Abstract
Numerous studies have detected a greater likelihood of excess weight gain with specific antiretrovirals (ARVs), particularly tenofovir alafenamide and integrase inhibitors, as compared with other agents and classes. The long-term implications and potential reversibility for individuals who have experienced substantial ARV-associated weight accumulation remain poorly understood. Furthermore, the underlying mechanism remains controversial: Is the explanation mitochondrial toxicity and weight suppression from the older agents or direct effects of the newer drugs on appetite, adipocytes, or other unintended targets? This review discusses proposed mechanisms and evidence to date and argues that the question about mechanism is highly clinically relevant because it carries significant implications for ARV management. The existing literature suggests that older ARVs, such as tenofovir disoproxil fumarate and efavirenz, suppress weight gain, but also that integrase inhibitors may stimulate excess weight gain through several plausible biologic pathways. Confirming the mechanisms of ARV-associated excess weight gain should be high priority for future research.
Keywords: antiretroviral therapy, body weight, drug-related side effects, HIV, obesity
Modern antiretroviral therapy (ART) regimens are typically anchored by an integrase strand transfer inhibitor (INSTI), frequently dolutegravir (DTG) or bictegravir (BIC), because these agents are overall better tolerated with higher durability and lower rates of virologic failure compared with other options [1–6]. The nucleoside reverse transcriptase inhibitor (NRTI) backbone often includes tenofovir alafenamide (TAF), which may confer reduced long-term side effects and other benefits compared with alternate NRTIs [7–10]. However, an unforeseen phenomenon has emerged with these contemporary antiretroviral (ARV) agents: excess weight gain. While many persons with HIV (PWH) gain weight after starting ART due to suppression of plasma HIV RNA levels and removal of the catabolic effects of viremia (a “return-to-health” process), a proportion of individuals gain amounts that surpass healthy levels. Studies of initiating ART and switching ART consistently identify an association between TAF as well as INSTIs, most predominantly DTG and BIG, and exaggerated weight gain [11–14]. While the long-term consequences are not fully understood, growing evidence shows that this leads to higher incidence of hyperglycemia, hypertension, and metabolic syndrome, along with elevated cardiovascular risk [15–21].
These findings create a frequent clinical conundrum: whether to switch to alternate ARV options if substantial weight gain has occurred while taking an INSTI and/or TAF. The question is difficult to answer because the mechanism of weight gain associated with these agents remains unknown. Furthermore, there is a paucity of data about composition of the excess weight (fat vs lean mass and visceral vs subcutaneous deposits) and about the reversibility of the weight gain once it has occurred. To date, evidence for reversibility is limited to case reports [22, 23].
Debate about the mechanism behind excess weight gain associated with specific ARVs is highly clinically relevant. The principal question: Do TAF and INSTIs cause weight gain directly? In other words, do they affect appetite or adipose cells in a way that causes weight accumulation? Alternatively, were the older agents, such as other NRTIs or drugs from the non-nucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor (PI) class, suppressing appetite or curbing weight accumulation, leading to weight gain following a switch to TAF or an INSTI because the suppressive effects were removed? In order to adequately counsel PWH about ART options, we must decipher whether the association with weight gain is secondary to direct, off-target side effects of TAF and the INSTIs vs previously underappreciated side effects of older agents.
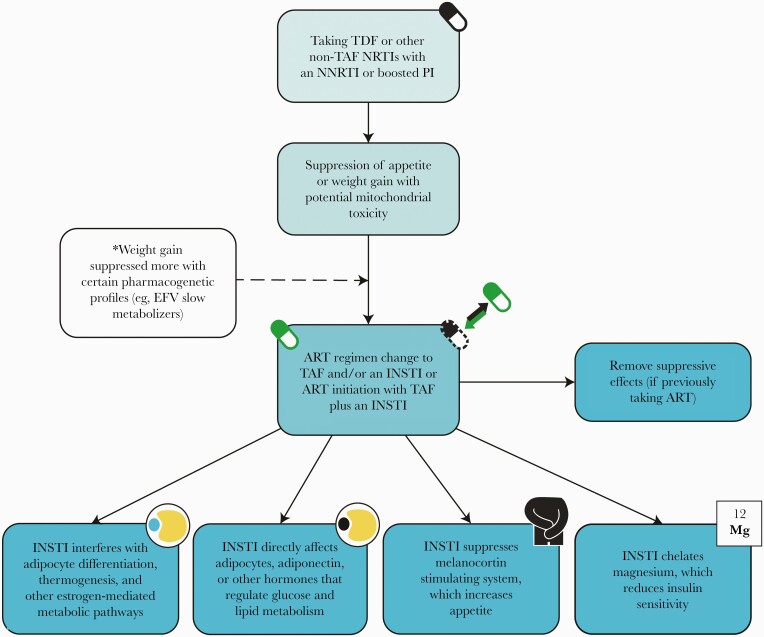
We aim to summarize what is known to date about potential mechanisms of ARV-associated weight gain. An integration of data from ART initiation trials, ART switch studies, pre-exposure prophylaxis (PrEP) trials, and other available literature demonstrates 2 principal findings: (1) tenofovir disoproxil fumarate (TDF), efavirenz (EFV), and other older ARVs indeed have weight suppressive effects and (2) there are several plausible means by which INSTIs could directly trigger weight accumulation, though none has been confirmed (Figure 1). We argue that the mechanisms matter because they significantly impact clinical decisions about ART and how we balance benefits vs risks of various options.
Figure 1.
Proposed mechanisms of TAF- and INSTI-mediated excess weight gain. All proposed mechanisms warrant further study for confirmation, and the explanation may be multifactorial. Abbreviations: ART, antiretroviral therapy; EFV, efavirenz; INSTI, integrase strand transfer inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
TENOFOVIR ALAFENAMIDE
It has long been known that TDF and TAF have disparate metabolic effects. Tenofovir disoproxil fumarate has lipid-lowering properties, the mechanism of which is not well described [24–27]. Lower plasma levels of active metabolites with TAF abrogate these lipid-lowering effects, and comparative trials of ART initiation and switch consistently find that TDF leads to lower lipid level fractions as compared with TAF; the scale of lipid changes observed with TAF is similar to that observed with other non-TDF agents [28–34]. Could biologic explanations for differential effects on lipids and weight be connected, or could lower lipid levels with TDF be explained by differential effects on weight?
