Abstract
Malignant catarrhal fever (MCF) was diagnosed by clinical signs and lesions in five out of six white-tailed deer (Odocoileus virginianus) in a North American zoo. The clinical signs and histopathological lesions in these deer were typical of MCF. Antibody to an epitope conserved among the MCF viruses was detected in the sera collected from the deer. PCR failed to amplify viral sequences from DNA extracted from peripheral blood leukocytes (PBL) and/or spleens of the deer with primers specific for ovine herpesvirus 2 (OHV-2) or specific for alcelaphine herpesvirus 1 (AHV-1). By using degenerate primers targeting a conserved region of a herpesviral DNA polymerase gene, a DNA fragment was amplified from the PBL or spleens of all six deer and sequenced. Alignment of the sequences demonstrated that the virus in the deer belongs to the Gammaherpesvirinae subfamily, exhibiting 82% identity to OHV-2, 71% to AHV-1, and 60% to a newly identified bovine lymphotropic herpesvirus. This virus, which causes classical MCF in white-tailed deer, is a newly recognized agent belonging to the MCF group of gammaherpesviruses. It is the third reported pathogenic MCF virus, genetically distinct but closely related to OHV-2 and AHV-1. The reservoir for the virus has not been identified.
Malignant catarrhal fever (MCF) is the clinical manifestation of the infection of certain ruminant species with one of a group of pathogenic gammaherpesviruses known as MCF viruses (4, 18). The disease is sporadic to occasionally epidemic and is distributed worldwide. Most domestic cattle and numerous exotic species, such as banteng (Bos javanicus) and gaur (Bos gaurus) (8) are susceptible to clinical disease. Bison, moose, and some species of deer are highly susceptible (4, 21). Susceptibility to MCF varies among species of deer, from moderate to low in some, such as fallow deer, to extremely high in others, such as white-tailed, axis, and Pere David's deer. The diseases represent a major economic constraint to enterprises that involve deer. It often devastates collections of deer in zoos and is considered by some to be the most important viral disease facing the farmed deer industry of New Zealand (21).
The disease syndromes associated with these viruses range from acute, severe inflammatory disease with a short clinical course to a more chronic syndrome. Occasionally, cattle recover and return to clinical normality (17). The acute disease is characterized by high fever, lymph node swelling, and widespread inflammation of mucosal surfaces (8, 18). Lymphoproliferation and vasculitis are the main histologic lesions (13, 14, 18).
There are two known pathogenic viruses that are etiologically associated with MCF (4). Sheep and wildebeest represent the respective reservoirs for these two MCF viruses, in which the infection is asymptomatic and almost universal. These two viruses are closely related antigenically and genetically. The two reservoirs have been recognized for many years. The virus that is endemic in and well adapted to wildebeest can be readily isolated and propagated in vitro (19). It was named alcelaphine herpesvirus 1 (AHV-1), based on the subfamily of the principal reservoir host, the wildebeest (25). The virus that is endemic in sheep (11), though never isolated, has nonetheless been designated ovine herpesvirus 2 (OHV-2) (25), solely on the basis of its base sequence relatedness to AHV-1 (3).
The recent development of molecular diagnostic assays has provided powerful tools for investigating how this group of complex viruses survives in nature. A serological assay, a competitive-inhibition enzyme-linked immunosorbent assay (CI-ELISA), was established based on a monoclonal antibody against a specific epitope conserved among all the MCF viruses examined, including those of sheep and wildebeest origin (10). This assay has become a useful epidemiological tool for studying MCF viral infection in a variety of ruminant species infected with viral strains that are heterogeneous at a base sequence level (9; H. Li, J. Keller, and T. B. Crawford, unpublished data). Development of PCR specific for the OHV-2 (2) or AHV-1 strains of MCF viruses has dramatically improved the accuracy of diagnosis of MCF in clinically infected animals (5, 15). Furthermore, the PCR assay using degenerate primers targeting highly conserved amino acid motifs (32) within the herpesviral DNA-directed DNA polymerase gene (31) has become an important tool for the identification of new herpesviruses that cause clinical or subclinical infection in animals (20, 24, 26). The present study describes the use of degenerate primers and base sequence analysis to identify a new strain of MCF virus. This virus, which represents the third known pathogenic virus in the MCF group, is closely related to OHV-2 and AHV-1 genetically and is highly virulent in white-tailed deer (Odocoileus virginianus).
