ABSTRACT
Background
It is unclear to what extent adjuvant dietary intervention can influence inflammation in rheumatoid arthritis (RA).
Objectives
The objective was to assess the effects of dietary manipulation on inflammation in patients with RA.
Methods
In a crossover design, participants [n = 50, 78% females, median BMI (in kg/m2) 27, median age 63 y] were randomly assigned to begin with either a 10-wk portfolio diet of proposed anti-inflammatory foods (i.e., a high intake of fatty fish, whole grains, fruits, nuts, and berries) or a control diet resembling a Western diet with a 4-mo washout in between. This report evaluates the secondary outcome markers of inflammation among participants with stable medication. Analyses were performed using a linear mixed ANCOVA model.
Results
There were no significant effects on CRP or ESR in the group as a whole. In those with high compliance (n = 29), changes in ESR within the intervention diet period differed significantly compared with changes within the control diet period (mean: –5.490; 95% CI: –10.310, –0.669; P = 0.027). During the intervention diet period, there were lowered serum concentrations of C-X-C motif ligand 1 (CXCL1) (mean: –0.268; 95% CI: –0.452, –0.084;P = 0.006), CXCL5 (mean: –0.278; 95% CI: –0.530, –0.026 P = 0.031), CXCL6 (mean: –0.251; 95% CI: –0.433, –0.069; P = 0.009), and tumor necrosis factor ligand superfamily member 14 (TNFSF14) (mean: –0.139; 95% CI: –0.275, –0.002; P = 0.047) compared with changes within the control diet period.
Conclusion
A proposed anti-inflammatory diet likely reduced systemic inflammation, as indicated by a decreased ESR in those who completed the study with high compliance (n = 29). These findings warrant further studies to validate our results, and to evaluate the clinical relevance of changes in CXCL1, CXCL5, CXCL6, and TNFSF14 in patients with RA.
Keywords: Rheumatoid arthritis, diet intervention, anti-inflammatory diet, Western diet, inflammation, biomarkers of inflammation
Introduction
Around 5% of the population suffers from an autoimmune disease (1). A common feature of autoimmune diseases is a life-long disabling effect on afflicted individuals, with an etiology that is largely unknown. Rheumatoid arthritis (RA), one of the most common autoimmune diseases, affects approximately 0.5–1% of the population in North America and Europe, though prevalence varies by geographical region (2). Symptoms of RA primarily include pain, swelling, and reduced function in peripheral joints. The chronic activation of inflammatory pathways also leads to a state of elevated systemic inflammation, which can increase the risk of comorbidities. Although pharmacological treatment of RA has improved substantially during the past decades, there is no cure and many patients still experience incomplete treatment response (3). A fear of side effects related to medical treatment and a belief that environmental factors modulate disease development and activity have been described for patients with RA (4), causing many patients to experiment with their lifestyle. In a Finnish survey, 50% of patients changed their diet after a RA diagnosis, many believing red meat and animal fats to be detrimental (5). There is growing interest in understanding the role of diet as a modulator of inflammatory activity. Several attempts have been made to determine beneficial foods and dietary patterns, such as the dietary inflammatory index (6) and the Mediterranean diet score (7). There is also some evidence from clinical trials on patients with RA that fish oil supplementation, fasting, and a Mediterranean-like diet pattern could reduce measures of disease activity and inflammation (8).
The rationale and primary aim of this study was to investigate whether a portfolio diet (compared with a typical Western diet), combining potential anti-inflammatory foods, could beneficially alter biomarkers of inflammation in patients with RA. We have previously demonstrated the effect of this diet on Disease Activity Score 28 joints erythrocyte sedimentation rate (DAS28-ESR), a recognized clinically relevant composite index of subjective and objective markers of disease activity (9). Here, we report the effects of the portfolio diet on biological markers of inflammation as secondary outcomes.
Methods
This report is an analysis of secondary outcomes; the main outcome of the Anti-inflammatory diet in Rheumatoid Arthritis (ADIRA) trial was DAS28-ESR, for which results have been published (9).
Ethical statement
This study was approved by the regional ethical review board in Gothenburg (976-16 and T519-17) and registered on clinicaltrials.gov as NCT02941055. Participants provided signed informed consent prior to enrollment and all procedures were performed according to the Helsinki Declaration.
Recruitment
Patients diagnosed with RA according to 1987 American College of Rheumatology and 2010 American College of Rheumatology/European League Against Rheumatism criteria (10) listed at Sahlgrenska University Hospital (Gothenburg, Sweden) were selected from the Swedish Rheumatology Quality Register (n = 1091). Those who resided in areas in the Gothenburg region where home delivery of food was possible (n = 774) were invited to participate. In total, 113 patients volunteered to take part in the study. After initial contact, 47 were deemed to not fulfill inclusion criteria, thus 66 volunteers were screened for inclusion and out of those, 50 were included in the study. Inclusion criteria were DAS28-ESR ≥2.6, unchanged disease modifying anti-rheumatic drug (DMARD) medication during the previous 8 wk, 18–75 y of age, and at least 2 y disease duration. Life threatening diseases, pregnancy or lactation, food allergies to components in the dietary intervention, inability to communicate verbally, and inability to understand study instructions were exclusion criteria.
