ketonic diet on women with PCOS. Fourteen obese women with PCOS followed a ketonic Mediterranean diet with plant extracts for 12 weeks. They could eat unlimited green leafy vegetables, cruciferous (vegetables), zucchini, cucumbers, and eggplants. The amount of meat, eggs and fish was limited. In addition, participants consumed four dietary supplements and liquid herbal extracts. Dietary supplements are high in protein and very low in carbohydrates. After 12 weeks, almost all measurements improved. Not only insulin was significantly reduced, but also cholesterol and triglycerides. Androgens as well as luteinizing hormone (LH) and the LH/FSH fraction decreased, which resulted in remission of hormonal abnormalities of PCOS.
DISCUSSION
According to the findings of our review, it seems that diet plays a very important role on the clinical picture and laboratory findings of PCOS. According to the included studies, the change in women’s diet brought positive results in terms of clinical appearance of the syndrome. More specifically, weight loss and reduction of other body measurements were observed in five out of seven studies. Six studies reported a decrease in fasting insulin and free testosterone. In one survey, a significant difference was found in the ultrasound image of the ovaries, while in another study a facilitation in the synthesis of inflammatory mediators was observed.
Based on the results of these studies, when women with PCOS changed their diet they lost weight and decreased their level of insulin, free testosterone, glucose, and fat. Also, most of the population showed a decrease in hyperandrogenism, cholesterol, triglycerides, LDL, LH and LH/FSH, hair loss, acne, and menstrual irregularities. In general, there was an improvement in the composition of inflammatory mediators and a better picture of the ovaries, with fewer follicles and a smaller ovary size.
Based on the review findings, a diet – which seems to help in the clinical and laboratory picture of the syndrome – contains fruits and vegetables low in glycemic index and usually non-starchy (such as artichokes, asparagus, bean sprouts, brussels sprouts, broccoli, cabbage, cauliflower, celery, cucumber, eggplant, mushrooms, onions, peppers, salad greens, spinach, tomato, turnips, zucchini, melons, berries like strawberries, raspberries, blackberries and blueberries, citrus fruits like oranges, tangerines, grapefruit and lemons, peaches, plums, apricots, cherries, and pears), low-fat dairy in small quantities, fish rich in Ù3 fatty acids, lean red meat and poultry (e.g., chicken, turkey) in small quantities, fatty acids (olive oil-olives, vegetable oils, fish oils), (almonds, pumpkin seeds, sunflower seeds, sesame seeds, poppy seeds), legumes, whole grain products and alcohol in moderate amount (150 mL of red wine per day). In 2020, Kazemi and his colleagues (13) concluded that the aMed and DASH diets, which are quite similar to each other, lead to a better image of the ovaries. Thus, the above variant of diet can lead to a better picture of PCOS. Along with this diet, hydration and light exercise is also necessary. These will strengthen the weight loss effort, which is quite difficult for women with PCOS.
It is well established that dietary changes can rapidly change the relative abundance of species making up the intestinal flora. Therefore, understanding the role of intestinal microbiota in the PCOS pathogenesis is essential, as the diet may act as a mediator factor between microbiota and PCOS clinical and laboratory appearance. For example, high-sugar foods may be one of the inducers of PCOS, by causing intestinal flora imbalance and triggering chronic inflammation, insulin resistance, and production of androgen. Gut microbiota dysbiosis can cause insulin resistance, which is closely linked to the occurrence of PCOS. Moreover, changes in the relative abundances of specific types of gut bacteria have been associated with clinical manifestations of PCOS (15). The use of microbiota-targeted agents in the treatment of PCOS has been recently discussed (15). Lastly, it is also necessary to explore the potential use of probiotics and fecal transplant therapies in the treatment of this condition (16). Recent studies have revealed that probiotics supplementation have favorable effects on the metabolic profile in women with PCOS (15).
CONCLUSION
According to the researching part of the dissertation, it seems that diet plays a very important role on the clinical picture and laboratory findings of PCOS.