MATERIALS AND METHODS
Cases and background.
A small zoo located in the North Central United States contained approximately 200 animals, including several nonruminant species, antelope, several species of deer (mule deer, Reeve's muntjac deer, white-lipped deer, and white-tailed deer), and both pygmy and domestic goats. At no time did the collection include wildebeest. Five white-tailed deer (four females and one male) died during January and February of 1999. Their ages ranged from 7 months to 8 years. A sixth clinically normal, 2-year-old female deer was euthanized as a precaution for the welfare of other hoofed stock on the grounds. All six animals were necropsied and examined histopathologically. Samples for laboratory tests were collected from these deer at necropsy. Four of six animals (animals 1, 2, 5, and 6) were examined for antiviral antibodies or antigens of epidemic hemorrhagic disease (EHD), bluetongue (BT), infectious bovine rhinotracheitis (IBR), bovine respiratory syncytial virus (BRSV), bovine viral diarrhea virus (BVDV), parainfluenza-3 virus (PI-3), and MCF viruses. Agar gel immunodiffusion tests were used for the detection of antibodies to EHD and BT viruses. The presence of IBR, BRSV, BVDV, and PI-3 viral antigens in the tissues (including lung, spleen, and kidney) was evaluated by indirect immunofluorescence assay. Antibody to MCF viruses was detected by CI-ELISA (10). Bacterial cultures were done on selected tissues from four deer. These tissues included intestine (deer 1 and 2), lung (deer 1, 3, and 4), and heart (deer 1, 2, and 3). DNA extracted from the PBL of four deer (deer 1, 2, 5, and 6) and from the spleens of all six deer, as well as DNA from the PBL of three clinically normal white-tailed deer from another zoo, was subjected to PCR amplification by using primers specific for OHV-2 and AHV-1 and degenerate primers for a conserved region of the herpesviral DNA polymerase gene. DNA extracted from the PBL of an axis deer with clinical sheep-associated malignant catarrhal fever (SA-MCF) which was confirmed by histopathology and OHV-2-specific PCR (12), was used as a control.
PCR.
Consensus primer PCR was performed using a set of primers directed at a region of the herpesviral DNA polymerase gene (31). The PCR amplification conditions were as described previously (26), with minor modifications. In the primary reaction, 1 μg of PBL DNA was subjected to thermocycling in a 50-μl reaction mixture with two upstream primers (DFA and ILK) and one downstream primer (KG1) (Table 1). The reaction mixture contained 10 mM Tris-HCl (pH 8.0); 50 mM KCl; 2 mM MgCl2; 2.5% dimethyl sulfoxide; 400 μM dATP, dCTP, dGTP, and dTTP (Boehringer Mannheim Co., Indianapolis, Ind.); 20 pmol of each primer; and 5 U of Taq DNA polymerase (Boehringer-Mannheim Co.). Thermal cycling conditions were 5 min at 94°C, followed by 45 cycles of 94°C (30 s), 46°C (1 min), and 72°C (1 min), followed by a final 7-min extension at 72°C. In the secondary reaction, 5 μl of the primary reaction product was amplified with one upstream primer (TGV) and one downstream primer (IYG) (Table 1) under the same conditions as in the primary reaction. Due to the difficulty of amplifying the viral sequences from some of the spleens with the above set of degenerate primers, more-specific PCR primers were designed based on the polymerase sequences obtained from OHV-2 and AHV-1 and the deer. A degenerate upstream primer (CON-EX) for the primary reaction was derived from sequences that were highly conserved in the analogous regions of both OHV-2 and AHV-1 (Table 1). In the secondary reaction, the upstream primer (CONS), which was also conserved among OHV-2 and AHV-1, was derived from the sequence amplified from the deer PBL DNA. The same downstream primer, DER, was used in both primary and secondary reactions. This primer was also derived from the sequence amplified from the deer PBL DNA. The protocol for these amplifications was the same as that described above for the consensus PCR, except that 40 pmol of each primer was used, and 2 μg of DNA was used in the primary reaction.
TABLE 1.