Study design
A crossover design was chosen to minimize interindividual variation in a heterogeneous population of patients with RA. Study staff randomly assigned the participants (allocation ratio 1:1) to begin with either intervention or control diet using a computer-generated list. The 10-wk diet periods were separated by a 4-mo washout period. The study ran in 2 batches, commencing in February 2017 and August 2017, respectively.
Dietary intervention
The dietary intervention has been described in detail elsewhere (9, 11, 12). In brief, the intervention diet had a nutritional profile similar to the Mediterranean diet, rich in whole grains and fatty fish, enriched with probiotics, and high in phytochemicals found in legumes, nuts, fruits, berries, and vegetables. However, instead of olive oil, canola oil was used. Advice was given to limit red meat consumption frequency to ≤3 times/wk and keep fruit, berry, and vegetable intake to ≥5 portions daily and to choose whole-grain products. Low-fat dairy products and use of margarine and vegetable oils for cooking were encouraged.
The control diet resembled a Western diet, being high in refined grains, red meat, and chicken, and low in fruit and vegetables. As snacks, protein bars and protein puddings, as well as quark, were included. Participants were advised to keep fish intake to ≤1 and red meat ≥5 times/wk. Advice was also given to keep intake of fruits, berries, and vegetables to ≤5 portions daily, as well to avoid probiotic products. Whole-fat dairy products and use of butter for cooking were encouraged.
Participants received a home-delivery of groceries with recipes and menus, which accounted for an intake of approximately 1100 kcal/d for 5 d/wk, designed to cover approximately half of the daily energy intake. Study staff urged participants to keep weight-stable and provided foods that were isocaloric between diets. In an attempt to blind participants to which study diet they were consuming, study staff consistently referred to the intervention diet as a “fiber diet,” and the control diet as “protein diet” in all communications.
Data collection
At screening, participants filled out questionnaires on medication usage, age, and demographic background as well as the RA-specific health assessment questionnaire (HAQ) (13). Waist-to-hip ratio and height were measured to the closest 0.5 cm and nonfasting blood samples were collected by venipuncture for analysis of erythrocyte sedimentation rate (ESR) and concentration of C-reactive protein (CRP). Measurements of CRP and ESR from screening were used as baseline values for the first diet period, but subsequent blood samples (and all serum samples) were collected from participants in the fasted state. Before and after each diet period, blood samples were collected by venipuncture, dietary intake was recorded in 3-d food records, weight was measured to the nearest 0.1 kg, with 1 kg subtracted to account for clothing. To estimate disease activity by DAS28-ESR, joint examinations were performed by nurses experienced in rheumatology.
During each dietary period, participants were urged to record any changes in medications. Furthermore, study staff interviewed participants by telephone asking if and to what extent each study meal had been consumed during the past week and calculated a score based on consumption in whole (2 points), in part (1 points), or not at all (0 points). This yielded a numerical score for each participant from 0 to 30. Participants reaching a score of >24 points (>80%) were considered compliant.
Laboratory analyses
Concentration of CRP and ESR were measured by routine analysis in fresh samples at Sahlgrenska University Hospital.
Serum was separated by leaving blood samples for 5 min in room temperature, 30 min in the refrigerator, and then centrifuging for 10 min at 2594 × g. Serum samples were stored at −80°C until analysis, then thawed to prepare aliquots for external analyses. Because sampling procedure affects the analysis of inflammation-related proteins in serum, only blood samples that were handled according to our strictest protocol, and were available from all study visits, were analyzed for inflammation-related proteins. This analysis included samples from 32 subjects.
To quantify inflammation-related proteins, a multiplex assay measuring relative concentrations of 92 inflammation-related proteins was deployed and analyzed externally by Olink Proteomics AB, using the Olink® Target 96 Inflammation panel (Olink Proteomics AB), as described elsewhere (14). In brief, pairs of oligonucleotide-labeled antibody probes bind to their targeted protein, and if the 2 probes are brought in close proximity, the oligonucleotides will hybridize in a pairwise manner. The addition of a DNA polymerase leads to a proximity-dependent DNA polymerization event, generating a unique PCR target sequence. The resulting DNA sequence is subsequently detected and quantified using a microfluidic real-time PCR instrument (Biomark HD, Fluidigm). Data are then quality controlled and normalized using an internal extension control and an interplate control, to adjust for intra- and interrun variation. The final assay readout is presented as a normalized protein expression value, which is an arbitrary unit on a log2-scale where a high value corresponds to a higher protein expression. If any of the internal controls deviates more than ±0.3 from the plate median, the sample fails quality control. All assay validation data are available on the manufacturer's website (www.olink.com). Data from the Olink analysis were included only on proteins for which ≥90% of the samples had results above the valid lower limit of detection and only on samples that passed quality control. This limited the quantification to 72 inflammation-related proteins (Supplemental Table 1).
Statistical analysis
Statistical analysis was performed with a linear mixed ANCOVA model by using IBM SPSS version 25. Fixed variables were dietary treatment (intervention or control diet), time period (first or second diet period), BMI (in kg/m2), and baseline value of each outcome variable. Individual participants were included as random effects. Residuals were inspected and variables with skewed distributions were transformed in order to comply with model assumptions. There was no correction for multiple hypothesis tests. The power analysis of the ADIRA trial was performed on the primary outcome DAS28-ESR. In order to detect a change of 0.6 units in DAS28-ESR with 90% power and α = 0.05, a sample size of 38 patients was needed, and to account for dropouts 50 additional patients were recruited.
