Abstract
Seven Ehrlichia strains (six HF strains and one Anan strain) that were obtained from laboratory mice by intraperitoneally inoculating homogenates of adult Ixodes ovatus collected in Japan were characterized. 16S rRNA sequences of all six HF strains were identical, and the sequences were 99.7, 98.2, and 97.7% identical to those of Anan strain, Ehrlichia chaffeensis (human monocytic ehrlichiosis agent), and E. muris, respectively. Partial GroEL amino acid sequencing also revealed that the six HF strains had identical sequences, which were 99.0, 98.5, and 97.3% identical to those of E. chaffeensis, the Anan strain, and E. canis, respectively. All HF strains were lethal to mice at higher dosages and intraperitoneal inoculation, whereas the Anan or E. muris strain induced only mild clinical signs. Light and electron microscopy of moribund mice inoculated with one of the HF strains revealed severe liver necrosis and the presence of numerous ehrlichial inclusions (morulae) in various organs. The study revealed that members of E. canis genogroup are naturally present in Ixodes ticks. HF strains that can cause severe illness in immunocompetent laboratory mice would be valuable in studying the pathogenesis and the roles of both cellular and humoral immune responses in ehrlichiosis caused by E. canis genogroup.
Ehrlichiae are obligatory intracellular bacteria that infect monocytes/macrophages, granulocytes, or platelets and cause a noncontagious, febrile systemic illness called ehrlichiosis in humans and in some varieties of domestic and wild animals (23–26). The severity of the disease varies from asymptomatic seroconversion to death, and severe morbidity is frequently documented. Ehrlichioses are now known as important emerging vector-borne zoonoses in the United States (1, 2, 4, 5, 23–26, 33). Five different species of Ehrlichia (E. chaffeensis, human granulocytic ehrlichiosis [HGE] agent, E. sennetsu, Venezuelan human Ehrlichia [VHE, a strain of E. canis], and E. ewingii) are now known to infect humans. Sennetsu fever in western Japan and in Malaysia is caused by E. sennetsu (8, 17, 34). Human monocytic ehrlichiosis (HME) in the United States is caused by E. chaffeensis (1), and asymptomatic human infection with VHE occurs in South America (21). HME in Europe and Africa (11, 18, 32) is probably caused by an Ehrlichia sp. closely related to E. chaffeensis. HGE in the United States and Europe is caused by an ehrlichial organism called HGE agent, which is closely related to E. equi and E. phagocytophila (5, 10). HGE caused by infection with another granulocytic Ehrlichia sp., E. ewingii (canine granulocytic Ehrlichia), was recognized in Missouri last year (4).
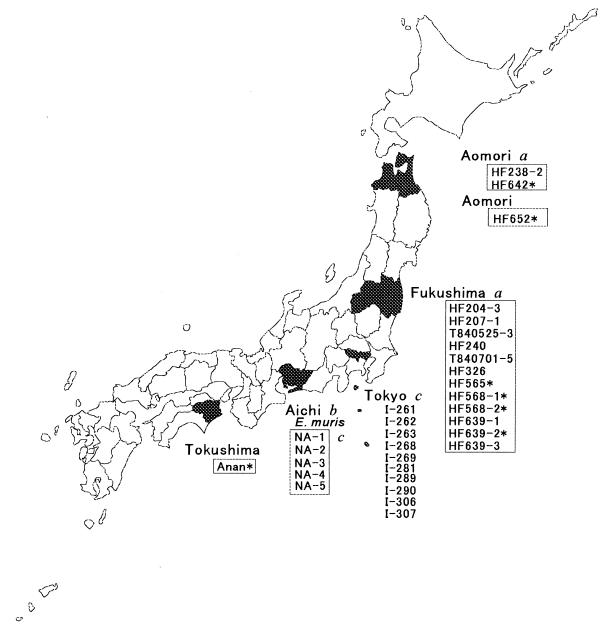
Ehrlichia spp. are transmitted to humans by specific species of infected ticks or trematodes from specific species of infected wild-animal reservoirs. For example, E. chaffeensis has been most commonly identified in the Lone Star tick (Amblyomma americanum) (3), and white-tailed deer are considered to be the major reservoir of E. chaffeensis (6, 15). The HGE agent has been found in the deer tick (Ixodes scapularis) (20, 31), and white-footed mice are considered to be the major reservoir of the HGE agent in northeastern and midwestern United States (31). Most Ehrlichia spp. characterized so far were isolated from domestic animals or humans. Only a few Ehrlichia spp. have been isolated from wild animals or vectors. Examination of Ehrlichia spp. in vectors and wild animals would provide an understanding of natural distribution and maintenance of ehrlichial organisms, as well as the diversity and evolution of ehrlichial populations. Such a study would provide a risk assessment for acquiring ehrlichial infection in particular geographic regions and, therefore, would facilitate proactive preventive measures. In 1983, Kawahara et al. isolated an infectious agent inducing splenomegaly in laboratory mice from a wild mouse (Eothenomys kageus) caught in central Japan. This organism was shown to be closely related to Ehrlichia canis by morphological and antigenic analysis (12), and 16S rRNA gene sequencing revealed it to be most closely related to E. chaffeensis. Since it is sufficiently distinct from any known Ehrlichia spp., it was designated E. muris (35). E. muris has been isolated from two additional species of wild mice in Metropolitan Tokyo and from Haemaphysalis flava ticks in Japan (14) (Fig. 1). Seroepidemiologic data suggested the exposure of humans and various wild animals to E. muris or related species in Japan (14).
FIG. 1.
Geographic region where Ehrlichia spp. were isolated from ticks or wild mice. Tick isolates are boxed. An asterisk indicates an isolate used for this study. Letters designate sources as follows: a, Fujita and Watanabe (7); b, Kawahara et al. (12); and c, Kawahara et al. (14).
