Abstract
Background
To date, cases of extraintestinal microsporidiosis have been increasingly reported in both otherwise healthy and immunocompromised individuals. Among them, microsporidial myositis is very rare. To the best of our knowledge, this is the first report of microsporidial myositis caused by Trachipleistophora hominis in a patient with human immunodeficiency virus (HIV) in Thailand.
Case report
A Thai man with HIV presented with fever and muscle pain at both anterior thighs and left arm for 3 months. Muscle biopsy was performed, and pathology exhibited neutrophil infiltration and focal aggregations of microsporidial spores. The 18S ribosomal RNA sequence revealed the species of this microsporidium as T hominis, and albendazole of 800mg/day was initiated. He gradually improved, and was discharged home 6 weeks after hospitalization.
Conclusions
To the best of our knowledge, this is the first report of microsporidial myositis caused by Trachipleistophora hominis in a person with HIV in Thailand.
Keywords: AIDS, HIV, Microsporidium, myositis, Thailand, Trachipleistophora, Trachipleistophora hominis
Microsporidia are eukaryotic obligate intracellular organisms that can infect a wide range of vertebrate and invertebrate hosts [1]. To date, there have been more than 200 genera and 1400 species in the phylum Microsporidia, with at least 15 species being identified as human pathogens [1]. There are a wide variety of clinical manifestations of human microsporidiosis ranging from localized (keratitis, diarrhea, cholangitis) to disseminated infection, depending on the species, the mode of transmission, and the preexisting host immune status [1, 2]. Modes of transmission include consumption, inhalation, direct inoculation to skin or mucous membrane, and transplacenta [2]. Currently, microsporidiosis is considered an emerging zoonotic and sapronotic disease. To date, cases of extraintestinal microsporidiosis have been increasingly reported in both otherwise healthy and immunocompromised individuals. Among them, microsporidial myositis is very rare [2, 3]. To the best of our knowledge, this is the first report of microsporidial myositis caused by Trachipleistophora hominis in a patient with human immunodeficiency virus (HIV) in Thailand.
CASE REPORT
A 45-year-old Thai man presented with fever and dull pain at the anterior thighs and left arm for 3 months. Pain continued despite administration of painkillers. Past medical history was insignificant. Examination revealed body temperature of 38.2°C, heart rate of 80 beats per minute, and blood pressure of 110/70mm Hg; presence of oral candidiasis; marked tenderness in both quadriceps and left biceps; and no hepatosplenomegaly or lymphadenopathy. Neurological examination was unremarkable except for mild weakness of both quadriceps femoris muscles.
Complete blood count analysis showed hemoglobin of 10.2g/dL, white blood cell count of 6460 cells/µL (82% neutrophils, 8% lymphocytes, 3% monocytes, and 7% eosinophils), and platelet count of 458000 cells/µL. Blood chemistry tests were normal except creatine phosphokinase of 947 U/L (normal range, 39–308 U/L). Blood anti-HIV was positive, with CD4 count of 12 cells/µL and HIV RNA load of 61600 copies/mL. Serum cryptococcal antigen was negative and chest radiograph was normal.