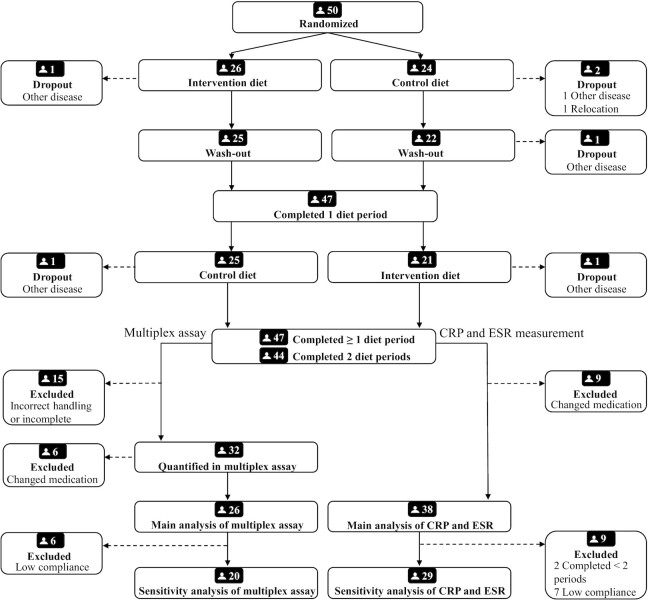
In order to avoid distortion of results due to changes in anti-inflammatory medication, participants who completely stopped or started a new DMARD or glucocorticoid treatment during the diet periods were excluded from analysis. In total, 38 participants completed ≥1 diet period (37 completed the intervention diet, 37 completed the control diet) without discontinued or new DMARD or glucocorticoid treatment (Figure 1). Quantification of inflammation-related proteins in the multiplex assay was performed on samples handled according to the strictest protocol; such samples were available from 26 participants who completed both diet periods.
FIGURE 1.
Flow chart of subject recruitment reported according to CONSORT. CRP and ESR was quantified in all participants’ samples. Quantifying relative concentrations of inflammation-related proteins in serum samples in the multiplex assay was done only if samples had been handled according to the strictest protocol and in participants whose samples were available from all visits. Participants with new or discontinued DMARD or glucocorticoid treatment were excluded from analyses, and only those who completed both diet periods with high compliance were selected for a sensitivity analysis. CRP, C-reactive protein; DMARD, disease modifying ant-rheumatic drug, ESR, erythrocyte sedimentation rate.
Sensitivity analysis
In an attempt to further explore the results, a sensitivity analysis was performed. In addition to excluding those who stopped or started a new DMARD or glucocorticoid treatment, only those who completed both diet periods and reported high compliance during both diet periods (>80%) were included in this analysis. Excluding participants with low compliance and those who discontinued any of the diet periods yielded 29 participants for the analysis of ESR and CRP, and 20 participants for analysis of inflammation-related proteins in the multiplex assay.
Carryover effect
In order to examine carryover effects, interaction between dietary treatment (intervention or control) and diet period (1 or 2) for CRP and ESR were tested. There were no significant interactions (P > 0.20) between diet period and treatment.
Group selection bias
In order to assess bias in group selections, baseline characteristics of participants were compared between those included and not included in analyses. Those included in analysis with the multiplex assay were compared with those not included, and participants who completed both diet periods with high compliance without new or discontinued DMARD or glucocorticoid treatment were compared with those who did not. Continuous variables were compared using Mann-Whitney U-test, whereas categorical variables were compared using Fishers Exact test.
Results
Participants
Overall, three-quarters of the participants were women and around half had a university-level education. The vast majority were nonsmokers of European descent and over half were treated with a conventional synthetic disease modifying antirheumatic drug (csDMARD) and about a third with a biological disease modifying antirheumatic drug (bDMARD) (Table 1). A majority of participants were middle-aged or older, had a moderate disease activity (defined by DAS28-ESR between 3.2 and 5.1), and were either overweight or obese (Table 1).
TABLE 1.
