Abstract
Following introduction of DNA interstrand cross-links (ICLs), mammalian cells display chromosome breakage or cell cycle delay with a 4N DNA content. To further understand the nature of the delay, previously described as a G2/M arrest, we developed a protocol to generate ICLs during specific intervals of the cell cycle. Synchronous populations of G1, S, and G2 cells were treated with photoactivated 4′-hydroxymethyl-4,5′,8-trimethylpsoralen (HMT) and scored for normal passage into mitosis. In contrast to what was found for ionizing radiation, ICLs introduced during G2 did not result in a G2/M arrest, mitotic arrest, or chromosome breakage. Rather, subsequent passage through S phase was required to trigger both chromosome breakage and arrest in the next cell cycle. Similarly, ICLs introduced during G1 did not cause a G1/S arrest. We conclude that DNA replication is required to elicit the cellular responses of cell cycle arrest and genomic instability after psoralen-induced ICLs. In primary human fibroblasts, the 4N DNA content cell cycle arrest triggered by ICLs was long lasting but reversible. Kinetic analysis suggested that these cells could remove up to ∼2,500 ICLs/genome at an average rate of 11 ICLs/genome/h.
Many clinically important chemotherapeutic chemicals can induce DNA interstrand cross-links (ICLs). These include mitomycin C (MMC), diepoxybutane, nitrogen and sulfur mustards, cisplatin, and photoactivated psoralens (21). ICLs pose a particular challenge to DNA repair systems since they involve both strands of DNA and cannot, therefore, be repaired using the redundant information in the complementary strand.
In Escherichia coli and yeast, the repair of ICLs involves the sequential action of several repair pathways. In E. coli, genetic and biochemical studies pointed to a recombinational-incisional repair (4, 13) in addition to a pathway involving a DNA glycosylase (28). In yeast, unlike in E. coli, double-strand breaks occur in response to ICLs. The repair of these breaks is dependent on the presence of RAD52 (homologous recombination repair) and RAD2 (excision repair) but not on RAD6 (mutagenic repair) (5, 12, 19).
Much less is known about the biology of cross-link repair in mammalian cells. Many of the studies in this field have focused on Fanconi anemia (FA) cells because of their sensitivity to cross-linking agents. The FA cellular phenotype is manifested as increased chromosome breakage (1) and a marked cell cycle delay with a 4N DNA content after introduction of ICLs (16). This delay has been also described as a G2/M checkpoint. It has therefore been suggested that the molecular basis of FA is a defect in the repair of ICLs (25), but to date no direct support for this hypothesis has been presented. After treatment with agents that induce ICLs, wild-type cells also display cell cycle delay with 4N DNA content and chromosome breakage, but the doses required are higher (10, 14).
In this study, we set out to determine the nature of the cell cycle delay with 4N DNA content in response to ICLs in wild-type human fibroblasts. We initially hypothesized that this delay was similar to that seen after ionizing radiation. In mammalian cells, ionizing irradiation during G2 activates a G2/M cell cycle checkpoint, thus providing time for DNA repair (11). Homologous recombination between the damaged and undamaged sister chromatids is suggested to be a mechanism for repair of radiation-induced DNA double-strand breaks (22, 29). In analogy, ICLs may also trigger a similar mechanism and undergo repair through recombination using the undamaged sister chromatid as a template. This hypothesis was supported by the genetic evidence that repair of ICLs in yeast and E. coli requires recombination functions and that increased rates of intrachromosomal recombination have been described after MMC treatment in mammalian cells (32). Two testable predictions resulted from the above hypothesis. First, ICLs introduced after completion of DNA replication would still trigger a G2/M cell cycle checkpoint, allowing time for repair. Second, ICLs would be preferentially repaired after replication is complete, i.e., as soon as sister chromatids are formed and become available for recombination.
Our experimental system relied on the use of primary human fibroblasts with intact cell cycle checkpoints and photoactivated 4′-hydroxymethyl-4,5′,8-trimethylpsoralen (HMT) as an ICL-inducing agent. We chose this protocol because it produced a measurable amount of ICLs without major cell death when the cross-linking regimen was applied as a pulse treatment and the cells were allowed to recover. HMT-induced growth arrest was examined as a function of the cell cycle position. We also determined the effects of ICLs on cell cycle progression in synchronized cells.
Unexpectedly, our results showed that human primary fibroblasts responded to ICLs by arresting only during DNA replication and not at the G2/M boundary. We found that passage through S phase was necessary for these lesions to induce a cellular response regardless of whether the damage was introduced pre- or post-DNA replication. An estimate of the ICL repair rate in human cells and a model for the cellular recognition of ICLs are presented.
MATERIALS AND METHODS
Cells and media.
Normal primary diploid fibroblasts were derived from human neonate foreskin samples. Two different cell lines from unrelated individuals (PD743.F and PD744.F) were used for all experiments and yielded similar and consistent results. Only PD743.F is described in this paper. Cells under passage 8 were employed for all the experiments described. Cells were maintained in α-modified Eagle medium (GIBCO/BRL) with 20% fetal calf serum (FCS) (Summit, Fort Collins, Colo.), 1× glutamine (GIBCO/BRL), and 0.1× penicillin-streptomycin (GIBCO/BRL) at 37°C and 5% CO2.
