Abstract
Plant water transport and its molecular components including aquaporins are responsive, across diverse time scales, to an extremely wide array of environmental and hormonal signals. These include water deficit and abscisic acid (ABA) but also more recently identified stimuli such as peptide hormones or bacterial elicitors. The present review makes an inventory of corresponding signalling pathways. It identifies some main principles, such as the central signalling role of ROS, with a dual function of aquaporins in water and hydrogen peroxide transport, the importance of aquaporin phosphorylation that is targeted by multiple classes of protein kinases, and the emerging role of lipid signalling. More studies including systems biology approaches are now needed to comprehend how plant water transport can be adjusted in response to combined stresses.
Introduction
As elaborate terrestrial living organisms, vascular plants have to maintain their water status as tightly as possible and throughout their life cycle (Steudle, 1992; Davies and Bennett, 2015). Particularly challenging processes include the expansion/growth of underground and aerial parts and the desiccation, imbibition, and germination of seeds and pollen. Most often, these processes occur under variable environmental conditions, including changes to water availability and temperature in the soil and atmosphere, which have marked impacts on the plant water status. The latter is primarily maintained through tight stomatal regulation controlling transpirational water flow (Tardieu et al., 2015). Osmotic adjustment and regulation of water permeability (hydraulics), which both contribute to water potential gradients and water movement intensity, provide a complementary and finely tuned regulation of plant water relations at the cell and tissue levels. In support of these general principles, a large body of studies has shown that plant water transport and its molecular components are responsive, across diverse time scales, to an extremely wide array of environmental and hormonal signals (Chaumont et al., 1998; Maathuis et al., 2003; Aroca et al., 2012; Maurel et al., 2015). Thus, plant water relations are under constant adjustment virtually in any physiological context.
Plant aquaporins, which are present in the plasma and intracellular membranes of most plant cells, play a central role in these processes by ensuring cell-to-cell water transport and to a lesser extent, single-cell osmotic regulation (Tyerman et al., 1999; Chaumont and Tyerman, 2014; Maurel et al., 2015). More generally, aquaporins have emerged as central membrane targets of environmental and hormonal signaling pathways acting on plant water relations. In addition, aquaporins are highly abundant proteins amenable to biochemical (proteomic) and cell biological approaches. Besides studies in the plant, their function and regulation can be easily studied after functional reconstitution (proteoliposomes) or heterologous expression (yeast, Xenopus oocytes). Thus, aquaporins are particularly relevant and convenient entry points for bottom-up dissections of plant signaling cascades.
Due to the central position of aquaporins in multiple hormonal and environmental responses determining the plant water status, the first aim of the present review was to inventory corresponding signaling pathways. Beyond the diversity of these pathways, a knowledge network with common or converging paths is now emerging. Conversely, the dedicated study of signaling pathways per se seems to reveal regulation states/contexts that escaped classical physiological analyses of plant water relations. A particularly challenging question is to understand how plants are able to perceive and respond to combined stresses.
Water deficit, abscisic acid, and related signals
Drought
Abscisic acid-dependent pathways
Whereas physiological links among drought stress, abscisic acid (ABA), and plant water transport have been identified for some time, it is only recently that the signaling mechanisms involved have started to be elucidated. Studies on epidermal peels from wild-type and aquaporin knockout Arabidopsis (Arabidopsis thaliana) plants have revealed that the plasma membrane aquaporin Plasma membrane Intrinsic Protein (PIP) 2;1 (AtPIP2;1) is required for ABA-induced stomatal closure, but dispensable during light- and CO2-induced stomatal movements (Grondin et al., 2015). Consistent with this, isolated guard cell protoplasts responded to exogenous ABA by a two-fold increase in osmotic water permeability that was strictly dependent on the functionality of AtPIP2;1. This aquaporin, which is known to be permeable to hydrogen peroxide (H2O2; Dynowski et al., 2008), was also required for ABA-induced accumulation of H2O2 in the guard cell interior (Rodrigues et al., 2017). These studies establish a dual water- and H2O2-transport function for guard cell plasma membrane aquaporins as players and targets of signaling processes triggering stomatal closure (Grondin et al., 2015; Rodrigues et al., 2017). More specifically, in vitro phosphorylation assays and transgenic plant analysis led to a model whereby Snf1-related protein kinase 2.6 (SnRK2.6)/Open stomata 1 (OST1), a protein kinase that plays a pivotal role in ABA signaling, is able to phosphorylate AtPIP2;1 at a specific cytosolic Ser residue (Ser121), with this phosphorylation being necessary for proper ABA-induced stomatal closure. SnRK2.6/OST1 also activates plasma membrane NADPH oxidases, which feed superoxide dismutases for production of apoplastic H2O2 (Maurel et al., 2016; Figure 1A). While the above-mentioned studies were performed on epidermal peels of wild-type and pip2;1 plants, we are concerned that intact leaves of a quadruple pip1;1 pip1;2 pip2;1 pip2;2 mutant showed normal responses to ABA (Ceciliato et al., 2019). We hypothesize that integration of guard cells within whole leaf physiology can compensate for the defects captured in isolated guard cells or protoplasts. We also note that, in contrast to stomata, ABA can downregulate aquaporin activity in bundle-sheaths (Shatil-Cohen et al., 2011) and roots (Rosales et al., 2019). Whereas the canonical ABA signaling machinery formed by RCAR/PYR/PYL receptors, protein Phosphatase 2C co-receptors acting on Class 2 Sucrose-NonFermenting Protein kinases (SnRK2s) is likely to be involved, in roots in particular (Rosales et al., 2019), the exact components providing these hydraulic regulations remain as yet unknown. Aquaporins and water transport can also be under ABA control through more indirect pathways. In Arabidopsis, ABA induced the expression of the membrane protein Tryptophan-rich sensory protein/translocator (TPSO), which physically interacts with AtPIP2;7 in the early secretory pathway, to trigger its degradation through the autophagic pathway, thereby reducing its water transport activity at the plasma membrane (Hachez et al., 2014b). In rice (Oryza sativa), the gene encoding a leucine-rich repeat receptor-like kinase (RLK) named Leaf Panicle 2 (LP2) is downregulated by drought and ABA, and acts as a negative regulator of drought response (Wu et al., 2015). Its action involves a molecular interaction at the plasma membrane with the drought-responsive aquaporins OsPIP1;1, OsPIP1;3, and OsPIP2;3.
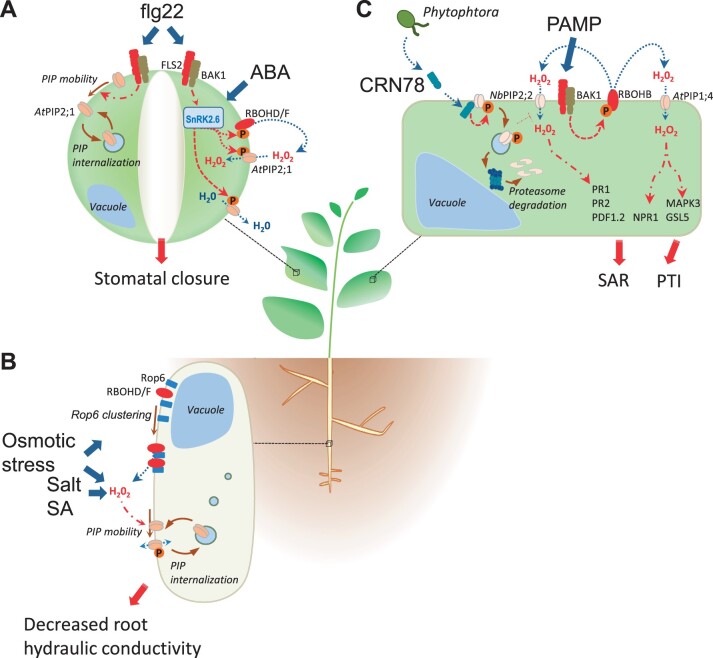
Figure 1.
