ABSTRACT
Background
Vitamin C may benefit bone as an antioxidant.
Objectives
This cross-sectional study evaluated associations between dietary, supplemental, and plasma vitamin C with bone mineral density (BMD) among Puerto Rican adults.
Methods
Diet was assessed by food-frequency questionnaire (n = 902); plasma vitamin C, measured in fasting blood (n = 809), was categorized as sufficient (≥50 μmol/L), insufficient (20–49 μmol/L), or low (<20 μmol/L). Associations between vitamin C and BMD (measured by DXA) were tested, with false discovery rate correction for multiple comparisons, and interactions by smoking, sex, and estrogen status. Least-squares mean BMDs were compared across tertiles of diet and plasma vitamin C.
Results
Participants’ mean age was 59 ± 7 y (range: 46–78 y), 72% were women, mean dietary vitamin C was 95 ± 62 mg/d, and plasma vitamin C ranged from 1.7 to 125 μmol/L. No associations were observed between dietary vitamin C and BMD (P-value range: 0.48–0.96). BMD did not differ by vitamin C supplement use (P-value range: 0.07–0.29). Total femur BMD was higher (P = 0.04) among plasma vitamin C–sufficient participants (mean: 1.06; 95% CI: 1.035, 1.076 g/cm2) compared with low plasma vitamin C participants (1.026; 0.999, 1.052 g/cm2) in adjusted models. Findings at the trochanter were similar (P = 0.04). Postmenopausal women without estrogen therapy, with sufficient plasma vitamin C, showed greater total femur BMD (1.004 ± 0.014 g/cm2) compared to those with low plasma vitamin C (0.955 ± 0.017 g/cm2; P = 0.001). Similar findings were observed at the trochanter (P < 0.001). No significant associations were observed among premenopausal women or those with estrogen therapy or men. Interactions with smoking status were not significant.
Conclusions
Dietary vitamin C was not associated with BMD. Low plasma vitamin C, compared with sufficiency, was associated with lower hip BMD, particularly among postmenopausal women without estrogen therapy. Future research is needed to determine whether vitamin C status is associated with change in BMD or reduction in fracture risk.
Keywords: Hispanic, bone mineral density, osteoporosis, vitamin C, Puerto Rican, diet
Introduction
Osteoporosis is a major health problem in the United States, with 50% of Americans >50 y of age presenting with clinically low bone mass (1). Approximately 8.9 million fractures each year are attributable to osteoporosis (2). Over the next 5 y, health care costs for fracture are projected to increase by 50% from the current $19 billion per year (3). The prevalence of osteoporosis differs across race/ethnic groups (4). Data from the NHANES 2005–2014 showed that Hispanic men and women had the second highest prevalence of osteoporosis (4.1%), following other races (including biracial, 5.9%); whereas non-Hispanic Blacks and non-Hispanic Whites showed lower prevalence (1.7% and 3.7%, respectively) (5). Among Hispanic origin groups, Puerto Rican men have been shown to have the highest prevalence of osteoporosis (8.6%), while Puerto Rican women (10.7%) were second to Mexican-American women (16%) (6). Therefore, it is imperative to identify modifiable risk factors to inform recommendations to prevent osteoporosis onset specific to Hispanic populations of different backgrounds.
Diet is a major modifiable risk factor for the prevention of bone loss among adults (7). Fruit and vegetable intake has been shown to be associated with higher bone mineral density (BMD) and lower the risk of hip fracture (8–14). One of the dietary components driving the relation between fruit and vegetable intakes and bone is hypothesized to be vitamin C (7). Vitamin C is an antioxidant, which functions to decrease oxidative stress, a harmful process that may lead to increased bone resorption (15). In addition, vitamin C is a cofactor in the hydroxylation of amino acids that produce collagen, the most abundant protein in bone. In animals, vitamin C deficiency has been linked to lower BMD (16–20). Previous literature in humans suggests that there is an association between dietary vitamin C and BMD (7, 21–24). However, some literature suggests that the positive association of vitamin C with BMD may depend on smoking status and estrogen use (22, 25–28). A limitation of previous work that may explain, in part, inconclusive results related to vitamin C and BMD includes lack of assessment of a biomarker of vitamin C status to objectively support estimated dietary intakes. To our knowledge, the only study that included plasma vitamin C, using data from the third NHANES (1988–1994), showed that higher plasma vitamin C was associated with greater BMD in men, premenopausal women, and postmenopausal women with a history of smoking and estrogen use (22). It is important to examine the association between plasma, as well as dietary vitamin C and bone, as it may better reflect actual exposure to this antioxidant, whereas food-frequency questionnaire (FFQ) assessment is a broad estimate of self-reported usual intake.
The objectives of the current study were to investigate the association of BMD with 1) dietary vitamin C (diet and supplements) and 2) plasma vitamin C (established biomarker) in a middle-aged and older adult Puerto Rican population. Furthermore, interactions by smoking status, sex, and estrogen status were tested, based on previous literature showing subgroup-specific results (22, 25, 27–30). As previous work in the Boston Puerto Rican Osteoporosis Study (BPROS) demonstrated that diet–bone relations differed by estrogen status (31), and that 27% of the BPROS reported current smoking (32), this study aimed to evaluate the relation of vitamin C with bone, stratified by sex and estrogen and smoking status. We hypothesized that 1) higher dietary vitamin C and use of vitamin C supplements would be related to greater BMD; 2) sufficient plasma vitamin C status would be associated with greater BMD, compared with low plasma vitamin C or insufficiency; and 3) these associations would be most evident among smokers and postmenopausal women.
Methods
Study population
Data from 902 BPROS participants, an ancillary study to the Boston Puerto Rican Health Study (BPRHS), were included (Supplemental Figure 1). The BPRHS is a longitudinal investigation of health disparities experienced by Puerto Rican adults living on the US mainland, described in detail elsewhere (32). Briefly, data were collected on sociodemographic, health and health behaviors, migration history, medication use, and stress by bilingual interviewers in participants’ homes. BPRHS participants were asked if they would like to participate in the BPROS.
The interview included questions on sunlight exposure and falls, BMD measures, and a fasting blood sample at the Metabolic Research Unit, Jean Mayer USDA Human Nutrition Research Center on Aging (HNRCA), Tufts University. All participants provided informed consent. The institutional review boards at Tufts University, Northeastern University and the University of Massachusetts–Lowell approved this study.
Measures of BMD
BMD (g/cm2) of the hip and lumbar spine was measured by DXA (GE-Lunar model Prodigy scanner; General Electric) at the Bone Metabolism Laboratory at the Tufts HNRCA. Measures were taken following standard procedures and the right hip was scanned, unless the participant reported a previous hip fracture or joint replacement. The root mean square precision was 1.31% for BMD measures of the femoral neck and 1.04% for the lumbar spine, as reported elsewhere (33). Each week, an external standard (aluminum spine phantom: Lunar Radiation Corp) was scanned to monitor stability of the DXA measures. A total of 25 participants’ lumbar spine (L2–L4) measures and 7 femoral neck measures were excluded from analyses, as determined by the study endocrinologist (BD-H) to have nonanatomical parts or extraskeletal calcification after reviewing all scans with T-scores >4.0.