Cell treatment and denaturation-renaturation gel electrophoresis.
Cells were seeded at 3,000 cells/cm2 and allowed to recover for 24 h before treatment. They were then preincubated in HMT (Sigma) in Hanks' balanced salt solution (HBSS; GIBCO/BRL) in the dark for 10 min and then irradiated for 20 min using a transilluminator (Ultra-Lum, Paramount, Calif.) with fixed wavelength (365 nm) (UVA) and at maximum intensity. Subsequently, cells were washed twice with HBSS at 15-min intervals and reirradiated for an additional 30 min. Following treatment, cells were allowed to recover at 37°C in complete medium. In all cases, control cells were irradiated with UVA but without any drug. The dose of UVA was 10 to 11 mW/cm2. DNA for denaturation-renaturation gel electrophoresis was isolated as described by Vos and Hanawalt (30). DNA samples were denatured in 0.4 N NaOH at 55°C for 10 min and then loaded onto the gel. The gel was probed with the human 28S rRNA gene, yielding a 17-kb band (24). Autoradiogram band intensities were measured using a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). Several experiments were performed and gave similar results. If ICLs are introduced randomly into the genome, the fraction of denaturable DNA molecules (those without ICLs) corresponds to the zero fraction (ICL0) of a Poisson distribution. We used this relationship to calculate the number of ICLs per genome from the measured percentage of nondenatured DNA (30).
Ionizing radiation was administered by exposing cells to various doses (5, 10, and 20 Gy) using a 131Cs source.
Cell growth assays.
Cells were seeded at 3,000 cells/cm2 and treated with the appropriate drug. At various time intervals, cells were harvested by trypsinization, resuspended in 1 ml of phosphate-buffered saline (PBS; GIBCO/BRL), diluted in Isoton II balanced electrolyte solution, and counted in a Coulter Counter model 21, using the Coulter Multisizer AccuComp software (version 1.19). Trypan blue exclusion was used to determine that all counted cells were viable. For the clonogenic assay, cells were seeded at 1,000 cells/100-mm-diameter plate and treated with HMT and UVA. Following recovery, clones were stained with methylene blue and counted.
BrdU labeling.
Cells were plated at 3,000 cells/cm2 and treated with different concentrations of HMT as described above. Before each time point, the cells were incubated with 20 μg of bromodeoxyuridine (BrdU; Sigma)/ml for 24 h and then fixed with 60% ethanol–9% formaldehyde–4% glacial acetic acid for 2 min. The cells were then washed three times with PBS for 2 min each and denatured in 0.07 N NaOH for 3 min. After three washes for 2 min each, cells were incubated in blocking solution (PBS with 10% FCS and 0.5% Tween 20) for 10 min. A fluorescein isothiocyanate-conjugated anti-BrdU antibody (Becton-Dickinson) was then added at a 1:10 dilution in blocking solution containing 1 μg of DAPI (4′,6′-diamidino-2-phenylindole; Sigma) per ml for 30 min. Cells were then washed three times with PBS and covered with an antifade solution (consisting of 0.233 g of DABCO (1,4-diazabicyclo[2,2,2]octane; Sigma), 90% glycerol, and 25 mM Tris, pH 8.0. The samples were viewed on a Zeiss Axiophot microscope. For each sample, four fields were observed and 100 cells/field were counted. The percentages of BrdU+ cells/field were then averaged.
Fluorescence-activated cell sorter (FACS) analysis.
Trypsinized cells were fixed in ice-cold 70% ethanol for 12 h or more and then resuspended in 1 ml of PBS containing 0.5 mg of RNase A and 50 μg of propidium iodide (Sigma)/ml. DNA distributions were determined using a FACScalibur (Becton Dickinson, Mountain View, Calif.) with a laser setting of 495 nm. Percentages of cells in G1, G2, and S phases were generated using the program ModFit (Verity Software House Inc.).
Synchronization procedures.
For synchrony in G1, cells were incubated in 0.5% FCS-containing medium for 48 h. For synchrony in S phase, cells were serum starved as described above for 48 h and then split into complete medium containing 1 mM hydroxyurea and incubated for 24 h (27). Cells were then released from hydroxyurea for 3 h, at which time most cells were in S phase. The same procedure was followed for cells in G2, except that the cells were released from hydroxyurea for 9 h (27). Two millimolar caffeine (Sigma) was added at the time of release from hydroxyurea.
Chromosome breakage and mitotic index analyses.
Cells were simultaneously exposed to a hypotonic solution (0.075 M KCL) and Colcemid (0.05 μg/ml) (Sigma) for 10 min and then treated with fresh fixative (3:1 methanol-acetic acid). Slides were made and stained with Wright's stain. One thousand cells were scored for mitoses, and 50 cells or the total number of mitoses of each culture were scored for chromosome breaks.
