Abstract
Background and objective:
Disease-specific outcomes following lung transplantation (LT) in patients with pulmonary Langerhans cell histiocytosis (PLCH) are not well established. We queried the Organ Procurement and Transplantation Network database to identify adult PLCH patients who had undergone LT in the United States.
Methods:
Overall survival data were analysed with Kaplan–Meier curves. Cox proportional hazard model was used to determine the effect of demographic, clinical and physiological variables on post-transplant survival.
Results:
A total of 87 patients with PLCH underwent LT in the United States between October 1987 and June 2017, accounting for 0.25% of the total LT during this period. The mean age at LT for PLCH patients was 49 years (range: 19–67 years), with a near equal gender distribution. Bilateral sequential LT was performed in 71 patients (82%). Pulmonary hypertension was present in 85% of patients, with a mean pulmonary artery pressure of 38.5 ± 14.1 mm Hg. The mean pre-transplant forced expiratory volume in 1 s (FEV1) was 41 ± 21% predicted and the mean 6-min walk distance was 221 ± 111 m. Median post-LT survival for PLCH patients was comparable to patients with other lung diseases (5.1 vs 5.5 years, P = 0.76). The actuarial Kaplan–Meier post-LT survival for PLCH patients was 85%, 65%, 49% and 22% at 1, 3, 5 and 10 years, respectively. Female sex (hazard ratio (HR): 0.40, 95% CI: 0.22–0.72), pre-transplant serum bilirubin (HR: 1.66, 95% CI: 1.23–2.26) and serum creatinine (HR: 4.03, 95% CI: 1.01–14.76) were independently associated with post-LT mortality in our cohort.
Conclusion:
Post-LT survival in patients with PLCH is similar to patients with other lung diseases and is significantly affected by patient gender.
Keywords: bronchiolitis obliterans, Langerhans cell histiocytosis, mortality, pulmonary Langerhans cell histiocytosis, pulmonary hypertension
INTRODUCTION
Lung transplantation (LT) is increasingly being employed in adult patients with advanced pulmonary diseases.1 Pulmonary Langerhans cell histiocytosis (PLCH) is a rare smoking-related cystic lung disease that can progress to respiratory failure and severe pulmonary hypertension (PH), but is an infrequent indication of LT worldwide, accounting for only 0.4% of adult primary LT from January 2004 to June 2015.2
PLCH commonly presents with cough, dyspnoea, ‘B’ symptoms or recurrent spontaneous pneumothorax; however, it may also be asymptomatic and present as an incidental radiographical finding. Therapeutic options for PLCH are limited, with smoking cessation being the cornerstone of treatment.3,4 Continued smoke exposure following diagnosis is associated with clinical deterioration and shorter survival, while smoking cessation is associated with decreased risk of lung function deterioration.5,6 Despite successful smoking cessation, a subset of patients with PLCH can progress to respiratory failure, PH and death.3,5,6 Median survival from the time of diagnosis in PLCH has been reported as 12.5–13 years,7,8 with a remarkable increase in mortality in the subset of patients who develop PH.9–11
In patients with advanced PLCH, LT is a viable management option; however, there are limited data regarding disease-specific outcomes following LT in patients with PLCH.12,13 We evaluated a national organ transplantation database to evaluate post-LT outcomes and determined the variables that impact post-LT survival in patients with PLCH.
METHODS
We obtained de-identified information regarding all lung transplants performed in the United States between 1 October 1987 and 30 June 2017 from the Organ Procurement and Transplantation Network (OPTN) database maintained by the United Network for Organ Sharing (UNOS). This national database contains information for every transplant that has occurred in the United States since 1 October 1987. We identified adult (age ≥ 18 years) PLCH patients from this database by using the following diagnosis codes: eosinophilic granulomatosis (code 414), Histiocytosis X (code 419) and pulmonary Langerhans cell histiocytosis (code 441). We also examined the records of patients who underwent LT with a primary diagnosis code of ‘other’ (code 999), and included patients with the following diagnoses in the PLCH cohort: Eosinophilic granulomatosis, Histiocytosis, Histiocytosis X, Langerhans cell histiocytosis, Pulmonary Langerhans Cell, Pulmonary Langerhans Cell Granulomatosis and Pulmonary histiocytosis. Institutional board review (IRB) and approval was not required for this analysis as our IRB deemed this project to be non-human subjects research.