Cellular responses to biotic or abiotic stimuli involving H2O2 and aquaporins. A, In guard cells (right), the SnRK2.6 protein kinase is a key component of ABA- and flg22-induced stomatal closure. flg22 is a PAMP recognized by the plasma membrane FLS2 receptor, which acts in concert with BAK1. Upon activation, SnRK2.6 phosphorylates two NADPH oxidases, RBOHD and RBOHF, and the AtPIP2;1 aquaporin. The latter facilitates the influx of H2O2 and the efflux of water, thereby promoting stomatal closure (Grondin et al., 2015; Rodrigues et al., 2017). fgl22 also enhances the lateral mobility of AtPIP2;1 at the guard cell plasma membrane (left), thereby promoting its internalization (Cui et al., 2021). B, The root hydraulic conductivity (Lpr) is strongly decreased in response to abiotic stresses such as osmotic and salt stress, or hormones such as SA. All these stimuli act through accumulation of H2O2 which lowers AtPIP2;1 phosphorylation, alters its plasma membrane mobility, and promotes its internalization (Boursiac et al., 2008; Wudick et al., 2015). In contrast, PIP phosphorylation promotes its trafficking to the plasma membrane. The increased apoplastic H2O2 production observed in response to an osmotic stress is mediated through the clustering in nanodomains of the Rop6 together with the RBOHD and F NADPH oxidases (Martiniere et al., 2019; Smokvarska et al., 2020). C, In mesophyll cells, PAMPs are perceived by plasma membrane receptors, which trigger, in combination with BAK1 co-receptor and other nonrepresented cellular components, the activation of RBOHB NADPH oxidase and, as a consequence, apoplastic accumulation of H2O2. The AtPIP1;4 aquaporin plays a key role in facilitating the diffusion of apoplastic H2O2 into the cytoplasm (Tian et al., 2016). The increase in cytoplasmic H2O2 concentration ultimately leads to activation of key proteins (Pathogenesis-Related protein 1 [PR1]; PR2; Plant Defensin 1.2; NPR1; Mitogen-Activated Protein Kinase 3; Glucan Synthase-like 5) involved in SAR or pathogen triggered immunity (PTI), as indicated. The figure also shows how the CRN78 effector of Phytophthora can penetrate the plant cell where it interacts with phosphorylated NbPIP2;2, to induce its internalization and further degradation, thereby preventing its role in PAMP signaling (Ai et al., 2021). Thick blue arrows and thick red arrows indicate initial effects of stimuli and their output responses, respectively; thin dotted blue line arrows refer to diffusion of the indicated molecules; red dotted line arrows represent protein phosphorylation by indicated protein kinases; red dashed-dotted line arrows and red dashed-dotted line blunt arrows describe indirect activation and inactivation pathways, respectively; brown line arrows indicate movements of proteins or membrane compartments.
ABA-independent pathways
Drought can also exert ABA-independent effects on aquaporin expression and function. For instance, several aquaporin gene promoters harbor Dehydration Responsive Elements (DRE) that are targetted by DRE binding transcription factors (Rae et al., 2011). Other classes of transcription factors (e.g. AP2/EREBP and ASR1) also confer drought-dependent expression on aquaporin genes (Ricardi et al., 2014; Zhu et al., 2014). Furthermore, induction under severe drought of ubiquitin-conjugating enzyme UBC32 and RING-type E3 ligase, which both interact with AtPIP2;1 and AtPIP2;2, leads to ubiquitination and degradation of these aquaporins, thereby preventing their negative impact on drought tolerance (Chen et al., 2021).
Functional interaction between an aquaporin (RhPIP2;1) and a membrane-tethered MYB protein named RhPTM was recently described in rose (Zhang et al., 2019b). In brief, dehydration stress-induced C-terminal phosphorylation of RhPIP2;1 at Ser273, which in turn led to membrane release and nuclear activation of RhPTM. RhPTM itself acts as a transcription factor with general growth inhibitory effects. This landmark work establishes an unexpected link between hydraulics and a transcriptionally determined metabolic control of growth that promotes water deficiency tolerance. Yet, the protein kinases/phosphatases that act on Ser273 of RhPIP2;1 and their drought-dependent activation/inhibition mechanisms remain to be identified. Similarly, some PIP aquaporins of Arabidopsis (e.g. AtPIP2;4) show enhanced phosphorylation as early as 5 min after exposure to a hypertonic stress (0.3 M mannitol; Stecker et al., 2014).
Cell biological approaches are now developed to address associated regulatory mechanisms. An improved super-resolution microscopic technique called single particle tracking Photo-Activated Localization Microscopy (sptPALM) was used to monitor the lateral mobility of proteins at the plasma membrane (LTi6a and AtPIP2;1) and tonoplast (AtTIP1;1; Hosy et al., 2015). In contrast to the other membrane proteins, AtPIP2;1 showed a low lateral mobility, was confined to membrane nanodomains and its mobility was enhanced upon a hyperosmotic stress. sptPALM further showed how reactive oxygen species (ROS) control specific diffusion and nano-organization of membrane cargo proteins (Martiniere et al., 2019). In particular, ROS initiated clustering of AtPIP2;1 and its removal from the plasma membrane. This process is achieved in part by clathrin-mediated endocytosis. It is linked to further aquaporin internalization and transfer into the endocytic pathways (Zwiewka et al., 2015). In the longer term, the same aquaporins are subjected to ubiquitination and further degraded (Lee et al., 2009) in a phosphorylation-dependent manner (Chen et al., 2021). In addition, a role of autophagic pathways was recently uncovered in Medicago truncatula under water deficit (Li et al., 2020b). A dehydrin (MtCAS31) was shown to act as a cargo receptor for the MtPIP2;7 aquaporin. MtCAS31 further promoted complex formation with the autophagy-related protein ATG8a to facilitate MtPIP2;7 degradation. This, in turn, reduced water loss under water deficit and improved drought tolerance (Li et al., 2020b). The possible relation of this mechanism with the ABA-dependent TPSO pathway (Hachez et al., 2014b) mentioned above is not yet clear.
Finally, a bottom-up approach was used to further explore the signaling mechanisms acting upstream of aquaporins in the Arabidopsis root under hyperosmotic stress. A RESPIRATORY BURST OXIDASE HOMOLOG (RBOH) pathway defined by two NADPH oxidases and an additional pathway involving apoplastic ascorbate and iron were found to mediate osmotic stress-induced ROS production (Martiniere et al., 2019). Furthermore, osmotic stress was shown to trigger interaction of a Rho GTPase, Rho-of-Plant 6 with the two NADPH oxidases (Smokvarska et al., 2020). This interaction occurs in specific plasma membrane nanodomains that are necessary and sufficient to transduce production of ROS and several plant adaptive responses to osmotic constraints (Figure 1B).
Salinity
Under salt stress, PIP aquaporins are subjected to cellular regulations that are reminiscent of those seen under a purely osmotic stress. In Arabidopsis roots for instance, a 100 mM NaCl treatment decreased C-terminal phosphorylation at Ser283 of AtPIP2;1 after 2–4 h (Prak et al., 2008). On a shorter timescale (30 min), a salt treatment also enhanced the lateral diffusion of AtPIP2;1 at the plasma membrane (Li et al., 2011) and its constitutive cycling (Luu et al., 2012). These movements lead to internalization of AtPIP2;1 from the plasma membrane, and its transfer to the prevacuolar and vacuolar compartments, a process that is mediated by ROS (Boursiac et al., 2008; Figure 1B) and is dependent on clathrin, phosphatidylinositol 3-kinase (PI3K), and PI4K (Ueda et al., 2016). These mechanisms may well be generalized: AtPIP2;7, another major aquaporin isoform in the Arabidopsis root, showed a combined transcriptional downregulation and protein internalization that resulted in a dramatic decrease in cell and tissue hydraulics (Pou et al., 2016). As in the case of osmotic stress, the primary signaling events triggering salt-dependent aquaporin regulation are not elucidated. Yet, salt-dependent expression of AtPIP2;3 and AtPIP2;5 is in part mediated by SnRK2s of subclass 1 (Kawa et al., 2020). Some of these protein kinases (SnRK2.4 and SnRK2.10) interact with and phosphorylate VARICOSE (VCS) and VCS RELATED (Soma et al., 2017; Kawa et al., 2020), two components of the mRNA decapping complex that catalyzes the first step of mRNA decay.