RESULTS
Rationale for the assay.
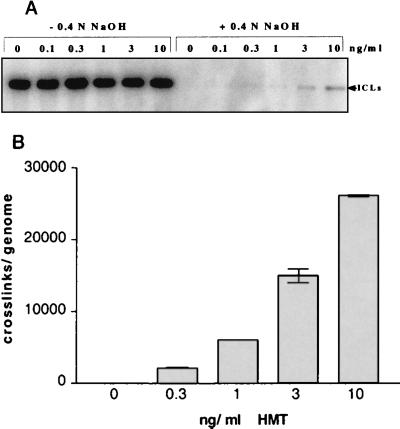
Quantitative estimates of the number of ICLs which are compatible with repair and cell survival of primary human cells have not been previously reported. We therefore wanted to establish a protocol that would provide a quantifiable level of ICLs as well as cell survival. Following treatment with MMC, diepoxybutane or HMT plus UVA irradiation (365 nm), we measured ICLs by denaturation-renaturation gel electrophoresis (30) and determined cell survival. Only HMT plus UVA provided a measurable level of DNA cross-links without death of >50% of cells. We found that a second UVA irradiation step (see Materials and Methods) was able to saturate the conversion of psoralen monoadducts into ICLs (Fig. 1A). Indeed, further in vitro UVA irradiation of DNA samples isolated from cells treated with our HMT-plus-UVA protocol did not produce additional cross-links on denaturing gels (data not shown). The quantitation of ICLs induced by different doses of HMT plus UVA showed a direct correlation between an increase in the HMT dose and an increase in the number of cross-links formed (Fig. 1B). It is important to mention that when HMT doses of <1 ng/ml were used and less than 6,000 ICLs/genome were present, the assay was at its detection limit (∼1% alkali-resistant DNA). Therefore, the numbers of cross-links measured in such samples were only approximate and were based on the assumption that the number of ICLs was proportional to the HMT dose.
FIG. 1.
(A) Detection of ICLs in the 17-kb 28S rRNA gene after treatment with 0.4 N NaOH. The covalent bond in cross-linked DNA made it resistant to alkaline denaturation and hence visible as a double-stranded DNA band. Increasing doses of HMT gave increasing levels of ICLs. (B) The numbers of ICLs/genome were calculated (see Materials and Methods and references therein) and plotted for each HMT dose. The graph shows a nearly linear dose response.
Cell growth in response to ICLs.
In contrast to ICLs induced by UVA alone, HMT-plus-UVA-induced ICLs caused a long growth arrest or delay (Fig. 2A). However, wild-type cells were able to recover from up to 0.3 ng of HMT/ml plus UVA after 8 ± 1 days. Upon resumption of growth the cells had no detectable cytogenetic abnormalities such as translocations or aneuploidy (200 metaphases examined; data not shown). Higher doses (>1 ng/ml or >6,000 ICLs) induced a “permanent” growth arrest (>20 days) but did not kill the cells, as indicated with trypan blue labeling. At even higher doses (>10 ng/ml or >26,000 ICLs/genome), cell death occurred within a few days. Importantly, the kinetics of recovery from low doses of HMT was not consistent with clonal expansion of a small subpopulation of cells but rather suggested a relatively homogeneous response of most of the cells in the population. This was confirmed by performing a time course of BrdU incorporation after the introduction of ICLs. Consistent with the measurement of cell numbers, the BrdU labeling index dropped dramatically within 2 days after ICL treatment. The index increased sharply to >30% at the same time as the cell number increased (Fig. 2B). Given that the average time to complete one cycle for human primary fibroblasts is ∼24 h, this indicated that the reinitiation of growth reflected nearly simultaneous recovery of most cells in the population and not clonal survival. In addition, a clonogenic survival assay was performed and showed that ∼60% of cells treated with 0.3 ng/ml indeed recovered and formed clones (Fig. 2C). Together, these data suggest that normal human fibroblasts have the capacity to remove approximately 2,500 ICLs/genome at an average rate of ∼11 ICLs/h (20). The BrdU labeling remained at 0 for the cells treated with 3 ng of HMT/ml plus UVA, thus further demonstrating that the constant cell number observed in these samples did not reflect a balance of continuing cell division and cell death.
FIG. 2.

(A) Effect of HMT plus UVA on the growth of PD743.F as a function of time. At 0.3 ng of HMT/ml, the cells were arrested for ∼8 days and then resumed entry into the cell cycle. At 1 to 3 ng of HMT/ml, cells remained arrested for the duration of the experiment. (B) Following treatment, cells were incubated with BrdU and labeled with an anti-BrdU antibody at various time points. As cells not treated with HMT (0 ng/ml) reached confluency, BrdU-positive cells decreased. Cells treated with 0.3 ng of HMT/ml were completely negative on day 7, followed by an abrupt increase in labeling at the time of recovery. At 3 ng/ml, cells remained unlabeled after they arrested. (C) Clonogenic survival assay of cells treated with 0, 0.15, 0.3, and 3 ng of HMT/ml and allowed to recover and form clones. With the exception of 3 ng of HMT/ml, all doses allowed 60% or more of cells to recover.