Demographic, pre-transplant, surgical, post-transplant and donor-related data from the PLCH cohort were collected for analysis from the OPTN database. Continuous variables included: age at LT, forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), 6-min walk (6 MW) distance, right heart catheterization (RHC) values, oxygen requirement, serum bilirubin, serum creatinine, body mass index (BMI) and ischaemic time. These were summarized using mean and SD. Non-continuous variables included gender, ethnicity, history of tobacco use, history of bronchiolitis obliterans syndrome (BOS), cytomegalovirus (CMV) status, type of transplant and chronic steroid use; these variables were analysed as number and percentage of total. Time spent on the waiting list was summarized using median and interquartile range. Donor age, gender and ethnicity were also collected. Presence or absence of PH was determined on the basis of mean pulmonary artery pressure (mPAP) ≥ 25 mm Hg determined via RHC.14
Survival analysis was performed using Kaplan–Meier curves and calculated for each primary LT recipient from the day of transplant until death or censor. We also performed a conditional survival analysis in which LT recipients who had died within the first 12 months following LT were excluded. Comparisons of Kaplan–Meier survival between different subgroups were conducted by the log-rank test. The primary outcome of interest for our analysis was the overall survival following LT in patients with PLCH compared with the overall post-LT survival in patients with other lung diseases. Finally, we employed a Cox proportional hazard model to determine the effect of selected pre-transplant demographic, clinical and physiological variables on post-LT survival. A two-sided P-value of <0.05 was used to indicate significance. Statistical analyses were performed using R (Institute for Statistics and Mathematics, Vienna, Austria, version 3.3.3).
RESULTS
Study population
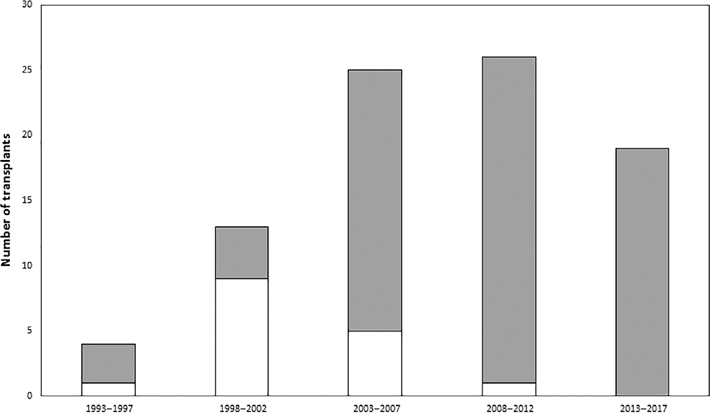
From 1 October 1987 to 30 June 2017, a total of 87 patients with PLCH underwent LT in the United States, accounting for 0.25% of the 31 902 total primary LT performed during this period (Fig. 1). The mean age at LT for the PLCH patients was 49 years (range: 19–67) and 51.7% were female recipients. The mean pre-LT FEV1 for our cohort was 41 ± 21%; over two-thirds of the cohort had pre-LT FEV1 < 50% predicted. Pre-LT 6 MW distance results were available for 66 patients; mean pre-LT 6 MW distance was 221 ± 111 m. Analysable RHC values were available for a total of 73 patients. PH was present in 85% of these patients (62/73), with mPAP of 38.5 ± 14.1 mm Hg. The mPAP was ≥35 mm Hg in 41 of 73 (56%) and was ≥45 mm Hg in 24 of 73 (33%) patients. Details regarding the demographic, clinical and physiological characteristics of the LT recipients and donors in our cohort are summarized in Tables 1 and 2.
Figure 1.
Histogram depicting the number of primary lung transplantation (LT) procedures performed in patients with pulmonary Langerhans cell histiocytosis (PLCH) in the United States. The first reported lung transplant for PLCH was performed in August 1993 and the most recent was performed in June 2017.  , Single LT;
, Single LT;  , bilateral sequential LT.
, bilateral sequential LT.
Table 1.
Demographic, clinical and physiological characteristics of our cohort
| Variable | Mean (SD) or n (%) |
|---|---|
|
| |
| Number of patients | 87 |
| Female | 45 (51.7%) |
| Ethnicity | |
| Caucasian | 82 (94.2%) |
| African American | 4 (4.6%) |
| Hispanic | 1 (1.2%) |
| Age at LT (years) | 49.4 (9.6) |
| Type of transplant | |
| Single | 16 (18.4%) |
| Bilateral | 71 (81.6%) |
| Ischaemic time, h (n = 78) | 5.31 (1.59) |
| History of tobacco use (n = 86) | |
| Yes | 73 (84.9%) |
| No | 13 (15.1%) |
| BMI (kg/m2) | 25.58 (4.5) |
| Chronic steroid use (n = 85) | |
| Yes | 37 (43.5%) |
| No | 45 (52.9%) |
| Unknown | 3 (3.6%) |
| Supplemental oxygen requirement pre-transplant (n = 82) | |
| Yes | 77 (93.9%) |
| No | 5 (6.1%) |
| Oxygen requirement (L/min) | 5 (3.72) |
| Total bilirubin (mg/dL) (n = 81) | 0.74 (1.07) |
| Serum creatinine (mg/dL) (n = 86) | 0.85 (0.22) |
| 6-Min walk distance, m (n = 66) | 221 (111) |
| Diabetes (n = 86) | |
| Yes | 11 (12.8%) |
| No | 75 (87.2%) |
| FEV1, % predicted (n = 83) | 41 (21) |
| FEV1 (% predicted) | |
| <50 | 58 (70%) |
| 50–70 | 14 (17%) |
| >70 | 11 (13%) |
| FVC, % predicted (n = 83) | 58.5 (19.9) |
| FVC (% predicted) | |
| <50 | 26 (31%) |
| 50–70 | 32 (39%) |
| >70 | 25 (30%) |
| mPAP, mm Hg (n = 73) | 38.5 ± 14.1 |
| mPAP (mm Hg) | |
| <25 | 11 (15.1%) |
| 25–35 | 21 (28.8%) |
| >35 | 41 (56.1%) |
| Cardiac output (L/min) (n = 66) | 5.09 (1.39) |
BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LT, lung transplantation; mPAP, mean pulmonary artery pressure.