A role of PIP aquaporins in ion transport, tentatively associated to the so-called Nonselective Cation Channels described by patch clamp in many cell types, was recently proposed in Arabidopsis and barley (Byrt et al., 2017; Tran et al., 2020). As thoughtfully discussed by (Tyerman et al., 2021), this activity may well be central during plant response to salinity stress. The ionic conductance of AtPIP2;1 is repressed by calcium and strongly enhanced upon C-terminal phosphorylation (Qiu et al., 2020). In barley, HvPIP2;8 shows similar regulation, and, in addition, its gene is induced in shoots of salt-stressed plants, specifically in a tolerant cultivar (Tran et al., 2020). The connections of these regulatory processes to upstream salt stress signaling events and, possibly, to water transport regulation remains to be investigated.
Other abiotic constraints and stimuli
Oxygen availability
Flooding stress, whether due to water logging or submergence, deeply impacts plant water relations by favoring a massive inflow of water through roots while transpiration is directly or indirectly impaired (Tan et al., 2018). Two pathways leading to aquaporin regulation have been uncovered in this context (Maurel and Nacry, 2020). On the short term, a pronounced cytosolic acidosis can be observed under oxygen (O2) deprivation due to the metabolic production of organic acids while a drop in cellular respiration and ATP production hampers efficient proton (H+) extrusion by H+-ATPases. This in turn induces protonation of a His residue located on the second cytosolic loop of PIPs, triggering channel gating. It is remarkable that this His residue is perfectly conserved in PIPs of all species, supporting the generality of the H+-dependent gating mechanism (Tournaire-Roux et al., 2003). We note that certain plant species do not show any effect of hypoxia on root hydraulic conductivity (Lpr). In the case of two lupin species (Lupinus angustifolius and Lupinus luteus), hypoxia induced a marked inhibition of cortical cell hydraulic conductivity but had no substantial effect at the whole root level, suggesting the predominance of an apoplastic pathway during water uptake (Bramley et al., 2010).
The second pathway was recently uncovered through quantitative genetic analysis of Arabidopsis root hydraulics. It is under the control of Hydraulic Conductivity of Root 1 (HCR1; Shahzad et al., 2016). HCR1 mediates root responses to hypoxia in relation to potassium (K+) availability, thereby acting as a negative regulator of Lpr and root aquaporins. HCR1 encodes a RAF-type MAP3K protein kinase that specifically accumulates in the presence of K+ and absence of O2. HCR1 enhances the abundance of RAP2.12, a key Ethylene Response Factor VII transcription factor that is stabilized under O2 starvation and triggers the core anaerobic transcriptional response. We have proposed that HCR1 phosphorylates RAP2.12 or one of its direct regulators in planta and thereby potentiates the most early plant responses to O2 starvation (Shahzad et al., 2016). However, the mode of aquaporin down-regulation through this pathway has not been elucidated.
Nitrogen
Nitrate (NO3−) is the major inorganic nitrogen (N) source absorbed by nonwetland plants and its uptake and metabolism are tightly associated with water utilization. Whereas ammonium has a rather negative effect on water absorption, NO3− was shown to enhance Lpr in multiple plant species. Although posttranslational regulation may also be involved, the increase in Lpr induced by NO3− was shown to correspond to upregulation of aquaporin expression (Gloser et al., 2007; Gorska et al., 2008). It has also been disputed whether NO3− acts directly or through its assimilation products. In Arabidopsis, the conserved Lpr response of a NO3− reductase mutant (nia) supports the first hypothesis (Li et al., 2016). Furthermore, characterization of various Arabidopsis genotypes, altered in NO3− transport, assimilation, or signaling and growing under various N supply conditions revealed a strong positive relationship between Lpr and NO3− accumulation, but in shoots rather than in roots (Li et al., 2016). This suggests a control of Lpr by putative long-distance signals generated in the shoots. Such systemic regulation of aquaporins was also observed in split-root experiments with rice plants subjected to heterogeneous NO3− treatments (Ishikawa-Sakurai et al., 2014). We also note that Lpr regulation by transpiration or wounding (Laur and Hacke, 2013; Ishikawa-Sakurai et al., 2014; Liu et al., 2014; Vandeleur et al., 2014) may also be mediated by shoot-borne signals acting on root hydraulics.
Although NRT1.1 is considered as the major NO3− sensor, the high-affinity NO3− transporter NRT2.1 is also thought to have a signaling role. In contrast with nrt1.1 plants, nrt2.1 plants showed a pronounced reduction in Lpr, by up to 30% (Li et al., 2016). Surprisingly, this regulation was independent of NO3− and hence, of NRT2.1 expression level. Thus, the signaling mechanisms involved in this newly discovered pathway remain fairly mysterious.
Cold
Both moderate cold and freezing challenge the plant water status. For instance, low temperature, which reduces Lpr due to a higher viscosity of water and aquaporin downregulation, causes water deficit and growth arrest in leaves (Lee et al., 2012). In maize, aquaporins seem to play a key role in the recovery of Lpr that is specifically observed in chilling tolerant genotypes (Aroca et al., 2005). Similarly, cold acclimation of Arabidopsis seedlings is accompanied by upregulation of two specific PIP genes (AtPIP1;4 and AtPIP2;5; Rahman et al., 2020), which also show mRNA accumulation in leaves under water deficit (Alexandersson et al., 2005). In rice, proper expression and function of Calcium-dependent Protein Kinase (CDPK) OsCPK17 appears to be key for plant adaptation to low-temperature stress (Almadanim et al., 2017). A comparative phosphoproteomic approach further identified OsPIP2;1 and OsPIP2;6 as putative targets of OsCPK17. A direct activation by OsCPK17 is further supported by in vitro assays showing that the two aquaporins can be phosphorylated by the protein kinase in a calcium-dependent manner. Since OsCPK17 also targets sugar metabolism, it was proposed that cold stress-activated OsCPK17 phosphorylates OsPIP2;1 and OsPIP2;6 to coordinate membrane water transport with cell osmotic regulation (Almadanim et al., 2017).
Circadian control
The circadian clock allows the fine tuning and synchronization of key physiological, metabolic, and signaling pathways, in phase with daily variations of environmental parameters. While circadian regulation of tissue hydraulics has been largely documented (Henzler et al., 1999), it is only recently that the corresponding modes of aquaporin regulation have been investigated. In roots of Arabidopsis and maize, the control of aquaporin expression seems to be largely transcriptional and depends on well-identified clock components such as ELF3 (Takase et al., 2011; Caldeira et al., 2014). In maize, the oscillation amplitude of aquaporin expression and plant hydraulic conductance was strongly enhanced under water deficit, while oscillating expression of the core circadian clock genes was not altered (Caldeira et al., 2014). Interestingly, this specific potentiation of the hydraulic outputs, which possibly depends on ABA, appears to be beneficial for cumulated plant growth under water deficit whereas steady tissue hydraulic properties are more appropriate under water replete conditions. These data show how adaptive responses of plant hydraulics to specific environmental variations rely on exquisite coupling to endogenous signaling mechanisms.
In contrast with roots, the circadian control of leaf hydraulics seems to be mediated through posttranslational modifications of proteins (Choudhary et al., 2015; Prado et al., 2019). In particular, the rosette hydraulic conductivity (Kros) of Arabidopsis shows circadian oscillations with a peak at midday, in phase with quantitative variations of PIP2 aquaporin phosphorylation (Prado et al., 2019). Remarkably, this modification is necessary but not sufficient for circadian control of Kros, which requires the additional contribution of 14-3-3 proteins (Prado et al., 2013, 2019). Two of these (GRF4 and GRF10) can physically interact with AtPIP2;1 and modulate its function in Xenopus oocytes depending on the phosphorylation status of the aquaporin C-terminal tail, with more pronounced effects on phosphomimetic than on phosphodeficient forms (Prado et al., 2019). Much remains to be learned about the protein kinase/protein phosphatase machinery that ensures oscillations in aquaporin phosphorylation and, possibly, a synchronized activation of 14-3-3 proteins.