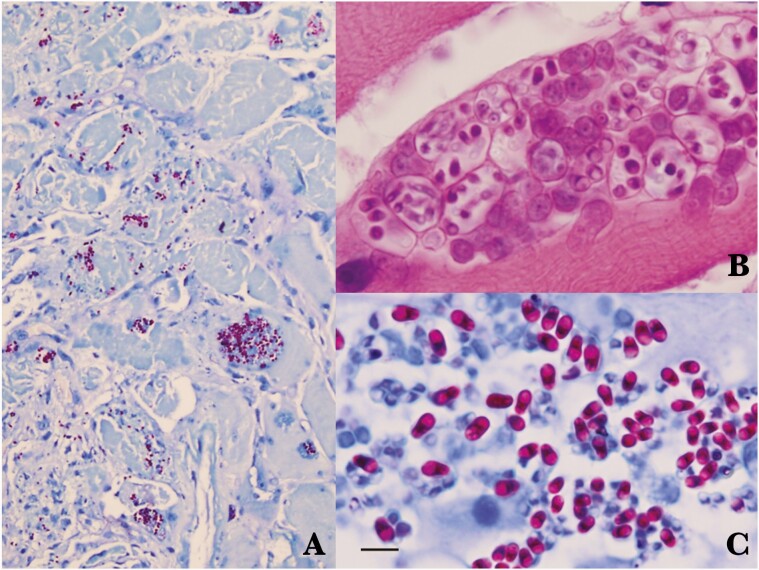
Antiretroviral therapy including tenofovir disoproxil fumarate, emtricitabine, and efavirenz, as well as cotrimoxazole, was initiated. Three days after hospitalization, suspicion of infectious myositis led to a biopsy of the left rectus femoris muscle; the pathology exhibited several foci of lymphocytic and eosinophilic infiltrates among endomysium, without any organisms demonstrated on Gram, acid-fast bacilli (AFB), modified AFB, Giemsa, periodic acid-Schiff, modified trichrome, and Gomori methenamine silver stains. A presumptive diagnosis of myositis caused by Toxoplasma gondii was made, and empiric treatment with pyrimethamine and clindamycin was begun for 2 weeks without response. Prednisolone of 50mg/day was added without improvement. A biopsy of right deltoid and both rectus femoris muscles was performed, and pathology demonstrated neutrophil infiltration and focal aggregations of microsporidial spores (1.5–2.5 × 3.2–4.2 µm in size) with a characteristic belt-like stripe and posterior vacuole within the 12 myofibers. These spores appeared as a pinkish-red color and ovoid shape on acid-fast stain (Figure 1). Unfortunately, the ultrastructures could not be studied via electron microscopic examination due to very poor quality of the formalin-fixed, paraffin-embedded (FFPE) biopsy specimen.
Figure 1.
Histopathology of the patient’s muscle tissues. A, Acid-fast stain showing inflammatory infiltration and endomysium reddish spores of Trachipleistophora hominis. B, Numerous spores inside sporophorous vesicles stained with hematoxylin and eosin. C, Acid-fast stain showing spores with belt-like structure and posterior vacuole. Bar = 5 µm.
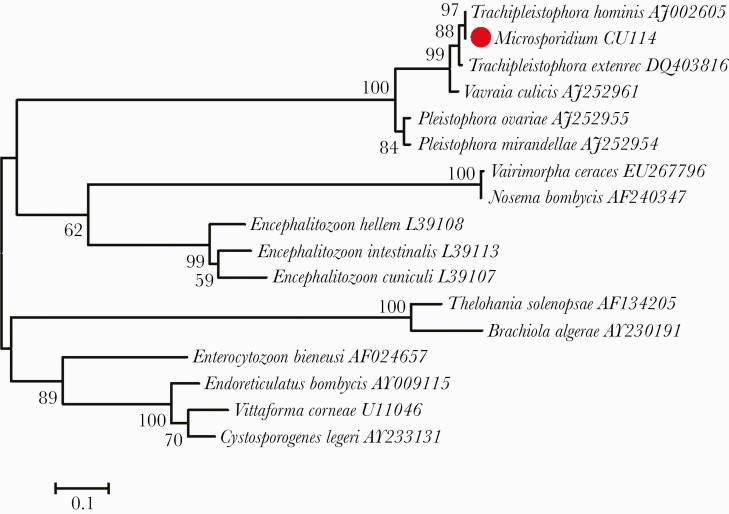
The definite identification of the species of the organism was performed by amplifying the 18S ribosomal RNA (rRNA) gene of microsporidia encompassing 1185bp of DNA extracted from FFPE muscle biopsy tissue using polymerase chain reaction technique, followed by direct sequencing of the amplicon as previously described [3]. A maximum-likelihood phylogenetic tree places the microsporidium infecting this patient (Microsporidium CU114) within the branch for Trachipleistophora hominis (GenBank accession number AJ002605), with this sequence having a 99.6% sequence identity with T hominis and a 1.78% sequence difference from Trachipleistophora extenrec (DQ403816). Although the phylogenetic tree suggests that T hominis is probably the causative agent in our patient, there is no 18S rRNA sequence for Trachipleistophora anthropophthera that would allow its comparison to Microsporidium CU114 (Figure 2) [4].
Figure 2.
Maximum-likelihood tree inferred from the 18S ribosomal RNA gene of representative microsporidia with GenBank accession numbers. The sequence of Trachipleistophora hominis from the patient in this study is marked with a red circle. The tree was constructed based on the general time-reversible model with a discrete gamma distribution for evolutionary rate differences among sites implemented in the MEGA version 6.0 program [4]. The percentages of bootstrap support >50%, determined from 1000 pseudoreplicates, are shown next to the branches.