Plasma vitamin C
A certified phlebotomist took a 12-h fasting blood sample at the participant's home the morning after the original interview, or as soon thereafter as possible. Aliquots were saved and stored at −80°C until being processed. Plasma vitamin C was measured with HPLC (34) with a 6% CV. Participants (n = 809) were categorized as having sufficient (≥50 μmol/L), insufficient (≥20 μmol/L, but <50 μmol/L), or low (<20 μmol/L) vitamin C status based on data suggesting that plasma vitamin C above acute deficiency, yet below optimal, results in nonspecific clinical symptomology (29, 35).
Dietary intake
Dietary intake was assessed at the 2-y follow-up appointment, the interview closest to the BMD measurement, with an FFQ adapted for use in Hispanic adults. The food list for the FFQ was developed using the National Cancer Institute/Block food-frequency format, but with data from the Hispanic Health and Nutrition Examination Survey dietary recalls for Puerto Rican adults. Following adjustment, the FFQ was shown to be an improved estimator of diet in this Hispanic population (36). The BPRHS FFQ has been validated against plasma carotenoids (37), vitamin E (38), vitamin B-6 (39), and vitamin B-12 (40) in Hispanic adults aged 60 y and older. Nutrient intakes were calculated from the scanned FFQ using the Nutrient Data System for Research software (NDS-R; Nutrition Coordinating Center). Participants with energy intake <600 or >4800 kcal/d were excluded from analyses (n = 23) as implausible.
Dietary variables (vitamin C and calcium) were energy adjusted using the residual method (41). Participants were categorized by tertile of energy-adjusted dietary vitamin C. Use of dietary supplements, including brand, dose, and frequency of intake, were captured by questionnaire. There was limited variation among users in intake of supplemental vitamin C (95% of the sample was taking 0–60 mg/d, an amount commonly found in multivitamin/mineral supplements); therefore, supplemental vitamin C was coded as taking a supplement containing vitamin C, yes/no.
Covariates
Covariates known to influence either vitamin C status or bone health were included in all statistical analyses and were measured at the same examination as the bone density measurements. If a covariate was missing at the bone visit, data from the 2-y follow-up examination to the BPRHS were used. These covariates included age (years), sex, menopause status, use of estrogen (women only), height (centimeters), BMI (kg/m2), physical activity [continuous score; range: 24.5 (none/little) to 60.5 (some)], education (< 8th grade, 8–12th grade, some college or more), total energy intake (kilocalories/day), smoking status [never, ever (former or current)], alcohol intake (none, moderate, heavy), total calcium intake (food + supplemental; milligrams/day), fasting high-sensitivity C-reactive protein (hs-CRP; milligrams/deciliter), fasting serum 25-hydroxyvitamin D [25(OH)D; nanograms/milliliter; n = 890], and diabetes status (fasting glucose ≥120 mg/dL or use of diabetes medication). Diabetes status was included as it has been shown to be an independent risk factor for poor bone health and fragility fracture, despite being associated with higher BMD (42–47). Height was measured with a SECA 214 Portable Stadiometer to the nearest centimeter and weight with a clinical scale (Toledo Weight Plate, Model I5S; Bay State and Systems, Inc.) to the nearest pound. Measures of height and weight were converted to meters and kilograms, respectively, to calculate BMI (kg/m2). Physical activity was assessed by questionnaire, modified from the Framingham Exercise and Physical Activity Questionnaire (48), and calculated as a weighted 24-h score of typical daily activity, based on hours spent doing heavy, moderate, light, or sedentary activity, including sleeping. Women were classified as either premenopausal or currently taking exogenous estrogen therapy or postmenopausal and not using exogenous estrogen therapy, based on the following self-reported variables: current estrogen use (yes or no) and menopausal status (menstrual periods stopped for 1 y; yes or no). Serum 25(OH)D concentration was measured with a 25I RIA Packard COBRA II Gamma Counter (DiaSorin, Inc.), with intra- and interassay CVs of 10.8% and 9.4%, respectively. Plasma hs-CRP was measured with a solid-phase, 2-site chemiluminescent immunometric assay (IMMULITE 1000, Seimens Medical Solutions Diagnostics, Los Angeles, CA), with intra- and interassay CVs of 4.2% and 4.8%, respectively. hs-CRP was included in models to account for the relation of inflammatory status with both bone and plasma vitamin C (49, 50). The Department of Health and Human Services (HHS) poverty guidelines for the year the participant was interviewed (2006–2011) were used to describe economic status. Self-reported family-unit size, total household income, and threshold dollar amount were used along with the year of interview to categorize participants as equal to or below, compared with above, the HHS poverty threshold. Five participants were excluded due to missing covariate information on education status and/or smoking.
Statistical analysis
Means ± SDs for continuous variables and proportion of participants for categorical variables were calculated. Differences between participants from the original BPRHS cohort who chose to participate in the BPROS, compared with those who did not participate, were assessed by t test (continuous variables) or chi-square (categorical variables). Pearson's correlation was used to test associations between dietary, supplemental, and total vitamin C with plasma vitamin C. Separate analyses were conducted for each BMD site (femoral neck, trochanter, total femur, and lumbar spine). Primary vitamin C exposure was modeled in 3 ways: 1) dietary vitamin C, 2) supplemental vitamin C use (yes/no), and 3) plasma vitamin C (continuous and by 3 status levels, as previously described). Multivariable general linear modeling was used to assess the relation between each vitamin C exposure (dietary vitamin C and supplemental vitamin C within 1 model, and plasma vitamin C in a separate model) with each BMD outcome. General linear modeling was used to compare least-squares BMD across tertile categories of dietary vitamin C, predefined clinical cutoffs of plasma vitamin C status, and by supplemental vitamin C use (yes/no) with Tukey's test for multiple comparisons. Initial models were adjusted for sex and estrogen status (with a composite 3-level variable), age, height, BMI, plus total energy intake in the diet-supplement models only (dietary vitamin C additionally energy adjusted via the residual method). Subsequent models were further adjusted for physical activity score, smoking status, alcohol intake, and level of education. Final models were further adjusted for total calcium intake, serum 25(OH)D, plasma hs-CRP, and diabetes status (yes/no). All regression models were tested for interaction with 1) sex and estrogen status, as BMD differs greatly by sex, in part due to androgen production, and 2) smoking status, as smoking is known to increase metabolism of vitamin C (51). Interactions between vitamin C exposure and sex-estrogen status (categorized as men, women with estrogen [premenopausal or taking hormone replacement therapy (HRT)], or women with little estrogen [postmenopausal and not taking HRT]) were tested in final models. Interactions between vitamin C exposure and smoking status (current vs. ever/never) were tested separately in final models. Models showing an interaction with P < 0.1 were stratified by their corresponding variable. The false discovery rate (FDR) method was applied to correct for multiple comparisons. In models without an interaction, P < 0.05 was considered statistically significant. All analyses were performed with SAS software (version 9.4; SAS Institute).