PCR primers: sequences and positions
| PCR and primer | Sequence (5′–3′) | Nucleotide positiona |
|---|---|---|
| Consensus | ||
| DFA | GAYTTYGCNAGYYTNTAYCC | 21231–21250 |
| ILK | TCCTGGACAAGCAGCARNYSGCNMTNAA | 21493–21520 |
| KG1 | GTCTTGCTCACCAGNTCNACNCCYTT | 21942–21967 |
| TGV | TGTAACTCGGTGTAYGGNTTYACNGGNGT | 21528–21556 |
| IYG | CACAGAGTCCGTRTCNCCRTADAT | 21732–21755 |
| Specificb | ||
| CON-EX | CAYAAYCTRTGCTACTCCAC | 21267–21286 |
| CONS | TGGCCTCGGGCATGCTGC | 21556–21573 |
| DER | GGGCGATTGACTCTTTATAA | 21689–21707 |
| OHV-2 | ||
| 556 | AGTCTGGGTATATGAATCCAGATGGCTCTC | 121692–121722c |
| 755 | AAGATAAGCACCAGTTATGCATCTGATAAA | 121312–121340 |
| 555 | TTCTGGGGTAGTGGCGAGCGAAGGCTTC | 121484–121510 |
| AHV-1 | ||
| C500-1 | TACGGGAGCCCTGACATTTCATCTCTTTTG | 73917–74014 |
| C500-2 | ATAACTGGTTGATGTGGCAGATGCATCTAT | 74292–74321 |
| C500-3 | TCTGGCCCGTGCTGCAGCAAGACTCTCAG | 73986–74014 |
| C500-4 | TATAGTAGAATCCCGTCTGAGTGGTAGCTG | 74230–74259 |
The OHV-2-specific PCR used was as previously described (2, 11). Primers 556 and 775 (Table 1) were used for the primary amplification, and primers 556 and 555 (Table 1) were used for the secondary amplification. The reaction mixtures and thermal cycling conditions were as previously described, except that 5 μl rather than 10 μl of amplified product from the primary reaction was used as a target for the secondary amplification.
PCR amplification specific for AHV-1 was performed by using a set of primers derived from the sequence of AHV-1 open reading frame (ORF) 50 (6), a region of the AHV-1 genome reported to be associated with virulence for rabbits (7). PCR mixtures and thermal cycling conditions were as described above for the OHV-2-specific PCR, except that the annealing temperature was 55°C. Primers C500-1 and C500-2 were used in the primary amplification, and primers C500-3 and C500-4 (Table 1) were used in the secondary amplification. The initial DNA sequence for AHV-1 ORF 50 was kindly provided by H. W. Reid (Moredun Research Institute, Edinburgh, United Kingdom). Ten microliters of the amplified PCR products from the final reaction was analyzed by 2% agarose gel electrophoresis and stained with ethidium bromide for product visualization.
Cloning, sequencing, and sequence analysis.
PCR amplification products were purified for cloning by either chloroform-isoamyl alcohol extraction or by extraction from gels using the Qiagen QIAquick Gel Extraction Kit (Qiagen, Valencia, Calif.). Purified amplicons were cloned into the pSTBlue-1 vector with the Novagen Perfectly Blunt Cloning Kit (Novagen, Madison, Wis.). Plasmid DNA was extracted by using a Qiagen QIAprep Spin Miniprep Kit (Qiagen). The identity of appropriate-sized inserts in the recombinant plasmids was confirmed by PCR amplification and EcoRI restriction digestion of the recombinant plasmids. The sequencing was carried out by Amplicon Express (Pullman, Wash.). Between two and five clones from each deer were selected for sequencing. DNA sequences and the amino acid translation products of 120- to 177-bp non-primer DNA sequences from the six deer were analyzed with GCG (version 10) software (Genetics Computer Group, Inc., Madison, Wis.). The portion of herpesviral DNA polymerase sequence obtained from the white-tailed deer herein has been deposited in the National Center for Biotechnology Information database (GenBank accession number AF181468).
RESULTS
Clinical signs and histopathology.