To date, we are not aware of clinical or laboratory studies that identify direct effects of TAF on hormones that control appetite and metabolism or direct effects on adipose tissue, though this has not been ruled out. That said, accumulating data from clinical trial comparisons suggest that the explanation for differential weight change when comparing TAF with other agents is more likely weight suppression from TDF or other non-TAF NRTI use, as opposed to TAF-induced weight accumulation. The data come from 3 main sources: (1) ART initiation trials, (2) ART switch studies, and (3) pre-exposure prophylaxis (PrEP) trials.
ART Initiation Trials
Findings from several studies of ART initiation indicate that TDF suppresses weight gain. In a pooled analysis of data from 8 randomized clinical trials (RCTs), the mean weight change was greatest with TAF as compared with TDF, abacavir (ABC), or zidovudine (ZDV) [35]. Initiation of TAF was also associated with elevated likelihood of >10% body weight gain compared with ZDV, whereas TDF and ABC were not, and TAF was associated with increased risk of >10% weight gain when compared with ABC or TDF. The model of weight change over time shows a clear continuum when comparing NRTIs, with weight gain from TAF > ABC > TDF > ZDV. Therefore, conclusions should not be as simple as “TAF causes weight gain,” and the source of this stratification of effects on weight requires further study. Is the explanation differential effects on mitochondria? Or gastrointestinal side effects from older agents that may not be fully appreciated by the patient or provider, such as reduced appetite? The answer is not clear, but the results suggest that older NRTIs may have a suppressive effect on weight; ZDV appears to have the most suppressive effects, which is logical given the drug’s known mitochondrial toxicity. The NRTIs TDF and, to a lesser degree, ABC appear to also suppress weight, though the precise explanation for the differential impact between certain agents requires further study.
Additional insights into potential mechanisms come from the AIDS Clinical Trials Group (ACTG) 5224s trial, a substudy of ACTG 5202, which randomized ART-naïve PWH to receive ABC/lamivudine (ABC/3TC) vs TDF/emtricitabine (TDF/FTC) plus EFV vs atazanavir/ritonavir (ATV/r) [36]. In the substudy, 56 participants underwent excisional fat biopsies at study entry and after 96 weeks of ART. Biopsy specimens were used to assess fat mitochondrial DNA (mtDNA) content plus levels of oxidative phosphorylation enzymes, including NADH dehydrogenase (complex I) and cytochrome c oxidase (complex IV). Notably, effects on these biological markers varied by NRTI. While TDF/FTC and ABC/3TC both led to decreases in fat mtDNA content, only TDF/FTC caused significant decreases in complex I and IV activity. The changes in enzyme levels indicate dysfunction of the mitochondrial respiratory chain induced by TDF but not by ABC. The investigators found that these changes to mitochondrial function consistently and inversely correlated with gains in subcutaneous and visceral abdominal fat (based on dual energy x-ray absorptiometry [DEXA] and computed tomography [CT] measurements). The authors cite that further research into the effects of NRTIs on fat apoptosis and oxidative stress are needed, as are longer and larger studies assessing the impact. Comparisons with TAF would also be informative.
A separate analysis of data that included ACTG 5202 results found that CYP2B6 genotype predicted weight change from EFV, with higher EFV levels suppressing weight, which will be discussed in more detail later [37]. Relevant to NRTI considerations, the association was present only when EFV was combined with TDF and not when combined with ABC. The authors conclude, “TDF exposure in the presence of higher efavirenz concentrations interferes with expected weight gain.” This influence of the NRTI was unexpected and supports the theory that TDF suppresses weight gain. The authors postulate that the explanation may be reduced appetite secondary to TDF.
Further evidence for weight suppression from TDF comes from trials that randomized PWH to initial ART with or without TDF. For instance, in the GEMINI 1 and 2 trials, treatment-naïve PWH received either DTG/3TC or DTG plus TDF/FTC [38]. Thus, the principal difference between study arms was presence or absence of TDF. The rate of virologic suppression at 48 weeks was similar between the 2 groups, but mean weight gain was lower in the group that received TDF compared with the group that did not (2.4kg vs 3.7kg, respectively), suggesting that TDF suppresses weight gain.
ART Switch Trials
Numerous ART switch trials demonstrate an increase in weight after PWH switch an alternate NRTI to TAF, though they do not necessarily address the question of mechanism, so they will not be reviewed in detail here [39–46]. However, a pooled analysis of switch trials offers important insights [47]. In this large study, switch from TDF to TAF was associated with greater weight gain than switch from ABC to TAF. Of the NRTI changes assessed, only TDF to TAF was associated with elevated risk of >10% weight gain. This supports the theory that the preswitch NRTI significantly influences the weight change that occurs following switch to TAF. Similar findings were seen in a retrospective observational study of >42000 PWH from 10 HIV clinics [48]. Of PWH with suppressed viral load who switched regimens, changing TDF to TAF led to higher risk of >3% weight gain after 48 weeks as compared with switching ABC to TAF, even after controlling for demographic factors, body mass index (BMI), CD4 count, and INSTI anchor drug.
Additionally, in an RCT in which investigators enrolled PWH who had suppressed viral loads while taking DTG plus TDF/FTC or TAF/FTC and randomized them to either continue the baseline regimen or switch to BIC/TAF/FTC, the median weight change was similar between study arms at 48 weeks [49]. However, when analyzed by baseline NRTI combination, the median weight change was significantly different between those taking TDF/FTC at enrollment compared with those taking TAF/FTC at enrollment (2.2kg vs 0.6kg, respectively). There was no difference when comparing BIC/TAF/FTC with DTG plus TAF/FTC. Thus, the cumulative evidence suggests that weight suppressive effects from TDF play an important role in the weight change that occurs after changing to TAF.