Results
Participants who did not join the BPROS were older (60.9 y vs. 58.7 y; P < 0.001) and more likely to have type 2 diabetes (47.8% vs. 40.4%; P = 0.03) compared with those who joined. Participation did not differ by sex (P = 0.91), smoking (P = 0.16), physical activity score (P = 0.42), or activities of daily living (no vs. some vs. considerable impairment; P= 0.34). The sample was majority postmenopausal women with overweight or obesity (Table 1). Approximately 55% exceeded the HHS poverty guidelines and only 44% had plasma vitamin C sufficiency. Mean dietary vitamin C was 95.6 ± 62.3 mg/d and 28% reported additional vitamin C intake from a supplement. The correlation between dietary and plasma vitamin C was r = 0.18 (P < 0.001), between supplemental and plasma vitamin C was r = 0.19 (P < 0.001), and between total vitamin C (diet + supplemental intake) and plasma vitamin C was r = 0.22 (P < 0.001).
TABLE 1.
Descriptive characteristics of participants from the Boston Puerto Rican Health Study, by tertile of dietary vitamin C1
| Dietary vitamin C tertile categories | |||
|---|---|---|---|
| Characteristics | Tertile 1 | Tertile 2 | Tertile 3 |
| Dietary vitamin C, mg/d | 30.4 ± 18.2 | 64.5 ± 8.1 | 124 ± 53 |
| Supplemental vitamin C, yes, n (%) | 68 (22) | 99 (32) | 86 (28) |
| Plasma vitamin C, μmol/L (n = 809) | 44.4 ± 23.5 | 49.2 ± 20.0 | 54.9 ± 20.1 |
| Women, n (%) | 202 (67) | 235 (77) | 225 (74) |
| Use of estrogen (women only), n (%) | 1 (0.5) | 2 (0.8) | 4 (1.8) |
| Age (range: 46–78 y), y | 57.5 ± 7.0 | 59.3 ± 7.6 | 59.3 ± 7.5 |
| BMI, kg/m2 | 32.0 ± 7.2 | 32.5 ± 6.0 | 32.2 ± 6.5 |
| Physical activity score2 | 31.7 ± 4.4 | 31.6 ± 4.5 | 31.9 ± 4.9 |
| Diabetes status, yes, n (%) | 111 (37) | 142 (47) | 105 (35) |
| Total energy intake, kcal/d | 2085 ± 874 | 1576 ± 654 | 1909 ± 749 |
| Total calcium intake, mg/d | 947 ± 488 | 894 ± 476 | 1031 ± 563 |
| Serum 25(OH)D, ng/mL | 19.1 ± 7.4 | 19.8 ± 7.3 | 19.5 ± 6.9 |
| Alcohol consumption, moderate, n (%) | 82 (28) | 77 (25) | 13 (4) |
| Smoking status, current, n (%) | 87 (29) | 51 (17) | 48 (16) |
| Postmenopausal (women only), n (%) | 170 (85) | 204 (88) | 201 (90) |
| Education | |||
| No schooling or <8th grade | 135 (45) | 163 (54) | 138 (45) |
| 8th–12th grade or GED | 120 (40) | 100 (33) | 113 (37) |
| Some college or more | 46 (15) | 41 (13) | 53 (17) |
| hs-CRP categories | |||
| 0 ≤ hs-CRP <1 | 44 (15) | 44 (15) | 58 (19) |
| 1 ≤ hs-CRP ≤3 | 95 (32) | 92 (31) | 80 (27) |
| 3 < hs-CRP <10 | 110 (37) | 96 (32) | 110 (37) |
| 10 ≤ hs-CRP | 47 (16) | 66 (22) | 52 (17) |
| Bone mineral density, g/cm2 | |||
| Femoral neck | 0.948 ± 0.146 | 0.930 ± 0.151 | 0.931 ± 0.136 |
| Total femur | 1.035 ± 0.163 | 1.022 ± 0.164 | 1.022 ± 0.144 |
| Trochanter | 0.838 ± 0.150 | 0.820 ± 0.148 | 0.823 ± 0.132 |
| Lumbar spine (L2–L4) | 1.159 ± 0.181 | 1.153 ± 0.180 | 1.156 ± 0.191 |
Values are means ± SDs or n (%); n = 902. GED, general educational development ; hs-CRP, high-sensitivity C-reactive protein; 25(OH)D, 25-hydroxyvitamin D.
Physical activity score modified from the Framingham Exercise and Physical Activity Questionnaire. In this sample, range 24.5 (none/little) to 60.5 (some).
Basic and fully adjusted models showed no significant association between dietary, supplemental, or plasma vitamin C and BMD at any site, following FDR correction for multiple comparisons (Table 2). Least-squares means BMD did not differ by tertile of dietary vitamin C (Table 3). Among individuals with sufficient plasma vitamin C, trochanter and total femur BMD values were significantly higher (0.855 ± 0.009 g/cm2; 1.056 ± 0.011 g/cm2) compared with those with low vitamin C (0.827 ± 0.012 g/cm2; 1.026 ± 0.014 g/cm2, respectively) (P = 0.04 at both sites, with FDR correction). No other significant differences in BMD were observed across plasma vitamin C groups (Table 3). Supplemental vitamin C use approached significance at the femoral neck (0.966 g/cm2; vs. no use: 0.947 g/cm2; P = 0.07) and total femur (1.058 g/cm2; no use: 1.038 g/cm2; P = 0.07) but was not significant at the trochanter or lumbar spine (P = 0.16 and 0.29, respectively).
TABLE 2.