The identification of characteristic microsporidial spores in tissue biopsy samples is an important diagnostic finding. Although the size of spores in the genus Trachipleistophora is similar to small yeasts, such as Histoplasma capsulatum, differentiation can be made by acid-fast staining (Figure 1) of tissue samples showing reddish belt-like structure and a distinct colorless posterior vacuole. Other stains that are useful for the identification of microsporidia that demonstrate these characteristic belt-like structure in spores in tissue include chromotrope 2R, Gram chromotrope, and Warthin-Starry stains.
The final diagnosis of T hominis myositis was made, and albendazole of 800mg/day was initiated. During hospitalization the patient developed hospital-acquired pneumonia with multiorgan dysfunction requiring antibiotic treatment, intubation with ventilatory support, and renal replacement therapy. However, he gradually improved and was discharged home after albendazole treatment for 6 weeks. He still underwent rehabilitation, and was seen for the last time 6 months after discharge when his CD4 count was 10 cells/µL and HIV RNA load was undetectable. Repeat muscle biopsy was not performed.
DISCUSSION
Microsporidial myositis is very rare. The true occurrence may be underestimated. To date, there have been approximately 20 reported cases in the literature [2, 3, 5–17]. The first case of myositis caused by Anncaliia connori (formerly Nosema connori) was reported in a 4-month-old athymic male infant in 1973 [6]. Most of the patients were male in the proportion of approximately 10:1 with the age ranging from 4 months to 67 years. Patients were from all continents except Europe; the 3 most common countries were the United States, Australia, and India. To date, there have been 2 cases of microsporidial myositis in Thailand (including the present case); the first case was caused by Endoreticularis-like species [3] and the current case was due to a microsporidium related to T hominis. The most common predisposing factor was immunodeficiency caused by HIV infection. Some patients had received organ transplantation, anti–tumor necrosis factor treatment for rheumatoid arthritis, and chemotherapy for hematologic malignancy. Only 2 patients seemed to be healthy without an immunodeficient state [3, 5].
Nine species of microsporidia have been reported as etiologic agents of microsporidial myositis: Anncaliia connori [6], Anncaliia algerae (formerly Nosema and Brachiola algerae) [7–10], Anncaliia vesicularum (formerly Brachiola vesicularum) [11], microsporidian species [12], a novel species closely related to Endoreticularis [3], Pleistophora ronneafiei [13], T hominis ([14–16] and present case), and Tubulinosema acridophagus [17]. Sequence variations in the 18S rRNA genes of various T hominis clinical isolates suggest that multiple strains of this species are pathogenic to humans.
Regarding microsporidiosis, a minority of the patients had myositis as the only manifestation (including the present case). Associated myocarditis was reported in 3 patients [6, 9, 16]. Other organs involved included paranasal sinus [7, 13], cornea [7], and brain parenchyma [9]. Most patients presented with asymmetrical polymyositis, with some having bulbar involvement.
Although the route of microsporidial infection in our patient is unknown, microsporidia are probably transmitted by ingestion of natural and municipal water contaminated with the infective spores. Some are zoonotic infections due to a broad host range of this organism [1, 2, 18]. Of the species capable of causing myositis, some have never been identified in any hosts other than humans, with some being found in insect hosts. Meanwhile, T hominis has been found only in human hosts [1, 15, 16]. Another species of Trachipleistophora, T anthropophthera, can cause disseminated infection and keratitis in humans, with unknown animal hosts as well [19]. Pleistophora ronneafiei has never been identified in animal hosts other than humans, even though Pleistophora species are parasites of fish and reptiles [1, 13]. Of 3 species of Anncaliia, A algerae and A connori have been reported only in human hosts, but A algerae has been found in mosquitoes as well [1, 13]. Endoreticularis species have been identified in lepidopteran insects while T acridophagus usually infects American grasshoppers (Schistocerca americana and Melanoplus species) [17]. The wide distribution of microsporidia in diverse genera of the animal kingdom could suggest that microsporidial myositis is mostly zoonotic infection.
In conclusion, to the best of our knowledge, this is the first report of microsporidial myositis caused by T hominis in a person with HIV in Thailand.