Table 2.
Characteristics of the 87 lung donors
| Variable | Mean (SD) or n (%) |
|---|---|
|
| |
| Donor age at LT, years | 34.6 (14.2) |
| Female | 45 (51.7%) |
| Donor ethnicity | |
| Caucasian | 59 (67.8%) |
| African American | 18 (20.7%) |
| Hispanic | 8 (9.2%) |
| Asian | 2 (2.3%) |
| D and R gender | |
| D female–R female | 31 (35.6%) |
| D female–R male | 14 (16.1%) |
| D male–R male | 28 (32.2%) |
| D male–R female | 14 (16.1%) |
| D and R CMV status (n = 79) | |
| Either D or R unknown | 8 (10.1%) |
| D+/R+ | 25 (31.6%) |
| D+/R− | 13 (16.5%) |
| D−/R+ | 23 (29.1%) |
| D−/R− | 10 (12.7%) |
CMV, cytomegalovirus; D, donor; LT, lung transplantation; R, recipient.
Transplant details
The 87 LT for PLCH were performed at 40 different centres, with 16 centres performing 1 transplant each and 24 centres performing the remaining 71 (median: 2 LT per centre (range: 1–5)). Mean graft cold ischaemic time was 5.32 ± 1.60 h. Bilateral sequential LT (BSLT) was performed for 82% (71/87) of all PLCH LT. Overall, the proportion of patients undergoing BSLT increased during the study duration (Fig. 1). There were no heart–lung transplants performed for PLCH during this period. Twenty patients were transplanted prior to the initiation of the lung allocation score (LAS) system in May 2005 and 67 patients were transplanted after the LAS system was instituted. Time spent on the LT waitlist was significantly shorter following institution of the LAS system compared to the pre-LAS time period (median time on waitlist: 292.5 days pre-LAS vs 84 days post-LAS, P = 0.003).
Study outcomes
One patient underwent LT in June 2017, did not have any recorded follow-up data and consequently was excluded from the survival analysis. Of the 86 patients included in survival analysis, 2 (2.3%) were lost to follow-up, 4 (4.6%) required re-transplantation, 30 (34.5%) were alive and 51 (58.6%) had died as of the data censor date of 8 September 2017.
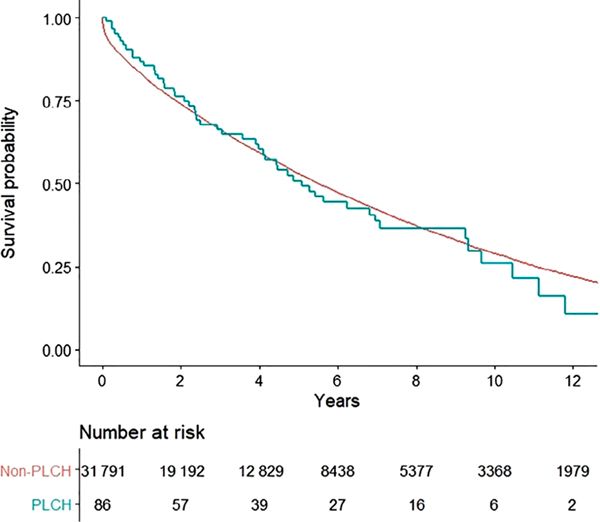
The overall survival following LT was similar in patients with PLCH as compared to other lung diseases (5.1 vs 5.5 years, P = 0.76; Fig. 2). The actuarial Kaplan–Meier post-LT survival for PLCH patients was 85%, 65%, 49% and 22% at 1, 3, 5 and 10 years, respectively. Overall conditional survival, assessed after excluding patients who had died in the first 12 months following LT, was also similar between PLCH patients and patients with other lung diseases (6.2 vs 7.1 years, P = 0.31; Fig. S1 in Supplementary Information).
Figure 2.
Kaplan–Meier curve demonstrating the probability of survival after primary lung transplantation (LT) in pulmonary Langerhans cell histiocytosis (PLCH) ( ) compared with the LT survival in non-PLCH (
) compared with the LT survival in non-PLCH ( ) patients. The comparisons were performed by using the log-rank test. There was no statistically significant difference between the two groups in median survival (5.1 vs 5.5 years, P = 0.76).
) patients. The comparisons were performed by using the log-rank test. There was no statistically significant difference between the two groups in median survival (5.1 vs 5.5 years, P = 0.76).