Multivariable associations between vitamin C (dietary, supplement intake, and plasma) and bone mineral density (g/cm2) at the hip and spine in men and women from the Boston Puerto Rican Health Study1
| Bone mineral density site (g/cm2) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Femoral neck (n = 902) | Trochanter (n = 902) | Total femur (n = 893) | Lumbar spine (n = 883) | |||||
| Exposure variable | β ± SE | P | β ± SE | P | β ± SE | P | β ± SE | P |
| Supplemental vitamin C (yes/no) | ||||||||
| Model 1 | 0.0113 ± 0.0094 | 0.72 | 0.0062 ± 0.0092 | 0.89 | 0.0107 ± 0.0103 | 0.72 | −0.0015 ± 0.0128 | 0.96 |
| Model 2 | 0.0100± 0.0094 | 0.72 | 0.0060 ± 0.0093 | 0.89 | 0.0101 ± 0.0103 | 0.74 | −0.0020 ± 0.0128 | 0.96 |
| Model 3 | 0.0184 ± 0.0100 | 0.60 | 0.0142 ± 0.0097 | 0.61 | 0.0215 ± 0.0108 | 0.48 | 0.0116 ± 0.0137 | 0.85 |
| Dietary vitamin C, mg/d | ||||||||
| Model 1 | −0.00004± 0.0001 | 0.91 | −0.00003 ± 0.0001 | 0.91 | −0.00003 ± 0.0001 | 0.91 | 0.00003 ± 0.0001 | 0.91 |
| Model 2 | −0.00006± 0.0001 | 0.89 | −0.00003 ± 0.0001 | 0.91 | −0.0004 ± 0.0001 | 0.90 | 0.00002 ± 0.0001 | 0.96 |
| Model 3 | −0.00006± 0.0001 | 0.89 | −0.00003± 0.0001 | 0.91 | −0.00003± 0.0001 | 0.91 | 0.00002 ± 0.0001 | 0.95 |
| Plasma vitamin C, μmol/L | ||||||||
| Model 1 | 0.0002 ± 0.0002 | 0.72 | 0.0003 ± 0.0002 | 0.60 | 0.0003 ± 0.0002 | 0.61 | −0.0020 ± 0.0189 | 0.97 |
| Model 2 | 0.0002± 0.0002 | 0.72 | 0.0003 ± 0.0002 | 0.60 | 0.0003 ± 0.0002 | 0.61 | −0.00004± 0.0003 | 0.99 |
| Model 3 | 0.0003 ± 0.0002 | 0.61 | 0.0005 ± 0.0002 | 0.48 | 0.0005 ± 0.0002 | 0.48 | 0.0002 ± 0.0003 | 0.91 |
Models adjusted for multiple comparisons with the false discovery rate correction method. Model 1: adjusted for sex and estrogen status, age (y), height (cm), BMI (kg/m2), total energy intake (diet + supplemental intake models only); dietary and supplemental vitamin C tested in the same model. Model 2: adjusted for model 1 and education (3 levels), alcohol (never, moderate, heavy), smoking (never/ever), and physical activity score. Model 3: adjusted for model 2 and total calcium intake (mg/d), serum 25(OH)D (ng/mL), plasma hs-CRP (mg/L), and diabetes status (yes/no). hs-CRP, high-sensitivity C-reactive protein; 25(OH)D, 25-hydroxyvitamin D.
TABLE 3.
Adjusted least-squares mean bone mineral density (g/cm2) at the hip and spine, by tertile of dietary vitamin C and plasma status in men and women from the Boston Puerto Rican Health Study1
| Measure of vitamin C | Dietary tertile 1 or plasma vitamin C <20 μmol/L | Dietary tertile 2 or plasma vitamin C 20–49 μmol/L | Dietary tertile 3 or plasma vitamin C ≥50 μmol/L | P-trend2 |
|---|---|---|---|---|
| Femoral neck bone mineral density (g/cm2) | ||||
| Dietary vitamin C | 0.955 ± 0.010 | 0.955 ± 0.011 | 0.951 ± 0.010 | 0.96 |
| Plasma vitamin C | 0.938 ± 0.013 | 0.955 ± 0.010 | 0.958 ± 0.010 | 0.26 |
| Trochanter bone mineral density (g/cm2) | ||||
| Dietary vitamin C | 0.845 ± 0.010 | 0.844 ± 0.010 | 0.843 ± 0.010 | 0.96 |
| Plasma vitamin C | 0.827 ± 0.012a | 0.837 ± 0.010a | 0.855 ± 0.009b | 0.04 |
| Total femur bone mineral density (g/cm2) | ||||
| Dietary vitamin C | 1.045 ± 0.011 | 1.046 ± 0.012 | 1.044 ± 0.011 | 0.96 |
| Plasma vitamin C | 1.026 ± 0.014a | 1.038 ± 0.011 | 1.056 ± 0.011b | 0.04 |
| Lumbar spine bone mineral density (g/cm2) | ||||
| Dietary vitamin C | 1.172 ± 0.015 | 1.179 ± 0.015 | 1.181 ± 0.015 | 0.91 |
| Plasma vitamin C | 1.159 ± 0.017 | 1.178 ± 0.014 | 1.184 ± 0.014 | 0.26 |
Values are least-squares means ± SEs. Models adjusted for: sex and estrogen status, age (y), height (cm), BMI (kg/m2), physical activity score, smoking status (ever/never), alcohol intake (never, moderate, heavy), education (3 levels), total energy intake (kcal/d), total calcium intake (mg/d), serum 25(OH)D (ng/mL), plasma hs-CRP (mg/L), and diabetes status (yes/no). Different superscript letters indicate significance between groups, P < 0.05 (least-squares means adjusted using Tukey's test). hs-CRP, high-sensitivity C-reactive protein; 25(OH)D, 25-hydroxyvitamin D.
P-trend models adjusted for multiple comparisons with the false discovery rate correction method.
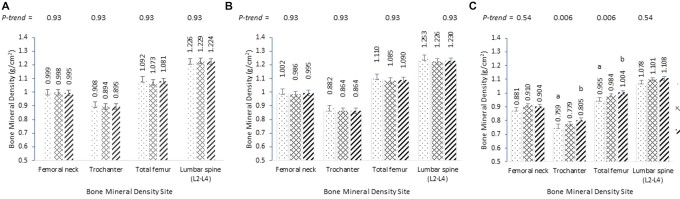
No interaction between sex-estrogen status and dietary vitamin C was observed at any BMD site (P-value range: 0.24–0.90). Interaction between sex-estrogen status and plasma vitamin C was observed at the trochanter and lumbar spine (each P = 0.05) and at the total femur (P = 0.06), but not at the femoral neck (P = 0.22). Following stratification by sex-estrogen status, the association between plasma vitamin C and BMD at the trochanter and total femur continued, although this was attenuated following FDR correction (P = 0.06, both sites) (Supplemental Table 1). Postmenopausal women not using estrogen with sufficient plasma vitamin C had significantly greater trochanter and total femur BMD (0.805 ± 0.012 g/cm2; 1.004 ± 0.014 g/cm2) than those with insufficient plasma vitamin C (0.759 ± 0.014 g/cm2, P < 0.001; 0.955 ± 0.017 g/cm2, P = 0.001, respectively, following Tukey's correction), but there were no differences in femoral neck or spine BMD (Figure 1). In addition, no significant trends or differences across plasma vitamin C groups were observed at any site among men, premenopausal women, or women taking estrogen therapy (Figure 1). No significant interaction between current smoking status, any vitamin C exposure, and any BMD site was observed (P-value range: 0.16–0.72).
FIGURE 1.
Least-squares mean bone mineral density (g/cm2) ± SE at the hip and spine across plasma vitamin C groups, by sex and estrogen status, from the Boston Puerto Rican Health Study. (A) men; (B) premenopausal women or using estrogen replacement therapy; (C) postmenopausal women not using estrogen therapy. Models were adjusted for height (cm), BMI (kg/m2), physical activity score, smoking status (ever/never), alcohol intake (never, moderate, heavy), education (3 levels), total energy intake (kcal/d), total calcium intake (mg/d), serum 25(OH)D (ng/mL), plasma hs-CRP (mg/L), and diabetes status (yes/no). Models were adjusted for multiple comparisons with the false discovery rate correction method. Different superscript letters indicate significance between groups, P < 0.05, following Tukey's adjustment for multiple comparisons. hs-CRP, high-sensitivity C-reactive protein; 25(OH)D, 25-hydroxyvitamin D.