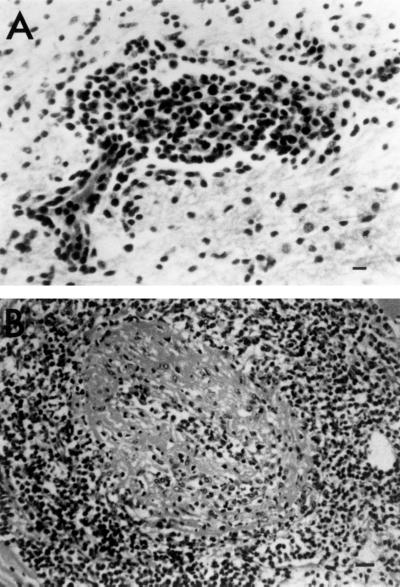
The first white-tailed deer became ill in December, 1998, and died after 3 weeks of illness. Over the next 2 months, an additional four deer also developed clinical signs and died, which represented a case fatality rate of 100%. Clinical signs in these five deer (deer 1 to 5) included serous ocular discharge, anorexia, depression, conjunctivitis, and periocular and nasal epithelial erosions, but no corneal opacity was observed in any of the animals. Gross lesions in deer 1 to 5 included fibrinous clots in the pericardial sac, mild to moderate random and irregular epicardial and myocardial pallor, accentuation of epicardial vessels, splenic serosal hemorrhages, splenomegaly, renal cortical infarcts, conjunctival hyperemia, lymphadenopathy of the mesenteric nodes, and zones of pallor in the pancreatic parenchyma. Deer 1 had perforating ulcers in the small intestine with fibrinous peritonitis, and deer 4 had severe mycotic pneumonia, respectively. The relationship between these lesions and MCF was not evident. Microscopic lesions in deer 1 to 5 were severe, extensive, and consistent. These lesions included various degrees of lymphocytic vasculitis and perivasculitis, fibrinoid arterial necrosis, and vascular thrombosis in the heart, adrenal gland, kidney, brain (deer 1) (Fig. 1A), liver, spleen, lung, pituitary gland, mesenteric lymph node, abomasum, ileum, pancreas, skeletal muscle, and thyroid gland. Lesions in two of the six animals (deer 3, heart; Fig. 1B) were consistent with a more chronic process (proliferative arteriopathy) similar to those described in previous reports of chronic MCF in cattle (17) and bison (27). These included endothelial cell hypertrophy, disruption of the internal elastic laminae, and occlusion of the vessel lumen due to adventitial smooth muscle hypertrophy and hyperplasia. Microscopic examination of tissues from deer 6 showed only mild, multifocal, lymphocytic interstitial nephritis. Although these changes were not specific for MCF, based on the confirmation of MCF virus as the cause of death in the previous herdmates and infection of this deer with the virus, these mild lesions could be interpreted as evidence of an early stage of MCF.
FIG. 1.
(A) Hypothalamus from deer 1 (hemotoxylin and eosin). Note the marked lymphocytic vasculitis and perivasculitis (bar, 20 μm). (B) Heart and muscular artery from deer 3 (hematoxylin and eosin). Note the marked lymphocytic perivasculitis, vasculitis, and fibrinoid necrosis (bar, 20 μm).
Serology, immunochemistry, and bacteriology.
Sera were available for four of six animals (deer 1, 2, 5, and 6). All four were negative for EHD and BT antibody but strongly positive for anti-MCF viral antibody by CI-ELISA (Table 2). Sera from three normal healthy white-tailed deer from another location were negative for anti-MCF viral antibody. Examination of frozen sections of spleen, kidney, and lung by immunofluorescence assay from all four animals yielded negative results for IBR, BRSV, BVDV, and PI-3. Bacterial culture of selected tissues from deer 1 to 4 yielded only mixed contaminants, with no significant bacterial pathogens. Tissues from animals 5 and 6 were not cultured.
TABLE 2.
Summary of diagnostic findings in six white-tailed deer from a North American zoo
| Case no. | Clinical sign | Histological lesion | Bacterial culture | Viral antibody or antigen
|
Viral DNA by PCR
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EHDa | BTa | IBRb | BRSb | BVDb | PI-3b | MCFc | OHV-2 | AHV-1 | HDPGd | ||||
| 1 | + | + | − | − | − | − | − | − | − | + | − | − | + |
| 2 | + | + | − | − | − | − | − | − | − | + | − | − | + |
| 3 | + | + | − | NDe | ND | ND | ND | ND | ND | ND | − | − | + |
| 4 | + | + | − | ND | ND | ND | ND | ND | ND | ND | − | − | + |
| 5 | + | + | ND | − | − | − | − | − | − | + | − | − | + |
| 6 | − | ±f | ND | − | − | − | − | − | − | + | − | − | + |
Detection of antibody by agar gel immunodiffusion.