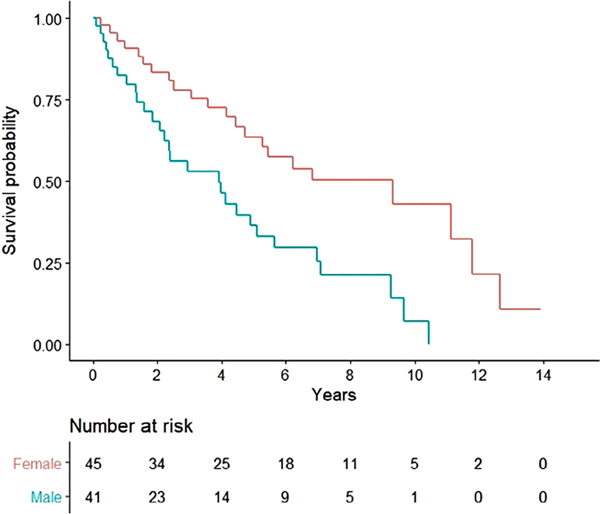
Subgroup analysis of the PLCH cohort showed improved survival in female transplant recipients compared with male recipients (9.3 vs 3.9 years, P = 0.0015; Fig. 3). Conditional survival, assessed after excluding patients who had died in the first 12 months following LT, also demonstrated improved survival in female LT recipients compared with their male counterparts (9.3 vs 4.5 years, P = 0.003; Fig. S2 in Supplementary Information). There was no difference in survival after segregating our cohort on the basis of type of LT (single vs bilateral: 5.4 vs 4.9 years, P = 0.69; Fig. S3 in Supplementary Information), presence or absence of PH (5.1 vs 7.1 years, P = 0.34; Fig. S4 in Supplementary Information) or the time of LT (pre-LAS vs post-LAS: 4.5 vs 5.6 years, P = 0.28; Fig. S5 in Supplementary Information). In addition to female sex, pre-transplant serum bilirubin (hazard ratio (HR): 1.66, 95% CI: 1.23–2.26) and serum creatinine (HR: 4.03, 95% CI: 1.01–14.76) were found to be independently associated with the risk of post-LT mortality (Table 3).
Figure 3.
Kaplan–Meier curve demonstrating the probability of survival after primary lung transplantation (LT) in pulmonary Langerhans cell histiocytosis (PLCH) patients after segregating on the basis of gender ( , female;
, female;  , male). The comparisons were performed by using the log-rank test. There was a statistically significant survival difference between the two groups in favour of females (median survival: 9.3 vs 3.9 years, P = 0.0015).
, male). The comparisons were performed by using the log-rank test. There was a statistically significant survival difference between the two groups in favour of females (median survival: 9.3 vs 3.9 years, P = 0.0015).
Table 3.
Results from Cox proportional hazard model analysing the association between selected pre-transplant variables and post-transplant mortality
| Covariate | HR | 95% CI | P-value |
|---|---|---|---|
|
| |||
| Single LT (vs bilateral LT) | 0.87 | 0.45–1.71 | 0.69 |
| Female (vs male) recipient | 0.40 | 0.22–0.72 | 0.002 |
| Recipient age | 1.00 | 0.97–1.03 | 0.87 |
| BMI | 1.00 | 0.94–1.06 | 0.89 |
| FEV1 | 1.00 | 0.99–1.02 | 0.51 |
| FVC | 0.99 | 0.99–1.01 | 0.93 |
| 6-Min walk | 0.99 | 0.99–1.00 | 0.10 |
| Mean pulmonary artery pressure | 1.00 | 0.98–1.03 | 0.76 |
| Cardiac output | 1.04 | 0.76–1.43 | 0.79 |
| Ischaemic time | 0.99 | 0.82–1.20 | 0.94 |
| Donor age | 1.00 | 0.97–1.20 | 0.87 |
| Serum bilirubin | 1.66 | 1.23–2.26 | 0.0008 |
| Serum creatinine | 4.03 | 1.01–14.76 | 0.035 |
BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HR, hazard ratio; LT, lung transplantation.
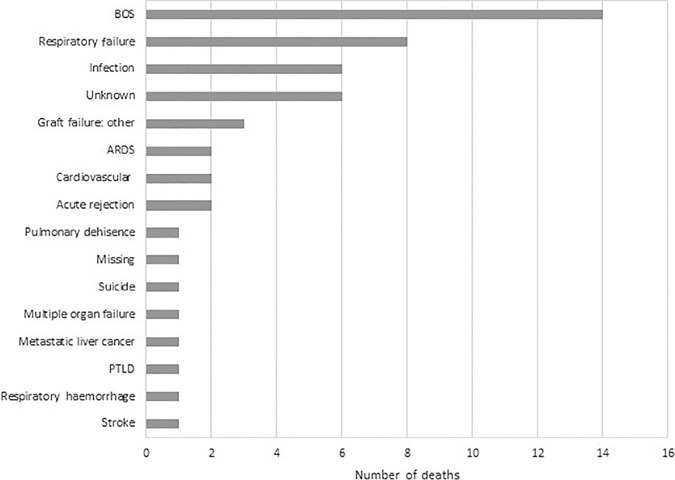
BOS was diagnosed in 45 of 78 patients for whom this variable was recorded (Table S1 in Supplementary Information). A total of 51 patients in our cohort had died following LT; post-LT time and cause of death were available in 44 of these patients (Table S2 in Supplementary Information). The leading primary causes of death were BOS (14/51, 27%), respiratory failure (8/51, 16%) and infections (6/51, 12%) (Fig. 4). Four patients required re-transplant at 5, 138, 203 and 498 days post-LT. The reasons for re-transplant were: primary graft failure (1/4), airway complications (1/4) and BOS (2/4).