Discussion
In a cohort of Puerto Rican adults, higher trochanter and total femur BMD was noted among individuals with sufficient plasma vitamin C, compared with those with low plasma vitamin C. Following stratification by estrogen status (men, women with or without endogenous or exogenous estrogen), higher hip BMD was observed in the sufficient plasma vitamin C group compared with the low vitamin C group only among postmenopausal women without estrogen use. Dietary intake or supplement use of vitamin C was not associated with BMD in this cohort. No interactions with current smoking status were observed.
There are inconsistencies in the literature surrounding the potential relation between dietary vitamin C and bone health. Although higher vitamin C intake has been associated with BMD in several studies (7, 11, 12, 22, 25, 28, 30, 52–59), it was not significantly associated in others ((26, 60–66). Recent meta-analyses suggest that greater vitamin C intake is associated with reduced risk of fracture (21), and that an increase of 50 mg/d of vitamin C intake may reduce risk of hip fracture by 5% (67). Conflicting evidence for the relation between vitamin C and bone may be due to variation in the following: 1) dietary assessment methods (24-h dietary recalls, FFQ, or food records), 2) measures of vitamin C exposure [diet alone, diet + supplements, supplement use (continuous or yes/no)], 3) bone outcomes (BMD at varying sites, fracture risk, osteoporosis status), and/or 4) baseline participant characteristics (age, sex, estrogen status). In the current study, greater dietary vitamin C (by FFQ) was not associated with BMD measured by DXA at any site among men or women with a mean age of 58 y, and is supported by a weak correlation between diet and plasma vitamin C. Diet estimation of vitamin C intake from foods explains only ∼12% of the variation in plasma vitamin C status (68).
Other factors that influence circulating vitamin C include body size, smoking, use of supplements, bioavailability, food-processing techniques, and disease status (69). The strength of association between vitamin C intake assessed by FFQ with plasma vitamin C was modest in a recent meta-analysis (r = 0.35) (68). The correlation between dietary and plasma vitamin C in the current sample was lower than previously reported (r = 0.18), likely due to the high prevalence of obesity, metabolic disease, and circulating inflammatory markers known to impact vitamin C status. Therefore, we also examined the association between BMD and plasma vitamin C, an objective measure of vitamin C status.
Differences in BMD were observed at multiple hip sites between individuals with low compared with sufficient plasma vitamin C; however, these observed differences were dependent on sex and estrogen status. Postmenopausal women not using estrogen therapy with sufficient plasma vitamin C (≥50 μmol/L) showed greater BMD at the hip compared with women in the same category with low plasma vitamin C (≤20 μmol/L). For each increase in 1 SD in T score, fracture risk declines on the order of 50%. Therefore, the differences observed in the current study are clinically meaningful, as postmenopausal women with sufficient plasma vitamin C showed ∼0.5-SD greater hip BMD compared with individuals with low plasma vitamin C.
Lower circulating estrogen leads to bone deterioration and progressive loss of bone mass over time (70). The impact of vitamin C on bone through reduction in oxidative stress and enhancement of collagen formation may be more apparent in women with lower estrogen due to accelerated bone loss. Conversely, men and women with endogenous and/or exogenous estrogen production may not respond as greatly to other external stimuli, such as plasma vitamin C, as they do not experience bone loss at the same accelerated rate. The interaction by estrogen status is corroborated by other work within the BPRHS cohort (31) and work in the Aberdeen Prospective Osteoporosis Screening Study (11), where dietary patterns and individual nutrients were associated with bone only among peri- and postmenopausal women. In contrast, data from the European Prospective Investigation into Cancer and Nutrition–Norfolk cohort showed that a plasma vitamin C concentration of 53–61 μmol/L, compared with 3–31 μmol/L, was significantly associated with a 65% reduction in hip fracture and 74% reduction in spine fracture risk among men only (71). Prospective associations between plasma vitamin C and risk of fracture among women may not have been detected because subgroup analyses by estrogen status was not tested (28% were pre- or perimenopausal; 21% were currently using HRT). Overall, there is a suggestion that adequate circulating vitamin C may be a lifestyle contributor to BMD, particularly among postmenopausal non–estrogen-using women. However, these associations are modest, may be influenced by subgroup size (lower sample sizes in men and women with estrogen may reduce statistical power), and require replication in other, larger cohorts.
We did not observe an interaction between vitamin C, BMD, and smoking status. In comparison, the Framingham Osteoporosis Study (FOS) showed a positive, cross-sectional association between vitamin C intake and BMD among never-smokers only, although the interaction was not observed in longitudinal analyses (7). It is important to note that participants of the FOS consumed significantly higher dietary (140 and 158 mg/d for men and women, respectively) and supplemental (81 and 95 mg/d for men and women, respectively) vitamin C compared with the BPROS (mean supplemental intake of ∼15 mg/d, regardless of smoking status). It is recommended that smokers consume 35 mg more vitamin C/d than nonsmokers due to the need to neutralize the additional insult of free radicals introduced by smoking (51). More studies are needed in larger cohorts with wide variation in supplemental vitamin C intake to assess the interaction between vitamin C, bone, and smoking status.
Vitamin C is a water-soluble vitamin that acts as a cofactor in the synthesis of collagen, carnitine, neurotransmitters, and hormones (51). It has been shown to benefit bone health, as it is required for the synthesis of collagen (a major component of the bone matrix), and due to its antioxidant properties, which may reduce oxidative stress during bone turnover (72). Animal models have shown that vitamin C supplementation prevents bone resorption (73) and reduces age-associated losses of BMD and trabecular bone volume (74) through the reduction in the ratio of Receptor activator of nuclear factor kappa-Β ligand (RANKL) and NF-κB cells to the total periodontal ligament cells (73), and by preventing oxidative stress (75). Translation of these results to human clinical trials is limited. In 1 trial of 75 patients with osteoporosis supplemented with 500 mg vitamin C/d for 90 d, bone turnover was reduced (observed increase in alkaline phosphatase and reduction in tartrate resistant acid phosphatase) alongside increases in antioxidant activity (increased serum superoxide dismutase and erythrocyte glutathione) (76). To our knowledge, no other well-controlled human trials have evaluated the effects of vitamin C supplementation on short- or long-term change in bone resorption or BMD.
The current study has several strengths, including being one of the first studies to evaluate the association between vitamin C status (by plasma biomarker) and BMD among a large sample of men and women and the only study among Puerto Rican adults. In addition, this study has a comprehensive assessment of sociodemographic, lifestyle, and health factors known to impact bone. Limitations to the use of the FFQ include potential under- or overreporting of food intake. The current study reduced potential bias by removing participants with extreme energy intakes and adjusting vitamin C intake and other nutrient intakes for total energy intake. This study cannot fully separate the associations of vitamin C with bone from that of other antioxidants and overall dietary quality. The use of plasma vitamin C provides an unbiased assessment of this dietary exposure, but a single measure of plasma vitamin C may not represent long-term dietary exposure. Furthermore, power to detect associations among men and premenopausal women was limited, due to small sample sizes within those groups. Last, while models controlled for known confounders, there remains the risk of residual confounding with any cross-sectional study. Causality between plasma vitamin C and its effect on bone can only be established in randomized controlled trials.