A multiplicity of endogenous signals
Auxin
Transcriptomic analyses in Arabidopsis have revealed a dramatic downregulation of aquaporin expression during lateral root formation (Péret et al., 2012). This effect is mediated in part by auxin which, more generally, downregulates most aquaporin expression in roots within 5–10 h, and, consequently, Lpr. Auxin also induces, after 24 h, a drop in turgor of cortical cells. Interestingly, mutant analyses have shown that all these molecular and physiological responses are mediated through Auxin Response Factor 7 (ARF7), a transcription factor that serves as a master regulator of lateral root formation and emergence. Yet, the down-stream targets of ARF7 in these responses remain to be identified. Our current understanding is that a finely tuned spatial and temporal control of PIP expression favors water entry into the lateral root primordium. This inflow is made at the expense of overlaying cells, thereby reducing their mechanical resistance and facilitating lateral root emergence.
Methyl jasmonate
Contrasting effects of Methyl jasmonate (MeJa) on root water transport have been reported by different groups. Sanchez-Romera et al. (2014) observed that exogenously supplied MeJA increases Lpr in bean, tomato, and Arabidopsis. These effects are associated with an increased phosphorylation of PIP2s and partly depend on ABA, which accumulates in response to MeJa. In beans, the fluoridone inhibitor counteracted the effects of MeJA on both ABA accumulation and Lpr. In contrast, exogenous MeJa induced a decrease in cell hydraulic conductivity in the cortex of Arabidopsis roots (Lee and Zwiazek, 2019). Surprisingly, fad3-2 and fad7-2 plants, which are defective in MeJa endogenous biosynthesis, were insensitive to this external treatment. These contrasting results suggest that effects of MeJa on root hydraulics are cell-type specific, somewhat indirect, and involve important hormonal cross-talks that remain to be uncovered.
Ethylene
The effects of ethylene on aquaporin function in various plant species and organs have revealed the contribution of multiple signaling pathways. In rose, ethylene acts as a repressor of flower opening. It negatively controls petal cell expansion through downregulation of RhPIP2;1 (Ma et al., 2008) and RhPIP1;1 (Chen et al., 2013), which functionally interacts with the former. Consistent with these negative hormonal effects, silencing of ethylene receptors (RhETRs) which, in the absence of the hormone function as repressors of ethylene effects, resulted in an ethylene-independent decrease in RhPIP1;1 expression (Chen et al., 2013). In Arabidopsis leaves, ethylene exerts positive effects on water transport through enhanced C-terminal phosphorylation of AtPIP2;1 (Qing et al., 2016). These posttranslational effects require the ETR-1 receptor and its interacting partner Constitutive Triple-Response 1 (CTR1). They are independent of the major transcriptional pathway involving Ethylene-Insensitive 1, and Ethylene-Insensitive 3-like, which would in turn mediate the transcriptional downregulation of PIPs, as mentioned above. Tentative regulatory networks acting on aquaporin phosphorylation, under the control of ethylene and through balanced effects on protein kinases and phosphatases, were inferred in part from a differential phosphoproteomics approach in Col-0 and ctr1 plants (Yang et al., 2013). Since ethylene enhances auxin accumulation, hormonal interactions will also have to be taken into account to comprehend the multiple pathways leading to ethylene-dependent aquaporin regulation (Yang et al., 2013; Qing et al., 2016).
Sugars
Sugars act as metabolites but also signaling molecules to regulate various whole plant and cellular processes such as photosynthesis and stomatal movements. With respect to tissue water transport, sucrose was shown to enhance Arabidopsis Lpr specifically in the dark, whereas it inhibited Lpr in the light, as did other osmotica (Di Pietro et al., 2013). Sucrose also induced changes in AtPIP2;6 phosphorylation (Niittylä et al., 2007), thereby providing a tentative link between light, sugars, and aquaporins. This link was corroborated by the high negative correlation existing between the expression of aquaporins and either exogenous glucose or the expression of Hexokinase 1 in over-expressing or knockout transgenic Arabidopsis (Kelly et al., 2017). Protein kinases that are involved in sucrose-induced phosphorylation responses, among which RLKs (e.g. Sucrose Induced Receptor Kinase 1 [SIRK1]) and receptor-like cytoplasmic kinases (RLCKs e.g. Brassinosteroid Signaling Kinase 8 [BSK8]) have been identified through typical changes in their phosphorylation status within the few minutes following sucrose resupply. Some aquaporins were subsequently identified as downstream targets of these protein kinases (Wu et al., 2013, 2014, 2019). For instance, molecular interactions of SIRK1 with AtPIP2;6 were established through pull down experiments and phosphorylation assays using an AtPIP2;6-specific peptide. The mesophyll protoplast water permeability of Arabidopsis lines showing different expression levels of active SIRK1 revealed that the RLK directly regulates aquaporins via phosphorylation in a sucrose-dependent manner (Wu et al., 2013). In addition, SIRK1 forms a complex with its co-receptor Qian Shou Kinase 1 (QSK1). SIRK1 shows autophosphorylation, subsequently trans-phosphorylates QSK1, which in turn enhances and stabilizes the interaction of aquaporins with the receptor kinase complex (Wu et al., 2019). Our understanding of BSK8 action on aquaporins is not as precise, but the RLCK possibly acts on another isoform, AtPIP2;7, that shows reduced phosphorylation in a bsk8 mutant. Although still incomplete, the whole set of data suggests intricate osmotic, metabolic, and signaling effects of sugars acting on aquaporins through multiple receptor pathways and time frames (Wu et al., 2014). At the whole plant level, the somewhat opposite effects of sugar on hydraulics depending on organs, timing, or environmental conditions surely reflect contrasting contexts where maintenance of growth or of water content dominates one another.
Peptidic hormones and other unknown ligands
The endodermis functions as a major barrier within the root, determining fundamental features of water and nutrient absorption from the soil (Doblas et al., 2017). These properties follow from the typical differentiation of endodermal cells, with an initial formation of Casparian strips made out of lignin and further deposition of suberin lamellas that wrap the whole cell. Casparian strips mostly block apoplastic solute movements and favor xylem loading by preventing solute backflow from the stele to the cortex. Suberin lamellas, rather, limit transcellular transport of nutrients and possibly water at the endodermis. These barriers, which are at the cross-roads of mineral nutrient and water uptake, are therefore under tight control by endogenous and environmental factors (Barberon et al., 2016). For instance, the loss of Casparian strip integrity is detected by diffusible vasculature-derived peptides CASPARIAN STRIP INTEGRITY FACTORS 1 and 2 (CIF1 and 2) which act through the SCHENGEN3 receptor-like kinase (Pfister et al., 2014; Doblas et al., 2017; Nakayama et al., 2017) to rebalance water and mineral nutrient uptake. More specifically, CIF1 and CIF2 induce a reduction in Lpr driven by the deactivation of aquaporins (Wang et al., 2019). They also limit ion leakage through deposition of suberin in endodermal cell walls. Inhibition of aquaporins seems to be a primary effect of CIFs, which in turn may promote suberin deposition. Interestingly, CIF1 and CIF2 act independently of ABA signaling, which can also be a potent regulator of aquaporins in endodermal cells.
Casparian strip deposition is guided by the local expression of a family of CAsparian Strip domain Proteins (CASPs). Related proteins named CASP-like are expressed throughout the plant. Four of these (CASPL1B1, CASPL1B2, CASPL1D1, and CASPL1D2) were identified as interacting with AtPIP2;1 (Bellati et al., 2016). Although the physiological relevance of these molecular interactions is not known, CASPL1B1 was shown to specifically activate phosphorylated AtPIP2;1 (Champeyroux et al., 2019), pointing to a putative composite regulatory pathway.
Biotic interactions
Plant immunity
In recent years, several landmark works have reported a crucial role of aquaporins in plant defense (Tian et al., 2016; Wang et al., 2018; Zhang et al., 2019a; Li et al., 2020a; Ai et al., 2021). AtPIP1;4 was identified as one of the PIPs, the expression of which is induced by infection with Pseudomonas syringae pv. Tomato (Tian et al., 2016). The use of intracellular and extracellular ROS dyes in yeast and transgenic Arabidopsis further revealed the capacity of AtPIP1;4 to mediate the diffusion of apoplastic H2O2 into the cytoplasm. This process represents a major step in the signaling response of plants to pathogen-associated molecular patterns (PAMPs), which activate RBOH NADPH oxidases present on the plasma membrane. More generally, analysis of knockout mutants and overexpressing plants revealed that AtPIP1;4 is necessary for PAMP-triggered immunity (Tian et al., 2016) and mediates systemic-acquired resistance (SAR) through a parallel pathway involving activation of NONINDUCER OF PR GENES1 (NPR1). These findings establish a major signaling role of PIPs in plant defense (Figure 1C). Interestingly, a mechanism allowing bacterial effectors to counteract this aquaporin signaling function was recently uncovered (Ai et al., 2021). In brief, a Crinkler 78 (CRN78) effector from Phytophthora displaying a protein kinase activity was shown to phosphorylate the C-terminal tail of several H2O2-transporting PIPs of soybean or Nicotiana benthamiana, thereby triggering their degradation through a proteasome-dependent pathway. This led to inhibition of H2O2-accumulation and reduced immunity of these plants when infected by Phytophthora oomycetes (Ai et al., 2021).