Figure 4.
Bar graph depicting the primary cause of mortality in the 51 PLCH patients who died after LT in our cohort. ARDS, acute respiratory distress syndrome; BOS, bronchiolitis obliterans syndrome; LT, lung transplantation; PLCH, pulmonary Langerhans cell histiocytosis; PTLD, post-transplant lymphoproliferative disorder.
DISCUSSION
The major findings from our study are: (i) PLCH is an uncommon indication for LT in the United States, with 87 transplants accounting for 0.25% of all adult primary LT from 1 October 1987 to 30 June 2017; (ii) median survival following LT for PLCH is 5.1 years and is similar to post-LT survival in other lung diseases; and (iii) post-LT survival in PLCH is significantly affected by the recipient gender, with females demonstrating a median survival of 9.3 years compared to 3.9 years in males.
PLCH is a rare, smoking-related diffuse cystic lung disease with a variable course, limited treatment options and the potential to progress to respiratory failure, severe PH and death. The exact incidence and prevalence of PLCH are not well known; however, the current prevalence of PLCH in the United States has been estimated to range between 3986 and 7972 patients.15 Since the first transplant for PLCH in 1993, there have been 87 total transplants, which equates to 3.6 transplants per year for PLCH, thus suggesting that a very small percentage of PLCH patients (<1%) progress to needing LT. Disease-specific outcome data following LT in PLCH is sparse; to our knowledge, post-LT outcomes in PLCH have been systematically evaluated only in one retrospective study consisting of 39 patients conducted in France.12 While there were differences in the type of transplants in the two studies (e.g. there were more heart–lung and single lung transplants in the French study as compared to our cohort), the overall survival estimates from our analysis are largely similar to the French study: 5-year survival rate of 57% in the French study compared to 5-year survival rate of 49% in our analysis. Our study included more than twice the number of subjects than the French study, and together these reports provide disease-specific data that can assist clinicians in counselling and decision-making when evaluating patients with PLCH.
In our analysis, females had decreased risk of mortality compared to male PLCH LT recipients (9.3 vs 3.9 years, HR: 0.40, 95% CI: 0.22–0.72, P = 0.002) (Table 3,Fig. 3). The exact reasons for this gender-based contrast in outcomes remain unknown. It is possible that this difference is purely due to chance given the overall small patient numbers in our cohort. However, it is worth noting that improved survival in females following LT has been previously reported in multiple studies,16–18 as well as the most recent International Society for Heart and Lung Transplantation (ISHLT) Registry analysis,19 although the magnitude of difference is markedly increased in our study compared to previous reports. Serum bilirubin and creatinine were also independently associated with increased risk of post-LT mortality in our cohort; however, the overall range of serum bilirubin and creatinine in our cohort was within the normal ranges in the vast majority of the subjects, and as such this relationship is of doubtful clinical significance.
Infections, graft failure and BOS are the most common causes of death following LT. If separated into early (within the first 12 months post-LT) versus late (>12 months post-LT) causes, infections and graft failure account for the majority of early deaths and BOS is the most common cause of late mortality.19 The results from our analysis are similar with infections and graft failure accounting for 63.6% of the deaths (7/11) in the first 12 months following LT, and BOS being the leading cause of mortality in the time period beyond the first 12 months (14/40, 35%) (Table S2 in Supplementary Information). Following LT, BOS is estimated to affect approximately half of the LT recipients at 5 years, and roughly three-fourths at 10 years.19 The overall prevalence of BOS in our cohort was similar to these estimates (Table S1 in Supplementary Information), thus lending further support to the importance of BOS as the primary cause of post-LT mortality and morbidity.
PH is a frequently encountered complication of PLCH, can be seen in both early as well as advanced cases and portends a poor prognosis.9–11 The exact mechanisms of development of PH in patients with PLCH are not well established; proposed hypotheses include direct invasion of the pulmonary arteries by immune cells, pulmonary vasculopathy due to activated immune system, hypoxic pulmonary vasoconstriction and vascular remodelling due to concomitant cigarette smoke exposure.11 PH was present in 85% of our cohort with 56% of the patients having severe PH (mPAP ≥ 35 mm Hg), similar to the estimates reported in the French PLCH LT cohort, where PH and severe PH were reported in 92% and 72% of the cohort, respectively.12 The prevalence of PH in our cohort was higher than other pre-LT chronic lung diseases such as cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis, sarcoidosis and lymphangioleiomyomatosis.20–24 Despite an increased prevalence of PH, the presence of PH did not seem to impact post-LT survival in patients with PLCH, similar to the majority of the other advanced lung diseases, with the exception of COPD.20–22,25
Recommendations regarding the optimal timing for patient referral and listing for LT in patients with CF, interstitial lung diseases, pulmonary vascular diseases and COPD have been provided in a recent ISHLT consensus statement.26 We are unable to provide disease-specific recommendations for PLCH patients with regards to the optimal timing of LT referral based on our analysis. We do, however, suggest that given the poor prognosis associated with the development of PH in patients with PLCH, and acceptable post-LT survival in PLCH patients with concomitant PH, consideration for LT referral is warranted in patients with PLCH and concomitant PH.