Cell cycle response to psoralen-induced ICLs.
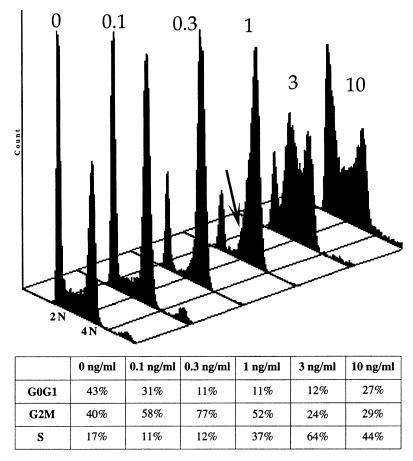
We next investigated the cell cycle stage at which HMT-plus-UVA-treated cells were arrested (Fig. 3). FACS analysis 24 h after treatment revealed that doses that produce more than 16,000 ICLs/genome (>3 ng of HMT/ml) resulted in an S-phase arrest (with a prominent shoulder in early S), followed by cell death, probably by apoptosis, as evidenced by the presence of a sub-G1 peak a few days later (data not shown). Doses that produced ∼6,000 to 16,000 ICLs/genome (1 to 3 ng of HMT/ml) caused the cells to arrest with an obvious intermediate DNA content, i.e., in S phase. Cells did not recover from the arrest within 15 days. At lower levels (less than approximately 2,500 ICLs/genome), however, cells arrested with an apparent 4N DNA content and after a prolonged arrest (∼8 days) resumed cycling. However, flow cytometry profiles showed that a shoulder of partially replicated DNA was present even at doses that resulted in a G2/M arrest (Fig. 3). Together with the obvious intermediate DNA content seen at higher doses, this observation suggested that the cells were also arrested in S phase (albeit with near-4N DNA content) and not in G2/M.
FIG. 3.
Flow cytometry analysis of PD743.F 24 h after treatment with HMT plus UVA. At 0.1 to 1 ng of HMT/ml, cells were arrested with a near-4N DNA content. A shoulder of unreplicated DNA was, however, apparent (arrow). At 3 and 10 ng of HMT/ml, cells were clearly arrested with an intermediate DNA content, i.e., in S phase. The table below shows the corresponding percentages of cells in G1, G2, and S phase.
DNA replication is required to cause cell cycle arrest after ICLs.
To determine whether ICLs are preferentially processed in G2, where fully formed sister chromatids are available as undamaged templates for recombinational repair, a synchronous population of G2 cells was treated with either HMT plus UVA or gamma irradiation (10 Gy). Seven hours later, most HMT-plus-UVA-treated cells had entered G1 (Fig. 4). In contrast to gamma-irradiated cells, which were immediately blocked at G2/M, G2 cells treated with HMT plus UVA passed through the first mitosis even after treatment with the highest doses of agents causing ICLs (Table 1). Since our flow cytometry studies on asynchronous cells had suggested that arrest due to HMT plus UVA occurred during DNA replication, we predicted that treatment of a synchronized population of S phase cells with HMT plus UVA would cause immediate cell cycle arrest in contrast to the effect on G2 cells. Indeed, cells treated during S phase did arrest and only entered mitosis (as determined by DAPI staining) if treated with caffeine, an agent that is known to override both S- and G2-phase checkpoints and permit S-phase-arrested cells to be scored in mitosis (26) (Table 2).
FIG. 4.
Synchronization and treatment of PD743.F cells in G2. (Left) cells were synchronized in G2 and treated with 3 ng of HMT/ml (generating 16,000 ICLs/genome). (Right) Seven hours after treatment, most cells have divided and entered G1. The table below shows the corresponding percentages of cells in G1, G2, and S phases.
TABLE 1.
Mitotic index and chromosome breakage analyses after treatment in G2
| Sample treatmenta | No. of mitoses/ 1,000 cells | No. of breaks/cell |
|---|---|---|
| 0 ng/ml | 60 | 0.1 |
| 0.3 ng/ml | 56 | 0.1 |
| 3 ng/ml | 51 | 0.3 |
| Gamma irradiation | 1 | >8b |
| 0 ng/ml + caffeine | 72 | 1.1 |
| 0.3 ng/ml + caffeine | 73 | 1.8 |
| 3 ng/ml + caffeine | 59 | 1.7 |
| Gamma irradiation + caffeine | 49 | 7.9 |
Cells were treated in G2 either with different doses of HMT (values with units of nanograms per milliliter) or with gamma irradiation at 10 Gy and then incubated with or without caffeine (2 mM) following treatment.
Very few cells were observed in mitosis.
TABLE 2.
