ABSTRACT
Background
Vegetarian-type dietary patterns have been associated with reducing the risk of developing diabetes and may function as an effective strategy for diabetes management.
Objectives
We aimed to examine the associations between adherence to plant-based diet indices and the risk of developing diabetes in the Boston Puerto Rican Health Study.
Methods
Puerto Rican adults (n = 646), aged 45–75 y and free of diabetes at baseline, were included. Dietary intake was assessed via a validated FFQ. Three plant-based dietary indices were calculated: an overall plant-based diet index (PDI), a healthful plant-based diet index (hPDI), and an unhealthful plant-based diet index (uPDI). Incident diabetes was defined as fasting plasma glucose ≥ 126 mg/dL (7.0 mmol/L), glycated hemoglobin ≥ 6.5% (48 mmol/mol), or use of hypoglycemic agents during follow-up. Cox proportional hazards were used to evaluate associations between the dietary patterns and incidence of diabetes, adjusting for potential confounders, such as age, sex, socioeconomic status, lifestyle factors, obesity, total energy intake, depressive symptomatology, and plasma concentrations of lipids.
Results
During a mean of 4.2 y of follow-up, we identified 134 diabetes cases. After adjustment for covariates, higher hPDI was associated with lower risk of developing diabetes (adjusted HR for the highest compared with the lowest tertile: 0.54; 95% CI: 0.31, 0.94; P-trend = 0.03). In contrast, the PDI and uPDI were not significantly associated with the risk of diabetes (P-trend > 0.3 for both).
Conclusions
The healthful plant-based dietary index, but not the total plant-based dietary index, was inversely associated with diabetes risk. These findings suggest that the quality of plant-based diets must be considered when recommending plant-based diets for the prevention of diabetes.
This trial was registered at clinicaltrials.gov as NCT01231958.
Keywords: Puerto Rican adults, diabetes, plant-based diet, dietary patterns, diet quality
Introduction
There is growing evidence that a healthy overall dietary pattern, rather than a focus on individual components, might be a better strategy for decreasing the risk of developing diabetes (1). Several studies have assessed the relation between vegetarian-type dietary patterns and the associated risk of developing diabetes (2–6). A defining feature of vegetarian diets is the absence of animal-based foods, specifically meat. Consuming meat products has been associated with a higher risk of diabetes, suggesting that meat may be an independent dietary risk factor (7–9). Furthermore, the American Diabetes Association has recommended plant-based eating patterns as an effective approach for disease management in individuals with diabetes (10). The shift to plant-based dietary patterns provides for an interesting approach to further characterize the role of the diet in the risk of developing diabetes.
A prospective analysis of 3 cohort studies in US men and women (the Nurses’ Health Studies and Health Professionals Follow-Up Study) conceptualized a graded plant-based dietary pattern based on 3 indices: an overall plant-based diet index (PDI), a healthful plant-based diet index (hPDI), and an unhealthful plant-based diet index (uPDI) (11). They found that the PDI and hPDI were associated with a lower risk of developing type 2 diabetes, whereas the uPDI was associated with increased risk (11). The population studied had significant socioeconomic homogeneity, potentially leading to biased results. Therefore, it is important to investigate if these results can be replicated in other diverse populations. To determine the applicability of these results we examined the relation between graded plant-based dietary patterns and diabetes risk in the population examined by the Boston Puerto Rican Health Study. Diabetes is prevalent in this population consisting of participants with a low socioeconomic status and a culturally based dietary pattern that differs considerably from that of the general US population (e.g., higher intake of rice, beans, and root vegetables and lower intake of fruit and other vegetables than non-Hispanic whites) (12–14). At baseline, the prevalence of type 2 diabetes was 39.5% in this cohort, identified by high fasting glucose concentration or use of hypoglycemic agents (12). We hypothesized that greater adherence to the hPDI would be associated with a lower incidence of diabetes, whereas greater adherence to the uPDI would be associated with a higher incidence of diabetes during ∼5 y of follow-up.