The 87 PLCH transplants were performed at 40 different centres over nearly three decades, with the majority being in the past 15 years. In 2005, the United States started to utilize the LAS system to determine the priority for the need of LT in patients on the transplant waitlist.27 The transition to LAS has resulted in a reduction in the time spent on LT waitlist as well as the waitlist mortality; however, the impact of the institution of the LAS system on post-LT mortality is not clear.28–31 Our results are consistent with these results and demonstrate a reduction in the time spent on LT waiting list from 292.5 to 84 days (P = 0.003) post-institution of the LAS system, with no impact on post-LT survival.
Our study is the first to evaluate LT for PLCH in the United States, is the largest study to date of patients with PLCH undergoing LT and provides disease-specific outcome data following LT in patients with this disease. The major limitations of our analysis are related to missing variables in the database that may be of relevance to the PLCH population. For instance, we were unable to determine the presence and extent of extra-thoracic disease, the effect of prior pleural symphysis procedures on post-LT outcomes and the risk of disease recurrence after LT. Despite this being the largest study to evaluate LT outcomes in PLCH, the overall sample size was small, thus limiting the ability to reach definitive conclusions on some of our analyses.
In conclusion, primary LT for advanced PLCH is associated with similar survival to patients with other lung diseases and remains a viable management option for patients with advanced disease. Recipient gender may impact survival with LT in patients with PLCH. Future research should focus on determining the variables that can help predict optimal timing for transplant referral in individual PLCH patients.
Supplementary Material
Figure S1 Kaplan–Meier curve demonstrating the probability of conditional survival, assessed after excluding patients who had died in the first 12 months following LT, in pulmonary Langerhans cell histiocytosis (PLCH) patients compared with non-PLCH patients.
Figure S2 Kaplan–Meier curve demonstrating the probability of conditional survival, assessed after excluding patients who had died in the first 12 months following LT, in female PLCH patients compared with male patients.
Figure S3 Kaplan–Meier curve demonstrating the probability of survival following primary lung transplantation (LT) in patients with PLCH after segregating the cohort on the basis of type of lung transplant: single LT versus bilateral sequential LT.
Figure S4 Kaplan–Meier curve demonstrating the probability of survival following primary lung transplantation in patients with PLCH after segregating the cohort on the basis of presence or absence of pulmonary hypertension, defined by mean pulmonary artery pressure ≥25 mm Hg determined by right heart catheterization.
Figure S5 Kaplan–Meier curve demonstrating the probability of survival following primary lung transplantation in patients with PLCH after segregating the cohort on the basis of time of lung transplant: before institution of the lung allocation score (LAS) system versus post-LAS.
Table S1 Details regarding the development of bronchiolitis obliterans post-lung transplantation in our cohort.
Table S2 Details regarding the timing and cause of death post-lung transplantation in our cohort.
SUMMARY AT A GLANCE.
We studied post-lung transplantation survival and clinical outcomes in patients with pulmonary Langerhans cell histiocytosis (PLCH) from a national transplantation database in the United States. Median post-transplant survival in patients with PLCH is approximately 5 years, similar to other chronic lung diseases undergoing transplantation, and significantly affected by patient gender.
Acknowledgements:
This work was supported in part by the Health Resources and Services Administration contract 234–2005-370011C. N.G. received salary support from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI), Grant R34HL138235. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of Department of Health and Human Services, nor does the mention of trade names of commercial products or organizations imply endorsement by the U.S. Government.
Abbreviations:
- 6 MW
6-min walk
- BOS
bronchiolitis obliterans syndrome
- BSLT
bilateral sequential LT
- CF
cystic fibrosis
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- HR
hazard ratio
- ISHLT
International Society for Heart and Lung Transplantation
- LAS
lung allocation score
- LT
lung transplantation
- mPAP
mean pulmonary artery pressure
- OPTN
Organ Procurement and Transplantation Network
- PH
pulmonary hypertension
- PLCH
pulmonary Langerhans cell histiocytosis
- RHC
right heart catheterization
Footnotes
Supplementary Information
Additional supplementary information can be accessed via the html version of this article at the publisher’s website.