In conclusion, dietary vitamin C and supplemental vitamin C were not related to BMD and no interactions with current smoking were observed. However, low plasma vitamin C (≤20 μmol/L) was associated with significantly lower hip BMD compared with a sufficient concentration (>50 μmol/L), driven by the significant association in non–estrogen-using postmenopausal women. Preclinical models support an effect of vitamin C on bone by reducing bone turnover and oxidative stress, and by increasing collagen formation. Future research is needed to determine whether plasma vitamin C status is associated with longitudinal change in BMD and reduction of fracture risk. Further well-controlled intervention studies that increase plasma vitamin C among insufficient individuals are needed to determine whether vitamin C intervention can positively influence bone health. Last, this paper highlights the need for future studies to evaluate metabolism of vitamin C and plasma vitamin C concentrations in Hispanic populations, who may be at increased risk for bone disease.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—KLT, KMM, and SEN: designed the research; KLT, SEN, and BDH: conducted research; KMM: analyzed data and wrote the manuscript; KMM, KLT, SEN, and BDH: interpreted data; KMM, SEN, KLT, and BDH: revised manuscript content; and all authors: take responsibility for approving the final version of manuscript and for the integrity of the data analysis and read and approved the final manuscript.
Notes
This work was supported by the National Heart, Lung, and Blood Institute grant P50-HL105185; National Institute on Aging grants P01-AG023394 and R01-AG055948; and a Mentored Career Development award NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) K01-AR067894 (to SEN). KMM, SEN, and KLT are supported by NIAMS 1R01AR072741-01A1.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents available on https://academic.oup.com/jn.
Abbreviations used: BMD, bone mineral density; BPRHS, Boston Puerto Rican Health Study; BPROS, Boston Puerto Rican Osteoporosis Study; FDR, false discovery rate; FFQ, food-frequency questionnaire; FOS, Framingham Osteoporosis Study; HHS, Department of Health and Human Services; HNRCA, Jean Mayer USDA Human Nutrition Research Center on Aging; HRT, hormone replacement therapy; hs-CRP, high-sensitivity CRP; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Kelsey M Mangano, Department of Biomedical and Nutritional Sciences, Center for Population Health, Zuckerberg College of Health Sciences, University of Massachusetts Lowell, Lowell, MA, USA.
Sabrina E Noel, Department of Biomedical and Nutritional Sciences, Center for Population Health, Zuckerberg College of Health Sciences, University of Massachusetts Lowell, Lowell, MA, USA.
Bess Dawson-Hughes, Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA.
Katherine L Tucker, Department of Biomedical and Nutritional Sciences, Center for Population Health, Zuckerberg College of Health Sciences, University of Massachusetts Lowell, Lowell, MA, USA.
Data Availability
Data are publicly available following approval by the Principal Investigator, KLT. Please contact the Center for Population Health at the University of Massachusetts, Lowell.
References
- 1. Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. Trends in osteoporosis and low bone mass in older US adults, 2005–2006 through 2013–2014. Osteoporos Int. 2017;28:1979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–33. [DOI] [PubMed] [Google Scholar]
- 3. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–75. [DOI] [PubMed] [Google Scholar]
- 4. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsai AJ. Disparities in osteoporosis by race/ethnicity, education, work status, immigrant status, and economic status in the United States. Eur J Intern Med. 2019;64:85–9. [DOI] [PubMed] [Google Scholar]
- 6. Noel SE, Mangano KM, Griffith JL, Wright NC, Dawson-Hughes B, Tucker KL. Prevalence of osteoporosis and low bone mass among Puerto Rican older adults. J Bone Miner Res. 2018;33:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sahni S, Hannan MT, Gagnon D, Blumberg J, Cupples LA, Kiel DP, Tucker KL. High vitamin C intake is associated with lower 4-year bone loss in elderly men. J Nutr. 2008;138:1931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tucker KL, Hannan MT, Kiel DP. The acid-base hypothesis: diet and bone in the Framingham Osteoporosis Study. Eur J Nutr. 2001;40:231–7. [DOI] [PubMed] [Google Scholar]
- 9. Byberg L, Bellavia A, Orsini N, Wolk A, Michaelsson K. Fruit and vegetable intake and risk of hip fracture: a cohort study of Swedish men and women. J Bone Miner Res. 2015;30:976–84. [DOI] [PubMed] [Google Scholar]
- 10. Chen YM, Ho SC, Woo JL. Greater fruit and vegetable intake is associated with increased bone mass among postmenopausal Chinese women. Br J Nutr. 2006;96:745–51. [PubMed] [Google Scholar]
- 11. Macdonald HM, New SA, Golden MH, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr. 2004;79:155–65. [DOI] [PubMed] [Google Scholar]
- 12. Prynne CJ, Mishra GD, O'Connell MA, Muniz G, Laskey MA, Yan L, Prentice A, Ginty F. Fruit and vegetable intakes and bone mineral status: a cross sectional study in 5 age and sex cohorts. Am J Clin Nutr. 2006;83:1420–8. [DOI] [PubMed] [Google Scholar]
- 13. Qiu R, Cao WT, Tian HY, He J, Chen GD, Chen YM. Greater intake of fruit and vegetables is associated with greater bone mineral density and lower osteoporosis risk in middle-aged and elderly adults. PLoS One. 2017;12:e0168906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am J Clin Nutr. 1999;69:727–36. [DOI] [PubMed] [Google Scholar]
- 15. Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–7. [DOI] [PubMed] [Google Scholar]
- 16. Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991;54:1135S–40S. [DOI] [PubMed] [Google Scholar]
- 17. Kipp DE, Grey CE, McElvain ME, Kimmel DB, Robinson RG, Lukert BP. Long-term low ascorbic acid intake reduces bone mass in guinea pigs. J Nutr. 1996;126:2044–9. [DOI] [PubMed] [Google Scholar]
- 18. Franceschi RT, Young J. Regulation of alkaline phosphatase by 1,25-dihydroxyvitamin D3 and ascorbic acid in bone-derived cells. J Bone Miner Res. 1990;5:1157–67. [DOI] [PubMed] [Google Scholar]
- 19. Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7:235–46. [DOI] [PubMed] [Google Scholar]