Mitotic index and chromosome breakage analyses after treatment in S phase (first mitosis) and in the G2 phase (second mitosis)
| Sample treatmenta | S
|
Second G2
|
||
|---|---|---|---|---|
| No. of mitoses/ 1,000 cells | No. of breaks/cell | No. of mitoses/ 1,000 cells | No. of breaks/cell | |
| 0 ng/ml | 54 | 0.1 | 37 | 0.1 |
| 0.3 ng/ml | 0 | NAb | 6 | 4.2 |
| 3 ng/ml | 0 | NA | 2 | >8 |
| 0 ng/ml + caffeine | 84 | 2.4 | 50 | 1.7 |
| 0.3 ng/ml + caffeine | 16 | >7.8 | 50 | >8 |
| 3 ng/ml + caffeine | 0 | NA | 43 | >7.8 |
Values of nanograms per milliliter are HMT doses.
NA, not applicable.
One potential explanation for the lack of G2/M arrest after introduction of ICLs during G2 is a very rapid and complete repair of the lesions during this phase of the cell cycle. Alternatively, cells could be resistant to the introduction of HMT-plus-UVA-induced ICLs during this phase of the cell cycle. To exclude these possibilities, we again treated cells with agents that induced ICLs in G2 and asked whether they could pass through the second mitosis after ICL induction (Table 2). Indeed, although cells traversed a normal first mitosis, passage through the second mitosis was blocked. Therefore, complete and rapid repair and lack of ICL formation during G2 could not explain the normal mitosis after ICL induction. Additionally, aberrant mitoses with chromosome breakage (see below) were observed following treatment with caffeine to override the block. FACS analysis further confirmed that the cells were arrested in S phase (intermediate DNA content) after successfully completing the first mitosis (data not shown). Together, these results showed that wild-type cells recognized the presence of ICLs in the context of DNA replication and that passage through S phase was required for triggering a cell cycle arrest.
ICL-induced chromosome breakage requires prior DNA replication.
Since chromosomal breakage is a common phenotype observed in cells treated with DNA cross-linking agents (2), the results described above prompted us to look for chromosomal aberrations in the mitosis following the introduction of ICLs in S phase compared to G2 phase. Consistent with our previous findings, cytogenetic evaluations of metaphase spreads of samples treated in G2 revealed the absence of chromosomal breakage in the first mitosis even at the highest HMT doses. In contrast, extensive chromosomal breakage resulted when gamma-irradiated cells were treated with caffeine to overcome the G2/M checkpoint (Table 1). In addition, cells treated with HMT plus UVA in S phase arrested immediately and, in the presence of caffeine, showed extensive chromosome breakage. Moreover, the second mitosis was blocked, and chromosomal breaks were observed when cells were treated with caffeine subsequent to the first mitosis (Table 2).
Cellular responses to ICLs induced during G1.
It is well known that a G1/S checkpoint is activated and the initiation of S phase is prevented when mammalian cells are subjected to high doses of gamma irradiation in G1 (17). In contrast, FACS analysis of primary fibroblasts treated with HMT plus UVA in G1 and then released into the cell cycle did not reveal any delay at the G1/S boundary (Fig. 5A). Furthermore, quantitative denaturation-renaturation gel electrophoresis of DNA samples isolated from cells retained in G1 indicated the absence of any strand incision near the cross-link even after several days. The number of measured ICLs remained constant during the length of the experiment (∼14,700, ∼15,100, and ∼15,700 ICLs/genome on days 1, 2, and 5, respectively). Additionally, cells retained in G1 and treated with agents inducing ∼16,000 ICLs/genome were healthy even 2 weeks after treatment, whereas cells treated in the proliferating cell cycle showed marked cell death (Fig. 5B). The latter observation suggests that cell death, a third cellular response to ICLs, also requires passage through S phase to be elicited.
FIG. 5.
(A) FACS analysis of PD743.F cells treated in G1 after serum starvation with either HMT plus UVA or ionizing radiation. Following treatment, cells were released into the cell cycle by serum addition and analyzed at three different time points. Untreated cells progress into the cell cycle as observed after 24 h and 40 h. Cells treated with HMT at 0.3 ng/ml (generating ∼2,500 ICLs/genome) also showed progress into S phase after 24 h, and by 40 h cells were arrested with a 4N DNA content. In contrast, cells treated with 5 and 10 Gy did not enter the cell cycle within these time points. (B) Comparison of cell viability in response to HMT-plus-UVA treatment of cycling cells (a) to that for cells retained in G1 by serum starvation (b). Approximately 2 weeks following treatment with 3 ng of HMT/ml (generating ∼16,000 ICLs/genome), cycling cells show a higher degree of cell death than cells retained in G1 by serum starvation.
DISCUSSION
The biology of cellular responses to ICLs is of relevance not only to cancer chemotherapy but also to the genetic disease FA and to the DNA repair field in general. Unfortunately, little is known about this process in mammalian cells. In this study, we sought to understand the cell cycle kinetics of ICL repair. We chose to work with primary fibroblasts with intact cell cycle checkpoints and a cross-linking regimen which permitted a pulse-like introduction of the damage and which was compatible with cell survival. This was possible with protocols using HMT and saturating UVA. We were able to generate ICLs during specific cell cycle intervals and to determine whether primary human cells responded differently to ICLs introduced during G1, S, or G2.