Methods
Study participants
Our analysis included participants enrolled in the ongoing longitudinal Boston Puerto Rican Health Study (NCT01231958), which began in 2004 (12, 13, 15). Objectives of the Boston Puerto Rican Health Study include examination of relations between stress, heart disease, physical and cognitive function, and bone outcomes in Puerto Rican adults living in the greater Boston area. The recruitment of self-identified Puerto Rican adults (aged 45–75 y) was conducted by door-to-door enumeration in randomly selected Hispanic-dense census blocks, with community-based recruitment approaches. The study included 1502 participants at baseline. We excluded participants with diabetes at baseline (fasting plasma glucose ≥ 126 mg/dL, glycated hemoglobin ≥ 6.5%, or use of hypoglycemic agents; n = 774) (16). We further excluded participants lost to follow-up (n = 39) and those with missing dietary intake data (n = 11) or with energy intakes <600 or >4800 kcal/d at baseline (n = 70) (13), leaving 646 participants for analyses (Supplemental Figure 1). The study protocols for the Boston Puerto Rican Health Study were approved by the institutional review board at Tufts Medical Center and before their participation in the study, all participants provided written informed consent.
Assessment of dietary intake and plant-based diet score
A validated semiquantitative FFQ developed and tested specifically for this population was used to assess dietary intake (17). The 3 plant-based diet indices evaluated were PDI, hPDI, and uPDI (11). A total of 18 defined food groups were denoted as either a healthy plant food group, a less healthy plant food group, or an animal-based food group (11). Plant foods were categorized as healthy or unhealthy based on existing knowledge of relations between the plant foods and health outcomes such as diabetes (11). The foods were then established in the 18 food groups as outlined by the plant-based diet indices of Satija et al. (11). Healthy plant food groups included vegetables, whole grains, legumes, fruits, vegetable oils, nuts, and tea/coffee (11). Unhealthy plant food groups included refined grains, fruit juices, sugar-sweetened beverages, potatoes, and sweets and desserts (11). The animal food group included fish, animal fats, eggs, dairy, seafood, meat, and miscellaneous animal-based foods (11). Individual food items were summed based on their frequencies of consumption and mixed dishes were deconstructed into individual food items and then added to the applicable food group. Each food group was ranked into quintiles and then allotted a positive score or a reverse score (11). For positive contribution to scores, participants in the highest quintile received a score of 5, whereas participants in the lowest quintile received a score of 1 (11). Reverse scores were defined for negative contributions: those in the highest quintile received a score of 1, whereas those in the lowest quintile received a score of 5 (11). For the hPDI, the healthy plant food group was assigned positive scores, and both the less healthy plant food group and the animal food group were assigned reverse scores (11). For the uPDI, the unhealthy plant food group was assigned a positive score and both the healthy plant food group and animal food group were assigned reverse scores (11). For the PDI, both the healthy and unhealthy plant food groups were assigned positive scores and the animal food group was assigned reverse scores (11).
In addition, we adapted the 3 plant-based diet indices to create an alternate PDI, alternate hPDI, and alternate uPDI. The food groups provisionally classified as plant food were excluded from the plant-based diet score. Tea/coffee was removed from the healthy plant food groups. Sweets/desserts and sugar-sweetened beverages were removed from the unhealthy plant food groups. The remaining 15 defined food groups were assigned to either the healthy plant food groups, the less healthy plant food groups, or the animal-based food group (11). The ranking and scoring for the 3 alternate plant-based diet indices were consistent with the previous plant-based diet scores.
Assessment of diabetes
The participants were asked to fast for 12 h preceding the morning blood draw, which was conducted by a certified phlebotomist in the participant's home. An enzymatic, kinetic reaction on the Olympus AU400e with Olympus glucose reagents (Olympus America Inc.) was used to measure serum glucose with a 2% intra-assay CV (13). Glycosylated hemoglobin was evaluated by a 2-step process where the final value was decided by a ratio of glycated hemoglobin to hemoglobin in conjunction with a conversion factor (13). Using the Roche Unimate hemoglobin A1c kit (Roche Diagnostics), whole-blood hemolysate was determined by latex-enhanced immunoturbidimetric assay to establish glycated hemoglobin, and by the colorimetric endpoint for hemoglobin, on the Cobas FARA (13). Information was collected on prescription and over-the-counter medications by having the participants show the bottles of medication they were currently taking. The use of hypoglycemic agents included any of the following medications: metformin, insulin, glinides, sulfonylureas, glitazones, and α-glucosidase inhibitors. For this study, diabetes status was defined as having fasting plasma glucose ≥ 126 mg/dL (7.0 mmol/L), glycated hemoglobin ≥ 6.5% (48 mmol/mol), or use of hypoglycemic agents (16).