REFERENCES
- 1.Valapour M, Lehr CJ, Skeans MA, Smith JM, Carrico R, Uccellini K, Lehman R, Robinson A, Israni AK, Snyder JJ et al. OPTN/SRTR 2016 Annual Data Report: lung. Am. J. Transplant 2018; 18(Suppl. 1): 363–433. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Edwards LB, Dipchand AI, Goldfarb SB, Kucheryavaya AY, Levvey BJ, Lund LH, Meiser B, Rossano JW, Stehlik J; International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J. Heart Lung Transplant. 2016; 35: 1170–84. [DOI] [PubMed] [Google Scholar]
- 3.Vassallo R, Harari S, Tazi A. Current understanding and management of pulmonary Langerhans cell histiocytosis. Thorax 2017; 72: 937–45. [DOI] [PubMed] [Google Scholar]
- 4.Gupta N, Vassallo R, Wikenheiser-Brokamp KA, McCormack FX. Diffuse cystic lung disease. Part I. Am. J. Respir. Crit. Care Med. 2015; 191: 1354–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elia D, Torre O, Cassandro R, Caminati A, Harari S. Pulmonary Langerhans cell histiocytosis: a comprehensive analysis of 40 patients and literature review. Eur. J. Intern. Med. 2015; 26: 351–6. [DOI] [PubMed] [Google Scholar]
- 6.Tazi A, de Margerie C, Naccache JM, Fry S, Dominique S, Jouneau S, Lorillon G, Bugnet E, Chiron R, Wallaert B et al. The natural history of adult pulmonary Langerhans cell histiocytosis: a prospective multicentre study. Orphanet J. Rare Dis. 2015; 10: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delobbe A, Durieu J, Duhamel A, Wallaert B. Determinants of survival in pulmonary Langerhans’ cell granulomatosis (histiocytosis X). Groupe d’Etude en Pathologie Interstitielle de la Societe de Pathologie Thoracique du Nord. Eur. Respir. J. 1996; 9: 2002–6. [DOI] [PubMed] [Google Scholar]
- 8.Vassallo R, Ryu JH, Schroeder DR, Decker PA, Limper AH. Clinical outcomes of pulmonary Langerhans’-cell histiocytosis in adults. N. Engl. J. Med. 2002; 346: 484–90. [DOI] [PubMed] [Google Scholar]
- 9.Le Pavec J, Lorillon G, Jais X, Tcherakian C, Feuillet S, Dorfmüller P, Simonneau G, Humbert M, Tazi A. Pulmonary Langerhans cell histiocytosis-associated pulmonary hypertension: clinical characteristics and impact of pulmonary arterial hypertension therapies. Chest 2012; 142: 1150–7. [DOI] [PubMed] [Google Scholar]
- 10.Chaowalit N, Pellikka PA, Decker PA, Aubry MC, Krowka MJ, Ryu JH, Vassallo R. Echocardiographic and clinical characteristics of pulmonary hypertension complicating pulmonary Langerhans cell histiocytosis. Mayo Clin. Proc. 2004; 79: 1269–75. [DOI] [PubMed] [Google Scholar]
- 11.Bois MC, May AM, Vassallo R, Jenkins SM, Yi ES, Roden AC. Morphometric study of pulmonary arterial changes in pulmonary Langerhans cell histiocytosis. Arch. Pathol. Lab. Med. 2018; 142: 929–37. [DOI] [PubMed] [Google Scholar]
- 12.Dauriat G, Mal H, Thabut G, Mornex JF, Bertocchi M, Tronc F, Leroy-Ladurie F, Dartevelle P, Reynaud-Gaubert M, Thomas P et al. Lung transplantation for pulmonary Langerhans’ cell histiocytosis: a multicenter analysis. Transplantation 2006; 81: 746–50. [DOI] [PubMed] [Google Scholar]
- 13.Saleem I, Moss J, Egan JJ. Lung transplantation for rare pulmonary diseases. Sarcoidosis Vasc. Diffuse Lung Dis. 2005; 22(Suppl. 1): S85–90. [PubMed] [Google Scholar]
- 14.Gupta N, Langenderfer D, McCormack FX, Schauer DP, Eckman MH. Chest computed tomographic image screening for cystic lung diseases in patients with spontaneous pneumothorax is cost effective. Ann. Am. Thorac. Soc. 2017; 14: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015; 46: 903–75. [DOI] [PubMed] [Google Scholar]
- 16.Demir A, Coosemans W, Decaluwe H, De Leyn P, Nafteux P, Van Veer H, Verleden GM, Van Raemdonck D. Donor-recipient matching in lung transplantation: which variables are important? Eur. J. Cardiothorac. Surg. 2015; 47: 974–83. [DOI] [PubMed] [Google Scholar]
- 17.Loor G, Brown R, Kelly RF, Rudser KD, Shumway SJ, Cich I, Holley CT, Quinlan C, Hertz MI. Gender differences in long-term survival post-transplant: a single-institution analysis in the lung allocation score era. Clin. Transplant. 2017; 31: e12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts DH, Wain JC, Chang Y, Ginns LC. Donor-recipient gender mismatch in lung transplantation: impact on obliterative bronchiolitis and survival. J. Heart Lung Transplant. 2004; 23: 1252–9. [DOI] [PubMed] [Google Scholar]
- 19.Chambers DC, Cherikh WS, Goldfarb SB, Hayes D Jr, Kucheryavaya AY, Toll AE, Khush KK, Levvey BJ, Meiser B, Rossano JW et al. ; International Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult lung and heart-lung transplant report-2018; focus theme: multiorgan transplantation. J. Heart Lung Transplant. 2018; 37: 1169–83. [DOI] [PubMed] [Google Scholar]