Fujita and Watanabe (7) isolated 14 Ehrlichia-like agents from Ixodes ovatus ticks collected in two northern prefectures in Japan from 1983 to 1994 (Fig. 1). In the present study, we characterized five isolates collected from 1993 to 1994, which were reported by Fujita and Watanabe (7), and two additional tick isolates that have not been reported previously. To accomplish this analysis, we examined genetic, antigenic, and ultrastructural features and concluded that the strains are most closely related to E. chaffeensis, followed by E. muris and E. canis. Histopathologic observations in infected immunocompetent mice suggests that these new strains would be valuable in studying disease mechanisms and the role of immune responses in ehrlichiosis caused by the E. canis genogroup.
MATERIALS AND METHODS
Ehrlichiae isolated from I. ovatus ticks.
Five isolates (HF565, HF568-1, HF568-2, HF639-2, and HF642) were reported previously (7), and two isolates (HF652 and Anan) were obtained in the present study. HF652 was isolated from I. ovatus collected in Aomori Prefecture in 1994, and Anan was isolated from I. ovatus collected in Tokushima Prefecture in 1994 (Fig. 1). All ticks were collected from vegetation with a standard 1-m2 flannel flag. Isolation of Ehrlichia spp. was done as described elsewhere (7). Briefly, ticks were soaked for 10 min in 70% ethanol with 0.1% povidone-iodine and then rinsed with phosphate-buffered saline (PBS, pH 7.2) with 0.5 to 1.0% calf serum (GIBCO, Grand Island, N. Y.). Of the pooled ticks, two to six—either male or female—were ground up with a depression slide glass and a glass pestle (Iuchi Co., Ltd., Osaka, Japan) or with a mortar and pestle in sucrose-phosphate-glutamate (0.0038 M KH2PO4–0.0072 M K2HPO4–0.0049 M l-glutamate–0.218 M sucrose [pH 7.2]) at 0.3 ml per tick. For isolation of infectious agents, 0.3 ml of the homogenate of each pool was inoculated intraperitoneally into one 6-week-old female ddY mouse (Funabashi Farm, Shizuoka, Japan). These isolates had been passaged through mice once or twice.
Analysis of 16S rRNA and GroEL sequences of strains.
DNA was extracted from the spleens harvested from mice infected with strains HF565, HF568-1, HF568-2, HF639-2, HF642, HF652, or Anan by using a blood kit (Qiagen, Inc., Valencia, Calif.). PCR amplifications and nucleotide sequencing of total 16S rRNA genes were performed as previously described (14).
PCR amplification and nucleotide sequencing of groEL genes in DNA from the spleens of mice infected with HF and Anan isolates were performed by the method of Sumner et al. (30). The primer pairs used were HS43 and HS45 (30), Cha792u (5′-GGTGATGGAACAACTACATG-3′) and Cha1429d (5′-CCWARCATRTCTTTTCTTCT-3′), and Cha1293u (5′-GARGTDDARGGTGAAGC-3′) and Cha1770d (5′-TTCAACAGCAGCTCTAGTTG-3′), which were designed based on E. chaffeensis and HS43-HS45-amplified products. The PCR products were sequenced by using the PCR primers.
Phylogenetic analysis of DNA and amino acid sequences.
Sequence data were prepared for analysis by using the AutoAssembler version 1.4 (Perkin-Elmer, Norwalk, Conn.). The corrected levels of divergences of nucleotide of the 16S rRNA gene and deduced amino acid sequences of GroEL were calculated by using the Genetic Information Processing Software GENETYX-WIN version 3.0 (Software Development Co., Tokyo, Japan) on a Power Mac 8500. A dendrogram was constructed by using the UPGMA method and data from the distance matrix.
Clinical signs, relative spleen size, and indirect fluorescence antibody (IFA) titer against E. muris.
The 10% homogenates of spleens prepared from each mouse infected with one of the six HF strains at days 9 to 10 postinfection (p.i.), just before death, at a 1:10 dilution and the 10% homogenate prepared from the mouse infected with the Anan strain at day 10 or 15 p.i. without dilution were inoculated intraperitoneally or subcutaneously at 0.2 ml/mouse into five BALB/c male mice (8 weeks old). As positive controls the homogenates from mice infected with three strains of E. muris AS145 (type strain [12]), I-268 (14), and NA-1 (14) at 10 days p.i. were inoculated into five mice each. The spleen homogenates used as inocula contained similar levels of ehrlichial DNA based on the band intensity of the PCR products, except for that of the Anan isolate. The band intensity of the Anan isolate in the 10% spleen homogenate corresponded to an ∼103 dilution of isolates from HF strains. To assess the ehrlichial amount in the inoculum, the PCR amplification of each of the strains was performed by using a thermal cycler (Sankojyunyaku Co., Ltd., Tokyo, Japan) and GeneAmp reagents (Takara, Kyoto, Japan). A pair of primers, EC9 (5′-AAGGATCCTACCTTGTTACGACTT-3′) and EC10 (5′-AATCTAGATTAGATACCCTDGTAGTCC-3′, in which D = A, T, or G) were used based on the bacterial sequence of 16S rRNA (1). Each sample was amplified for 3 cycles at 94°C (1 min), 48°C (2 min), and 66°C (1 min 30 s), followed by 94°C for 9 min and 25 cycles at 88°C (1 min), 52°C (2 min), and 68°C (1 min 30 s). Products were electrophoresed through a 1.5% agarose gel to assess amplification efficiency. A 733-bp fragment was obtained after the amplification of DNA templates from all isolates.
The clinical signs of inoculated mice were observed daily until death or ≤20 days p.i. The relative spleen indexes (in grams per 100 g of body weight) were compared among all mice inoculated intraperitoneally on days 8 and 10 p.i. Antibody titers were compared for all mice inoculated subcutaneously on day 20 p.i. Antibody titers against E. muris, the HGE agent, and E. sennetsu were measured by the IFA test (12, 29). Various tissues, including the liver, spleen, thymus, and bone marrow, were collected at days 7 to 10 from mice inoculated intraperitoneally with HF565 or Anan strain, fixed in 10% buffered formalin solution (pH 7.4), and embedded in paraffin. Sections were stained with hematoxylin-eosin and Giemsa.