Assessment of covariates
Trained bilingual interviewers collected information on age, sex, education level, household income, marital status, alcohol history, smoking history, medication use, and acculturation. The income-to-poverty ratio was calculated using year-specific poverty thresholds and guidelines from the US Department of Health and Human Services. To quantify physical activity, the modified Paffenbarger questionnaire from the Harvard Alumni Activity Survey was used to estimate a physical activity score (18, 19). The physical activity score was determined by the sum of hours expended on usual 24-h activities, including sedentary, light, moderate, or heavy activity and sleeping, multiplied by weighing factors that correspond to the rate of oxygen consumption correlated with the activities. Psychological acculturation was assessed with a scale determining psychological attachment toward either US or Puerto Rican culture (20). The Center for Epidemiology Studies Depression (CES-D) Scale was used to evaluate depressive symptomatology (21, 22). Height, weight, and blood pressure were measured in duplicate during the survey and the averages were obtained for each measurement. BMI (in kg/m2) was calculated using weight (kg) divided by height (m) squared. Hypertension status was defined as mean systolic blood pressure ≥ 140 mm Hg, mean diastolic blood pressure ≥ 90 mm Hg, or use of antihypertensive medications. Plasma triglycerides (mmol/L) were analyzed from EDTA plasma with an enzymatic endpoint reaction on the Olympus AU400e with Olympus triglycerides reagents (OSR6133) (Olympus America Inc.). Antilipemic agents were defined by the use of any of the following medications: HMG CoA reductase and omega-3 fatty acid supplements.
Statistical analysis
Baseline descriptive statistics were assessed for baseline characteristics of the participants stratified by tertile of the plant-based diet indices. Values for categorical covariates are shown as percentages, and for continuous covariates as means ± SEs, adjusted for age and sex. Food group intake was assessed by tertile of the 3 plant-based diet indices and presented as means ± SDs.
Cox proportional hazards were used to calculate HRs and 95% CIs to determine associations between the plant-based diet indices and the incidence of diabetes as a binary outcome in participants without diabetes. The proportional hazard assumption was satisfied. We first adjusted for age and sex (model 1); then for age, sex, total energy (kcal), and BMI (model 2); then further adjusted for education level, marital status, income-to-poverty ratio, total energy intake, smoking status, alcohol frequency, physical activity score, psychological acculturation score, depressive symptomatology, BMI, hypertension status, plasma triglyceride concentration, and antilipemic agents (model 3). Further, secondary analyses were conducted using the 3 alternate plant-based diet indices. There was a concern for overadjustment in our models, thus sensitivity analyses were conducted by excluding covariates, including hypertension status, plasma triglyceride concentration, and antilipemic agents, that may act as mediators on the pathway linking the plant-based dietary indices to diabetes. Statistical analyses were conducted using SAS version 9.4 (SAS Institute).
Results
Age- and sex-adjusted baseline characteristics are presented by PDI (Table 1), hPDI (Supplemental Table 1), and uPDI tertiles (Supplemental Table 2). Participants with higher PDI were more likely to have higher total energy intake and higher physical activity scores (Table 1). On the other hand, participants with higher hPDI were older, more likely to be women, nonsmokers, and current users of lipid-lowering medications. They were also less likely to be heavy alcohol drinkers (Supplemental Table 1). Supplemental Table 3 presents actual intake of the food groups by PDI, hPDI, and uPDI tertiles.
TABLE 1.