Baseline data of participants who completed ≥1 diet period without discontinued or new bDMARD or glucocorticoid treatment, grouped by inclusion in multiplex inflammation-related protein quantification1
| Intervention-control (n = 13) | Control-intervention (n = 13) | Not included (n = 12) | |
|---|---|---|---|
| Female | 9 (69) | 10 (77) | 10 (83) |
| Age, y | 62 (55, 63) | 66 (48, 72) | 70 (61, 73) |
| Parental origin | |||
| Europe | 12 (92) | 13 (100) | 10 (83) |
| Africa | 0 (0) | 0 (0) | 1 (8) |
| Asia | 1 (8) | 0 (0) | 1 (8) |
| Nonsmoker | 11 (85) | 13 (100) | 12 (100) |
| Employment status | |||
| Not employed | 2 (15) | 7 (54) | 9 (75) |
| Employed <15 hr/wk | 1 (8) | 0 (0) | 0 (0) |
| Employed 16–30 hr/wk | 3 (23) | 1 (8) | 0 (0) |
| Employed 31–40 hr/wk | 3 (23) | 3 (23) | 1 (7) |
| Employed > 40 hr/wk | 4 (31) | 2 (15) | 2 (17) |
| Educational level | |||
| Junior high school | 1 (8) | 0 (0) | 4 (33) |
| 2 y senior high school | 1 (8) | 3 (23) | 4 (33) |
| ≥3 y senior high school | 1 (8) | 2 (15) | 2 (17) |
| College or university | 10 (77) | 8 (62) | 2 (17) |
| Medication usage | |||
| bDMARD | 4 (31) | 5 (38) | 6 (50) |
| csDMARD | 10 (77) | 9 (69) | 9 (75) |
| No DMARD | 2 (15) | 2 (15) | 1 (8) |
| Anthropometric measures | |||
| BMI | 27.1 (23.6, 32.8) | 26.4 (24.2, 29.9) | 27.7 (24.2, 33.5) |
| Waist-hip ratio | 0.84 (0.78, 0.98) | 0.85 (0.83, 0.92) | 0.82 (0.80, 0.88) |
| Laboratory data | |||
| DAS28-ESR | 3.9 (3.2, 4.7) | 3.2 (2.9, 4.5) | 3.6 (3.0 4.4) |
| HAQ | 0.38 (0.13, 1.19) | 0.38 (0.13, 1.31) | 0.69 (0.31, 1.06) |
| CRP, mg/L) | 2 (1, 4) | 5 (1, 6) | 3 (1, 5) |
| ESR, mm/hr) | 20 (13, 27) | 14 (8, 26) | 18 (10, 23) |
| WBC, 109/L) | 5.1 (4.3, 5.7) | 6.3 (5.1, 7.6) | 5.6 (4.8, 6.4) |
| Trombocytes, 109/L) | 250 (240, 310) | 280 (250, 410) | 240 (220, 280) |
| Dietary intake | |||
| Energy, kcal/d) | 1900 (1600, 2200) | 1800 (1400, 2100) | 1800 (1200, 2300) |
| Fat, E%) | 38 (31, 42) | 41 (36, 45) | 35 (32, 37) |
| Saturated fatty acids, E%) | 16 (14, 17) | 15 (13, 16) | 13 (11, 14) |
| Protein, E%) | 16 (14, 18) | 15 (14, 20) | 15 (15, 22) |
| Carbohydrate, E%) | 42 (39, 47) | 38 (36, 42) | 46 (39, 52) |
| Fiber, g/d) | 19 (14, 22) | 15 (13, 20) | 19 (15, 21) |
| Vitamin D, μg/d) | 3 (2, 6) | 5 (4, 10) | 6 (5, 8) |
| Selenium, μg/d) | 35 (32, 40) | 48 (41, 75) | 51 (42, 74) |
| Folate, μg/d) | 260 (210, 330) | 270 (220, 320) | 220 (190, 280) |
1Values are medians (25th, 75th percentiles) or n (%) unless otherwise indicated. bDMARD, biological disease modifying antirheumatic drug; CRP, C-reactive protein; csDMARD, conventional synthetic disease modifying antirheumatic drug; DMARD, disease modifying antirheumatic drug; DAS28-ESR, Disease Activity Score-28 erythrocyte sedimentation rate; ESR, erythrocyte sedimentation rate; HAQ, health assessment questionnaire; WBC, white blood cell count.
Adverse effects
In the group as a whole (n = 38), there were 15 reports of gastrointestinal discomfort, with 11/15 during the intervention diet period. Among the patients in whom inflammation-related proteins were measured (n = 26), there were 9 reports of gastrointestinal discomfort, of which 7/9 were during the intervention diet period.
Group selection bias
Participants without new or discontinued DMARD or glucocorticoid therapy who continued both diet periods with high compliance (n = 29), had lower waist-to-hip ratio (P = 0.006), and a higher educational level (P = 0.030) but did not otherwise differ from the rest of the participants (n = 18). Among those participants whose samples were selected for multiplex analysis (n = 32), leucocyte concentration was lower (P = 0.024) than the rest of the participants (n = 15). Furthermore, in those participants included compared with those not included in the multiplex analysis, the percentages of energy intake from total and saturated fat were higher (P = 0.027 and P = 0.027, respectively), whereas the percentage of energy intake from carbohydrates was lower (P = 0.040).
Effects of diet on clinically validated markers of inflammation
There were no effects of diet on CRP (P = 0.125) or ESR (P = 0.059) in the main analysis (Table 2). There was, however, a significant increase in ESR during the control diet period. In the sensitivity analysis, ESR was lowered during the intervention diet period compared with during the control diet period (mean between-period difference: –5.490 mm/h; 95% CI: –10.310, –0.669; P = 0.027).
TABLE 2.