Two RCTs that examined outcomes after a switch to the 2-drug maintenance ART regimen DTG/3TC merit attention because ultimately they assess the effects of including vs excluding TAF. In the TANGO study, individuals with suppressed viral loads while taking TAF-based 3-drug ART were enrolled and randomized to continue their current regimen or switch to DTG/3TC [50]. After 144 weeks, the adjusted mean change in weight from baseline was not statistically different between the 2 arms, suggesting that dropping TAF from a regimen does not lead to a substantial change in weight and that TAF is relatively “weight neutral.” Interestingly, despite similar weight trajectories in the 2 groups, participants who continued TAF did have higher lipid parameters at follow-up, so TAF may have an effect on serum lipids that is independent of weight, though the incidence of other metabolic consequences, like insulin resistance and metabolic syndrome, remained equivalent. When results of the TANGO study are juxtaposed with findings from a trial called SALSA, notable comparisons emerge. In SALSA, individuals taking any standard suppressive ART regimen were enrolled and randomized to switch to DTG/3TC or continue the baseline regimen [51]. After 48 weeks, greater increases in weight and BMI were observed in the DTG/3TC group compared with the group that continued their baseline regimen. There may be multiple reasons that this outcome differs from that of TANGO, but one is likely that in SALSA 44% of participants in the switch arm stopped TDF. Thus, stoppage of TAF does not seem to impact weight significantly, while stoppage of TDF often leads to an increase in weight.
The precise influence of ABC on weight change has not been fully elucidated. As described above, there seems to be a spectrum of weight gain in which ABC initiation leads to greater likelihood of weight gain than TDF but lower likelihood of weight gain than TAF [35], and in the large retrospective switch study 27% of individuals switching ABC to TAF gained >3% body weight (less than the 40% who gained this proportion of body weight with the TDF-to-TAF switch, but still a sizeable group) [48]. We hypothesize that ABC also has some weight suppressive effects, though not as much as TDF, and this should be confirmed in future work. If this holds true, it is possible that the explanation is either gastrointestinal side effects of ABC that affect appetite and calorie consumption or differential effects on mitochondria or adipocyte function.
PrEP Trials
We can learn about patterns of weight change associated with TDF vs TAF by examining outcomes of PrEP RCTs. In the DISCOVER trial, which included cisgender men who have sex with men (MSM) and transgender women (TGW), participants were randomized to daily TDF/FTC or TAF/FTC. After 48 weeks, there was a statistically significant difference in weight gain (1.1kg with TAF/FTC vs no change with TDF/FTC) [52]. However, it is difficult to draw conclusions about the mechanism. Comparisons of the INSTI cabotegravir (CAB) with TDF/FTC are more informative. In the HPTN 083 trial, which included cisgender MSM and TGW, participants received either intramuscular (IM) CAB plus oral placebo or oral TDF/FTC with IM placebo [53]. Results demonstrated greater overall weight gain for individuals who received IM CAB; however, the difference was driven by weight decrease in the TDF/FTC arm, which occurred predominantly during the first 48 weeks. Similarly, in the iPREX trial, which compared TDF/FTC with placebo, weight increase was lower and fat accumulation less in the TDF/FTC arm [54]. In this trial, total body weight and fat mass changes were significantly different between TDF/FTC and placebo, but lean body mass change was not statistically different. Together, these results suggest that TDF has a weight suppressive effect, which drives differences when comparing TDF with other agents.
Interestingly, a case report describes rapid weight increase in a person without HIV after switching TDF/FTC to TAF/FTC for PrEP [23]. The individual then experienced weight loss after returning to TDF/FTC. While not confirmative, data suggest that this is due to TDF suppressing weight as opposed to TAF causing an increase in weight, but further study to confirm the mechanism, including the effects of TDF vs TAF on mitochondria and the appetite–weight axis, would help to inform clinical decision-making. Better understanding of the reversibility of TAF-associated weight accumulation is needed, and the risks of TDF must be considered, including long-term renal and bone side effects.
Summary of Data on TAF and Weight Gain Mechanisms
Integrating the data from ART initiation, switch, and PrEP trials, we find differential likelihood of weight change with various NRTIs. While direct effects of TAF on weight have not been fully excluded and require further study, the preponderance of findings to date suggest that the primary explanation is a continuum of weight suppressive effects within the NRTI class, such that the NRTI backbone at ART initiation or pre–ART switch affects the likelihood of excess weight accumulation. The primary limitation is that the findings to date rely on clinical trial and retrospective observational comparisons. Unlike with INSTIs, as we will discuss, we are unable to identify laboratory studies assessing the effects of TAF vs other NRTIs on hormones that control appetite or regulate weight; such laboratory studies would be valuable to the field.
INTEGRASE STRAND TRANSFER INHIBITORS
Data demonstrating INSTI-associated excess weight gain have most consistently implicated DTG and BIC; fewer studies have demonstrated an association with raltegravir (RAL) or elvitegravir/cobicistat (EVG/c) [55–64]. Several analyses have found that individuals born female have greater likelihood of INSTI-associated weight gain than individuals born male [16, 56, 61]. When considering mechanisms by which INSTIs may promote weight gain, we can again integrate data from ART initiation studies, switch trials, and PrEP RCTs. In addition, several groups have proposed or evaluated specific theories based on in vitro studies, animal models, or laboratory analyses.
ART Initiation Studies
In the pooled analysis of data from ART initiation trials, at 96 weeks the participants who initiated DTG- or BIC-containing regimens experienced greater weight gain than those who started EVG/c [35]. The investigators found higher odds of >10% weight change with any INSTI as compared with the NNRTI EFV. These findings on their own do not speak to a mechanism for weight gain associated with INSTIs, though another intriguing finding from the analysis was that the NNRTI rilpivirine (RPV) was associated with greater weight gain and higher odds of >10% weight gain than EFV. This begs the question, are the findings secondary to INSTIs causing weight gain or EFV suppressing weight?