Baseline characteristics by tertile of PDI among Boston Puerto Rican participants without diabetes at baseline 1
| Tertile 1 | Tertile 2 | Tertile 3 | P | |
|---|---|---|---|---|
| n | 204 | 243 | 199 | |
| PDI, median (range) | 48 (35–51) | 54 (52–57) | 61 (58–71) | |
| Age,2 y | 54.9 ± 0.5 | 55.5 ± 0.5 | 56.0 ± 0.5 | 0.143 |
| Sex | 0.164 | |||
| Men | 30.9 | 28.4 | 24.6 | |
| Women | 69.1 | 71.6 | 75.4 | |
| Education | 0.674 | |||
| <5th grade | 17.2 | 17.9 | 19.6 | |
| 5th–8th grade | 25.0 | 22.5 | 23.6 | |
| 9th–12th grade | 45.1 | 42.1 | 36.2 | |
| Some college or more | 12.7 | 17.5 | 20.6 | |
| Marital status | 0.544 | |||
| Married/living as married, spouse in HH | 28.9 | 30.0 | 33.2 | |
| Married, spouse not in HH | 4.4 | 0.83 | 4.5 | |
| Divorced/separated | 38.7 | 47.5 | 34.7 | |
| Widowed | 12.3 | 11.3 | 11.6 | |
| Never married | 15.7 | 10.4 | 16.1 | |
| Income-to-poverty ratio5 | 132 ± 16.6 | 156 ± 15.4 | 166 ± 16.9 | 0.1443 |
| Total energy,5 kcal/d | 1909 ± 60.8 | 2351 ± 56.4 | 2562 ± 62.7 | <0.0013 |
| Smoking status | 0.654 | |||
| Never (<100 cigarettes in entire life) | 41.7 | 44.9 | 45.2 | |
| Smoked in the past, but not currently | 30.9 | 28.8 | 27.1 | |
| Currently smoke | 27.5 | 26.3 | 27.6 | |
| Alcohol frequency | 0.634 | |||
| None within past year | 50.3 | 49.4 | 50.5 | |
| Moderate | 39.9 | 36.4 | 43.9 | |
| Heavy | 9.9 | 14.2 | 5.6 | |
| Physical activity score5 | 31.8 ± 0.4 | 32.8 ± 0.3 | 32.8 ± 0.4 | 0.043 |
| Psychological acculturation score5 | 17.9 ± 0.5 | 19.2 ± 0.5 | 19.1 ± 0.5 | 0.093 |
| Depressive symptomatology score5 | 19.7 ± 0.9 | 18.7 ± 0.9 | 18.6 ± 1.0 | 0.413 |
| BMI,5 kg/m2 | 29.5 ± 0.4 | 29.6 ± 0.4 | 30.0 ± 0.5 | 0.413 |
| Hypertension | 0.304 | |||
| No hypertension | 41.7 | 46.3 | 46.9 | |
| Hypertension | 58.3 | 53.8 | 53.1 | |
| Plasma triglycerides,5 mmol/L | 1.7 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.1 | 0.793 |
| Use of antilipemic agents | 0.824 | |||
| Not taking medications | 77.0 | 76.5 | 77.9 | |
| Taking medications | 23.0 | 23.5 | 22.1 |
Values are means ± SEs or percentages. Baseline diabetes status was defined as fasting plasma glucose ≥ 126 mg/dL (7.0 mmol/L), glycated hemoglobin ≥ 6.5% (48 mmol/mol), or use of hypoglycemic agents. The PDI was defined by 18 food groups according to healthy, less healthy, and animal food groups. For all tertiles, a higher score is indicative of greater adherence to the dietary pattern. HH, household; PDI, overall plant-based diet index.
Adjusted for sex.
P-trend was calculated by using the median value of each PDI tertile as a continuous variable for continuous variables.
P-difference was calculated using the median value of each PDI tertile as a continuous variable for categorical variables.
Adjusted for age and sex.
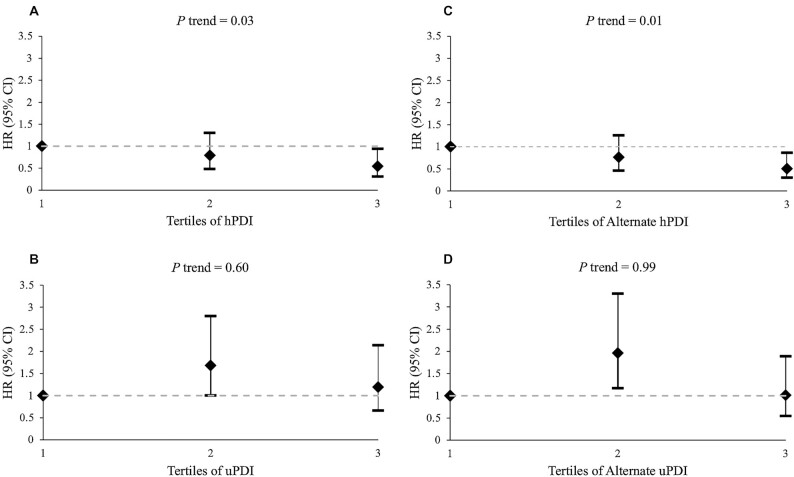
During a mean of 4.2 y of follow-up, we documented 134 cases for incident diabetes. An inverse nonsignificant trend between the PDI and risk of diabetes was observed (HR for the highest compared with lowest tertile: 0.79; 95% CI: 0.48, 1.30; P-trend = 0.36) (Table 2). Higher hPDI was associated with a significantly lower risk of diabetes after adjustment for covariates (HR for the highest compared with lowest tertile: 0.54; 95% CI: 0.31, 0.94; P-trend = 0.03) (Figure 1, Supplemental Table 4). We did not observe a significant association between the uPDI and risk of diabetes (HR for the highest compared with lowest tertile: 1.19; 95% CI: 0.66, 2.14; P-trend = 0.60) (Figure 1, Supplemental Table 4). Removing tea/coffee from the healthy plant food groups and removing sweets/desserts and sugar-sweetened beverages from the unhealthy plant food groups did not substantially change the results for the alternate PDI and uPDI (Table 2, Figure 1, Supplemental Table 5). Higher alternate hPDI was significantly associated with a lower risk of diabetes (HR for the highest compared with lowest tertile: 0.50; 95% CI: 0.30, 0.86; P-trend = 0.01) (Figure 1, Supplemental Table 5). Excluding hypertension status, plasma triglyceride concentration, and antilipemic agents did not change results materially (Model 4) (Table 2, Supplemental Tables 4, 5). Further, we did not observe any significant interactions between the plant-based dietary indices and age, sex, BMI, or income-to-poverty ratio, in relation to diabetes risk (P > 0.05 for all) (data not shown).