- 20.Hayes D Jr, Higgins RS, Kirkby S, McCoy KS, Wehr AM, Lehman AM, Whitson BA. Impact of pulmonary hypertension on survival in patients with cystic fibrosis undergoing lung transplantation: an analysis of the UNOS registry. J. Cyst. Fibros. 2014; 13: 416–23. [DOI] [PubMed] [Google Scholar]
- 21.Hayes D Jr, Tumin D, Budev MM, Tobias JD, St John RC, Kukreja J. Adverse outcomes associated with pulmonary hypertension in chronic obstructive pulmonary disease after bilateral lung transplantation. Respir. Med. 2017; 128: 102–8. [DOI] [PubMed] [Google Scholar]
- 22.Hayes D Jr, Black SM, Tobias JD, Kirkby S, Mansour HM, Whitson BA. Influence of pulmonary hypertension on patients with idiopathic pulmonary fibrosis awaiting lung transplantation. Ann. Thorac. Surg. 2016; 101: 246–52. [DOI] [PubMed] [Google Scholar]
- 23.Shorr AF, Helman DL, Davies DB, Nathan SD. Pulmonary hypertension in advanced sarcoidosis: epidemiology and clinical characteristics. Eur. Respir. J. 2005; 25: 783–8. [DOI] [PubMed] [Google Scholar]
- 24.Ussavarungsi K, Hu X, Scott JP, Erasmus DB, Mallea JM, Alvarez F, Lee AS, Keller CA, Ryu JH, Burger CD. Mayo clinic experience of lung transplantation in pulmonary lymphangioleiomyomatosis. Respir. Med. 2015; 109: 1354–9. [DOI] [PubMed] [Google Scholar]
- 25.Fitton TP, Kosowski TR, Barreiro CJ, Chan V, Patel ND, Borja MC, Orens JB, Conte JV. Impact of secondary pulmonary hypertension on lung transplant outcome. J. Heart Lung Transplant. 2005; 24: 1254–9. [DOI] [PubMed] [Google Scholar]
- 26.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, Lederer DJ, Mulligan MJ, Patterson GA, Singer LG et al. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2015; 34: 1–15. [DOI] [PubMed] [Google Scholar]
- 27.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA et al. Development of the new lung allocation system in the United States. Am. J. Transplant. 2006; 6: 1212–27. [DOI] [PubMed] [Google Scholar]
- 28.Maxwell BG, Levitt JE, Goldstein BA, Mooney JJ, Nicolls MR, Zamora M, Valentine V, Weill D, Dhillon GS. Impact of the lung allocation score on survival beyond 1 year. Am. J. Transplant. 2014; 14: 2288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Shiboski SC, Golden JA. Impact of the lung allocation score on lung transplantation for pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2009; 180: 468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gries CJ, Mulligan MS, Edelman JD, Raghu G, Curtis JR, Goss CH. Lung allocation score for lung transplantation: impact on disease severity and survival. Chest 2007; 132: 1954–61. [DOI] [PubMed] [Google Scholar]
- 31.Lancaster TS, Miller JR, Epstein DJ, DuPont NC, Sweet SC, Eghtesady P. Improved waitlist and transplant outcomes for pediatric lung transplantation after implementation of the lung allocation score. J. Heart Lung Transplant. 2017; 36: 520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Kaplan–Meier curve demonstrating the probability of conditional survival, assessed after excluding patients who had died in the first 12 months following LT, in pulmonary Langerhans cell histiocytosis (PLCH) patients compared with non-PLCH patients.
Figure S2 Kaplan–Meier curve demonstrating the probability of conditional survival, assessed after excluding patients who had died in the first 12 months following LT, in female PLCH patients compared with male patients.
Figure S3 Kaplan–Meier curve demonstrating the probability of survival following primary lung transplantation (LT) in patients with PLCH after segregating the cohort on the basis of type of lung transplant: single LT versus bilateral sequential LT.
Figure S4 Kaplan–Meier curve demonstrating the probability of survival following primary lung transplantation in patients with PLCH after segregating the cohort on the basis of presence or absence of pulmonary hypertension, defined by mean pulmonary artery pressure ≥25 mm Hg determined by right heart catheterization.
Figure S5 Kaplan–Meier curve demonstrating the probability of survival following primary lung transplantation in patients with PLCH after segregating the cohort on the basis of time of lung transplant: before institution of the lung allocation score (LAS) system versus post-LAS.
Table S1 Details regarding the development of bronchiolitis obliterans post-lung transplantation in our cohort.
Table S2 Details regarding the timing and cause of death post-lung transplantation in our cohort.