Several analyses of pharmacogenetics suggest an answer. Multiple investigators have assessed the effects of individual genetic variations on weight change with EFV in treatment initiation and switch studies. For example, researchers compared weight change after treatment initiation with EFV or DTG based on CYP2B6 genetic loss-of-function polymorphism, which leads to higher EFV concentrations [65]. Based on 342 participants receiving DTG and 168 receiving EFV, the CYP2B6 EFV metabolizer genotype significantly influenced likelihood of weight gain while taking EFV; extensive metabolizers gained similar amounts of weight to the group taking DTG. This suggests that slow or intermediate metabolizers of EFV gain less weight while taking that medication and thus gain more weight after a switch to an INSTI like DTG (discussed further in “ART Switch Trials” below).
It has been argued that the exaggerated weight gain identified when comparing INSTIs with other classes may stem from viral kinetics and faster viral load reduction with INSTIs, leading to faster or more pronounced return-to-health changes. However, several studies that analyzed differences in weight over time after initiation of INSTIs vs other agents found differential weight gain at time points when the viral suppression rates were equivalent, so this seems unlikely to be the explanation [55, 62].
ART Switch Trials
Investigators assessed weight gain 48 weeks after switch from EFV to an INSTI in 2 cohorts (61 individuals from an observational cohort and 462 from a clinical trial cohort) and evaluated whether the CYP2B6 and UGT1A1 genotypes were associated with weight change following the ART switch (both genotypes are associated with ART metabolism and drug levels) [37]. Interestingly, the results demonstrated that CYP2B6 EFV slow metabolizers experienced significantly greater weight gain after the switch from EFV to EVG or RAL, but not DTG. In the pooled analysis of data from multiple clinical trials that enrolled PWH with suppressed viral loads and randomized participants to maintain baseline ART or switch, the greatest risk of excess weight gain (>10% by week 48) occurred with a switch from EFV to RPV or EVG/c. Participants switching off EFV or TDF experienced the greatest weight gain [47]. From these trials, it appears that EFV suppresses weight gain, especially for individuals with pharmacogenomic profiles associated with higher drug levels, and this influences weight change after switch to a different NNRTI or an INSTI.
In 1 switch study, investigators followed numerous cardiovascular and metabolic biomarkers after an ART change, and some of the outcomes are highly relevant. The study, a subanalysis of the NEAT022 trial, in which participants were randomized to either switch from a boosted PI to DTG or continue the boosted PI, demonstrated greater weight gain at 48 weeks in the group that switched to DTG [66]. The investigators compared levels of plasma adiponectin, a chemokine produced by adipose tissue that is involved in regulation of glucose and lipid metabolism, and found that adiponectin levels decreased following the switch to DTG. This decrease could cause insulin resistance and weight gain. However, a possible alternative explanation is that adiponectin is downregulated as an effect of weight gain from another cause. Furthermore, controversy exists in the literature about the role of adiponectin in weight regulation, so this finding is intriguing but requires further exploration.
PrEP Trials
Several findings from PrEP trials are worth noting. First, in the phase 2a HPTN 077 trial, individuals at low risk for HIV infection were randomized to long-acting, injectable CAB vs placebo. The investigators did not find a difference in weight change, BMI change, fasting glucose, or fasting lipid parameters between the groups after 41 weeks [67]. This argues that the INSTI does not cause weight gain directly and, when combined with results of HPTN 083 (previously discussed), suggests that the weight difference seen in PrEP RCTs is driven by weight loss from TDF/FTC.
Other Analyses
Researchers assessed the impact of INSTIs on adipose tissue phenotype and function using a mixed methods study of 14 macaques without SIV infection and 19 PWH treated or not treated with an INSTI [68]. Biopsies of subcutaneous and visceral adipose tissue were performed to assess fibrosis, adipogenesis, oxidative stress, mitochondrial function, and insulin sensitivity after exposure to DTG or RAL. The results demonstrated elevated fibrosis, adipocyte size, and adipogenic marker expression in subcutaneous and visceral adipose tissue from macaques exposed to INSTIs. Similarly, higher levels of fibrosis were detected in tissue from PWH treated with an INSTI compared with PWH not receiving an INSTI. In particular, DTG was associated with greater extracellular matrix production and lipid accumulation in adipose stem cells and adipocytes in vitro; RAL was associated to a lesser degree. In sum, DTG and RAL appeared proadipogenic and prolipogenic and promoted oxidative stress, mitochondrial dysfunction, and insulin resistance. These direct effects may contribute to weight gain and should be confirmed.
An investigation that employed a mouse model similarly compared effects of various ARVs on mitochondrial function as well as adipocyte differentiation [69]. Researchers treated primary pre-adipocyte cells from female mice with DTG, BIC, or the NNRTI doravirine (DOR) for 8 days during differentiation. The INSTIs DTG and BIC mildly induced differentiation into white adipocytes and significantly suppressed differentiation into brown adipocytes. The INSTIs also caused downregulation of enzymes responsible for thermogenesis in brown adipocytes and affected other mitochondrial enzymes. Assays that specifically examined the effects of DTG found that it inhibited cellular oxygen consumption and energy expenditure and interfered with estrogen-mediated metabolic pathways. On the other hand, DOR did not affect mitochondrial activity or fat cell differentiation. These findings demonstrate differential effects between INSTIs and NNRTIs on adipocyte differentiation and thermogenesis and raise potential explanations for the greater propensity for INSTI-associated weight gain for cisgender women because some effects may be estrogen mediated.
Another laboratory-based theory merits attention. There is in vitro evidence that DTG inhibits binding of radio-labeled alpha-melanocyte-stimulating hormone to the human recombinant melanocortin 4 receptor (MC4R), which helps regulate energy homeostasis and food intake [70, 71]. Deficiency or blockage of MC4R has been associated with obesity. Investigators proposed that INSTIs directly interfere with the melanocortin signaling system, causing an orexigenic (appetite stimulating) response [72]. The authors present an elegant description and figure detailing potential ways (at least 4) by which this could occur. However, another group tested and refuted this theory [73]. They evaluated the effects of INSTIs on MC4R in an in vitro cell-based assay and found that INSTIs indeed antagonize MC4R, but only at supratherapeutic concentrations, and thus concluded that this is unlikely to explain INSTI-induced weight gain. Further studies to corroborate these findings are warranted.