Detection of antigens in tissues by indirect immunofluorescence assay.
Detection of antibody by competitive inhibition ELISA.
Amplification of a DNA fragment from herpesviral DNA polymerase gene.
ND, not done.
The tissue contained mild lesions.
PCR amplification and sequence analysis.
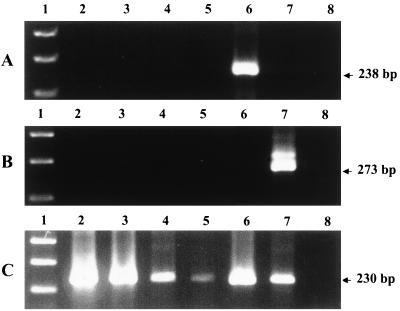
Both PCR assays with primers specific for OHV-2 or AHV-1 failed to amplify DNA sequences from either PBL (deer 1, 2, 5, and 6) or from spleen (deer 1 to 6) (Fig. 2). Consensus PCR, targeting a portion of the herpesviral DNA polymerase gene, amplified a 230-bp DNA fragment from PBL of four animals (deer 1, 2, 5, and 6) (Fig. 2). These amplified products were cloned and sequenced, and the sequences were used to create a primer that was specific for this deer-derived virus. With this primer (DER) and two other primers (CON-EX and CONS), a 155-bp DNA fragment was amplified from the spleens of all six deer. This deer-herpesvirus-specific PCR did not amplify the sequence from OHV-2 or AHV-1. Amplification of DNA from the three normal healthy white-tailed deer with all three primer sets was negative (data not shown).
FIG. 2.
Agarose gel electrophoresis of ethidium bromide-stained PCR products amplified from different DNA samples with primers specific for OHV-2 (A) and AHV-1 (B) and consensus primers for the herpesviral DNA polymerase gene (C). Lane 1, 100-bp DNA ladder; lanes 2 to 5, DNA extracted from PBL of white-tailed deer 1, 2, 5, and 6; lane 6, DNA extracted from an axis deer with clinical OHV-2 MCF; lane 7, DNA from AHV-1 (Minnesota isolate); lane 8, no-DNA control.
Alignment of sequences of the amplicons derived from the various deer DNA samples revealed that all six sequences were completely identical but distinct from the analogous OHV-2 or AHV-1 sequences in this region. The sequence of the consensus PCR product amplified from an axis deer with clinical SA-MCF was identical to the published OHV-2 DNA polymerase gene sequence except for a 1-bp mismatch (Fig. 3A). The base sequences of the herpesviral polymerase gene fragments from the white-tailed deer were 82% identical to OHV-2, 71% identical to AHV-1, and 60% identical to a newly identified bovine lymphotropic herpesvirus (BLHV) (26). The predicted amino acids coded for by the base sequence derived from the white-tailed deer were 81% identical to OHV-2, 72% identical to AHV-1, and only 53% identical to BLHV (Fig. 3B).
FIG. 3.
(A) Comparison of the nucleotide sequences of a region of the herpesviral DNA polymerase gene derived from white-tailed deer 1 from a North American zoo with the same region of OHV-2 from an axis deer with clinical SA-MCF, AHV-1, and BLHV. The black boxes represent nucleotides that vary from the sequence of OHV-2. (B) Comparison of the translated amino acid sequences of the same gene region as that shown in panel A. The white, light, white-framed, and black boxes represent identical residues, conservative substitutions, somewhat-similar residues, and dissimilar residues, respectively. The OHV-2, AHV-1, and BLHV DNA polymerase sequences used here were obtained from GenBank, the National Center for Biotechnology Information database. The accession numbers are as follows: OHV-2, AF031812; AHV-1, AF031809; and BLHV, AF031808.