ICLs do not trigger an immediate G2/M arrest or chromosome breakage.
For bacteria and yeast, genetic experiments have suggested an important role for homologous recombination in ICL repair (12). Thus, we hypothesized that the G2/M delay observed in mammalian cells after treatment with cross-linking agents represents a checkpoint which allows time for ICL repair by homologous recombination between sister chromatids. If this is true, ICLs introduced during G2 would be predicted to be less toxic to cells and to be preferentially repaired compared to ICLs introduced during G1 or early S.
Contrary to this model, however, even high doses of HMT plus UVA (resulting in ∼16,000 ICLs/genome) did not prevent mitosis when cells were treated in G2. Moreover, no chromosome breakage was observed during the mitosis immediately following treatment. This lack of G2/M delay, however, did not signify rapid repair of all the cross-links because treated cells arrested in the next S phase following mitosis. The present data do not, however, exclude the possibility that the majority of ICLs are repaired in G2 but that a minority of irreparable ICLs, tolerated by the mitotic machinery, cause a block in the next S phase.
The DNA content of the arrested cells depended on the amount of ICLs and varied between >2 and 4N. We therefore believe that our data are most consistent with the interpretation that the “4N” DNA content of cells arrested by ICLs was caused by incomplete DNA replication, i.e., an arrest late in S phase. The apparent 4N DNA can be explained by the scarcity of ICLs (2,300 ICLs/genome = 1 ICL/2.5 × 106 bp), which are less frequent than origins of replication (1/2 × 105 bp) (9), and by the fact that flow cytometry is not sensitive enough to detect very small amounts of unreplicated DNA. Importantly, others have also observed a similar 4N DNA content arrest in synchronized lymphoblasts and epithelial cells by using MMC (15). Although they interpreted their findings as a G2/M rather than a late-S-phase delay, their results indicate that this phenomenon is not exclusive to HMT plus UVA as a cross-linking agent.
Our present data cannot conclusively distinguish between an S-phase checkpoint and a passive mechanical block to replication presented by ICLs. Both interpretations, however, are compatible with the fact that the structure of an ICL makes it an obvious obstacle to replication.
ICLs do not trigger a G1/S arrest.
Previous reports have indicated that ICLs in actively transcribed genes are preferentially repaired compared to transcriptionally silent loci (31). This observation implies the repair of DNA cross-links during the G1 phase of the cell cycle, when housekeeping genes are transcribed. Additionally, if DNA double-strand breaks are structural intermediates in ICL repair, they would induce a p53-mediated G1/S cell cycle delay (18). We therefore sought to determine whether HMT-plus-UVA treatment during G1 could generate a G1/S delay similar to that observed after ionizing radiation. Our results showed that even doses of HMT plus UVA which caused a long-lasting (>8-day) mitotic arrest with 4N DNA content caused no G1/S delay. Furthermore, no ICL incision was detected in cells held in G1 by serum starvation. These results suggest that efficient repair of ICLs does not occur during the G1 phase of the cell cycle of fibroblasts.
Rate of ICL repair in wild-type human fibroblasts.
Our study is the first to report an estimate of the number of ICLs which can be removed by primary mammalian cells. Wild-type human fibroblasts were able to recover from ICL damage and reenter the cell cycle without chromosomal abnormalities. In multiple independent experiments, asynchronous fibroblasts from different individuals showed remarkable consistency in the time of reentry into the cell cycle after ICL treatment. In all cases, it took 8 ± 1 days after the introduction of ∼2,500 ICLs/genome. Therefore, under the assumption of a constant repair rate, it can be estimated that ∼11 ICLs/genome/h are removed.
Previously, by using MMC and various transformed cell lines, others have reported much faster rates of ICL repair (8, 30, 33). However, in those studies, only the initial incision of the ICL was measured and only short-term assays performed at 48 h after ICL induction were used (3). Moreover, long-term cell survival was not reported, and our data indicate that the very high number of ICLs induced in those studies would have been incompatible with prolonged cell survival.
Conclusions for mammalian ICL recognition and repair.
The introduction of psoralen-induced ICLs during G1 resulted neither in a prolonged G1/S delay nor in any incision of these ICLs. Additionally, those ICLs generated during G2 also did not delay the subsequent mitosis or result in chromosomal breakage. Therefore, our data indicate that ICLs may not be sensed and hence not repaired in either G1 or G2 in human primary fibroblasts. Rather, DNA replication appears to be required to induce cell cycle arrest and/or chromosomal breakage in response to ICLs. Our results are consistent with earlier work performed with plants (7). In those studies, it was shown that all cytogenetic abnormalities, seen after treatment with nitrogen mustard, were due to so-called DNA misreplication. The lack of an immediate G2/M arrest following ICLs suggests that mammalian cells, and perhaps all eukaryotes, may not utilize the undamaged sister chromatid as a template for ICL repair. It is important to mention, however, that although these conclusions may be true for all cross-linking agents, our experiments were only performed using HMT plus UVA.