Case Reports
Regardless of the mechanism, the phenomenon of INSTI-associated weight gain seems clinically significant. A related consequence may be higher propensity for hyperglycemia. One case report describes onset of significant hyperglycemia following a switch to DTG [74], and a small case series describes 3 PWH who developed hyperglycemia and ketoacidosis within months of switching to BIC/FTC/TAF [75]. In a report of an individual who developed diabetes mellitus following switch to DTG-anchored ART, the authors presented an intriguing theory about INSTIs and weight [76]. They proposed that by chelating magnesium and manganese, which are required for the process of viral integration, INSTIs may have the unintended, off-target effect of increasing insulin resistance. Thus, the chemical structure and intended effects of INSTIs on magnesium may have unintended effects on blood sugars, insulin sensitivity, and thereby weight. That said, other investigations found no changes to glucose homeostasis following switch to an INSTI, even though weight gain occurred [77]. So, this theory is plausible but not yet validated.
Summary of Data on INSTIs and Weight Gain Mechanisms
The constellation of findings on INSTIs and weight gain mechanisms is different than that for TAF. For the INSTIs, several plausible biologic mechanisms for direct effects have been proposed. The literature to date suggests that the preswitch anchor drug plays a role and that older anchor drugs like EFV likely suppress weight, particularly for individuals with certain genetic profiles, but also that INSTIs may directly stimulate weight accumulation through several proposed pathways. Future research should assess these specific pathways, clarify whether this is an INSTI class effect or is unique to the newer INSTIs, and continue to examine the long-term clinical consequences as well as pros vs cons of switching an INSTI to an alternative option.
CONCLUSIONS
The mechanisms responsible for TAF- and INSTI-associated excess weight gain remain incompletely understood. Our interpretation of the literature to date is that the mechanism of excess weight gain from TAF and INSTIs is most likely different, credible theories about direct effects from INSTIs exist but must be corroborated, and it is likely that older agents like TDF and EFV suppress weight more than previously realized. Clinical trials like ACTG 5391 [78] will add insight into whether switching from an INSTI to DOR, with or without a switch from TAF to TDF, leads to reversal of weight gain; a case report describes weight loss when switching an INSTI-TAF combination back to TDF with an NNRTI, so this is plausible [22]. Additional trials, like DEFINE, will assess whether switching from an INSTI to a boosted PI is beneficial for individuals who have experienced rapid and significant weight gain while taking an INSTI with TAF; this will add further insights into the pros and cons of such a switch, particularly isolating the outcome of switching off an INSTI as both the intervention and comparator arms will continue taking TAF [79]. However, ongoing research is also needed to confirm the mechanisms and further explore differences based on birth sex and genetic factors. Until we better understand the phenomenon of ARV-associated excess weight gain, clinicians will need to carefully monitor patients for adverse metabolic or cardiovascular outcomes and counsel those who have gained substantial weight while taking TAF and/or an INSTI on healthy lifestyle habits, together with what is known and unknown about the potential benefits and risks of switching ART.
Acknowledgments
The authors would like to thank Kody Keckler at Cook County Health for assistance developing the figure.
Author contributions. B.R.W. performed the initial literature search, compilation of findings, and manuscript preparation. G.D.H. contributed additional resources, description and interpretation of findings, and insights for the figure.
Patient consent. This review article does not include factors necessitating patient consent.
Financial support. None.
Potential conflicts of interest. B.R.W.: none. G.D.H.: institutional research grants: Gilead, Lilly, Viiv, Janssen, Proteus; consultancy/advisory board: Trio Health, Gilead, Viiv, Janssen, Lilly. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Davy-Mendez T, Eron JJ, Zakharova O, et al. . Increased persistence of initial treatment for HIV infection with modern antiretroviral therapy. J Acquir Immune Defic Syndr 2017; 76:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruciani M, Parisi SG.. Dolutegravir based antiretroviral therapy compared to other combined antiretroviral regimens for the treatment of HIV-infected naive patients: a systematic review and meta-analysis. PLoS One 2019; 14:e0222229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanters S, Vitoria M, Doherty M, et al. . Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta-analysis. Lancet HIV 2016; 3:e510–20. [DOI] [PubMed] [Google Scholar]
- 4. Nickel K, Halfpenny NJA, Snedecor SJ, Punekar YS.. Comparative efficacy, safety and durability of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: an update on a systematic review and network meta-analysis. BMC Infect Dis 2021; 21:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nance RM, Vannappagari V, Smith K, et al. . Virologic failure among people living with HIV initiating dolutegravir-based versus other recommended regimens in real-world clinical care settings. J Acquir Immune Defic Syndr 2019; 81:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calmy A, Tovar Sanchez T, Kouanfack C, et al. ; New Antiretroviral and Monitoring Strategies in HIV-infected Adults in Low-Income Countries (NAMSAL) ANRS 12313 Study Group. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV 2020; 7:e677–87. [DOI] [PubMed] [Google Scholar]