Notes
Author contributions. A. B. described the details of the case. T. A. provided pathological details of the muscle biopsy specimens. C. P. and S. J. performed the molecular tests and interpreted the results. C. S. advised on the diagnosis and treatment of the case, and wrote the first draft of the manuscript. All authors read and critically revised the first and subsequent drafts of the manuscript and approved the final version.
Patient consent statement. Informed consent was obtained from the patient for this publication.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ramanan P, Pritt BS.. Extraintestinal microsporidiosis. J Clin Microbiol 2014; 52:3839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Didier ES. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop 2005; 94:61–76. [DOI] [PubMed] [Google Scholar]
- 3. Suankratay C, Thiansukhon E, Nilaratanakul V, et al. Disseminated infection caused by novel species of microsporidium, Thailand. Emerg Infect Dis 2012; 18:302–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tamura K, Stecher G, Peterson D, et al. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 2013; 30:2725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sundaram TG, Aggarwal A, Ganguly S, et al. Microsporidial myositis in adult-onset immunodeficiency: case-based review. Rheumatol Int 2019; 39:1995–2003. [DOI] [PubMed] [Google Scholar]
- 6. Margileth AM, Strano AJ, Chandra R, et al. Disseminated nosematosis in an immunologically compromised infant. Arch Pathol 1973; 95:145–50. [PubMed] [Google Scholar]
- 7. Field AS, Paik JY, Stark D, et al. Myositis due to the microsporidian Anncaliia (Brachiola) algerae in a lung transplant recipient. Transpl Infect Dis 2012; 14:169–76. [DOI] [PubMed] [Google Scholar]
- 8. Watts MR, Chan RC, Cheong EY, et al. Anncaliia algerae microsporidial myositis. Emerg Infect Dis 2014; 20:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boileau M, Ferreira J, Ahmad I, et al. Successful treatment of disseminated Anncaliia algerae microsporidial infection with combination fumagillin and albendazole. Open Forum Infect Dis 2016; 3:ofw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sutrave G, Maundrell A, Keighley C, et al. Anncaliia algerae microsporidial myositis, New South Wales, Australia. Emerg Infect Dis 2018; 24:1528–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cali A, Takvorian PM, Lewin S, et al. Brachiola vesicularum, n. g., n. sp., a new microsporidium associated with AIDS and myositis. J Eukaryot Microbiol 1998; 45:240–51. [DOI] [PubMed] [Google Scholar]
- 12. Patel AK, Patel KK, Chickabasaviah YT, et al. Microsporidial polymyositis in human immunodeficiency virus-infected patients, a rare life-threatening opportunistic infection: clinical suspicion, diagnosis, and management in resource-limited settings. Muscle Nerve 2015; 51:775–80. [DOI] [PubMed] [Google Scholar]
- 13. Cali A, Takvorian PM.. Ultrastructure and development of Pleistophora ronneafiei n. sp., a microsporidium (Protista) in the skeletal muscle of an immune-compromised individual. J Eukaryot Microbiol 2003; 50:77–85. [DOI] [PubMed] [Google Scholar]
- 14. Chupp GL, Alroy J, Adelman LS, et al. Myositis due to Pleistophora (Microsporidia) in a patient with AIDS. Clin Infect Dis 1993; 16:15–21. [DOI] [PubMed] [Google Scholar]
- 15. Field AS, Marriott DJ, Milliken ST, et al. Myositis associated with a newly described microsporidian, Trachipleistophora hominis, in a patient with AIDS. J Clin Microbiol 1996; 34:2803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curry A, Beeching NJ, Gilbert JD, et al. Trachipleistophora hominis infection in the myocardium and skeletal muscle of a patient with AIDS. J Infect 2005; 51:e139–44. [DOI] [PubMed] [Google Scholar]
- 17. Choudhary MM, Metcalfe MG, Arrambide K, et al. Tubulinosema sp. microsporidian myositis in immunosuppressed patient. Emerg Infect Dis 2011; 17:1727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathis A, Weber R, Deplazes P.. Zoonotic potential of the microsporidia. Clin Microbiol Rev 2005; 18:423–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pariyakanok L, Jongwutiwes S.. Keratitis caused by Trachipleistophora anthropopthera. J Infect 2005; 51:325–8. [DOI] [PubMed] [Google Scholar]