Modeled estimates of developments in clinically validated markers of inflammation within and between diet periods among patients with RA who did not discontinue or start any new disease modifying antirheumatic drug or glucocorticoid therapy1
| Intervention mean change (95% CI) | Control mean change (95% CI) | Difference between diet periods2 | 95% CI | P value | |
|---|---|---|---|---|---|
| Clinical markers of inflammation in participants completed ≥1 diet period regardless of compliance3 | |||||
| CRP,4 mg/L | –0.042 (–0.167, 0.082) | 0.09 (–0.034, 0.215) | –0.133 | –0.304, 0.039 | 0.125 |
| ESR, mm/h | –0.709 (–3.485, 2.067) | 3.071 (0.303, 5.838) | –3.779 | –7.710, 0.152 | 0.059 |
| Clinical markers of inflammation in participants completing both diet periods with high compliance5 | |||||
| CRP,4 mg/L | –0.058 (–0.215, 0.100) | 0.097 (–0.058, 0.251) | –0.154 | –0.362, 0.054 | 0.136 |
| ESR, mm/h | –1.504 (–4.991, 1.982) | 3.985 (0.566, 7.404) | –5.490 | –10.310, –0.669 | 0.027 |
Participants completing ≥1 diet period. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; RA, rheumatoid arthritis.
Intervention-control, change during period values.
Analyzed by use of a linear mixed model with period, treatment, BMI, and baseline value as fixed effects and subject as random effect, n = 38.
To comply with model assumptions, log10-transformed values were used.
Analyzed by use of a linear mixed model with period, treatment, BMI, and baseline value as fixed effects and subject as random effect, n = 29.
Exploratory analysis of biomarkers related to inflammation
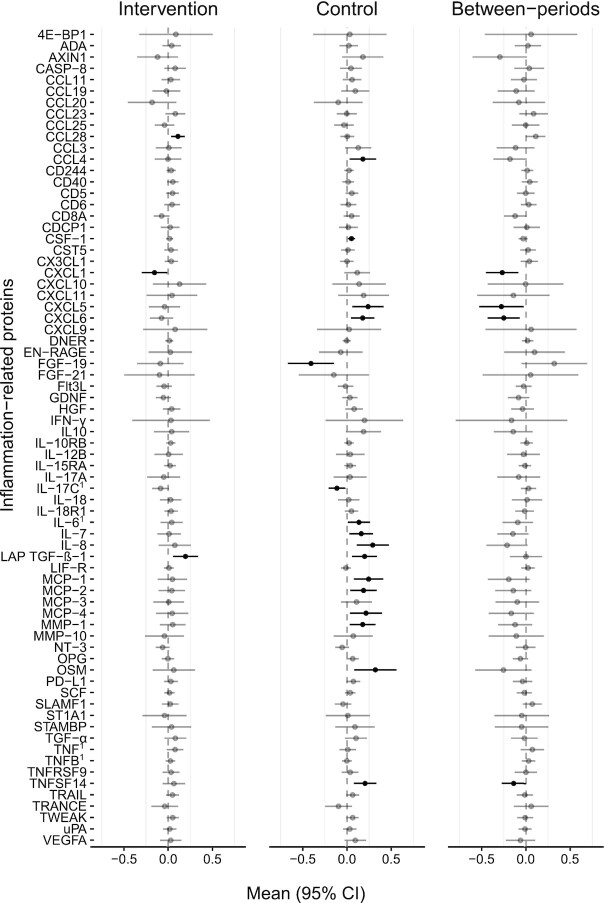
C-X-C motif chemokine ligand1 (CXCL1), CXCL5, CXCL6, and tumor necrosis factor ligand superfamily member 14 (TNFSF14) were significantly lower during the intervention diet period than during the control diet period in the main analysis (Figure 2 and Supplemental Table 2). No other significant between diet-period effects were identified (Figure 2 and Supplemental Table 2).
FIGURE 2.
Changes in concentrations of inflammation-related proteins within and between dietary periods measured in participants completing at least one diet period who did not discontinue or start any new disease modifying anti-rheumatic drug or glucocorticoid therapy, n = 26. Black colored lines denotes P < 0.05. Concentrations are presented in an arbitrary, semiquantitative log2 scale that is valid for comparison of relative concentrations between different time points within individuals, analyzed using a linear mixed model with period, treatment, BMI, and baseline value as fixed effects and subject as random effect. See Supplemental Table 1 for abbreviations. 1Analyzed and presented on a log10 scale in order to comply with model assumptions.
In the sensitivity analyses, results for CXCL1 and CXCL6 remained significantly lower during the intervention diet period than during the control diet period (Supplemental Figure 1 and Supplemental Table 3). Additionally, glial cell line–derived neurotrophic factor (GDNF) differed significantly between diet periods, with a lower value during the intervention diet period than during the control diet period (Supplemental Figure 1 and Supplemental Table 3).
Discussion
This investigation examined the hypothesis that dietary manipulation using a proposed anti-inflammatory portfolio diet will further decrease inflammation in patients with RA during stable and adequate antirheumatic pharmacological treatment. To our knowledge, this is the most comprehensive analysis of effects on biomarkers of inflammation from dietary manipulation in patients with RA in a randomized controlled trial.
The clinically validated markers of inflammation (CRP and ESR) were unchanged by the diet in the main analysis, but among participants who reported high compliance and who completed both diet periods, a significant treatment effect on ESR was seen. This highlights controlling for compliance as a key priority in studies on effects of dietary intervention in humans.