- 7. Schwarze-Zander C, Piduhn H, Boesecke C, et al. . Switching tenofovir disoproxil fumarate to tenofovir alafenamide in a real life setting: what are the implications? HIV Med 2020; 21:378–85. [DOI] [PubMed] [Google Scholar]
- 8. Tao X, Lu Y, Zhou Y, et al. . Efficacy and safety of the regimens containing tenofovir alafenamide versus tenofovir disoproxil fumarate in fixed-dose single-tablet regimens for initial treatment of HIV-1 infection: a meta-analysis of randomized controlled trials. Int J Infect Dis 2020; 93:108–17. [DOI] [PubMed] [Google Scholar]
- 9. Aloy B, Tazi I, Bagnis CI, et al. . Is tenofovir alafenamide safer than tenofovir disoproxil fumarate for the kidneys? AIDS Rev 2016; 18:184–92. [PubMed] [Google Scholar]
- 10. Gibson AK, Shah BM, Nambiar PH, Schafer JJ.. Tenofovir alafenamide. Ann Pharmacother 2016; 50:942–52. [DOI] [PubMed] [Google Scholar]
- 11. Eckard AR, McComsey GA.. Weight gain and integrase inhibitors. Curr Opin Infect Dis 2020; 33:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kolakowska A, Maresca AF, Collins IJ, Cailhol J.. Update on adverse effects of HIV integrase inhibitors. Curr Treat Options Infect Dis 2019; 11:372–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scévola S, Tiraboschi JM, Podzamczer D.. Nothing is perfect: the safety issues of integrase inhibitor regimens. Expert Opin Drug Saf 2020; 19:683–94. [DOI] [PubMed] [Google Scholar]
- 14. Lake JE, Trevillyan J.. Impact of Integrase inhibitors and tenofovir alafenamide on weight gain in people with HIV. Curr Opin HIV AIDS 2021; 16:148–51. [DOI] [PubMed] [Google Scholar]
- 15. McCann K, Shah S, Hindley L, et al. . Implications of weight gain with newer anti-retrovirals: 10-year predictions of cardiovascular disease and diabetes. AIDS 2021; 35:1657–65. [DOI] [PubMed] [Google Scholar]
- 16. Venter WDF, Sokhela S, Simmons B, et al. . Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV 2020; 7:e666–76. [DOI] [PubMed] [Google Scholar]
- 17. Rebeiro PF, Jenkins CA, Bian A, et al. . Risk of incident diabetes mellitus, weight gain, and their relationships with integrase inhibitor-based initial antiretroviral therapy among persons with HIV in the US and Canada. Clin Infect Dis. 2021;73:e2234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nolan NS, Adamson S, Reeds D, O’Halloran JA.. Bictegravir-based antiretroviral therapy-associated accelerated hyperglycemia and diabetes mellitus. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah S, Hill A.. Risks of metabolic syndrome and diabetes with integrase inhibitor-based therapy: republication. Curr Opin HIV AIDS 2021; 16:106–14. [DOI] [PubMed] [Google Scholar]
- 20. Schafer JJ, Sassa KN, O’Connor JR, et al. . Changes in body mass index and atherosclerotic disease risk score after switching from tenofovir disoproxil fumarate to tenofovir alafenamide. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galdamez R, García JA, Fernández M, et al. . Short-term increase in risk of overweight and concomitant systolic blood pressure elevation in treatment-naïve persons starting INSTI-based antiretroviral therapy. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Max B, DeMarais P.. Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide discontinuation and return to normal weight. Int J STD AIDS 2021; 32:92–5. [DOI] [PubMed] [Google Scholar]
- 23. Selvaraj SV, Bares SH, Havens JP.. Acute weight gain after switch to emtricitabine/tenofovir alafenamide for human immunodeficiency virus pre-exposure prophylaxis. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santos JR, Saumoy M, Curran A, et al. ; Tenofovir/emtricitabine inflUence on LIPid metabolism (TULIP) Study Group. The lipid-lowering effect of tenofovir/emtricitabine: a randomized, crossover, double-blind, placebo-controlled trial. Clin Infect Dis 2015; 61:403–8. [DOI] [PubMed] [Google Scholar]
- 25. Valantin MA, Bittar R, de Truchis P, et al. ; TOTEM trial group. Switching the nucleoside reverse transcriptase inhibitor backbone to tenofovir disoproxil fumarate + emtricitabine promptly improves triglycerides and low-density lipoprotein cholesterol in dyslipidaemic patients. J Antimicrob Chemother 2010; 65:556–61. [DOI] [PubMed] [Google Scholar]
- 26. Tungsiripat M, Kitch D, Glesby MJ, et al. . A pilot study to determine the impact on dyslipidemia of adding tenofovir to stable background antiretroviral therapy: ACTG 5206. AIDS 2010; 24:1781–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fabbiani M, Bracciale L, Doino M, et al. . Lipid-lowering effect of tenofovir in HIV-infected patients. J Antimicrob Chemother 2011; 66:682–3. [DOI] [PubMed] [Google Scholar]
- 28. Huhn GD, Shamblaw DJ, Baril JG, et al. . Atherosclerotic cardiovascular disease risk profile of tenofovir alafenamide versus tenofovir disoproxil fumarate. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Milinkovic A, Berger F, Arenas-Pinto A, Mauss S.. Reversible effect on lipids by switching from tenofovir disoproxil fumarate to tenofovir alafenamide and back. AIDS 2019; 33:2387–91. [DOI] [PubMed] [Google Scholar]
- 30. Ikeda M, Wakabayashi Y, Okamoto K, et al. . Changing trends in lipid profile and biomarkers of renal function and bone metabolism before and after switching from tenofovir disoproxil fumarate to tenofovir alafenamide: a prospective observational study. AIDS Res Ther 2021; 18:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Squillace N, Ricci E, Menzaghi B, et al. ; CISAI Study Group. The effect of switching from tenofovir disoproxil fumarate (TDF) to tenofovir alafenamide (TAF) on liver enzymes, glucose, and lipid profile. Drug Des Devel Ther 2020; 14:5515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kauppinen KJ, Kivelä P, Sutinen J.. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide significantly worsens the lipid profile in a real-world setting. AIDS Patient Care STDS 2019; 33:500–6. [DOI] [PubMed] [Google Scholar]