TABLE 2.
Incidence of diabetes by tertile of PDI among participants without baseline diabetes 1
| Tertile 1 | Tertile 2 | Tertile 3 | P-trend 2 | |
|---|---|---|---|---|
| n (person-years) | 204 (746) | 243 (938) | 199 (796) | |
| PDI, median (range) | 48 (35–51) | 54 (52–57) | 61 (58–71) | |
| Cases | 44 | 48 | 42 | |
| Crude incidence rate/1000 person-years | 59.0 | 51.2 | 52.8 | |
| Model 13 | 1.00 (Ref.) | 0.77 (0.50, 1.17) | 0.77 (0.50, 1.19) | 0.26 |
| Model 24 | 1.00 (Ref.) | 0.80 (0.52, 1.23) | 0.77 (0.49, 1.22) | 0.29 |
| Model 35 | 1.00 (Ref.) | 0.86 (0.54, 1.38) | 0.79 (0.48, 1.30) | 0.36 |
| Model 46 | 1.00 (Ref.) | 0.84 (0.53, 1.35) | 0.77 (0.47, 1.27) | 0.32 |
| Alternate PDI5,7 | 1.00 (Ref.) | 0.83 (0.50, 1.36) | 0.84 (0.52, 1.34) | 0.45 |
GED, General Educational Development; PDI, overall plant-based diet index.
P-trend was calculated using the median value of each PDI or alternate PDI tertile as a continuous variable.
Adjusted for age and sex.
Adjusted for age, sex, total energy (kcal), and BMI (kg/m2).
Adjusted for age, sex, education (<5th grade, 5th–8th grade, 9th–12th grade or GED, some college or more), marital status (married/living as married/spouse in household, married and spouse not in household, divorced/separated, widowed, or never married), income-to-poverty ratio (total household income/threshold dollar amount), total energy (kcal), smoking status (<100 cigarettes in entire life, past but not current, or current), alcohol frequency (none, moderate, or heavy), physical activity score, psychological acculturation score, depressive symptomatology score, BMI (kg/m2), hypertension (y/n), plasma cholesterol (mmol/L), and antilipemic agents (y/n).
Adjusted for age, sex, education (<5th grade, 5th–8th grade, 9th–12th grade or GED, some college or more), marital status (married/living as married/spouse in household, married and spouse not in household, divorced/separated, widowed, or never married), income-to-poverty ratio (total household income/threshold dollar amount), total energy (kcal), smoking status (<100 cigarettes in entire life, past but not current, or current), alcohol frequency (none, moderate, or heavy), physical activity score, psychological acculturation score, depressive symptomatology score, and BMI (kg/m2).
Alternate PDI was calculated by removing tea and coffee from the healthy plant food groups and removing sweets and desserts, and sugar-sweetened beverages, from the unhealthy plant food groups.
FIGURE 1.
Adjusted HRs with 95% CIs for incidence of diabetes by tertile of hPDI (A), uPDI (B), alternate hPDI (C), or alternate uPDI (D). Models adjusted for age, sex, education (<5th grade, 5th–8th grade, 9th–12th grade or General Educational Development, some college or more), marital status (married/living as married/spouse in household, married and spouse not in household, divorced/separated, widowed, or never married), income-to-poverty ratio (total household income/threshold dollar amount), total energy (kcal), smoking (<100 cigarettes in entire life, past but not current, or current), alcohol frequency (none, moderate, or heavy), physical activity score, psychological acculturation score, depressive symptomatology score, BMI (kg/m2), hypertension (y/n), plasma cholesterol (mmol/L), and antilipemic agents (y/n). Alternate hPDI and uPDI were calculated by removing tea and coffee from the healthy plant food groups and removing sweets and desserts, and sugar-sweetened beverages, from the unhealthy plant food groups. hPDI, healthful plant-based diet index; uPDI, unhealthful plant-based diet index.