Electron microscopy.
The spleen and liver samples from mice intraperitoneally inoculated with strain HF565 at days 7 and 9 p.i. or with Anan strain at day 10 p.i. were cut into 1-mm-thick cubes and fixed and processed as described elsewhere (12). Ultrathin sections were stained with uranyl acetate and lead citrate, and the stained sections were examined with a Philips model 300 electron microscope at 60 kV.
Nucleotide sequence accession numbers.
The GenBank nucleotide sequence accession numbers for the 16S rRNAs used for comparison in this study are as follows: E. chaffeensis, M73222; E. canis, M73221; E. ewingii, M73227; E. sennetsu, M73225; E. muris, type strain AS145, U15527; strain I-268, AB013008; and strain NA-1, AB013009. The nucleotide sequences of strain HF565 and strain Anan have been deposited in the GenBank data library under accession numbers AB024928 and AB028319, respectively. The accession numbers of groEL sequences used for sequence comparisons are Z15160 for Bartonella bacilliformis, U13638 for Cowdria ruminantium, L10917 for E. chaffeensis, U88092 for E. sennetsu, X07850 for Escherichia coli, U96728 for HGE agent, U96727 for E. equi, U96731 for E. canis, and U96729 for E. phagocytophila. Nucleotide sequences of strain HF-565 and strain Anan have been deposited in the GenBank data library under accession numbers AB032712 and AB032711, respectively.
RESULTS
16S rRNA gene and partial GroEL amino acid sequences and phylogenetic analysis.
All ehrlichial strains isolated from wild mice or ticks in Japan, including three strains of E. muris AS145 (type strain, 11), I-268 isolated from an Apodemus speciosus wild mouse in Metropolitan Tokyo (14), or NA-1 isolated from a H. flava tick in Aichi Prefecture (14), are shown in Fig. 1. A total of 16 strains were isolated from adult I. ovatus: 12 strains in Fukushima Prefecture from 1983 to 1994, 3 strains in Aomori Prefecture from 1984 and 1994, and 1 strain in Tokushima Prefecture in 1994 (Fig. 1) (7). Four Fukushima strains (HF565, HF568-1, HF568-2, and HF639-2), two Aomori strains (HF642 and HF652), and one Tokushima strain (Anan) were examined in the present study.
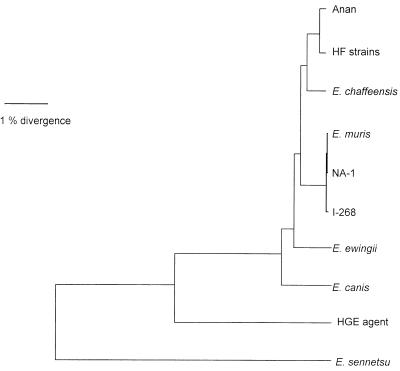
Of the 1,449-bp 16S rRNA gene sequences compared, all HF strains had identical base sequences and between Anan and HF strains, only three bases were different (Table 1). Anan and HF strains belonged to the E. canis genogroup and were far apart from the E. phagocytophila (the HGE agent) and E. sennetsu genogroups (Table 1 and Fig. 2). The HF strains were closest to E. chaffeensis, even more so than E. muris: HF strains and E. muris differ from E. chaffeensis by 19 and 29 bases, respectively (Table 1).
TABLE 1.
Levels of similarity and evolutionary distances between 16S rRNA gene sequences
| Organism(s) | Nucleotide substitution distances and nucleotide sequence similaritya
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HF strains | Anan | E. chaffeensis | E. muris | NA-1 | I-268 | E. ewingii | E. canis | HGE | E. sennetsu | |
| HF strains | 0.0028 | 0.0111 | 0.0094 | 0.0100 | 0.0094 | 0.0174 | 0.0212 | 0.605 | 0.1112 | |
| Anan | 99.72 | 0.0105 | 0.0090 | 0.0096 | 0.0090 | 0.0170 | 0.0208 | 0.601 | 0.1108 | |
| E. chaffeensis | 98.21 | 98.21 | 0.0111 | 0.0117 | 0.0111 | 0.0129 | 0.0167 | 0.560 | 0.1067 | |
| E. muris | 97.65 | 97.65 | 97.72 | 0.0006 | 0.0006 | 0.0176 | 0.0214 | 0.602 | 0.1114 | |
| NA-1 | 97.58 | 97.72 | 97.65 | 99.93 | 0.0009 | 0.0182 | 0.0220 | 0.608 | 0.1120 | |
| I-268 | 97.52 | 97.52 | 97.58 | 99.79 | 99.72 | 0.0176 | 0.0214 | 0.607 | 0.1114 | |
| E. ewingii | 97.31 | 97.38 | 97.17 | 96.55 | 96.48 | 96.41 | 0.0193 | 0.596 | 0.1108 | |
| E. canis | 96.62 | 96.69 | 96.96 | 95.79 | 95.72 | 95.65 | 97.79 | 0.629 | 0.1196 | |
| HGE | 89.73 | 89.67 | 89.67 | 89.26 | 89.19 | 89.12 | 89.67 | 89.53 | 0.9860 | |
| E. sennetsu | 83.13 | 83.34 | 83.76 | 83.51 | 83.44 | 83.37 | 83.16 | 83.09 | 82.94 | |
The values in the upper right portion of the table are the average numbers of substitutions per sequence position (evolutionary distances), adjusted as described for multiple substitutions at individual positions and calculated for 1,449 positions which could be aligned unambiguously. The values in the lower left portion of the table are the levels of fractional nucleotide identity between the sequences. HF strains: HF565, HF568-1, HF568-2, HF639-2, HF642, and HF652.
FIG. 2.
Phylogenetic relationships between Anan, HF, and other Ehrlichia strains based on 16S rRNA gene sequence comparisons. HF strains included HF565, HF568-1, HF568-2, HF639-2, HF642, and HF652.