- 33. Lagoutte-Renosi J, Flammang M, Chirouze C, et al. . Real-life impact on lipid profile of a switch from tenofovir disoproxil fumarate to tenofovir alafenamide in HIV-infected patients. Curr HIV Res 2021; 19:84–9. [DOI] [PubMed] [Google Scholar]
- 34. Lacey A, Savinelli S, Barco EA, et al. ; UCD ID Cohort Study. Investigating the effect of antiretroviral switch to tenofovir alafenamide on lipid profiles in people living with HIV. AIDS 2020; 34:1161–70. [DOI] [PubMed] [Google Scholar]
- 35. Sax PE, Erlandson KM, Lake JE, et al. . Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McComsey GA, Daar ES, O’Riordan M, et al. . Changes in fat mitochondrial DNA and function in subjects randomized to abacavir-lamivudine or tenofovir DF-emtricitabine with atazanavir-ritonavir or efavirenz: AIDS Clinical Trials Group study A5224s, substudy of A5202. J Infect Dis 2013; 207:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leonard MA, Zinhle C, Bradford Y, et al. . Efavirenz pharmacogenetics and weight gain following switch to integrase inhibitor-containing regimens. Clin Infect Dis. 2021;73:e2153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cahn P, Madero JS, Arribas JR, et al. . Durable efficacy of dolutegravir (DTG) plus lamivudine (3TC) in antiretroviral treatment-naïve adults with HIV-1 infection- 3-year results from the GEMINI studies. P018. Paper presented at: HIV Glasgow Conference; 5–8 October 2020; Glasgow, Scotland. Available at: http://www.hivglasgow.org/wp-content/uploads/2020/11/P018_Cahn.pdf. Accessed 15 June 2021. [Google Scholar]
- 39. Shokoohi M, Gupta M, Crouzat F, et al. . Changes in renal and metabolic indices after switching from tenofovir disoproxil fumarate- to tenofovir alafenamide-containing ART among individuals with HIV in Canada: a retrospective study. Int J STD AIDS. 2021;32:861–71. [DOI] [PubMed] [Google Scholar]
- 40. Mallon PW, Brunet L, Hsu RK, et al. . Weight gain before and after switch from TDF to TAF in a U.S. cohort study. J Int AIDS Soc 2021; 24:e25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Surial B, Mugglin C, Calmy A, et al. ; Swiss HIV Cohort Study. Weight and metabolic changes after switching from tenofovir disoproxil fumarate to tenofovir alafenamide in people living with HIV: a cohort study. Ann Intern Med 2021; 174:758–67. [DOI] [PubMed] [Google Scholar]
- 42. Kanda N, Okamoto K, Okumura H, et al. . Outcomes associated with treatment change from tenofovir disoproxil fumarate to tenofovir alafenamide in HIV-1-infected patients: a real-world study in Japan. HIV Med 2021; 22:457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Łomiak M, Stępnicki J, Mikuła T, Wiercińska-Drapało A.. Weight and body mass index increase after switch from tenofovir disoproxil fumarate to tenofovir alafenamide fumarate-containing treatment in an antiretroviral therapy-experienced group. Int J STD AIDS 2021; 32:570–7. [DOI] [PubMed] [Google Scholar]
- 44. Lahiri CD, Xu Y, Wang K, et al. . Weight and body mass index change after switching to integrase inhibitors or tenofovir alafenamide among women living with HIV. AIDS Res Hum Retroviruses 2021; 37:461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taramasso L, Berruti M, Briano F, Di Biagio A.. The switch from tenofovir disoproxil fumarate to tenofovir alafenamide determines weight gain in patients on rilpivirine-based regimen. AIDS 2020; 34:877–81. [DOI] [PubMed] [Google Scholar]
- 46. Gomez M, Seybold U, Roider J, et al. . A retrospective analysis of weight changes in HIV-positive patients switching from a tenofovir disoproxil fumarate (TDF)- to a tenofovir alafenamide fumarate (TAF)-containing treatment regimen in one German university hospital in 2015-2017. Infection 2019; 47:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Erlandson KM, Carter CC, Melbourne K, et al. . Weight change following antiretroviral therapy switch in people with viral suppression: pooled data from randomized clinical trials. Clin Infect Dis 2021; 73:1440–51. [DOI] [PubMed] [Google Scholar]
- 48. McComsey GA, Sax PE, Althoff KN, et al. . Weight change in suppressed people with HIV (PWH) switched from either tenofovir disoproxil fumarate (TDF) or abacavir (ABC) to tenofovir alafenamide (TAF). Abstract LB-7. Paper presented at: ID Week Conference 2020; 20–25 October2020; Virtual. Available at: https://www.eventscribe.net/2020/IDWeek/fsPopup.asp?Mode=presInfo&PresentationID=798013. Accessed 15 June 2021. [Google Scholar]
- 49. Sax PE, Rockstroh JK, Luetkemeyer AF, Tazdanpanah Y, Ward D, Trottier B.. Switching to bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV. Clin Infect Dis. 2021;73:e485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Wyk J, Ait-Khaled M, Santos J, et al. . Week 144 in the TANGO study, comparing a switch to Dovato (DTG/3TC) versus maintenance of TAF-based regimens. Paper presented at: 11th IAS Conference on HIV Science; 18–21 July2021; Virtual. [Google Scholar]
- 51. Llibre JM, Alves Brites C, Cheng CY, et al. . Switching to the 2-drug regimen of Dovato (dolutegravir/lamivudine) fixed-dose combination is non-inferior to continuing a 3-drug regimen through 48 weeks in a randomized clinical trial (SALSA). Paper presented at: 11th IAS Conference on HIV Science; 18–21 July2021; Virtual. [Google Scholar]
- 52. Hare CB, Coll J, Ruane P, et al. . The phase 3 DISCOVER study: daily F/TAF or F/TDF for HIV preexposure prophylaxis. Abstract 104LB. Paper presented at: CROI Conference; 4–7 March 2019; Seattle, WA. Available at: https://www.croiconference.org/abstract/phase-3-discover-study-daily-ftaf-or-ftdf-hiv-preexposure-prophylaxis/. Accessed 15 June 2021. [Google Scholar]
- 53. Landovitz RJ, Donnell D, Clement M, et al. . HPTN 083 final results: pre-exposure prophylaxis containing long-acting injectable cabotegravir is safe and highly effective for cisgender men and transgender women who have sex with men. Abstract #OAXLB01. Paper presented at: AIDS 2020 Conference; 6–10 July 2021; Virtual. Available at: https://aids2020.org/wp-content/uploads/2020/07/HIV-Highlights-Press-Conference-Abstracts.pdf. Accessed 15 June 2021. [Google Scholar]