The diet, which seems to help in the clinical and laboratory picture of the syndrome, contains fruits and vegetables with a low GI and usually non-starchy, low-fat dairy in small quantities, fish rich in Ω-3 fatty acids, lean red meat and poultry in small quantities, fatty acids, legumes, whole grain products and alcohol in moderation.
This review had some limitations. Initially, only surveys that had been conducted during 2011 and 2021 were used, not also from previous years. Also, the review was not based on the types of PCOS, but in general on the endocrine disorder. Another limitation is that the review involves research in which the intervention was some kind of dietary plan, and not taking dietary supplements.
Future research should involve a larger population with PCOS and a longer duration, during which a specific diet would be given to participants. It would also be important to compare the effects of diet to those of mild exercise and dietary supplementation. Another idea for future research would be to compare various diets to the different types of this syndrome and laboratory results. Finally, a comparison could be made between diet and fertility of women with PCOS.
Conflict of interests: none declared.
Financial support: none declared.
FIGURE 1.
Flow diagram illustrating the article filtering process
Contributor Information
Maria XENOU, Department of Midwifery, University of West Attica, Athens, Greece.
Kleanthi GOUROUNTI, Department of Midwifery, University of West Attica, Athens, Greece.
References
- 1.McCartney CR, Marshall JC. Clinical practice. Polycystic Ovary Syndrome. NEJM. 2016;375:54–64. doi: 10.1056/NEJMcp1514916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertility and Sterility. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Bellver J, Rodríguez-Tabernero L, Robles A, et al . Polycystic ovary syndrome throughout a woman's life. J Assist Reprod Genet. 2018;35:25–39. doi: 10.1007/s10815-017-1047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melo AS, Ferriani RA, Navarro PA. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics (Sao Paulo, Brazil) 2015;11:765–769. doi: 10.6061/clinics/2015(11)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giampaolino P, Foreste V, Di Filippo C, et al. Microbiome and PCOS: State-of-Art and Future Aspects. Int J Mol Sci. 2021;22:2048. doi: 10.3390/ijms22042048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pohlmeier AM, Phy JL, Watkins P, et al. Effect of a low-starch/low-dairy diet on fat oxidation in overweight and obese women with polycystic ovary syndrome. Applied physiology, nutrition, and metabolism. 2014;39:1237–1244. doi: 10.1139/apnm-2014-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phy JL, Pohlmeier AM, Cooper JA, et al. Low Starch/Low Dairy Diet Results in Successful Treatment of Obesity and Co-Morbidities Linked to Polycystic Ovary Syndrome (PCOS). Journal of Obesity & Weight Loss Therapy. 2015;5:259. doi: 10.4172/2165-7904.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szczuko M, Zapałowska-Chwyć M, Maciejewska D, et al. Significant Improvement Selected Mediators of Inflammation in Phenotypes of Women with PCOS after Reduction and Low GI Diet. Mediators of Inflammation. 2017;5489523 doi: 10.1155/2017/5489523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrea L, Arnone A, Annunziata G, et al. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients. 2019;11:2278. doi: 10.3390/nu11102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shishehgar F, Mirmiran P, Rahmati M, et al. Does a restricted energy low glycemic index diet have a different effect on overweight women with or without polycystic ovary syndrome? BMC Endocrine Disorders. 2019;19:93. doi: 10.1186/s12902-019-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kazemi M, Jarrett BY, Vanden Brink H, et al. Obesity, Insulin Resistance, and Hyperandrogenism Mediate the Link between Poor Diet Quality and Ovarian Dysmorphology in Reproductive-Aged Women. Nutrients. 2020;12:1953. doi: 10.3390/nu12071953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paoli A, Mancin L, Giacona MC, et al. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. Journal of Translational Medicine. 2020;18:104. doi: 10.1186/s12967-020-02277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He FF, Li YM. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. Journal of Ovarian Research. 2020;13:73. doi: 10.1186/s13048-020-00670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J. The Human Microbiome Project: Extending the definition of what constitutes a human. National Human Genome. Research Institute. 2020.