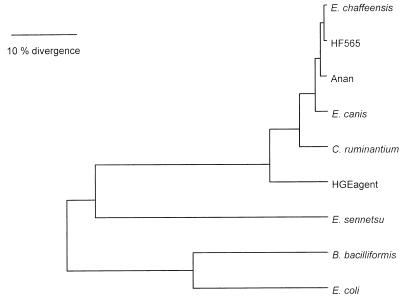
Of the 409 deduced amino acid sequences of GroEL compared, all six HF strains had the identical amino acid sequence (Table 2). All strains belonged to E. canis genogroup and were far from the E. phagocytophila (the HGE agent) or E. sennetsu genogroup (Table 2 and Fig. 3). HF strains were closest to E. chaffeensis, followed by the Anan strain and E. canis in the dendrogram (Fig. 3). Between HF strains and E. chaffeensis, four amino acids were different, and between HF and Anan strains six amino acids were different (Table 2).
TABLE 2.
Levels of similarity and evolutionary distances between amino acid sequences of groEL proteins
| Organism(s) | Amino acid substitution distances and amino acid sequence similaritya
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| HF strains | Anan | E. chaffeensis | E. canis | C. ruminantium | HGE agent | E. sennetsu | B. bacilliformis | E. coli | |
| HF strains | 0.0202 | 0.0100 | 0.0288 | 0.0714 | 0.1485 | 0.6106 | 0.6394 | 0.7243 | |
| Anan | 98.54 | 0.0274 | 0.0462 | 0.0888 | 0.1659 | 0.6280 | 0.6568 | 0.7417 | |
| E. chaffeensis | 99.02 | 97.56 | 0.0276 | 0.0702 | 0.1473 | 0.6094 | 0.6382 | 0.7231 | |
| E. canis | 97.32 | 95.85 | 97.07 | 0.0760 | 0.1531 | 0.6152 | 0.6440 | 0.7289 | |
| C. ruminantium | 93.17 | 91.72 | 93.66 | 92.68 | 0.1629 | 0.6250 | 0.6538 | 0.7387 | |
| HGE agent | 86.83 | 85.37 | 86.34 | 86.10 | 84.63 | 0.6693 | 0.6981 | 0.7830 | |
| E. sennetsu | 58.29 | 56.83 | 57.80 | 57.56 | 57.80 | 54.39 | 0.7054 | 0.7903 | |
| B. bacilliformis | 57.18 | 55.72 | 56.69 | 57.69 | 55.83 | 54.85 | 54.22 | 0.3623 | |
| E. coli | 53.16 | 51.70 | 53.16 | 52.67 | 52.91 | 50.24 | 51.82 | 71.46 | |
The values in the upper right portion of the table are the average numbers of substitutions per sequence position (evolutionary distances) calculated for 409 positions which could be aligned unambiguously. The values in the lower left portion of the table are the levels of fractional amino acid identity between sequences. HF strains: HF565, HF568-1, HF568-2, HF639-2, HF642, and HF652.
FIG. 3.
Phylogenetic relationships generated with UPGMA based on an alignment of the first 409 amino acid sequences of GroEL of HF and Anan strains and other members of the tribe Ehrlichieae.
Antibody reactivity.
Antibody titers against E. muris were examined by the IFA test in the sera collected on day 20 p.i. from mice inoculated subcutaneously. The titers of sera from mice inoculated with all I. ovatus isolates were comparable with three strains of E. muris: AS145, I-268, and NA-1 (Table 3). There was no cross-reactivity of these sera with E. sennetsu or the HGE agent. Because E. muris, E. chaffeensis, E. canis, VHE, and E. ewingii are antigenically highly cross-reactive to each other (1, 2, 4, 12, 14, 21, 23–26), this result is in agreement with the 16S rRNA gene and GroEL sequencing data showing that all I. ovatus isolates examined belong to the E. canis genogroup rather than the E. phagocytophila or E. sennetsu genogroup.
TABLE 3.
Characteristics of Ehrlichia strains isolated from ticks and wild mice
| Ehrlichia strain | Tick or mouse source | Collection site | Lethality of BALB/c (no. of mice dead/total no.)a | Relative spleen size (g)b | Antibody titer to E. murisc |
|---|---|---|---|---|---|
| HF565 | I. ovatus | Fukushima | 5/5 | 0.99 ± 0.35 | 3.20 ± 0.75 |
| HF568-1 | I. ovatus | Fukushima | 5/5 | 0.87 ± 0.26 | 2.40 ± 1.36 |
| HF568-2 | I. ovatus | Fukushima | 5/5 | 1.17 ± 0.38 | 2.80 ± 1.47 |
| HF639-2 | I. ovatus | Fukushima | 5/5 | 1.21 ± 0.41 | 2.40 ± 1.36 |
| HF642 | I. ovatus | Aomori | 5/5 | 0.96 ± 0.15 | 3.00 ± 0.63 |
| HF652 | I. ovatus | Aomori | 5/5 | 1.11 ± 0.45 | 2.40 ± 0.80 |
| Anan | I. ovatus | Aomori | (0/5) | (2.68 ± 0.22) | (3.00 ± 0.63) |
| E. muris | E. kageus | Tokushima | 0/5 | 1.96 ± 0.34 | 2.80 ± 0.75 |
| I-268 | A. speciosus | Tokyo | 0/5 | 2.41 ± 0.32 | 3.67 ± 1.10 |
| NA-1 | H. flava | Aichi | 0/5 | 2.81 ± 0.51 | 3.60 ± 1.02 |
No. dead/total mice inoculated intraperitoneally with isolates. The mice were observed by day 20 postinoculation.
Spleen weights, in grams per 100 g of body weight, were determined on days 8 to 10 postinoculation. Values are the means ± the standard deviation (n = 5). A value of 0.42 ± 0.04 g/100 g of body weight (n = 5) was determined for the control mouse.