- 20. Dixon MC, Yeaman SJ, Agius L, Day CP. Transforming growth factor beta increases the activity of phosphatidate phosphohydrolase-1 in rat hepatocytes. Biochem Biophys Res Commun. 1997;230:365–9. [DOI] [PubMed] [Google Scholar]
- 21. Malmir H, Shab-Bidar S, Djafarian K. Vitamin C intake in relation to bone mineral density and risk of hip fracture and osteoporosis: a systematic review and meta-analysis of observational studies. Br J Nutr. 2018;119:847–58. [DOI] [PubMed] [Google Scholar]
- 22. Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol. 2001;154:427–33. [DOI] [PubMed] [Google Scholar]
- 23. Stunes AK, Syversen U, Berntsen S, Paulsen G, Stea TH, Hetlelid KJ, Lohne-Seiler H, Mosti MP, Bjornsen T, Truls Ret al. . High doses of vitamin C plus E reduce strength training-induced improvements in areal bone mineral density in elderly men. Eur J Appl Physiol. 2017;117:1073–84. [DOI] [PubMed] [Google Scholar]
- 24. Sahni S, Hannan MT, Gagnon D, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total and supplemental vitamin C intake on the risk of hip fracture—a 17-year follow-up from the Framingham Osteoporosis Study. Osteoporos Int. 2009;20:1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998;63:183–9. [DOI] [PubMed] [Google Scholar]
- 26. Leveille SG, LaCroix AZ, Koepsell TD, Beresford SA, Van Belle G, Buchner DM. Dietary vitamin C and bone mineral density in postmenopausal women in Washington State, USA. J Epidemiol Community Health. 1997;51:479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, Senin U, Pacifici R, Cherubini A. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88:1523–7. [DOI] [PubMed] [Google Scholar]
- 28. Morton DJ, Barrett-Connor EL, Schneider DL. Vitamin C supplement use and bone mineral density in postmenopausal women. J Bone Miner Res. 2001;16:135–40. [DOI] [PubMed] [Google Scholar]
- 29. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415–23. [DOI] [PubMed] [Google Scholar]
- 30. Martinez-Ramirez MJ, Palma Perez S, Delgado-Martinez AD, Martinez-Gonzalez MA, De la Fuente Arrillaga C, Delgado-Rodriguez M. Vitamin C, vitamin B12, folate and the risk of osteoporotic fractures: a case-control study. Int J Vitam Nutr Res. 2007;77:359–68. [DOI] [PubMed] [Google Scholar]
- 31. Noel SE, Mangano KM, Mattei J, Griffith JL, Dawson-Hughes B, Bigornia S, Tucker KL. Dietary Approaches to Stop Hypertension, Mediterranean, and Alternative Healthy Eating indices are associated with bone health among Puerto Rican adults from the Boston Puerto Rican Osteoporosis Study. Am J Clin Nutr. 2020;111:1267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tucker KL, Mattei J, Noel SE, Collado BM, Mendez J, Nelson J, Griffith J, Ordovas JM, Falcon LM. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health. 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. White J, Harris SS, Dallal GE, Dawson-Hughes B. Precision of single vs bilateral hip bone mineral density scans. J Clin Densitom. 2003;6:159–62. [DOI] [PubMed] [Google Scholar]
- 34. Behrens WA, Madere R. A highly sensitive high-performance liquid chromatography method for the estimation of ascorbic and dehydroascorbic acid in tissues, biological fluids, and foods. Anal Biochem. 1987;165:102–7. [DOI] [PubMed] [Google Scholar]
- 35. German Nutrition S. New reference values for vitamin C intake. Ann Nutr Metab. 2015;67:13–20. [DOI] [PubMed] [Google Scholar]
- 36. Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol. 1998;148:507–18. [DOI] [PubMed] [Google Scholar]
- 37. Bermudez OI, Ribaya-Mercado JD, Talegawkar SA, Tucker KL. Hispanic and non-Hispanic white elders from Massachusetts have different patterns of carotenoid intake and plasma concentrations. J Nutr. 2005;135:1496–502. [DOI] [PubMed] [Google Scholar]
- 38. Gao X, Martin A, Lin H, Bermudez OI, Tucker KL. alpha-Tocopherol intake and plasma concentration of Hispanic and non-Hispanic white elders is associated with dietary intake pattern. J Nutr. 2006;136:2574–9. [DOI] [PubMed] [Google Scholar]
- 39. Ye X, Maras JE, Bakun PJ, Tucker KL. Dietary intake of vitamin B-6, plasma pyridoxal 5'-phosphate, and homocysteine in Puerto Rican adults. J Am Diet Assoc. 2010;110:1660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kwan LL, Bermudez OI, Tucker KL. Low vitamin B-12 intake and status are more prevalent in Hispanic older adults of Caribbean origin than in neighborhood-matched non-Hispanic whites. J Nutr. 2002;132:2059–64. [DOI] [PubMed] [Google Scholar]
- 41. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S.; discussion: 9S–31S. [DOI] [PubMed] [Google Scholar]
- 42. Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL. Risk of fracture in women with type 2 diabetes: the Women's Health Initiative Observational Study. J Clin Endocrinol Metab. 2006;91:3404–10. [DOI] [PubMed] [Google Scholar]
- 43. Kilpadi KL, Eldabaje R, Schmitz JE, Ehler B, Thames TA, Joshi AP, Simmons JW 3rd, Michalek JE, Fajardo RJ. Type 2 diabetes is associated with vertebral fractures in a sample of clinic- and hospital-based Latinos. J Immigr Minor Health. 2014;16:440–9. [DOI] [PubMed] [Google Scholar]
- 44. Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR; Study of Osteoporotic Features Research Group . Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86:32–8. [DOI] [PubMed] [Google Scholar]
- 45. Schwartz AV, Sellmeyer DE, Strotmeyer ES, Tylavsky FA, Feingold KR, Resnick HE, Shorr RI, Nevitt MC, Black DM, Cauley JAet al. . Diabetes and bone loss at the hip in older black and white adults. J Bone Miner Res. 2005;20:596–603. [DOI] [PubMed] [Google Scholar]
- 46. Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, Donaldson MG, Cauley JA, Harris TB, Koster Aet al. . Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305:2184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res. 2009;24:702–9. [DOI] [PubMed] [Google Scholar]
- 48. Albanes D, Conway JM, Taylor PR, Moe PW, Judd J. Validation and comparison of eight physical activity questionnaires. Epidemiology. 1990;1:65–71. [DOI] [PubMed] [Google Scholar]
- 49. Ishii S, Cauley JA, Greendale GA, Crandall CJ, Danielson ME, Ouchi Y, Karlamangla AS. C-reactive protein, bone strength, and nine-year fracture risk: data from the Study of Women's Health Across the Nation (SWAN). J Bone Miner Res. 2013;28:1688–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jafarnejad S, Boccardi V, Hosseini B, Taghizadeh M, Hamedifard Z. A meta-analysis of randomized control trials: the impact of vitamin C supplementation on serum CRP and serum hs-CRP concentrations. Curr Pharm Des. 2018;24:3520–8. [DOI] [PubMed] [Google Scholar]