DISCUSSION
Amplification of highly conserved gene regions such as the DNA polymerase gene of herpesviruses (31) has provided a powerful tool for detecting and identifying previously unrecognized members of this family in diseased tissue samples (20, 24, 26). Analysis of the base sequences of this gene fragment revealed that the sequences of the fragments from all six white-tailed deer were 100% identical to each other, 82% identical to OHV-2, and 71% identical to AHV-1. The phylogenetic tree for both published sequences and this new herpesviral DNA polymerase gene sequence was generated by the Seqweb program (GCG, Inc.) (data not shown). This clearly indicates the assignment of this virus as a member of the Gammaherpesvirinae subfamily (1, 30, 31) that is distinct from, but closely related to, the two other known pathogenic MCF viruses, OHV-2 and AHV-1. Collectively, the clinical and histological aspects of the disease, the serological response to MCF virus, and the PCR results indicate that this herpesvirus, which has not been identified previously, was associated with the MCF outbreak in white-tailed deer in the zoo. The factors that triggered the outbreak at that time could not be identified. The zoo management reported no unusual movement or introduction of new animal species.
Clinical MCF has been reported in at least 13 species of deer (21). The disease in deer usually presents in an acute or peracute manner, often without characteristic signs (21). In a previous report of an OHV-2-associated MCF outbreak in a petting zoo, all of the affected deer expired within 24 to 48 h after onset of clinical signs, and none developed overt clinical signs of MCF (12). In the present outbreak, however, all five deer developed typical symptoms of MCF, and some of them lived for as long as 3 weeks following the onset of clinical signs before succumbing to the infection. The histological lesions in two of these deer resembled those described previously in chronic OHV-2-associated MCF in cattle (17). The explanation for the more chronic nature of the disease in two of these deer is not yet known. Vascular thrombosis and infarction was somewhat more prominent in these deer than is usually observed in MCF, particularly in cattle. Whether or not this observation is related to the more prolonged clinical course accompanied by significant proliferative arteriopathy seen in these cases is not clear.
A reasonable speculation would be that strains of the MCF viruses exist that vary significantly in their virulence for a given host. Evidence is beginning to accumulate to support this concept. AHV-2 and -3 and hippotragine herpesvirus 1 are herpesviral isolates from hartebeest, topi, and roan antelope, respectively (16, 22), that have been called MCF viruses based on similarities in restriction fragment length polymorphism and neutralizing epitopes to AHV-1 (28, 29). These viruses are virtually avirulent, however, for any known species by natural transmission modes (23). Moreover, recent evidence generated in this laboratory supports the concept that domestic goats are endemically infected with another distinct but closely related gammaherpesvirus for which no etiological role in disease has been ascribed at this time (Li et al., unpublished data). In contrast to these viruses, the agent in the white-tailed deer is highly virulent, at least in this species. Whether or not the agent found in the white-tailed deer is also pathogenic for cattle, bison, or other ruminants is not known. However, we have never observed a recognized case of MCF in cattle or bison in which either OHV-2 or AHV-1 viral DNA could not be detected, which was the initial observation in the present deer cases. The reservoir host for this virus must first be identified before the transmissibility and virulence of the agent for various susceptible ruminant species can be systematically examined.
The reservoir host has not been identified, since a complete sampling of all the animals in the zoo environment that existed at the time the cases occurred was not possible in retrospect. Nomenclature for this virus has therefore not been developed, pending recognition of the carrier species. The newly-developed molecular tools, such as the monoclonal antibody-based CI-ELISA assay for antibody to the epitope conserved among MCF viruses and PCR using both species-specific primers and degenerate primers for herpesviral genes, offer the hope of identifying the reservoir host(s) for this pathogenic herpesvirus and a better understanding of the diversity of this group of gammaherpesviruses in nature.
ACKNOWLEDGMENTS
This work was supported by USDA-Agricultural Research Service grant CWU 5348-32000-013-00D.
We thank Dongyue Zhuang and Lori Fuller for excellent technical assistance. We greatly appreciate the help of Lowell Kappmeyer on the sequence alignment graphs. We also thank the Red River Zoological Society for assistance with epidemiological information and sample collection.