ESR determination is a rather simple and readily available laboratory test that—along with CRP—is the recommended clinical measure for the determination of acute-phase reactants in the clinical care of patients with RA (15). As reported in a recently published review, ESR is a nonspecific marker of inflammation in general (16). The data in our trial do not permit us to draw any conclusions on the mechanism by which the treatment diet lowered ESR in this patient population. Several foods in the intervention diet might act in an anti-inflammatory manner. For example, ω-3 fatty acids from fatty fish can act as a competitive substrate with arachidonic acid for the cyclooxygenase, lipoxygenase, and cytochrome P450 enzymes, yielding less inflammatory eicosanoids, and they may also act as substrates for synthesis of proresolving lipid mediators. In addition, a high intake of fruits, berries, vegetables, nuts, and seeds containing phytochemicals may potentially dampen oxidative stress, which in turn could reduce general inflammatory activity. It is also possible that the higher fiber intake (through whole grains and less processed foods) coupled with probiotics affected the microbiota and increased the production of short-chain fatty acids, which could exert an anti-inflammatory effect (17).
Studies with similar dietary interventions in patients with RA are rare. McKellar et al. used cooking classes as a method to reach a Mediterranean-like diet among participants in socially deprived areas but found no effects on inflammation (18). In comparison, our approach of supplying foods and controlling for compliance and medication likely yields higher precision in examining efficacy. Skoldstam et al. noted a decrease in CRP after a Mediterranean diet compared with a control diet in patients with RA, but there was no effect on ESR (19). However, the concurrent weight loss seen in the study by Skoldstam et al. complicates the interpretation. The same research group has since published a follow-up investigation based on pooled data, which indicate effects beyond weight reduction in interventions with lacto-vegetarian, vegan, or Mediterranean diets (20). Furthermore, bDMARDs, powerful anti-inflammatory agents that were used by about a third of participants in ADIRA, were uncommon when Skoldstam et al. carried out their study.
The chemokines CXCL1, CXCL5, and CXCL6, known for their neutrophil chemoattractant effects at the site of injury, infection, or inflammation (21), decreased significantly in the main analysis. In studies on synovial fibroblasts isolated from patients with RA, CXCL1 is indicated to stimulate an inflammatory response and upregulate IL-6 expression (22). Previously, higher concentration of CXCL1 was reported in plasma and synovial fluids from patients with RA compared with healthy volunteers, and CXCL1 is thus suggested to play a mediating role in neutrophil recruitment into the inflamed joint (23). Previous research also found increased CXCL1 expression linked to poor survival rates in cancer (24). Further, increased circulating concentration of CXCL5 has been found in patients with RA compared with healthy controls (25). Thus, lower circulating concentrations of CXCL1, CXCL5, and CXCL6 as observed in the current study probably reflect reduced systemic inflammation.
TNFSF14 decreased significantly with the intervention diet compared with the control diet in the main analysis. Previously, higher concentration of TNFSF14 has been found in patients with RA in comparison with healthy controls (26). Furthermore, studies indicate a role of TNFSF14 as an osteoclast-inducing protein promoting the progression of bone destruction in RA (26, 27).
The sensitivity analysis yielded similar significant effects in CXCL1 and CXCL6 as did the main analysis (Supplemental Figure 1). In addition, GDNF decreased significantly during the intervention diet period compared with the control diet period. As recently reviewed by Morel et al. (28), GDNF is produced by glial cells and binds primarily to GDNF family coreceptor α1 (GFRα1), expressed in a wide range of tissues. GDNF is described as having neuroprotective effects as well as regenerative effects on epithelial tissue upon infection or damage (28). Data on serum protein levels of GDNF in patients with RA are scarce, but one investigation has shown lower concentrations in plasma from patients with active RA compared with healthy controls (29). Thus, a lowered level of GDNF in serum could translate to decreased activation of inflammation-resolving pathways.
The ADIRA trial has several unique strengths. First, along with dietary advice, easily prepared foods were supplied to the participants’ homes free of charge, which likely contributed to the high reported compliance. Second, we employed a crossover design to reduce the effects of inter-individual variation and maximize the statistical power from the available sample size. We also implemented a 4-mo washout period, which we believe to be sufficient to normalize effects from the prior dietary period. Of the most importance for evaluating dietary effect, rather than effects related to energy balance, is that the participants were weight stable during the study.
This study also has some limitations. First, the generalizability can be questioned, because those participants who completed both diet periods with high compliance with stable medication had higher educational levels and a lower waist-to-hip ratios than the participants who did not. The study design with provided food items may also be difficult to achieve in other populations, specifically in outpatient settings (i.e., patient compliance might be affected). Moreover, our study population was mainly highly educated, middle aged, or older females of European descent. Additionally, those who completed both diet periods with high compliance and stable medication had an even higher educational level as well as a lower waist-to-hip ratio than the rest of the participants. It is possible that effects from dietary manipulation may differ in younger, more diverse, or less educated populations. Second, our investigation examined markers of inflammation in blood, and in serum isolated from blood, taken by venipuncture. As such, our results likely reflect systemic inflammation, or at least proteins exhibiting systemic effects. It is theoretically possible to examine local samples, such as for example synovial fluid, to explore the environment around the joints. However, due to procedural limitations and in consideration to participant comfort, we found it most suitable to collect blood samples. Third, our power analysis and subsequent sample size was constructed to detect relevant effects on DAS28-ESR, not for analysis of biomarkers of inflammation. For this report, sample size was further reduced because the full set of serum samples was not used to quantify biomarkers. Although we consider that our procedure of only analyzing correctly handled samples increases the reliability of our findings, the resulting lower sample size might have decreased the probability of detecting statistically significant differences. There is also a risk of bias; those with correctly handled samples did have lower leucocyte concentration as well as a slightly skewed macronutrient composition in their diet compared with those not included. Finally, as the Olink panel analysis was performed without correction for multiple testing, we view the results presented here as an exploratory report on the potential effects of diet on markers of inflammation.