Samples were inoculated subcutaneously, and the sera were collected at day 20. Values (1/10 log2) are the means ± the standard deviation (n = 5).
Mouse pathogenicity.
When ca. 10% spleen of the homogenate of mice containing each strain was intraperitoneally inoculated, starting ca. day 7 p.i., all mice inoculated with six HF strains developed clinical signs of ruffled fur, inactivity, anorexia, dehydration, and weight loss, and they died from days 8 through 10 p.i. (Table 3). The lethality of the HF strains was dependent on the dosage and route of inoculation. With a dilution of up to 102 of the inoculum, all mice inoculated intraperitoneally died at days 8 to 10 p.i., but at a 104 dilution all mice survived. With the subcutaneous route, two of five mice died on days 8 to 10 p.i., but with >10-fold dilutions of inoculum all mice survived for more than 20 days p.i., at which time the experiment was terminated. The mice inoculated intraperitoneally with the Anan strain without dilution developed mild clinical signs similar to those inoculated with the E. muris strains (12, 13). Mice inoculated subcutaneously with the Anan isolate did not develop any significant clinical signs. None of mice inoculated intraperitoneally with the three strains of E. muris AS145, I-268, or NA-1 died during the 20-day p.i. period (Table 3). By PCR, ehrlichial DNAs present in 10% of the spleen homogenates were similar among all strains, including E. muris strains, except the Anan strain. The Anan strain in the spleen was approximately 1/103 of the HF565 strain in the spleen. Lack of death with the Anan strain was, however, not caused by small amounts of organisms in the inoculum. When the Anan strain at the same DNA level as that of the HF565 strain was intraperitoneally inoculated into mice, it did not kill them (data not shown).
At necropsy, mice inoculated with all strains developed splenomegaly. When spleen sizes were compared among intraperitoneally inoculated mice on days 8 to 10 p.i., the degrees of splenomegaly were greater in the following order: I-268, NA-1, E. muris (AS145) > Anan > all HF strains (Table 3). The mean relative spleen size of uninfected mice was 0.42 ± 0.04 (n = 5); therefore, there were seven- to twofold increases in the spleen size. The spleens were dull dark red and tender in HF strain-inoculated mice. In contrast, the tissues were less dark and firm in mice inoculated with Anan or E. muris strains.
Light and electron microscopy of organs of infected mice.
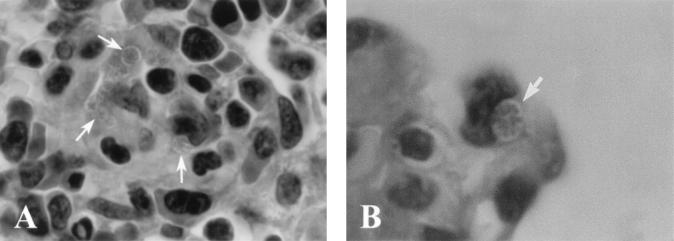
By light microscopy of paraffin sections numerous dark-blue (Giemsa)- or purple (hematoxylin-eosin)-stained cocci in round inclusions (morulae) or by light microscopy of Epon-embedded 1-μm sections light-blue (toluidine blue)-stained organisms could be visualized. These organisms were frequently observed in the cytoplasms of mononuclear cells in the lumen and along the wall of small blood vessels in the spleen, liver, thymus, lung, bone marrow, and large intestine of mice intraperitoneally inoculated with strain HF565 on days 7 and 9 p.i. (Fig. 4). Prominent neutrophilic inflammatory cell infiltration was not evident in any of these organs. In the spleen, the red pulp was expanded, the white pulp was disorganized, and the follicles were not significantly activated. Thymic severe lymphoid depletion as seen in E. risticii-infected mice (27) was not evident in the mice infected with HF strain. The liver showed severe necrosis, especially around the central veins. Morulae were not seen in the necrotic area. In contrast, morulae were not detectable in the spleen of Anan strain-infected mice on days 10 and 15 p.i. The spleens of mice inoculated with Anan strain had increased cellularlity and well-developed follicles even at day 7 p.i. (data not shown).
FIG. 4.
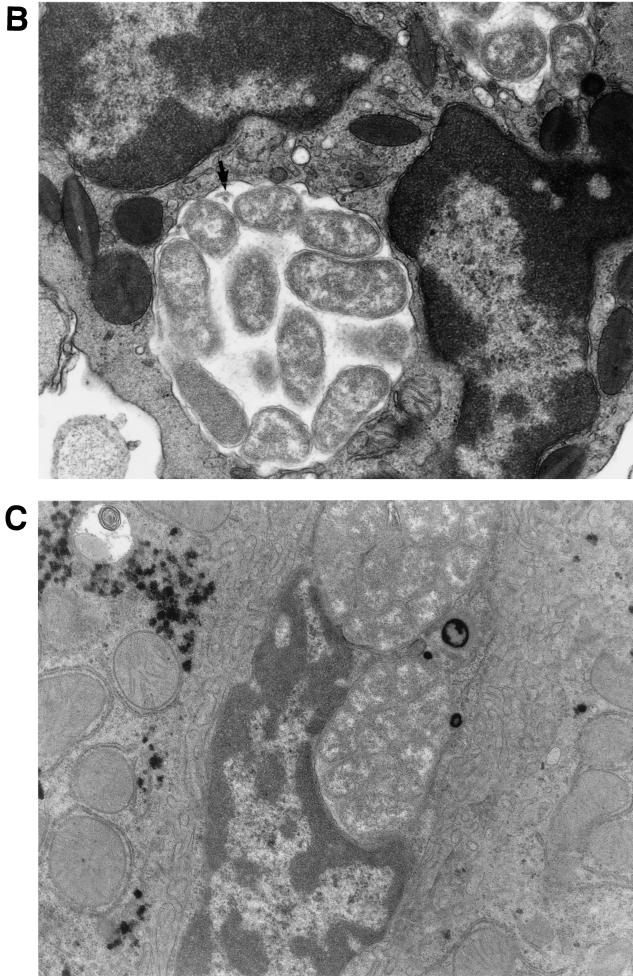
Light micrographs of paraffin-embedded section of the thymus (A) and the lung (B) from the mice intraperitoneally inoculated with HF565 strain at day 9 p.i. Note several morulae packed with many ehrlichial organisms (colonies of ehrlichiae) along the capillary (arrows) (A) [magnification, ×1,070]) and in the capillary of the alveolar wall (arrows) (B) [magnification, ×1,700]). Hemtoxylin and eosin staining was used.