Discussion
In this sample of self-identified Puerto Rican adults living in Massachusetts, greater adherence to the hPDI was associated with a lower risk of developing diabetes (P-trend = 0.03). Greater adherence to the PDI appeared to be protective in the development of incident diabetes (P-trend = 0.36).
The observed inverse association between the hPDI and diabetes risk is consistent with other epidemiologic studies (11, 23). Results from 3 prospective US cohorts found that, after pooled multivariable adjustments, the HR comparing the 2 extreme deciles of the hPDI was 0.55 (95% CI: 0.51, 0.59) for risk of incident type 2 diabetes (11). Similar significant inverse associations between the hPDI and diabetes risk were also observed in the Singapore Chinese Health Study; after multivariable adjustment, the HR for the highest compared with the lowest quintile of the hPDI was 0.81 (95% CI: 0.75, 0.89) (23). Previous clinical trials have shown that a vegetarian or vegan diet could significantly increase insulin sensitivity, improve glycemic control, and lower plasma lipid concentrations and body weight, in comparison with other more conventional diets (24–26). Collectively, these observations confirm the broad applicability of adherence to the hPDI diet decreasing the risk of developing diabetes.
These findings have important public health implications, given the disproportionately high prevalence of diabetes in the Puerto Rican population (12, 27). In addition, among Boston Puerto Rican study participants, higher psychological acculturation by US orientation was associated with higher diet quality as assessed by higher scores for the Alternate Healthy Eating Index-2010 (AHEI-2010) and the Mediterranean Diet Score (MeDS) (28); whereas, lower acculturation by a psychological-based scale, years living in the mainland United States, and a language-based scale was associated with greater adherence to traditional dietary patterns (28). However, adjustment for the psychological acculturation score in the analyses did not materially change the observed association between hPDI and diabetes risk. Many of the traditional foods consumed by this population, including beans, oatmeal, and nuts, are also found in the healthful plant-based dietary pattern (11, 29). This is, therefore, a starting point for encouraging increased use of healthy plant-based foods in this population. By providing education on the health benefits and identification of culturally significant hPDI foods, one can encourage the transition to an hPDI diet. Ultimately this dietary change has the potential to reduce the prevalence of diabetes in this population.
We observed a nonsignificant trend between a higher PDI and lower risk of diabetes (adjusted HR: 0.79, for the 2 extreme tertiles). This inverse relation is consistent with previous studies conducted in Asian and non-Hispanic white populations. In the Singapore Chinese Health Study (n = 45,411), the multivariable-adjusted HR comparing the highest with the lowest quintile for the PDI was 0.83 (95% CI: 0.76, 0.92) for diabetes risk (P-trend < 0.001) (23). Further, the Rotterdam Study (n = 6798) reported an HR of 0.87 (95% CI: 0.79, 0.99) linked to a risk of diabetes with a higher plant-based dietary index (30). Similar results were observed in 3 prospective cohort studies consisting of 69,949 older women from the Nurses’ Health Study I, 90,239 middle-aged women from the Nurses’ Health Study II, and 40,539 men from the Health Professionals Follow-Up Study (11). The nonsignificant results in our study are likely due to the relatively small sample size and the small number of diabetes cases. As a result of the small number of incident diabetes cases and relatively small starting baseline cohort of 1502 study participants, our study may lack the statistical power to detect significant associations of diet as depicted by the PDI and uPDI with diabetes risk. However, the uPDI in our study demonstrated an observed positive association with diabetes risk although this trend was not significant. Despite the null findings observed for the PDI and uPDI scores, our study was still able to detect significant associations between diabetes risk and the hPDI.