- 54. Glidden DV, Mulligan K, McMahan V, et al. . Metabolic effects of preexposure prophylaxis with coformulated tenofovir disoproxil fumarate and emtricitabine. Clin Infect Dis 2018; 67:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ruderman SA, Crane HM, Nance RM, et al. . Brief report: weight gain following ART initiation in ART-naïve people living with HIV in the current treatment era. J Acquir Immune Defic Syndr 2021; 86:339–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lake JE, Wu K, Bares SH, et al. . Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis 2020; 71:e471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Verboeket SO, Boyd A, Wit FW, et al. ; AGEhIV Cohort Study Group. Generally rare but occasionally severe weight gain after switching to an integrase inhibitor in virally suppressed AGEhIV cohort participants. PLoS One 2021; 16:e0251205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mounzer K, Brunet L, Hsu R, et al. . Changes in BMI associated with antiretroviral regimen switch among treatment-experienced virologically suppressed people living with HIV in the United States. AIDS Res Hum Retroviruses. 2021; 37:852– 61. [DOI] [PubMed] [Google Scholar]
- 59. Goldberg RN, Kania AT, Michienzi SM, et al. . Weight gain in incarcerated individuals living with HIV after switching to integrase strand inhibitor-based therapy. J Int Assoc Provid AIDS Care. 2021;20:2325958221996860. doi:10.1177/2325958221996860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Caniglia EC, Shapiro R, Diseko M, et al. . Weight gain during pregnancy among women initiating dolutegravir in Botswana. EClinicalMedicine 2020; 29-30:100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bourgi K, Jenkins CA, Rebeiro PF, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc 2020; 23:e25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bourgi K, Rebeiro PF, Turner M, et al. . Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020; 70:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Norwood J, Turner M, Bofill C, et al. . Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 2017; 76:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Menard A, Meddeb L, Tissot-Dupont H, et al. . Dolutegravir and weight gain: an unexpected bothering side effect? AIDS 2017; 31:1499–500. [DOI] [PubMed] [Google Scholar]
- 65. Griesel R, Maartens G, Chirehwa M, et al. . CYP2B6 genotype and weight differences between dolutegravir and efavirenz. Clin Infect Dis. 2020;ciaa1073. doi:10.1093/cid/ciaa1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. González-Cordón A, Assoumou L, Moyle G, et al. ; NEAT022 Study Group. Switching from boosted PIs to dolutegravir decreases soluble CD14 and adiponectin in high cardiovascular risk people living with HIV. J Antimicrob Chemother 2021; 76:2380–93. [DOI] [PubMed] [Google Scholar]
- 67. Landovitz RJ, Zangeneh SZ, Chau G, et al. . Cabotegravir is not associated with weight gain in human immunodeficiency virus-uninfected individuals in HPTN 077. Clin Infect Dis 2020; 70:319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gorwood J, Bourgeois C, Pourcher V, et al. . The integrase inhibitors dolutegravir and raltegravir exert proadipogenic and profibrotic effects and induce insulin resistance in human/simian adipose tissue and human adipocytes. Clin Infect Dis 2020; 71:e549–60. [DOI] [PubMed] [Google Scholar]
- 69. Jung I, Jin S, Tu-Sekine B, et al. . Integrase inhibitors target mitochondria in brown adipocytes disrupting thermogenesis. Abstract 515. Paper presented at: CROI Conference; 6–10 March 2021; Virtual. [Google Scholar]
- 70. European Medicines Agency. European Medicines Agency assessment report. Dolutegravir (Tivicay). Available at: www.ema.europa.eu/documents/assessment-report/tivicay-epar-public-assessment-report_en.pdf. Accessed 13 June 2021.
- 71. Hill A, Waters L, Pozniak A.. Are new antiretroviral treatments increasing the risks of clinical obesity? J Virus Erad 2019; 5:41–3. [PMC free article] [PubMed] [Google Scholar]
- 72. Domingo P, Villarroya F, Giralt M, Domingo JC.. Potential role of the melanocortin signaling system interference in the excess weight gain associated to some antiretroviral drugs in people living with HIV. Int J Obes (Lond) 2020; 44:1970–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McMahon C, Trevaskis JL, Carter C, et al. . Lack of an association between clinical INSTI-related body weight gain and direct interference with MC4 receptor (MC4R), a key central regulator of body weight. PLoS One 2020; 15:e0229617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McLaughlin M, Walsh S, Galvin S.. Dolutegravir-induced hyperglycaemia in a patient living with HIV. J Antimicrob Chemother 2018; 73:258–60. [DOI] [PubMed] [Google Scholar]
- 75. Nolan NS, Adamson S, Reeds D, O’Halloran JA.. Bictegravir-based antiretroviral therapy-associated accelerated hyperglycemia and diabetes mellitus. Open Forum Infect Dis 2021; 8:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fong PS, Flynn DM, Evans CD, Korthuis PT.. Integrase strand transfer inhibitor-associated diabetes mellitus: a case report. Int J STD AIDS 2017; 28:626–8. [DOI] [PubMed] [Google Scholar]
- 77. Ibrahim F, Samarawickrama A, Hamzah L, et al. ; BESTT Trial Team. Bone mineral density, kidney function, weight gain and insulin resistance in women who switch from TDF/FTC/NNRTI to ABC/3TC/DTG. HIV Med 2021; 22:83–91. [DOI] [PubMed] [Google Scholar]
- 78. ACTG 5391 trial. Available at: https://clinicaltrials.gov/ct2/show/NCT04636437. Accessed 13 June 2021.
- 79. DEFINE trial. Available at: https://clinicaltrials.gov/ct2/show/NCT04442737. Accessed 18 September 2021.