A signaling role for aquaporins during plant defense was also uncovered for AtPIP2;1 in guard cells, with this aquaporin being necessary for flg22-induced stomatal closure. In this case, a pathway was proposed, whereby the bacterial PAMP flg22 binds to its receptor flg22-sensitive 2 (FLS2) leading to the successive activation of the BRI1-Associated Kinase 1 (BAK1) co-receptor and SnRK2.6 protein kinase. This in turn phosphorylates AtPIP2;1 at Ser121 to promote both water and H2O2 transport as shown in the context of ABA-induced stomatal closure (Rodrigues et al., 2017; Figure 1A). Flagellin (flg22) also enhanced lateral diffusion of AtPIP2;1 at the guard cell plasma membrane through effects on microtubules, resulting in its internalization to the cell (Cui et al., 2021). It is not yet clear whether these effects contribute to the primary role of AtPIP2;1 in stomatal closure or reflect a feedback inhibition of its function due to flg22-induced ROS accumulation. Interestingly, AtPIP2;1 dynamics at the plasma membrane of adjacent (subsidiary) cells showed a distinct dependency on the cytoskeleton and were somewhat insensitive to flg22 (Cui et al., 2021). Thus, cell-specific regulation of aquaporins probably contributes to an integrated control of water exchange between cell types during stomatal movements.
PAMPs such as chitin are produced during plant attack by fungi and insects. Aquaporin-mediated water transport in leaf tissues such as the mesophyll and bundle sheath was shown to be downregulated by chitin (Attia et al., 2020). This inhibition could contribute to plant defense by hampering vascular pathogen propagation in xylem vessels and by promoting stomatal closure through long-distance hydraulic signaling. Although the mode of aquaporin regulation by chitin remains as yet unknown, the contribution of two chitin receptor-like kinases, chitin elicitor receptor kinase 1 and lysine motif receptor kinase 5, could be established (Attia et al., 2020).
Recent work also points to another molecular role of aquaporin during bacterial infection. For instance, rice OsPIP1;3 and Arabidopsis AtPIP1;4 molecularly interact with the bacterial protein harpin (Hpa1) which is secreted by Gram-negative pathogenic bacteria. Thus, PIPs serve as receptors for harpins at the plant plasma, which in turn mediate the delivery of effector proteins (such as PthXo1) into the plant cell cytoplasm (Li et al., 2015b, 2019). Along the same lines, AtPIP2;7 was recently shown to form a molecular complex with the protease domain of the tobacco etch potyvirus NIa protein (Martinez et al., 2016). Due to its numerous additional connections to stress responses, the aquaporin seems to be a preferential target during viral infection.
The overall data show that aquaporins are at the heart of the arms race between plants and their aggressors. On the one hand, aquaporins help plants resist pathogens by contributing to defense signaling pathways and by preventing their ingress through promotion of stomatal closure (Zhang et al., 2019a; Li et al., 2020a). On the other hand, some aquaporins can be hijacked by bacterial or viral pathogenic machineries, thereby promoting pathogen propagation.
Myccorhizae
Aquaporins also contribute to positive plant–microbe interactions whereby they play important roles in nutrient transport and abiotic stress responses. In particular, arbuscular mycorrhizal symbiosis can notably improve the plant water status. A fine dialog exists between the fungus and the host plants, leading to precise transcriptional and posttranslational regulation of plant aquaporins, particularly under drought (Quiroga et al., 2019). Similarly, the sub-cellular expression of specific tonoplast aquaporins seems to be subtly targeted in N-fixing symbiotic nodules (Gavrin et al., 2014). Although strong hormonal interplays are suspected, the signaling pathways leading to these regulations are as yet unknown.
Commonalities of signaling pathways
The survey above provides a broad but scattered knowledge of signaling processes targeting cell and tissue hydraulics in plants (Table 1). Yet, a few main principles do emerge from this information.
Table 1.
Molecular signaling mechanisms involved in aquaporin regulation under abiotic and biotic stresses and by endogenous signals
| Type of Signal | Stimulus | Signaling Intermediate | Molecular Mechanism on Aquaporins | Aquaporin Regulation | References |
|---|---|---|---|---|---|
| Abiotic | Drought (ABA- dependent) | SnRK2.6 | AtPIP2;1 loop B phosphorylation | Up-regulated activity | Grondin et al. (2015); Rodrigues et al. (2017) |
| LP2 | Interaction with OsPIP1;1, OsPIP1;3 and OsPIP2;3 | Downregulated activity | Wu et al. (2015) | ||
| Drought (ABA- independent) | RhPTM | Interaction with RhPIP2;1 | ND | Zhang et al. (2019a, 2019b) | |
| TPSO | ND | AtPIP2;7 protein degradation | Hachez et al. (2014b) | ||
| MtCas31/MtATG8 | MtCas31 as a cargo receptor for MtPIP2;7 | Protein degradation by autophagy | Li et al. (2020b) | ||
| Osmotic | ND | Increased C-ter phosphorylation of AtPIP2;4 | ND | Stecker et al. (2014) | |
| RBOHs and ROP6 | Membrane lateral mobility | Clustering in nano-domains | Hosy et al. (2015); Martiniere et al. (2019); Smokvarska et al. (2020) | ||
| clathrin | ND | Protein internalization | Zwiewka et al. (2015) | ||
| Rma1H1 | AtPIP2;1 ubiquitination | Protein degradation | Lee et al. (2009) | ||
| UBC32 | AtPIP2;1 ubiquitination | Protein degradation | Chen et al. (2021) | ||
| Salinity | clathrin, PI3K, PI4K, SnRK2.4, SnRK2.10 | C-ter phosphorylation of PIPs; enhanced mRNA decay | Protein internalization and down-regulated activity | Prak et al. (2008) Boursiac et al. (2008); Ueda et al., (2016); Pou et al., (2016); Kawa et al. (2020) | |
| O2 Availability | cytosolic protons | His protonation of PIPs | Downregulated activity | Tournaire-Roux et al. (2003) | |
| HCR1 | ND | Downregulated activity | Shahzad et al. (2016) | ||
| N | NRT2;1 | ND | Enhanced gene expression, protein accumulation and upregulated activity | Li et al. (2016); Ishikawa-Sakurai et al. (2014) | |
| Cold | ND | AtPIP1;4 and AtPIP2;5 gene expression | Upregulation of gene expression | Rahman et al. (2020) | |
| OsCPK17 | OsPIP2;1 and OsPIP2;6 C-ter phosphorylation | Upregulated activity | Almadanim et al. (2017) | ||
| Circadian Clock | 14-3-3 | AtPIP2;1 C-ter phosphorylation | Upregulated activity | Prado et al. (2019) | |
| ELF3 | AtPIP1;2 and AtPIP2;1 gene expression | Oscillating activity | Caldeira et al. (2014); Takase et al. (2011) | ||
|
| |||||
| Biotic | flg22 | BAK1 and SnRK2.6 | Increased AtPIP2;1 loop B phosphorylation | Upregulated activity | Rodrigues et al. (2017) |
| Phytophtora sojae | CRN78 | GmPIP2;13 and NbPIP2;2 phosphorylation | Protein degradation | Ai et al. (2021) | |
| Chitin | CERK1 and LYK5 | ND | Upregulated activity | Attia et al. (2020) | |
| Xanthomonas oryzae | Harpin | Interaction with OsPIP1;3 and AtPIP1;4 | ND | Li et al. (2015b); Li et al. (2019) | |
|
| |||||
| Endogenous | Auxin | ARF7 | PIP gene expression | Downregulated activity | Péret et al. (2012) |
| MeJa | ND | PIP2 C-ter phosphorylation | Upregulated activity | Lee and Zwiazek (2019) | |
| Ethylene | RhETRs | RhPIP1;1 and RhPIP2;1 gene expression | Downregulated gene expression | Ma et al. (2008); Chen et al. (2013) | |
| ETR1 and CTR1 | AtPIP2;1 C-ter phosphorylation | Upregulated activity | Yang et al. (2013); Qing et al. (2016) | ||
| Sucrose | SIRK1, QSK1 | AtPIP2;6 C-ter phosphorylation | Upregulated activity | Niittylä et al. (2007); Wu et al. (2013, 2014, 2019) | |
| BSK8 | AtPIP2;7 C-ter phosphorylation | ND | Wu et al. (2014) | ||
| CIF1 and CIF2 | SGN3 | ND | Downregulated activity | Wang et al. (2019) | |
| Other | CASPL1B1 | Physical interaction with AtPIP2;1 | Upregulated activity | Champeyroux et al. (2019) | |
Whereas transcriptional control is definitely key for long-term adjustment of plant hydraulics in response to environmental or developmental cues, posttranslational mechanisms seem to dominate short term (minutes and hours) regulations, as these are more tailored for fast and reversible responses to the multiple factors (including water availability) acting on the plant water status. Here, we discuss a few common players of the latter regulatory mechanisms.