Two main possibilities for the repair of ICLs exist. First, the removal of ICLs during the next S phase may involve deletion of the lesion followed by religation, as was suggested for FA cells. This process would always result in the loss of genetic information and may be mutagenic (6). Alternatively, it is conceivable that ICL repair involves interchromosomal mitotic recombination (repair by gene conversion) rather than recombination between sister chromatids. The existence of such a pathway has recently been documented for double-strand break repair (23).
DNA replication is required to trigger the classic cellular responses to ICLs including both chromosome breakage and arrest with 4N DNA content. We therefore propose a model in which at least the initial steps of mammalian ICL recognition and repair occur exclusively in S phase (Fig. 6).
FIG. 6.
A model for the cellular response to ICLs. When ICLs are introduced prior to replication, DNA synthesis stalls at the lesion. This unreplicated DNA triggers a cell cycle arrest, whereby cells do not enter mitosis unless caffeine is added to the cells. As a result of an aberrant mitosis, chromosome breaks form. Conversely, if ICLs are introduced in G2 (post-replication DNA cross-link), the cells are unable to recognize the lesion, and can enter a normal mitosis. Cell cycle arrest will only result when the cells undergo DNA replication again.
ACKNOWLEDGMENTS
We thank Mike Liskay for his critical reading of the manuscript, Andrew Buermeyer, Stefan Lanker, Matt Thayer, Alan D'Andrea, and Cynthia Timmers for useful discussions, and Kara Manning for help in the preparation of the manuscript.
This work was supported by NHLBI program project grant 1PO1HL48546 to M.G.
REFERENCES
- 1.Auerbach A D, Wolman S R. Susceptibility of Fanconi's anaemia fibroblasts to chromosome damage by carcinogens. Nature. 1976;261:494–496. doi: 10.1038/261494a0. [DOI] [PubMed] [Google Scholar]
- 2.Bempong M A, Trower E C. Sensitivity of rat testes to inhibitors of nucleic acid synthesis. III. The inheritance of mitomycin C-induced structural rearrangements of chromosomes. J Hered. 1975;66:285–289. doi: 10.1093/oxfordjournals.jhered.a108631. [DOI] [PubMed] [Google Scholar]
- 3.Bessho T, Mu D, Sancar A. Initiation of DNA interstrand cross-link repair in humans: the nucleotide excision repair system makes dual incisions 5′ to the cross-linked base and removes a 22- to 28-nucleotide-long damage-free strand. Mol Cell Biol. 1997;17:6822–6830. doi: 10.1128/mcb.17.12.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng S, Van Houten B, Gamper H B, Sancar A, Hearst J E. Use of psoralen-modified oligonucleotides to trap three-stranded RecA-DNA complexes and repair of these cross-linked complexes by ABC excinuclease. J Biol Chem. 1988;263:15110–15117. [PubMed] [Google Scholar]
- 5.de Andrade H H, Marques E K, Schenberg A C, Henriques J A. The PSO4 gene is responsible for an error-prone recombinational DNA repair pathway in Saccharomyces cerevisiae. Mol Gen Genet. 1989;217:419–426. doi: 10.1007/BF02464912. [DOI] [PubMed] [Google Scholar]
- 6.Escarceller M, Buchwald M, Singleton B K, Jeggo P A, Jackson S P, Moustacchi E, Papadopoulo D. Fanconi anemia C gene product plays a role in the fidelity of blunt DNA end-joining. J Mol Biol. 1998;279:375–385. doi: 10.1006/jmbi.1998.1784. [DOI] [PubMed] [Google Scholar]
- 7.Evans H J, Scott D. The induction of chromosome aberrations by nitrogen mustard and its dependence on DNA synthesis. Proc R Soc Lond B. 1969;173:491–512. doi: 10.1098/rspb.1969.0073. [DOI] [PubMed] [Google Scholar]
- 8.Gruenert D C, Cleaver J E. Repair of psoralen-induced cross-links and monoadducts in normal and repair-deficient human fibroblasts. Cancer Res. 1985;45:5399–5404. [PubMed] [Google Scholar]
- 9.Hamlin J L. Mammalian origins of replication. Bioessays. 1992;14:651–659. doi: 10.1002/bies.950141002. [DOI] [PubMed] [Google Scholar]
- 10.Heinrich M C, Hoatlin M E, Zigler A J, Silvey K V, Bakke A C, Keeble W W, Zhi Y, Reifsteck C A, Grompe M, Brown M G, Magenis R E, Olson S B, Bagby G C. DNA cross-linker-induced G2/M arrest in group C Fanconi anemia lymphoblasts reflects normal checkpoint function. Blood. 1998;91:275–287. [PubMed] [Google Scholar]
- 11.Hong J H, Gatti R A, Huo Y K, Chiang C S, McBride W H. G2/M-phase arrest and release in ataxia telangiectasia and normal cells after exposure to ionizing radiation. Radiat Res. 1994;140:17–23. [PubMed] [Google Scholar]
- 12.Jachymczyk W J, von Borstel R C, Mowat M R, Hastings P J. Repair of interstrand cross-links in DNA of Saccharomyces cerevisiae requires two systems for DNA repair: the RAD3 system and the RAD51 system. Mol Gen Genet. 1981;182:196–205. doi: 10.1007/BF00269658. [DOI] [PubMed] [Google Scholar]