Conclusion
In conclusion, our results indicate that a Mediterranean-like diet intervention with proposed anti-inflammatory foods compared with a Western diet reduced the systemic inflammation in patients with RA that had a high compliance to the dietary intervention. These findings need to be interpreted carefully given the risk of type 1 error due to multiple hypothesis tests. The results warrant further studies to validate our findings and to evaluate the clinical relevance of changes in CXCL1, CXCL5, CXCL6, GDNF, and TNFSF14 in patients with RA.
Supplementary Material
Acknowledgments
We acknowledge the work of the nurses Anneli Lund and Marie-Louise Andersson at the Clinical Rheumatology Research center at Sahlgrenska University Hospital, and the statistician David Bock at the Health Metrics Unit. We also would like to acknowledge the work from the home-food delivery chain mat.se.
The authors’ responsibilities were as follows—AW, HML, LB, IG: designed the study; EH, HML, LB, AW, ATW: conducted the research; EH: performed the statistical analysis, analyzed the data, wrote the first draft, and had primary responsibility for the final content; all authors: helped interpret the data; and all authors; read and approved the final manuscript.
Notes
Supported funds from the Swedish government under the ALF agreement (award number ALFGBG-716341), the Swedish Research Council for Health, Working Life and Welfare (FORTE) (award number 2017-00318), the Inger Bendix Foundation, the Lennander Foundation, the Sahlgrenska University Hospital Foundations, and the Gothenburg Region Foundation for Rheumatology Research (GSFR).
Author disclosures: The authors report no conflicts of interest. PCC is an Associate Editor on the Journal of Nutrition and played no role in the Journal's evaluation of the manuscript.
Supplemental Tables 1–3 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ADIRA, Anti-inflammatory Diet In Rheumatoid Arthritis; bDMARD, biological disease–modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CCL28, C-C motif chemokine 28; CCL23, C-C motif chemokine 23; CRP, C-reactive protein; CXCL1, C-X-C motif chemokine 1; CXCL5, C-X-C motif chemokine 5; CXCL6, C-X-C motif chemokine 6; DMARD, disease-modifying antirheumatic drug; DAS28-ESR, Disease activity score 28 joints erythrocyte sedimentation rate; ESR, erythrocyte sedimentation rate; GDNF, glial cell line–derived neurotrophic factor; HAQ, health assessment questionnaire; RA, rheumatoid arthritis; TNFSF14, tumor necrosis factor ligand superfamily member 14.
Contributor Information
Erik Hulander, Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Linnea Bärebring, Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Anna Turesson Wadell, Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Inger Gjertsson, Department of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Philip C Calder, Faculty of Medicine, University of Southampton and NIHR Southampton Biomedical Research Centre, University Hospital Southampton NHS Foundation Trust, Southampton, United Kingdom.
Anna Winkvist, Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Helen M Lindqvist, Department of Internal Medicine and Clinical Nutrition, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
References
- 1. Hayter SM, Cook MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev. 2012;11(10):754–65. [DOI] [PubMed] [Google Scholar]
- 2. Minichiello E, Semerano L, Boissier M-C. Time trends in the incidence, prevalence, and severity of rheumatoid arthritis: a systematic literature review. Joint Bone Spine. 2016;83(6):625–30. [DOI] [PubMed] [Google Scholar]
- 3. Yu C, Jin S, Wang Y, Jiang N, Wu C, Wang Q, Tian X, Li M, Zeng X. Remission rate and predictors of remission in patients with rheumatoid arthritis under treat-to-target strategy in real-world studies: a systematic review and meta-analysis. Clin Rheumatol. 2019;38(3):727–38. [DOI] [PubMed] [Google Scholar]
- 4. Palominos PE, Gasparin AA, de Andrade NPB, Xavier RM, da Silva Chakr RM, Igansi F, Gossec L. Fears and beliefs of people living with rheumatoid arthritis: a systematic literature review. Advanc Rheumatol. 2018;58(1):1. doi: 10.1186/s42358-018-0001-4. [DOI] [PubMed] [Google Scholar]
- 5. Salminen E, Heikkilä S, Poussa T, Lagström H, Saario R, Salminen S. Female patients tend to alter their diet following the diagnosis of rheumatoid arthritis and breast cancer. Prev Med. 2002;34(5):529–35. [DOI] [PubMed] [Google Scholar]
- 6. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets. 2014;14(4):245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Philippou E, Petersson SD, Rodomar C, Nikiphorou E. Rheumatoid arthritis and dietary interventions: systematic review of clinical trials. Nutr Rev. 2021;79(4):410–28. [DOI] [PubMed] [Google Scholar]