First, ROS plays a central signaling role in such regulation of plant water transport (Figure 1). They are produced in response to numerous stimuli including osmotic, salt and cold stress, nutrient deprivation, and hormones (salicylic acid [SA] and ABA). Although several ROS generating systems may operate at the plasma membrane (Martiniere et al., 2019), RBOH NADPH oxidases seem to occupy a central position in this device (Li et al., 2011; Martiniere et al., 2019). By enhancing constitutive cycling of aquaporins at the plasma membrane and shifting the equilibrium toward endocytosis, ROS promote aquaporin internalization, thereby reducing their density at the cell surface and, as a consequence, cell hydraulic conductivity (Boursiac et al., 2008; Wudick et al., 2015). As suggested by recent work in guard cells (Cui et al., 2021), this mechanism could provide a feedback regulation in contexts where aquaporins contribute to the initial steps of ROS signaling.
The sub-cellular trafficking and regulated degradation of aquaporins also appear to be a main component of plant responses to environmental stimuli. Syntaxins and other traffic proteins, such as adaptor proteins, play well-established roles in these processes (Besserer et al., 2012; Hachez et al., 2014a; Pertl-Obermeyer et al., 2016). While some internalized aquaporins accumulate in prevacuolar compartments (Luu et al., 2012), a stress–response pathway under the control of TPSO (Hachez et al., 2014b) can relay the above-mentioned trafficking to promote aquaporin degradation. The autophagic pathways described in M. truncatula under water deficit (Li et al., 2020b) may also operate under other stressing conditions. In addition, two recent works have pointed to a control by phosphorylation of ubiquitin-dependent degradation of aquaporins in plants under abiotic or biotic stress (Ai et al., 2021; Chen et al., 2021). This mechanism, which allows control of aquaporin abundance, further expands the multiple, and possibly opposite, roles of phosphorylation in controlling aquaporin function.
In relation to the central role of phosphorylation, aquaporins are known to be targeted by multiple classes of protein kinases. This feature holds promises to further decipher aquaporin signaling pathways in bottom-up approaches (Figure 2). CDPKs have long been proposed as major players of aquaporin regulation (Johnson and Chrispeels, 1992; Johansson et al., 1996). Yet, there are very few cases where their role was formally established (Almadanim et al., 2017; Ji et al., 2017). We also note that CDPKs can be involved in upstream steps and therefore indirectly act on aquaporin regulation (Li et al., 2015a). Due to their key role during stomatal movements (Yip Delormel and Boudsocq, 2019), it will be worth exploring the action of CDPKs on guard cell aquaporins in closer detail.
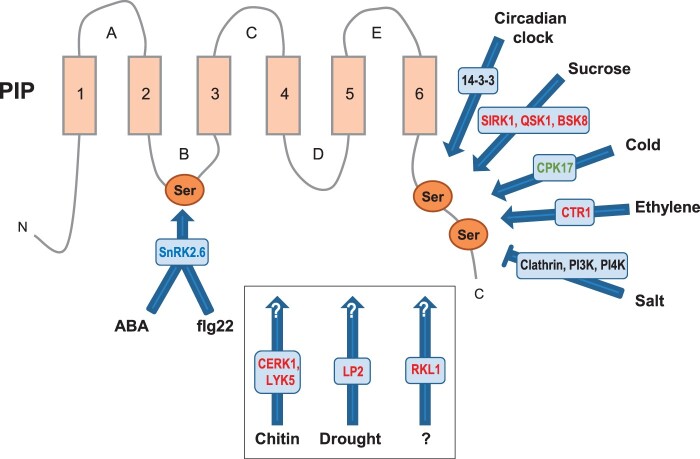
Figure 2.
Signaling pathways converging on PIP phosphorylation. The figure represents a prototypal PIP aquaporin with its transmembrane domains (1–6) and extracellular (A, C, and E) or cytosolic (B and D) loops. The indicated stimuli lead to enhanced (arrows) or reduced (blunt arrow) phosphorylation of Ser residues in loop B or C-terminal tail. The inset shows three pathways acting on unknown PIP phosphorylation sites (white question marks). Signaling intermediates are indicated with the following color code: SnRK2: blue; CDPK, green; RLK, red; others, black. Note that the indicated protein kinases do not necessarily act directly on the indicated sites. For more details and references see text and Table 1.
SnRK2s represent another potentially important class of protein kinases acting on plant aquaporins. Besides SnRK2.6 (OST1), which mediates ABA- and flg22-dependent activation of AtPIP2;1 in guard cells (Grondin et al., 2015; Rodrigues et al., 2017), other SnRK2s may delineate additional ABA- or osmotic stress-dependent pathways acting on aquaporins. In support for this, functional expression of plant signaling pathways in yeast using a transcriptional readout indicated that four SnRK2s initially thought to be specific for osmotic responses can also contribute to ABA signaling (Ruschhaupt et al., 2019). Thus, the model of ABA-dependent regulation of AtPIP2;1 may be extended to additional pairs of SnRK2s and PIPs.
RLKs constitute another major a class of serine/threonine kinases that perceive environmental and extracellular developmental signals and transduce them via their intracellular kinase domain. While their role in aquaporin regulation in the endodermis (SGN3) and during sugar response (SIRK1 and BSK8) can serve as models, we anticipate a role for RLKs in many other cellular contexts including water stress (see “Outstanding questions”). For instance, two RLKs from rice (LP2, OsRLCK311) were shown to interact with PIPs in vivo (Wu et al., 2015; Sade et al., 2020), but the functional role of these interactions was not definitively elucidated. Moreover, Arabidopsis RKL1, the activating signals of which remain to be identified, was shown to molecularly interact with AtPIP2;1 (Bellati et al., 2016). Co-expression of AtPIP2;1 with RKL1 in Xenopus oocytes resulted in enhanced AtPIP2;1 water transport activity by an as yet unknown mechanism (Bellati et al., 2016). RLKs may also regulate aquaporins by noncanonical mechanisms. For instance, co-expression of FERONIA and AtPIP2;1 in Xenopus oocytes leads to inactivation of the latter (Bellati et al., 2016). This inactivation seems to require the protein kinase activity of FERONIA and is favored by two C-terminal phosphorylation sites of AtPIP2;1 that, in contrast, have been described as activating sites. Thus, these phosphorylated residues may favor the FERONIA–AtPIP2;1 interaction, which in turn would lead to aquaporin inhibition.
Finally, CBL-interacting protein kinases represent major regulators of plant nutrition and response to abiotic stresses (Dong et al., 2020). It is rather surprising that, despite efforts from several laboratories including ours, no aquaporin has ever been identified as one of their numerous substrates. In addition, protein phosphatases, which surely play a key role in phosphorylation-dependent regulation of aquaporins, will deserve specific attention in future studies.