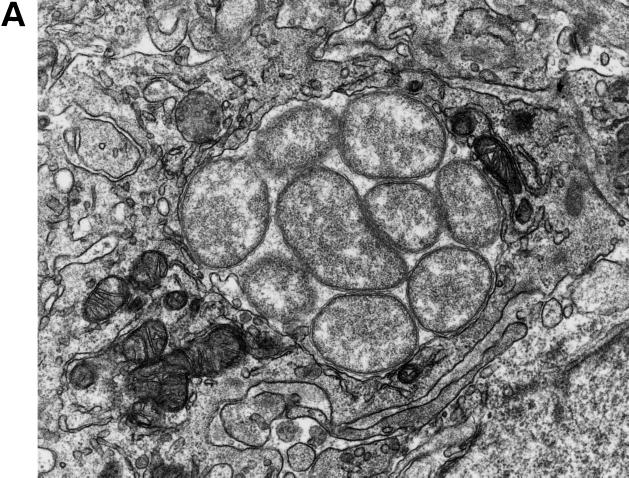
Under an electron microscope, numerous ehrlichial organisms were detected in membrane-bound inclusions in the cytoplasm of monocytes, macrophages, and eosinophils but not in neutrophils in the red pulp of the spleen or in Kupffer cells in the liver from mice inoculated with strain HF565 (Fig. 5). The organisms were generally round, but some were pleomorphic and variable in size, ranging from 0.4 to 1.0 μm in length and 0.2 to 0.7 μm in diameter. Each organism was bound by two membranes, and the outer membranes were ruffled (Fig. 5). Significant amounts of ribosomes and a fine meshwork of DNA strands were evident (Fig. 5). As noted previously in E. canis inclusions in DH82 cells (23) and in various E. chaffeensis strains (25), the intramorular matrix contained tiny fibrillar materials in some morulae (Fig. 5). Intramorular tubules of approximately 25 nm in diameter, which are found in the inclusions of several strains of E. chaffeensis in DH82 cells (25), were occasionally seen (one is indicated Fig. 5B). As noted in E. chaffeensis (25), occasional association of mitochondria with ehrlichial inclusions was seen (Fig. 5A and B). These findings are in agreement with the 16S rRNA and groEL gene sequencing data showing that HF565 strain belongs to E. canis genogroup. Ehrlichial organisms were not detectable in the spleens of Anan strain-infected mice by electron microscopy.
FIG. 5.
Electron micrographs of morulae of HF565 in the cytoplasm of a monocyte (A) and an eosinophil (B) in the blood vessel in the spleen and in a Kupffer cell in the liver (C) from the mouse at day 7 p.i. Note the numerous pleomorphic coccobacilli enveloped in two layers of membranes embedded in a fine filamentous matrix in the membrane-bound inclusion in panel A. An intramorular tubule is indicated by the arrow in panel B. The inclusions are tightly packed with ehrlichiae without intramorular space in the Kupffer cells adhered to the endothelial layer lining the sinusoid. Magnifications: ×24,200 (A), ×21,000 (B), and ×16,400 (C).
DISCUSSION
16S rRNA genes and GroEL amino acid sequences, antigenic cross-reactivities, and ultrastructural features indicate that our I. ovatus isolates in Japan belong to the E. canis genogroup and are most closely related to E. chaffeensis. E. chaffeensis has been found so far primarily in Amblyomma americanum and, less frequently, in Dermacentor variabilis in the United States (3). A small number of the I. scapularis ticks tested were negative for E. chaffeensis (3). Most Ehrlichia spp. found in Ixodes spp. in the United States and Europe are Ehrlichia spp. belonging to E. phagocytophila, E. equi, and the HGE agent genogroup (24). However, a partial 16S rRNA gene sequence identical to that of E. muris was recently found in Ixodes persulcatus ticks in Perm, Russia (22). Therefore, Ixodes spp. can be naturally infected by two distinct ehrlichial genogroups. I. ovatus is a common tick found throughout Japan (38). The adult-stage tick bites humans and large animals, and the nymphal- and larval-stages tick bite small mammals such as rodents (38). Whether, like E. muris, wild mice are reservoirs of these I. ovatus isolates is yet unknown. Two species of ticks have so far been found infected with Ehrlichia spp. in Japan. We previously isolated E. muris from H. flava ticks (14). According to Fujita and Watanabe (7), the ehrlichial infection rate for I. ovatus (16 of 439 ticks examined) is higher than for H. flava (0 of 351 ticks examined).
By electron microscopy HF565 organisms, which belong to the monocytotropic ehrlichiae, were detected in eosinophils. It is unlikely that I. ovatus was coinfected with granulocytotropic Ehrlichia sp., since mouse neutrophils were not infected and our serologic and gene sequencing data did not reveal any contamination of granulocytotropic Ehrlichia sp. in the inoculum. Ehrlichial organisms were previously seen in neutrophils in the blood of HME patients (16, 19). Therefore, although this is a rare event and monocytes are the primary hosts, in severe infections some granulocytes are infected with the E. canis genogroup. Whether this occurs with granulocytes from uninfected healthy hosts in vitro or due to alterations that occur in granulocytes in infected hosts is unknown.