REFERENCES
- 1.Avise J C. Molecular markers, natural history and evolution. New York, N.Y: Chapman & Hall Inc.; 1994. [Google Scholar]
- 2.Baxter S I F, Pow I, Bridgen A, Reid H W. PCR detection of the sheep-associated agent of malignant catarrhal fever. Arch Virol. 1993;132:145–159. doi: 10.1007/BF01309849. [DOI] [PubMed] [Google Scholar]
- 3.Bridgen A, Reid H W. Derivation of DNA clone corresponding to the viral agent of sheep-associated malignant catarrhal fever. Res Vet Sci. 1991;50:38–44. doi: 10.1016/0034-5288(91)90051-o. [DOI] [PubMed] [Google Scholar]
- 4.Crawford T B, O'Toole D, Li H. Malignant catarrhal fever. In: Howard J L, editor. Current veterinary therapy IV: food animal practice. Philadelphia, Pa: The W. B. Saunders Co.; 1998. pp. 306–309. [Google Scholar]
- 5.Crawford T B, Li H, O'Toole D. Diagnosis of malignant catarrhal fever by PCR using formalin-fixed, paraffin-embedded tissues. J Vet Diagn Investig. 1999;11:111–116. doi: 10.1177/104063879901100201. [DOI] [PubMed] [Google Scholar]
- 6.Ensser A, Pflanz R, Fleckenstein B. Primary structure of the alcelaphine herpesvirus 1 genome. J Virol. 1997;71:6517–6525. doi: 10.1128/jvi.71.9.6517-6525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handley J A, Sargan D R, Herring A J, Reid H W. Identification of a region of the alcelaphine herpesvirus-1 genome associated with virulence for rabbits. Vet Microbiol. 1995;47:167–181. doi: 10.1016/0378-1135(95)00105-j. [DOI] [PubMed] [Google Scholar]
- 8.Heuschele W P. Malignant catarrhal fever: a review of a serious disease hazard for exotic and domestic ruminants. Zool Garten NF. 1988;58:123–133. [Google Scholar]
- 9.Li H, Shen D T, Jessup D A, Knowles D P, Gorham J R, Thorne T, O'Toole D, Crawford T B. Prevalence of antibody to malignant catarrhal fever virus in wild and domestic ruminants by competitive-inhibition ELISA. J Wildl Dis. 1996;32:437–443. doi: 10.7589/0090-3558-32.3.437. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Shen D T, Knowles D P, Gorham J R, Crawford T B. Competitive-inhibition enzyme-linked immunosorbent assay for antibody in sheep and other ruminants to a conserved epitope of malignant catarrhal fever virus. J Clin Microbiol. 1994;32:1674–1679. doi: 10.1128/jcm.32.7.1674-1679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Shen D T, O'Toole D, Knowles D P, Gorham J R, Crawford T B. Investigation of sheep-associated malignant catarrhal fever virus infection in ruminants by PCR and competitive inhibition enzyme-linked immunosorbent assay. J Clin Microbiol. 1995;33:2048–2053. doi: 10.1128/jcm.33.8.2048-2053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Westover W C, Crawford T B. Sheep-associated malignant catarrhal fever in a petting zoo. J Zoo Wildl Med. 1999;30:408–412. [PubMed] [Google Scholar]
- 13.Liggitt H D, DeMartini J C. The pathomorphology of malignant catarrhal fever. I. Generalized lymphoid vasculitis. Vet Pathol. 1980;17:59–73. doi: 10.1177/030098588001700107. [DOI] [PubMed] [Google Scholar]
- 14.Liggitt H D, DeMartini J C. The pathomorphology of malignant catarrhal fever. II. Multisystemic epithelial lesions. Vet Pathol. 1980;17:74–84. doi: 10.1177/030098588001700108. [DOI] [PubMed] [Google Scholar]
- 15.Muller-Doblies U U, Li H, Hauser B, Adler H, Ackermann M. Field validation of laboratory tests for clinical diagnosis of sheep-associated malignant catarrhal fever. J Clin Microbiol. 1998;36:2970–2972. doi: 10.1128/jcm.36.10.2970-2972.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mushi E Z, Karstad L, Jessett D M. Isolation of bovine malignant catarrhal fever virus from ocular and nasal secretions of wildebeest calves. Res Vet Sci. 1980;29:168–171. [PubMed] [Google Scholar]