Lipid signaling represents another emerging component targeting aquaporins (see “Outstanding questions”). For instance, genetic alteration of phosphoinositides (phosphatidyl inositol [4,5] bisphosphate: PtdInsP2) in tobacco (Nicotiana tabacum) increased the osmotic water permeability and aquaporin activity of protoplasts isolated from suspension cells (Ma et al., 2015). The lipid effects may operate through direct interaction with aquaporins or through indirect cellular effects of PtdInsP2. For instance, interaction of PtdInsP2 with TPSO is required for interaction with AtPIP2;7 and its subsequent degradation (Jurkiewicz et al., 2020). A role for lipid signaling in PIP regulation is also suggested by the physical interaction between AtPIP2;1 and two phospholipases D (PLDδ and PLDγ1) (Bellati et al., 2016). In plants, PLDs and their enzymatic product phosphatidic acid (PA) play key roles in cellular responses to hormonal and abiotic stimuli. Along these lines, AtPIP2;1 and AtPIP2;2 were identified as PA-binding proteins (McLoughlin et al., 2013) but the functional effects of these interactions remain to be elucidated.
In complement to these targeted studies, we believe that systems biology approaches will help uncover previously unidentified signaling pathways targeting aquaporins. For instance, protein interaction networks involving aquaporins have pointed to links with clusters associated with brassinosteroid signaling (Bellati et al., 2016). A genome-wide search for membrane–protein complexes has revealed other clusters that are enriched in aquaporins and have potential roles in stimulus-dependent regulation of water transport (Jones et al., 2014). Finally, a close inspection of aquaporin gene position in gene regulation networks will for sure reveal other modes of developmental or environmental regulation of aquaporins (Alexandersson et al., 2010).
Perspectives
Whereas this overview principally deals with chemical signals, whether abiotic or hormonal, there is still much to be learned about how physical cues lead to aquaporin regulation (see “Outstanding questions”). Particularly, challenging seems the direct link between tissue hydraulics and transpiration that may well be mediated through hydraulic signals, or in other words, pressure-dependent regulation of aquaporins (Laur and Hacke, 2013; Pou et al., 2013). Such dependency may also be on the basis of wound-induced alterations in root or leaf hydraulic conductivity (Liu et al., 2014; Vandeleur et al., 2014). Plant defense is another emerging context in which aquaporin regulation will have to be investigated. While the plant water status is crucial for resilience of plants under attacks, it is also a key target for pathogens to facilitate their proliferation within the plant (Xin et al., 2016). We now have some ideas on how aquaporins serve as both signaling components and targets during innate immunity (Zhang et al., 2019a). In contrast, the action of effectors on aquaporins and possible resistance mechanisms associated to these are just emerging (Ai et al., 2021).
Beyond regulation of water transport in a strict sense, we realize that mechanisms targeting other aquaporin transport functions may have some relevance for plant water relations or share similarities with mechanisms targeting the latter. For instance, aquaporin-mediated CO2 transport is regulated through interaction with βCA4 carbonic anhydrase (Wang et al., 2016), indicating that the various molecular functions of aquaporin interactants and their ability to associate aquaporin regulation to upstream signaling cascades still represent a gold mine of information. Also, the ion transport of AtPIP2;1, which is strongly dependent on divalent cations and pH (Byrt et al., 2017), confirms that both Ca2+ and H+ are definitely crucial second messengers acting on aquaporin activity.
Because plants are continuously exposed to combinations of environmental constraints, we realize that our analytic representation of single signaling pathways remains largely inappropriate. Although we emphasized how conserved pathways and their cross talks ultimately act on aquaporins, our knowledge of these processes remains very poor. More generally, basic information on how plant water transport is adjusted in response to combined stresses is still lacking. This large field of investigation, combining fundamental plant physiology and systems biology, is now necessary to properly comprehend the amazing capacity of plants to acclimate to a large array of stressful environments.
Acknowledgments
The authors thank their colleagues of the Aqua team for fruitful discussions.
Funding
This work was supported in part by the Agence Nationale de la Recherche (ANR- 18-CE92-0055).
Conflict of interest statement. None declared.
Advances
ABA-dependent and independent signaling pathways act on aquaporins in plants under water deficit.
Aquaporin-mediated water transport is targeted by multiple abiotic, biotic, or endogenous stimuli; recently identified stimuli include peptide hormones and PAMPs.
ROS play a central signaling role in regulating water transport in plants by acting on aquaporin subcellular dynamics, localization, and/or phosphorylation.
Plasma membrane aquaporins exhibit a dual water- and H2O2-transport function, acting as players in and targets of signaling processes.
Phosphorylation of aquaporins, which interferes with their gating, trafficking, and stability, is a major target of plant signaling cascades, and multiple classes of protein kinases that act on distinct phosphorylation sites have been identified.
Outstanding questions
Is aquaporin-mediated H2O2 transport a common process in plant cell signaling?
Which cellular signals trigger RLK-dependent aquaporin regulation?
What is the role of lipid signaling in water transport regulation?
What are the mechanisms for coupling physical stimuli to aquaporin regulation?
How do aquaporins contribute to plant defense? What are their active roles in plant immunity, and how can they be hijacked by pathogens?
What are the effects of combined stresses on plant water transport, and which signaling pathways are involved?
All authors contributed to the literature survey. C.M. wrote the article which was revised by all other authors. C.T.R., L.V., and V.S. designed the figures and table.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Christophe Maurel (christophe.maurel@cnrs.fr).
References
- Ai G, Xia Q, Song T, Li T, Zhu H, Peng H, Liu J, Fu X, Zhang M, Jing M, et al. (2021) A Phytophthora sojae CRN effector mediates phosphorylation and degradation of plant aquaporin proteins to suppress host immune signaling. PLoS Pathog 17: e1009388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersson E, Danielson JA, Råde J, Moparthi VK, Fontes M, Kjellbom P, Johanson U (2010) Transcriptional regulation of aquaporins in accessions of Arabidopsis in response to drought stress. Plant J 61: 650–660 [DOI] [PubMed] [Google Scholar]
- Alexandersson E, Fraysse L, Sjovall-Larsen S, Gustavsson S, Fellert M, Karlsson M, Johanson U, Kjellbom P (2005) Whole gene family expression and drought stress regulation of aquaporins. Plant Mol Biol 59:469–484 [DOI] [PubMed] [Google Scholar]
- Almadanim MC, Alexandre BM, Rosa MTG, Sapeta H, Leitao AE, Ramalho JC, Lam TT, Negrao S, Abreu IA, Oliveira MM (2017) Rice calcium-dependent protein kinase OsCPK17 targets plasma membrane intrinsic protein and sucrose-phosphate synthase and is required for a proper cold stress response. Plant Cell Environ 40:1197–1213 [DOI] [PubMed] [Google Scholar]
- Aroca R, Amodeo G, Fernandez-Illescas S, Herman EM, Chaumont F, Chrispeels MJ (2005) The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol 137:341–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroca R, Porcel R, Ruiz-Lozano JM (2012) Regulation of root water uptake under abiotic stress conditions. J Exp Bot 63: 43–57 [DOI] [PubMed] [Google Scholar]
- Attia Z, Dalal A, Moshelion M (2020) Vascular bundle sheath and mesophyll cells modulate leaf water balance in response to chitin. Plant J 101:1368–1377 [DOI] [PubMed] [Google Scholar]
- Barberon M, Vermeer JE, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, et al. (2016) Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164: 447–459 [DOI] [PubMed] [Google Scholar]