Ehrlichia spp. can be subdivided into three genetically distinct groups (genogroups). Two ehrlichial genogroups have been isolated in Japan to date. E. sennetsu is the first ehrlichial organism discovered in Japan. It was isolated from the blood of patients with “Hyuga fever” or “Kagami fever,” which was endemic in Kyushu, Southern Japan, in the 1950s (8, 17). SF agent, which belongs to E. sennetsu genogroup (36), had originally been isolated in Japan in 1962 from Stellantchasmus falcatus metacercarial parasites on gray mullet fish in Kyushu (9). The second genogroup organisms isolated were the E. muris strains (12, 14) and I. ovatus strains described here. The third E. phagocytophila genogroup has not yet been identified in Japan.
HF strains, when inoculated intraperitoneally, were more virulent in immunocompetent laboratory mice than any other ehrlichial strains we examined. Because all six strains obtained at different times in different geographic regions had similar levels of virulence, it is unlikely that the viruses or other agents are contaminated in all of these isolates. We have tried to cultivate HF and Anan strains by using various cell lines. We have not cultivated any of them yet, but we have not found any contamination by bacteria, viruses, or parasites in these isolates. Haemobartonella or Eperythrozoon spp. were not detectable on a blood smear or by a PCR based on the 16S rRNA gene sequences we determined (28). Therefore, it is unlikely that other contaminating agents are responsible for the virulence of the HF strains.
Of significance is the striking difference in mouse pathogenicity between HF strains and the Anan strain, despite the closeness of their 16S rRNA gene sequences. Massive ehrlichial proliferation was evident in immunocompetent mice with HF stains, whereas proliferation of the Anan strain-like E. muris appears to be held in check. As previously noted with E. muris infection of mice (13), this may be due to active immune stimulation with the Anan strain, as evidenced by increased cellularlity and follicle stimulation in the spleen. On the other hand, with lethal dosages of HF strain, the spleen structure was disorganized and follicle stimulation was not evident. With the HF strain widespread prominent liver necrosis similar to that previously noted in fatal HME (D. H. Walker, J. P. Taylor, J. S. Blie, and C. Dearden, Abstr. 89th Annu. Meet. Am. Soc. Microbiol. 1989, abstr. D76, p. 95, 1989) or in SCID/beige mice experimentally infected with E. chaffeensis (37) was seen. In connection with this observation, one of the characteristic laboratory findings of HME as well as with HGE is increased liver enzyme activities. What gene products of HF strain are responsible for this fulminant infection in immunocompetent mice is unknown. These two isolates, i.e., HF and Anan, the closest relatives of E. chaffeensis, may provide a valuable comparative immunocompetent mouse disease model for understanding pathogenesis and roles of immune responses in ehrlichiosis caused by the E. canis genogroup.
ACKNOWLEDGMENTS
We thank Fumihiko Mahara for kindly providing accommodations for tick collection in Anan City, Tokushima, Japan.
A part of the study was supported by the grant RO1 AI30010 from the National Institutes of Health.
REFERENCES
- 1.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Greene C E, Jones D C, Dawson J E. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int J Syst Bacteriol. 1992;42:299–302. doi: 10.1099/00207713-42-2-299. [DOI] [PubMed] [Google Scholar]
- 3.Anderson B E, Sims K G, Olson J G, Childs J M, Piesman J F, Happ C M, Maupin G O, Johnson B J B. Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1993;49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- 4.Buller R S, Arens M, Hmiel S P, Paddock C, Sumner J W, Rikihisa Y, Unver A, Graudreauls-Keener M, Marinian F A, Liddell A M, Schmulewitz N, Storch G. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. New Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 5.Chen S-M, Dumler J S, Bakken J S, Walker D. Identification of granulocytic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:585–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewing S A, Dawson J E, Kokan A A, Barker R W, Warner C K, Panciera R J, Fox C J, Kocan K M, Blouin E F. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Ambryomma americanum (Acari: Ixodidae) J Med Entomol. 1995;32:368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- 7.Fujita H, Watanabe Y. Ehrlichial organisms isolated from Ixodes ovatus ticks and field rodents in Japan. Ann Rep Ohara Hosp. 1994;37:13–17. . (In Japanese.) [Google Scholar]
- 8.Fukuda T, Kitao T, Keida Y. Studies on the causative agent of “Hyuganetsu” disease. I. Isolation of the agent and its inoculation trial in human beings. Med Biol. 1954;32:200–209. . (In Japanese.) [Google Scholar]
- 9.Fukuda T, Sasahara T, Kito T. Studies on the causative agent of “Hyuganetsu” disease. XI, Characteristics of rickettsia-like organism isolated from metacercaria of Stellantchasmus falcatus. J Jpn Assoc Infect Dis. 1973;53:713–716. doi: 10.11150/kansenshogakuzasshi1970.47.474. . (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 10.Goodman J L, Nelson C, Vitale B, Madigan J E, Dumler J S, Kurtti T J, Munderloh U G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. [DOI] [PubMed] [Google Scholar]
- 11.Guerrero A, Fishbein D, Mesa E, Escudero R. Human infection by Ehrlichia canis in Spain. Med Clin. 1991;96:236–237. [PubMed] [Google Scholar]