- 13.Jones B K, Yeung A T. Repair of 4,5′,8-trimethylpsoralen monoadducts and cross-links by the Escherichia coli UvrABC endonuclease. Proc Natl Acad Sci USA. 1988;85:8410–8414. doi: 10.1073/pnas.85.22.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser T N, Lojewski A, Dougherty C, Juergens L, Sahar E, Latt S A. Flow cytometric characterization of the response of Fanconi's anemia cells to mitomycin C treatment. Cytometry. 1982;2:291–297. doi: 10.1002/cyto.990020505. [DOI] [PubMed] [Google Scholar]
- 15.Kruyt F A, Dijkmans L M, Arwert F, Joenje H. Involvement of the Fanconi's anemia protein FAC in a pathway that signals to the cyclin B/cdc2 kinase. Cancer Res. 1997;57:2244–2251. [PubMed] [Google Scholar]
- 16.Kubbies M, Schindler D, Hoehn H, Schinzel A, Rabinovitch P S. Endogenous blockage and delay of the chromosome cycle despite normal recruitment and growth phase explain poor proliferation and frequent edomitosis in Fanconi anemia cells. Am J Hum Genet. 1985;37:1022–1030. [PMC free article] [PubMed] [Google Scholar]
- 17.Kuerbitz S J, Plunkett B S, Walsh W V, Kastan M B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci USA. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu V F, Boubnov N V, Weaver D T. Cell cycle checkpoints and repair of ionizing radiation damage. Stem Cells. 1995;13(Suppl. 1):117–128. [PubMed] [Google Scholar]
- 19.Magana-Schwencke N, Henriques J A, Chanet R, Moustacchi E. The fate of 8-methoxypsoralen photoinduced crosslinks in nuclear and mitochondrial yeast DNA: comparison of wild-type and repair-deficient strains. Proc Natl Acad Sci USA. 1982;79:1722–1726. doi: 10.1073/pnas.79.6.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meniel V, Magana-Schwencke N, Averbeck D. Preferential repair in yeast after induction of interstrand cross-links by 8-methoxypsoralen plus UVA. Mutat Res. 1995;329:121–130. doi: 10.1016/0027-5107(95)00023-c. [DOI] [PubMed] [Google Scholar]
- 21.Metzler M. DNA adducts of medicinal drugs: some selected examples. J Cancer Res Clin Oncol. 1986;112:210–215. doi: 10.1007/BF00395914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resnick M A, Moore P D. Molecular recombination and the repair of DNA double-strand breaks in CHO cells. Nucleic Acids Res. 1979;6:3145–3160. doi: 10.1093/nar/6.9.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson C, Moynahan M E, Jasin M. Double-strand break repair by interchromosomal recombination: suppression of chromosomal translocations. Genes Dev. 1998;12:3831–3842. doi: 10.1101/gad.12.24.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Sasaki M S. Is Fanconi's anaemia defective in a process essential to the repair of DNA cross links? Nature. 1975;257:501–503. doi: 10.1038/257501a0. [DOI] [PubMed] [Google Scholar]
- 26.Schlegel R, Pardee A B. Caffeine-induced uncoupling of mitosis from the completion of DNA replication in mammalian cells. Science. 1986;232:1264–1266. doi: 10.1126/science.2422760. [DOI] [PubMed] [Google Scholar]
- 27.Tobey R A, Valdez J G, Crissman H A. Synchronization of human diploid fibroblasts at multiple stages of the cell cycle. Exp Cell Res. 1988;179:400–416. doi: 10.1016/0014-4827(88)90279-0. [DOI] [PubMed] [Google Scholar]
- 28.Van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990;54:18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vispe S, Cazaux C, Lesca C, Defais M. Overexpression of Rad51 protein stimulates homologous recombination and increases resistance of mammalian cells to ionizing radiation. Nucleic Acids Res. 1998;26:2859–2864. doi: 10.1093/nar/26.12.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vos J M, Hanawalt P C. Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell. 1987;50:789–799. doi: 10.1016/0092-8674(87)90337-0. [DOI] [PubMed] [Google Scholar]
- 31.Vos J M, Wauthier E L. Differential introduction of DNA damage and repair in mammalian genes transcribed by RNA polymerases I and II. Mol Cell Biol. 1991;11:2245–2252. doi: 10.1128/mcb.11.4.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y Y, Maher V M, Liskay R M, McCormick J J. Carcinogens can induce homologous recombination between duplicated chromosomal sequences in mouse L cells. Mol Cell Biol. 1988;8:196–202. doi: 10.1128/mcb.8.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youssoufian H. Cytoplasmic localization of FAC is essential for the correction of a prerepair defect in Fanconi anemia group C cells. J Clin Investig. 1996;97:2003–2010. doi: 10.1172/JCI118635. [DOI] [PMC free article] [PubMed] [Google Scholar]