Our study confirmed the association of the hPDI diet with decreased risk of incident diabetes in a representative sample of Puerto Rican adults living in the Boston area. The results in the Puerto Rican adult population are consistent with prior studies conducted in different populations, demonstrating the broad applicability of the hPDI diet across various cultures and socioeconomic classes. We adjusted for potential confounders, including age, sex, socioeconomic status, lifestyle factors, obesity, total energy intake, depression, and plasma concentrations of lipid profiles. Further, the FFQ used to capture dietary intake in this study has previously been validated and adapted for this population. In addition, by investigating an overall plant-based dietary index, a healthful plant-based dietary index, and an unhealthful plant-based dietary index, we were able to examine graded plant-based dietary patterns to identify beneficial and opposing effects for the incidence of diabetes.
There are several potential limitations to our study. First, generalizability of the results may be limited owing to the specific cultural context of this Puerto Rican cohort. In addition, the semiquantitative FFQ captured self-reported dietary intake which leads to inevitable errors in measurement, which may attenuate the association toward the null if the misclassification is nondifferential. Likewise, the plant-based dietary patterns were assessed based on baseline dietary measures, which may not accurately predict diabetes incidence over time. Further, another limitation is the scoring for the 3 plant-based diet indices which consider all components to contribute an equal weight, yet the impact of each plant food may differentially influence the outcome of diabetes. Subsequently, the small sample size limited further analyses of the individual components of the 3 plant-based diet indices. Correspondingly, the participants in our analyses were more likely to be women. The interactions between the plant-based diet exposures and sex, concerning the risk of diabetes, were tested and found to be not significant. However, this could be due to the lack of statistical power to detect the small-to-moderate gender difference. Finally, because the study is observational, the analyses may be predisposed to residual confounding despite controlling for a wide spectrum of confounders. Therefore, it is important to continue to test the associations between plant-based dietary patterns and the risk of diabetes among other populations, particularly other Hispanic groups with different dietary patterns.
In summary, the current study suggests the favorable effects of a healthful plant-based dietary pattern on the risk of developing diabetes. The findings of this study suggest that adhering to a diet comprising healthy plant-based foods decreases the risk of developing diabetes in a Puerto Rican population and might be an independent modifiable risk factor.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—XG, KLT, BM, and CH: designed the research; XG, JIK, LA-S, and ACF: analyzed the data and interpreted the results; XG and ACF: wrote the manuscript; XG: had primary responsibility for the final content and is the guarantor of the contents of this article and this work; and all authors: contributed meaningfully to the study and critical reviewing of the manuscript and read and approved the final manuscript.
Notes
The Boston Puerto Rican Health Study was supported by the NIH under National Institute on Aging grant P01 AG023394 and National Heart, Lung, and Blood Institute grant P50 HL105185. ACF is supported by National Center for Advancing Translational Sciences grants TL1 TR002016 and UL1 TR002014.
Author disclosures: the authors report no conflicts of interest.
Supplemental Figure 1 and Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Abbreviations used: hPDI, healthful plant-based diet index; PDI, overall plant-based diet index; uPDI, unhealthful plant-based diet index.
Contributor Information
Ashley C Flores, Department of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA.
Christopher Heron, Department of Family Medicine, Penn State Health Family and Community Medicine Residency at Mount Nittany Medical Center, State College, PA, USA.
Jung In Kim, Department of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA; Department of Statistics, The Pennsylvania State University, University Park, PA, USA.
Bryan Martin, Department of Family Medicine, Penn State Health Family and Community Medicine Residency at Mount Nittany Medical Center, State College, PA, USA.
Laila Al-Shaar, Department of Public Health Sciences, The Pennsylvania State University College of Medicine, Hershey, PA, USA.
Katherine L Tucker, Department of Biomedical and Nutritional Sciences, University of Massachusetts Lowell, Lowell, MA, USA.
Xiang Gao, Department of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA.