- 12.Kawahara M, Suto C, Rikihisa Y, Yamamoto S, Tsuboi Y. Characterization of ehrlichial organisms isolated from a wild mouse. J Clin Microbiol. 1993;31:89–96. doi: 10.1128/jcm.31.1.89-96.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawahara M, Suto C, Shibata S, Futohashi M, Rikihisa Y. Impaired antigen-specific responses and enhanced polyclonal stimulation in mice infected with Ehrlichia muris. Microbiol Immunol. 1996;40:575–581. doi: 10.1111/j.1348-0421.1996.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 14.Kawahara M, Tadahiko I, Suto C, Shibata S, Rikihisa Y, Hata K, Hirai K. Comparison of Ehrlichia muris strains isolated from wild mice and ticks and serologic survey of humans and animals with E. muris as antigen. J Clin Microbiol. 1999;37:1123–1129. doi: 10.1128/jcm.37.4.1123-1129.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lockhart J M, Davidson W R, Stallknecht D E, Dawson J E, Howerth E W. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681–1686. doi: 10.1128/jcm.35.7.1681-1686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda K, Markowitz N, Hawley R C, Ristic M, Cox D, McDade J E. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:856–853. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 17.Misao T, Kobayashi Y. Studies on infectious mononucleosis (glandular fever). I. Isolation of etiologic agent from blood, bone marrow and lymph node of a patient with infectious mononucleosis by using mice. Tokyo Iji Shinshi. 1954;71:683–686. (In Japanese.) [Google Scholar]
- 18.Moraris J D, Dawson J E, Greene C, Filipe A R, Galhardas L C, Bacellar F. First European cases of ehrlichiosis. Lancet. 1991;338:633–634. doi: 10.1016/0140-6736(91)90644-5. [DOI] [PubMed] [Google Scholar]
- 19.Paddock C D, Sumner J W, Shore M, Bartley D C, Elie D C, McQuade J G, Martin C R, Goldsmith C S, Childs J E. Isolation and characterization of Ehrlichia chaffeensis strains from patients with fatal ehrlichiosis. J Clin Microbiol. 1997;35:2496–2502. doi: 10.1128/jcm.35.10.2496-2502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pancholi P, Kolbert C P, Mitchell P, Reed K D, Dumler J S, Bakken J S, Telford III S R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 21.Perez M, Rikihisa Y, Bohi W. Ehrlichia canis-like agent isolated from a man in Venezuela: antigenic and genetic characterization. J Clin Microbiol. 1996;34:2133–2139. doi: 10.1128/jcm.34.9.2133-2139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravyn M D, Korenberg E I, Oeding J A, Kovalevskii Y V, Johnson R C. Monocytic Ehrlichia in Ixodes persulcatus ticks from Perm, Russia. Lancet. 1999;353:722–723. doi: 10.1016/s0140-6736(98)05640-2. [DOI] [PubMed] [Google Scholar]
- 23.Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rikihisa Y. Ehrlichiae. In: Kazar J, Tomen R, editors. Proceedings of the 5th International Symposium on Rickettsiae and Rickettsial Diseases Slovak Academy of Sciences, Bratislava, Slovak Republic. Bratislava, Slovak Republic: International Society of Rickettsiae and Rickettsial Diseases; 1996. pp. 272–286. [Google Scholar]
- 25.Rikihisa Y. Clinical and biological aspects of infections caused by Ehrlichia chaffeensis. Microb Infect. 1999;3:1–10. doi: 10.1016/s1286-4579(99)80053-7. [DOI] [PubMed] [Google Scholar]
- 26.Rikihisa Y. Ehrlichiae of veterinary importance. In: Raoult D, Brouqui P, editors. Rickettsiae and rickettsial diseases at the turn of the third millenium. Paris, France: Elsevier; 1999. pp. 393–405. [Google Scholar]
- 27.Rikihisa Y, Johnson G J, Burger C J. Reduced immune responsiveness and lymphoid depletion in mice infected with Ehrlichia risticii. Infect Immun. 1987;55:2215–2222. doi: 10.1128/iai.55.9.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rikihisa Y, Kawahara M, Wen B, Kociba G, Fuerst P, Kawamori F, Suto C, Shiba S, Futohashi M. Western immunoblot analysis of Haemobartonella muris and comparison of 16S rDNA sequences of H. muris. H. felis, and Eperythrozoon suis. J Clin Microbiol. 1997;35:823–829. doi: 10.1128/jcm.35.4.823-829.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rikihisa Y, Zhi N, Wormser G, Wen B, Horowitz H W, Hechemy K E. Direct isolation and cultivation of human granulocytic ehrlichia from a human patient. J Infect Dis. 1997;175:210–213. doi: 10.1093/infdis/175.1.210. [DOI] [PubMed] [Google Scholar]
- 30.Sumner J W, Nicholson W L, Massung R F. PCR Amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Telford S R, III, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhaa I J, MacLean J D, Greene C R, Fishbein D B. A case of human ehrlichiosis acquired in Mali: clinical and laboratory findings. Am J Trop Med Hyg. 1992;46:161–164. doi: 10.4269/ajtmh.1992.46.161. [DOI] [PubMed] [Google Scholar]
- 33.Walker D H, Dumler J S. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss E, Dasch G A, Williams J C, Kang Y-H. Biological properties of the genus Ehrlichia: substrate utilization and energy metabolism. In: Williams J C, Kakoma I, editors. Ehrlichiosis: a vector-borne disease of animals and humans. Boston, Mass: Kluwer Academic Publishers; 1990. pp. 59–67. [Google Scholar]
- 35.Wen B, Rikihisa Y, Mott J, Fuerst P A, Kawahara M, Suto S. Ehrlichia muris sp. nov., a new species of Ehrlichia, identified on the basis of 16S rRNA base sequence, serological, morphological, and biological characteristics. Int J Syst Bacteriol. 1995;45:250–254. doi: 10.1099/00207713-45-2-250. [DOI] [PubMed] [Google Scholar]
- 36.Wen B, Rikihisa Y, Yamamoto S, Kawabata N, Fuerst P A. Characterization of SF agent, an Ehrlichia sp. isolated from the fluke Stellantchasmus falcatus, by 16S rRNA base sequence, serological, and morphological analyses. Int J Syst Bacteriol. 1996;46:149–154. doi: 10.1099/00207713-46-1-149. [DOI] [PubMed] [Google Scholar]
- 37.Winslow G M, Yager E, Shilo K D, Collins N, Chu F K. Infection of the laboratory mouse with the intracellular pathogen Ehrlichia chaffensis. Infect Immun. 1998;66:3892–3899. doi: 10.1128/iai.66.8.3892-3899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi N, Kitaoka S. Ixodes ovatus Neumann. In: Ehara S, editor. Illustrations of the mites and ticks of Japan. Zenkoku Nosonkyoika Kyokai, Japan. (In Japanese.) 1980. pp. 149–155. [Google Scholar]