- 17.O'Toole D, Li H, Williams D, Miller D, Crawford T B. Chronic and recovered cases of sheep-associated malignant catarrhal fever in cattle. Vet Rec. 1997;140:519–524. doi: 10.1136/vr.140.20.519. [DOI] [PubMed] [Google Scholar]
- 18.Plowright W. Malignant catarrhal fever virus. In: Dinter Z, Morein B, editors. Virus infections of ruminants. 1st ed. New York, N.Y: Elsevier Science Publishers BV; 1990. pp. 123–150. [Google Scholar]
- 19.Plowright W, Ferris R D, Scott G R. Blue wildebeest and the aetiological agent of bovine malignant catarrhal fever. Nature. 1960;188:1167–1169. doi: 10.1038/1881167a0. [DOI] [PubMed] [Google Scholar]
- 20.Quackenbush S L, Work T M, Balazs G H, Casey R N, Rovnak J, Chaves A, du Toit L, Baines J D, Parrish C R, Bowser P R, Casey J W. Three closely related herpesviruses are associated with fibropapillomatosis in marine turtles. Virology. 1998;246:392–399. doi: 10.1006/viro.1998.9207. [DOI] [PubMed] [Google Scholar]
- 21.Reid H W. The biology of a fatal herpesvirus infection of deer (malignant catarrhal fever) In: Brown R D, editor. The biology of deer. New York, N.Y: Springer-Verlag; 1992. pp. 93–100. [Google Scholar]
- 22.Reid H W, Bridgen A. Recovery of a herpesvirus from a roan antelope (Hippotragus equinus) Vet Microbiol. 1991;28:269–278. doi: 10.1016/0378-1135(91)90081-p. [DOI] [PubMed] [Google Scholar]
- 23.Reid H W, Rowe L. The attenuation of a herpesvirus (malignant catarrhal fever virus) isolated from hartebeest (Alcelaphus buselaphusz cokei, Gunther) Res Vet Sci. 1973;15:144–146. [PubMed] [Google Scholar]
- 24.Richman L K, Montli R J, Garber R L, Kennedy M A, Lehnhardt J, Hildebrandt T, Schmitt D, Hardy D, Alcendor D J, Hayward G S. Novel endotheliotropic herpesviruses fatal for Asian and African elephants. Science. 1999;283:1171–1176. doi: 10.1126/science.283.5405.1171. [DOI] [PubMed] [Google Scholar]
- 25.Roizman B. The family Herpesviridae: an update. Arch Virol. 1992;123:425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- 26.Rovnak J, Quackenbush S L, Reyes R A, Baines J D, Parrish C R, Casey J W. Detection of a novel bovine lymphotropic herpesvirus. J Virol. 1998;72:4237–4242. doi: 10.1128/jvi.72.5.4237-4242.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultheiss P C, Collins J K, Austgen L E, DeMartini J C. Malignant catarrhal fever in bison, acute and chronic cases. J Vet Diagn Investig. 1998;10:255–262. doi: 10.1177/104063879801000305. [DOI] [PubMed] [Google Scholar]
- 28.Seal B S, Klieforth R B, Welch W H, Heuschele W P. Alcelaphine herpesvirus 1 and 2 SDS-PAGE analysis of virion polypeptides, restriction endonuclease analysis of genomic DNA and virus replication restriction in different cell types. Arch Virol. 1989;106:301–320. doi: 10.1007/BF01313959. [DOI] [PubMed] [Google Scholar]
- 29.Seal B S, Heuschele W P, Klieforth R B. Prevalence of antibodies to alcelaphine herpesvirus-1 and nucleic acid hybridization analysis of viruses isolated from captive exotic ruminants. Am J Vet Res. 1989;50:1447–1453. [PubMed] [Google Scholar]
- 30.Sneath P H A, Sokal R R. Numerical taxonomy. W. H. San Francisco, Calif: Freeman & Co.; 1973. [Google Scholar]
- 31.VanDevanter D R, Warrener P, Bennett L, Schultz E R, Coulter S, Garber R L, Rose T M. Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol. 1996;34:1666–1671. doi: 10.1128/jcm.34.7.1666-1671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilks A F, Kurban R R, Hovens C M, Ralph S J. The application of the polymerase chain reaction to cloning members of the protein tyrosine kinase family. Gene. 1989;85:67–74. doi: 10.1016/0378-1119(89)90465-4. [DOI] [PubMed] [Google Scholar]