References
- 1. Salas-Salvadó J, Martinez-González M, Bulló M, Ros E. The role of diet in the prevention of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21(Suppl 2):B32–48. [DOI] [PubMed] [Google Scholar]
- 2. Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care. 2009;32:791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnard ND, Katcher HI, Jenkins DJ, Cohen J, Turner-McGrievy G. Vegetarian and vegan diets in type 2 diabetes management. Nutr Rev. 2009;67:255–63. [DOI] [PubMed] [Google Scholar]
- 4. Snowdon DA, Phillips RL. Does a vegetarian diet reduce the occurrence of diabetes?. Am J Public Health. 1985;75:507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu THT, Pan W-H, Lin M-N, Lin C-L. Vegetarian diet, change in dietary patterns, and diabetes risk: a prospective study. Nutr Diabetes. 2018;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trapp CB, Barnard ND. Usefulness of vegetarian and vegan diets for treating type 2 diabetes. Curr Diab Rep. 2010;10:152–8. [DOI] [PubMed] [Google Scholar]
- 7. Vang A, Singh PN, Lee JW, Haddad EH, Brinegar CH. Meats, processed meats, obesity, weight gain and occurrence of diabetes among adults: findings from Adventist Health Studies. Ann Nutr Metab. 2008;52:96–104. [DOI] [PubMed] [Google Scholar]
- 8. Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med. 2004;164:2235–40. [DOI] [PubMed] [Google Scholar]
- 9. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94:1088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Diabetes Association. 3. Prevention or delay of type 2 diabetes: S tandards of Medical Care in Diabetes — 2021. Diabetes Care. 2021;44:S34–9. [DOI] [PubMed] [Google Scholar]
- 11. Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13:e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mattei J, Bigornia SJ, Sotos-Prieto M, Scott T, Gao X, Tucker KL. The Mediterranean diet and 2-year change in cognitive function by status of type 2 diabetes and glycemic control. Diabetes Care. 2019;42:1372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bermúdez OI, Falcón LM, Tucker KL. Intake and food sources of macronutrients among older Hispanic adults: association with ethnicity, acculturation, and length of residence in the United States. J Am Diet Assoc. 2000;100:665–73. [DOI] [PubMed] [Google Scholar]
- 15. Berkowitz SA, Gao X, Tucker KL. Food-insecure dietary patterns are associated with poor longitudinal glycemic control in diabetes: results from the Boston Puerto Rican Health Study. Diabetes Care. 2014;37:2587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Diabetes Association. 2. Classification and diagnosis of diabetes: S tandards of Medical Care in Diabetes — 2021. Diabetes Care. 2021;44:S15–33. [DOI] [PubMed] [Google Scholar]
- 17. Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148:507–18. [DOI] [PubMed] [Google Scholar]
- 18. Paffenbarger RS Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–75. [DOI] [PubMed] [Google Scholar]
- 19. Paffenbarger RS Jr, Hyde RT, Wing AL, Lee I-M, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–45. [DOI] [PubMed] [Google Scholar]
- 20. Marin G, Gamba RJ. A new measurement of acculturation for Hispanics: the Bidimensional Acculturation Scale for Hispanics (BAS). Hisp J Behav Sci. 1996;18:297–316. [Google Scholar]
- 21. Mackinnon A, McCallum J, Andrews G, Anderson I. The Center for Epidemiological Studies Depression scale in older community samples in Indonesia, North Korea, Myanmar, Sri Lanka, and Thailand. J Gerontol B Psychol Sci Soc Sci. 1998;53:P343–P52. [DOI] [PubMed] [Google Scholar]
- 22. Miller TQ, Markides KS, Black SA. The factor structure of the CES-D in two surveys of elderly Mexican Americans. J Gerontol B Psychol Sci Soc Sci. 1997;52:S259–69. [DOI] [PubMed] [Google Scholar]
- 23. Chen G-C, Koh W-P, Neelakantan N, Yuan J-M, Qin L-Q, van Dam RM. Diet quality indices and risk of type 2 diabetes mellitus: the Singapore Chinese Health Study. Am J Epidemiol. 2018;187(12):2651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnard ND, Cohen J, Jenkins DJ, Turner-McGrievy G, Gloede L, Green A, Ferdowsian H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr. 2009;89:1588S–96S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kahleova H, Matoulek M, Malinska H, Oliyarnik O, Kazdova L, Neskudla T, Skoch A, Hajek M, Hill M, Kahle Met al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med. 2011;28(5):549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. CDC. National Diabetes Statistics Report, 2020. Atlanta, GA: CDC, US Department of Health and Human Services; 2020. p. 12–15. [Google Scholar]
- 28. Mattei J, McClain AC, Falcón LM, Noel SE, Tucker KL. Dietary acculturation among Puerto Rican adults varies by acculturation construct and dietary measure. J Nutr. 2018;148:1804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mattei J, Sotos-Prieto M, Bigornia SJ, Noel SE, Tucker KL. The Mediterranean diet score is more strongly associated with favorable cardiometabolic risk factors over 2 years than other diet quality indexes in Puerto Rican adults. J Nutr. 2017;147:661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Z, Zuurmond MG, van der Schaft N, Nano J, Wijnhoven HAH, Ikram MA, Franco OH, Voortman T. Plant versus animal based diets and insulin resistance, prediabetes and type 2 diabetes: the Rotterdam Study. Eur J Epidemiol. 2018;33:883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.