- 9. Vadell AKE, Bärebring L, Hulander E, Gjertsson I, Lindqvist HM, Winkvist A. Anti-inflammatory diet in rheumatoid arthritis (ADIRA)—a randomized, controlled crossover trial indicating effects on disease activity. Am J Clin Nutr. 2020;111(6):1203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MDet al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. [DOI] [PubMed] [Google Scholar]
- 11. Winkvist A, Barebring L, Gjertsson I, Ellegard L, Lindqvist HM. A randomized controlled cross-over trial investigating the effect of anti-inflammatory diet on disease activity and quality of life in rheumatoid arthritis: the Anti-inflammatory Diet in Rheumatoid Arthritis (ADIRA) study protocol. Nutr J. 2018;17(1):44. doi: 10.1186/s12937-018-0354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hulander E, Bärebring L, Turesson Wadell A, Gjertsson I, Calder PC, Winkvist A, Lindqvist HM. Diet intervention improves cardiovascular profile in patients with rheumatoid arthritis: results from the randomized controlled cross-over trial ADIRA. Nutr J. 2021;20(1):9. doi: 10.1186/s12937-021-00663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ekdahl C, Eberhardt K, Andersson SI, Svensson B. Assessing disability in patients with rheumatoid arthritis. Use of a Swedish version of the Stanford health assessment questionnaire. Scand J Rheumatol. 1988;17(4):263–71. [DOI] [PubMed] [Google Scholar]
- 14. Assarsson E, Lundberg M, Holmquist G, Björkesten J, Thorsen SB, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt Get al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. 2014;9(4):e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Radner H, Chatzidionysiou K, Nikiphorou E, Gossec L, Hyrich KL, Zabalan C, van Eijk-Hustings Y, Williamson PR, Balanescu A, Burmester GRet al. 2017 EULAR recommendations for a core data set to support observational research and clinical care in rheumatoid arthritis. Ann Rheum Dis. 2018;77(4):476–9. [DOI] [PubMed] [Google Scholar]
- 16. Tishkowski K, Gupta V. Erythrocyte sedimentation rate. StatPearls. Treasure Island, Fl: StatPearls Publishing LLC., 2021. [PubMed] [Google Scholar]
- 17. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. doi: 10.1016/b978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 18. McKellar G, Morrison E, McEntegart A, Hampson R, Tierney A, Mackle G, Scoular J, Scott JA, Capell HA. A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann Rheum Dis. 2007;66(9):1239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skoldstam L, Hagfors L, Johansson G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62(3):208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skoldstam L, Brudin L, Hagfors L, Johansson G. Weight reduction is not a major reason for improvement in rheumatoid arthritis from lacto-vegetarian, vegan or Mediterranean diets. Nutr J. 2005;4(1):15. doi: 10.1186/1475-2891-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338(7):436–45. [DOI] [PubMed] [Google Scholar]
- 22. Hou S-M, Chen P-C, Lin C-M, Fang M-L, Chi M-C, Liu J-F. CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res Ther. 2020;22(1):251. doi: 10.1186/s13075-020-02331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koch AE, Kunkel SL, Shah MR, Hosaka S, Halloran MM, Haines GK, Burdick MD, Pope RM, Strieter RM. Growth-related gene product alpha. A chemotactic cytokine for neutrophils in rheumatoid arthritis. J Immunol. 1995;155(7):3660. [PubMed] [Google Scholar]
- 24. Zhang Z, Chen Y, Jiang Y, Luo Y, Zhang H, Zhan Y. Prognostic and clinicopathological significance of CXCL1 in cancers: a systematic review and meta-analysis. Cancer Biol Ther. 2019;20(11):1380–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, Pope RM, Walz A, Strieter RM. Epithelial neutrophil activating peptide-78: a novel chemotactic cytokine for neutrophils in arthritis. J Clin Invest. 1994;94(3):1012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edwards JR, Sun SG, Locklin R, Shipman CM, Adamopoulos IE, Athanasou NA, Sabokbar A. LIGHT (TNFSF14), a novel mediator of bone resorption, is elevated in rheumatoid arthritis. Arthritis Rheum. 2006;54(5):1451–62. [DOI] [PubMed] [Google Scholar]
- 27. Ishida S, Yamane S, Nakano S, Yanagimoto T, Hanamoto Y, Maeda-Tanimura M, Toyosaki-Maeda T, Ishizaki J, Matsuo Y, Fukui Net al. The interaction of monocytes with rheumatoid synovial cells is a key step in LIGHT-mediated inflammatory bone destruction. Immunology. 2009;128(1pt2):e315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morel L, Domingues O, Zimmer J, Michel T. Revisiting the role of neurotrophic factors in inflammation. Cells. 2020;9(4):865. doi: 10.3390/cells9040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petersen LE, Baptista TSA, Molina JK, Motta JG, do Prado A, Piovesan DM, de Nardi T, Viola TW, Vieira ÉLM, Teixeira ALet al. Cognitive impairment in rheumatoid arthritis: role of lymphocyte subsets, cytokines and neurotrophic factors. Clin Rheumatol. 2018;37(5):1171–81.. doi: 10.1007/s10067-018-3990-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.