- 51. Levine M Padayatty SJ.. Vitamin C. In: Ross AC, B CaballeroCousins RJ, Tucker KL, Ziegler TR, editors. Modern nutrition in health and disease. 11th ed.Baltimore (MD): Lippincott Williams and Wilkins; 2014. [Google Scholar]
- 52. Ilich JZ, Brownbill RA, Tamborini L. Bone and nutrition in elderly women: protein, energy, and calcium as main determinants of bone mineral density. Eur J Clin Nutr. 2003;57:554–65. [DOI] [PubMed] [Google Scholar]
- 53. Kim DE, Cho SH, Park HM, Chang YK. Relationship between bone mineral density and dietary intake of beta-carotene, vitamin C, zinc and vegetables in postmenopausal Korean women: a cross-sectional study. J Int Med Res. 2016;44:1103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim YA, Kim KM, Lim S, Choi SH, Moon JH, Kim JH, Kim SW, Jang HC, Shin CS. Favorable effect of dietary vitamin C on bone mineral density in postmenopausal women (KNHANES IV, 2009): discrepancies regarding skeletal sites, age, and vitamin D status. Osteoporos Int. 2015;26:2329–37. [DOI] [PubMed] [Google Scholar]
- 55. Liu ZM, Leung J, Wong SY, Wong CK, Chan R, Woo J. Greater fruit intake was associated with better bone mineral status among Chinese elderly men and women: results of Hong Kong Mr. Os and Ms. Os studies. J Am Med Dir Assoc. 2015;16:309–15. [DOI] [PubMed] [Google Scholar]
- 56. Park HM, Heo J, Park Y. Calcium from plant sources is beneficial to lowering the risk of osteoporosis in postmenopausal Korean women. Nutr Res. 2011;31:27–32. [DOI] [PubMed] [Google Scholar]
- 57. Rivas A, Romero A, Mariscal-Arcas M, Monteagudo C, Lopez G, Lorenzo ML, Ocana-Peinado FM, Olea-Serrano F. Association between dietary antioxidant quality score (DAQs) and bone mineral density in Spanish women. Nutr Hosp. 2012;27:1886–93. [DOI] [PubMed] [Google Scholar]
- 58. Yang YJ, Kim J. Factors in relation to bone mineral density in Korean middle-aged and older men: 2008–2010 Korea National Health and Nutrition Examination Survey. Ann Nutr Metab. 2014;64:50–9. [DOI] [PubMed] [Google Scholar]
- 59. Zhang J, Zhang K, Shi H, Tang Z. A cross-sectional study to evaluate the associations between hypertension and osteoporosis in Chinese postmenopausal women. Int J Clin Exp Med. 2015;8:21194–200. [PMC free article] [PubMed] [Google Scholar]
- 60. Casale M, von Hurst PR, Beck KL, Shultz S, Kruger MC, O'Brien W, Conlon CA, Kruger R. Lean mass and body fat percentage are contradictory predictors of bone mineral density in pre-menopausal pacific island women. Nutrients. 2016;8:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sasaki S, Yanagibori R. Association between current nutrient intakes and bone mineral density at calcaneus in pre- and postmenopausal Japanese women. J Nutr Sci Vitaminol. 2001;47:289–94. [DOI] [PubMed] [Google Scholar]
- 62. Chan R, Woo J, Leung J. Effects of food groups and dietary nutrients on bone loss in elderly Chinese population. J Nutr Health Aging. 2011;15:287–94. [DOI] [PubMed] [Google Scholar]
- 63. Kaptoge S, Welch A, McTaggart A, Mulligan A, Dalzell N, Day NE, Bingham S, Khaw KT, Reeve J. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporos Int. 2003;14:418–28. [DOI] [PubMed] [Google Scholar]
- 64. New SA, Robins SP, Campbell MK, Martin JC, Garton MJ, Bolton-Smith C, Grubb DA, Lee SJ, Reid DM. Dietary influences on bone mass and bone metabolism: further evidence of a positive link between fruit and vegetable consumption and bone health?. Am J Clin Nutr. 2000;71:142–51. [DOI] [PubMed] [Google Scholar]
- 65. Wang MC, Luz Villa M, Marcus R, Kelsey JL. Associations of vitamin C, calcium and protein with bone mass in postmenopausal Mexican American women. Osteoporos Int. 1997;7:533–8. [DOI] [PubMed] [Google Scholar]
- 66. Wolf RL, Cauley JA, Pettinger M, Jackson R, Lacroix A, Leboff MS, Lewis CE, Nevitt MC, Simon JA, Stone KLet al. . Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women's Health Initiative. Am J Clin Nutr. 2005;82:581–8. [DOI] [PubMed] [Google Scholar]
- 67. Sun Y, Liu C, Bo Y, You J, Zhu Y, Duan D, Cui H, Lu Q. Dietary vitamin C intake and the risk of hip fracture: a dose-response meta-analysis. Osteoporos Int. 2018;29:79–87. [DOI] [PubMed] [Google Scholar]
- 68. Dehghan M, Akhtar-Danesh N, McMillan CR, Thabane L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr J. 2007;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carr AC, Rowe S. Factors affecting vitamin c status and prevalence of deficiency: a global health perspective. Nutrients. 2020;12:1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Brown JP, Albert C, Nassar BA, Adachi JD, Cole D, Davison KS, Dooley KC, Don-Wauchope A, Douville P, Hanley DAet al. . Bone turnover markers in the management of postmenopausal osteoporosis. Clin Biochem. 2009;42:929–42. [DOI] [PubMed] [Google Scholar]
- 71. Finck H, Hart AR, Lentjes MA, Jennings A, Luben RN, Khaw K-T, Welch AA. Cross-sectional and prospective associations between dietary and plasma vitamin C, heel bone ultrasound, and fracture risk in men and women in the European Prospective Investigation into Cancer in Norfolk cohort. Am J Clin Nutr. 2015;102:1416–24. [DOI] [PubMed] [Google Scholar]
- 72. Chin KY, Ima-Nirwana S. Vitamin C and bone health: evidence from cell, animal and human studies. Curr Drug Targets. 2018;19:439–50. [DOI] [PubMed] [Google Scholar]
- 73. Sanbe T, Tomofuji T, Ekuni D, Azuma T, Tamaki N, Yamamoto T. Oral administration of vitamin C prevents alveolar bone resorption induced by high dietary cholesterol in rats. J Periodontol. 2007;78:2165–70. [DOI] [PubMed] [Google Scholar]
- 74. Zhu LL, Cao J, Sun M, Yuen T, Zhou R, Li J, Peng Y, Moonga SS, Guo L, Meckhanick JIet al. . Vitamin C prevents hypogonadal bone loss. PLoS One. 2012;7:e47058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arslan A, Orkun S, Aydin G, Keles I, Tosun A, Arslan M, Caglayan O. Effects of ovariectomy and ascorbic acid supplement on oxidative stress parameters and bone mineral density in rats. Libyan J Med. 2011;6.1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chavan SN, More U, Mulgund S, Saxena V, Sontakke AN. Effect of supplementation of vitamin C and E on oxidative stress in osteoporosis. Indian J Clin Biochem. 2007;22:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are publicly available following approval by the Principal Investigator, KLT. Please contact the Center for Population Health at the University of Massachusetts, Lowell.