- Bellati J, Champeyroux C, Hem S, Rofidal V, Krouk G, Maurel C, Santoni V (2016) Novel aquaporin regulatory mechanisms revealed by interactomics. Mol Cell Proteomics 15: 3473–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besserer A, Burnotte E, Bienert GP, Chevalier AS, Errachid A, Grefen C, Blatt MR, Chaumont F (2012) Selective regulation of maize plasma membrane aquaporin trafficking and activity by the SNARE SYP121. Plant Cell 24: 3463–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y, Boudet J, Postaire O, Luu DT, Tournaire-Roux C, Maurel C (2008) Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J 56: 207–218 [DOI] [PubMed] [Google Scholar]
- Bramley H, Turner NC, Turner DW, Tyerman SD (2010) The contrasting influence of short-term hypoxia on the hydraulic properties of cells and roots of wheat and lupin. Function Plant Biol 37: 183–193 [Google Scholar]
- Byrt CS, Zhao M, Kourghi M, Bose J, Henderson SW, Qiu J, Gilliham M, Schultz C, Schwarz M, Ramesh SA, et al. (2017) Non-selective cation channel activity of aquaporin AtPIP2;1 regulated by Ca2+ and pH. Plant Cell Environ 40: 802–815 [DOI] [PubMed] [Google Scholar]
- Caldeira CF, Jeanguenin L, Chaumont F, Tardieu F (2014) Circadian rhythms of hydraulic conductance and growth are enhanced by drought and improve plant performance. Nat Commun 5: 5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceciliato PHO, Zhang J, Liu Q, Shen X, Hu H, Liu C, Schaffner AR, Schroeder JI (2019) Intact leaf gas exchange provides a robust method for measuring the kinetics of stomatal conductance responses to abscisic acid and other small molecules in Arabidopsis and grasses. Plant Methods 15: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champeyroux C, Bellati J, Barberon M, Rofidal V, Maurel C, Santoni V (2019) Regulation of a plant aquaporin by a Casparian strip membrane domain protein-like. Plant Cell Environ 42: 1788–1801 [DOI] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Herman EM, Chrispeels MJ (1998) Characterization of a maize tonoplast aquaporin expressed in zones of cell division and elongation. Plant Physiol 117: 1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F, Tyerman SD (2014) Aquaporins: highly regulated channels controlling plant water relations. Plant Physiol 164: 1600–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Liu R, Wu Y, Wei S, Wang Q, Zheng Y, Xia R, Shang X, Yu F, Yang X, et al. (2021) ERAD-related E2 and E3 enzymes modulate the drought response by regulating the stability of PIP2 aquaporins. Plant Cell doi:10.1093/plcell/koab141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Yin X, Wang L, Tian J, Yang R, Liu D, Yu Z, Ma N, Gao J (2013) Involvement of rose aquaporin RhPIP1;1 in ethylene-regulated petal expansion through interaction with RhPIP2;1. Plant Mol Biol 83: 219–233 [DOI] [PubMed] [Google Scholar]
- Choudhary MK, Nomura Y, Wang L, Nakagami H, Somers DE (2015) Quantitative circadian phosphoproteomic analysis of Arabidopsis reveals extensive clock control of key components in physiological, metabolic, and signaling pathways. Mol Cell Proteomics 14: 2243–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Zhao Y, Lu Y, Su X, Chen Y, Shen Y, Lin J, Li X (2021) In vivo single-particle tracking of the aquaporin AtPIP2;1 in stomata reveals cell type-specific dynamics. Plant Physiol 185: 1666–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WJ, Bennett MJ (2015) Achieving more crop per drop. Nat Plants 1: 15118. [DOI] [PubMed] [Google Scholar]
- Di Pietro M, Vialaret J, Li G, Hem S, Rossignol M, Maurel C, Santoni V (2013) Coordinated post-translational responses of aquaporins to abiotic and nutritional stimuli in Arabidopsis roots. Mol Cell Proteomics 12: 3886–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblas VG, Geldner N, Barberon M (2017) The endodermis, a tightly controlled barrier for nutrients. Curr Opin Plant Biol 39: 136–143 [DOI] [PubMed] [Google Scholar]
- Dong Q, Bai B, Almutairi BO, Kudla J (2020) Emerging roles of the CBL-CIPK calcium signaling network as key regulatory hub in plant nutrition. J Plant Physiol 257: 153335. [DOI] [PubMed] [Google Scholar]
- Dynowski M, Schaaf G, Loque D, Moran O, Ludewig U (2008) Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J 414: 53–61 [DOI] [PubMed] [Google Scholar]
- Gavrin A, Kaiser BN, Geiger D, Tyerman SD, Wen Z, Bisseling T, Fedorova EE (2014) Adjustment of host cells for accommodation of symbiotic bacteria: vacuole defunctionalization, HOPS suppression, and TIP1g retargeting in Medicago. Plant Cell 26: 3809–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloser V, Zwieniecki MA, Orians CM, Holbrook NM (2007) Dynamic changes in root hydraulic properties in response to nitrate availability. J Exp Bot 58: 2409–2415 [DOI] [PubMed] [Google Scholar]
- Gorska A, Ye Q, Holbrook NM, Zwieniecki MA (2008) Nitrate control of root hydraulic properties in plants: translating local information to whole plant response. Plant Physiol 148: 1159–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin A, Rodrigues O, Verdoucq L, Merlot S, Leonhardt N, Maurel C (2015) Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 27:1945–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Laloux T, Reinhardt H, Cavez D, Degand H, Grefen C, De Rycke R, Inze D, Blatt MR, Russinova E, et al. (2014a) Arabidopsis SNAREs SYP61 and SYP121 coordinate the trafficking of plasma membrane aquaporin PIP2;7 to modulate the cell membrane water permeability. Plant Cell 26: 3132–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachez C, Veljanovski V, Reinhardt H, Guillaumot D, Vanhee C, Chaumont F, Batoko H (2014b) The Arabidopsis abiotic stress-induced TSPO-related protein reduces cell-surface expression of the aquaporin PIP2;7 through protein-protein interactions and autophagic degradation. Plant Cell 26: 4974–4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzler T, Waterhouse RN, Smyth AJ, Carvajal M, Cooke DT, Schaffner AR, Steudle E, Clarkson DT (1999) Diurnal variations in hydraulic conductivity and root pressure can be correlated with the expression of putative aquaporins in the roots of Lotus japonicus. Planta 210: 50–60 [DOI] [PubMed] [Google Scholar]
- Hosy E, Martiniere A, Choquet D, Maurel C, Luu DT (2015) Super-resolved and dynamic imaging of membrane proteins in plant cells reveal contrasting kinetic profiles and multiple confinement mechanisms. Mol Plant 8: 339–342 [DOI] [PubMed] [Google Scholar]
- Ishikawa-Sakurai J, Hayashi H, Murai-Hatano M (2014) Nitrogen availability affects hydraulic conductivity of rice roots, possibly through changes in aquaporin gene expression. Plant Soil 379: 289–300 [Google Scholar]
- Ji R, Zhou L, Liu J, Wang Y, Yang L, Zheng Q, Zhang C, Zhang B, Ge H, Yang Y, et al. (2017) Calcium-dependent protein kinase CPK31 interacts with arsenic transporter AtNIP1;1 and regulates arsenite uptake in Arabidopsis thaliana. PLoS One 12: e0173681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Larsson C, Ek B, Kjellbom P (1996) The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell 8: 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KD, Chrispeels MJ (1992) Tonoplast-bound protein kinase phosphorylates tonoplast intrinsic protein. Plant Physiol 100: 1787–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Xuan Y, Xu M, Wang RS, Ho CH, Lalonde S, You CH, Sardi MI, Parsa SA, Smith-Valle E, et al. (2014) Border control–a membrane-linked interactome of Arabidopsis. Science 344: 711–716 [DOI] [PubMed] [Google Scholar]
- Jurkiewicz P, Senicourt L, Ayeb H, Lequin O, Lacapere JJ, Batoko H (2020) A plant-specific N-terminal extension reveals evolutionary functional divergence within translocator proteins. iScience 23: 100889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa D, Meyer AJ, Dekker HL, Abd-El-Haliem AM, Gevaert K, Van De Slijke E, Maszkowska J, Bucholc M, Dobrowolska G, De Jaeger G, et al. (2020) SnRK2 Protein Kinases and mRNA Decapping Machinery Control Root Development and Response to Salt. Plant Physiol 182: 361–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G, Sade N, Doron-Faigenboim A, Lerner S, Shatil-Cohen A, Yeselson Y, Egbaria A, Kottapalli J, Schaffer AA, Moshelion M, et al. (2017) Sugar and hexokinase suppress expression of PIP aquaporins and reduce leaf hydraulics that preserves leaf water potential. Plant J 91: 325–339. [DOI] [PubMed] [Google Scholar]
- Laur J, Hacke UG (2013) Transpirational demand affects aquaporin expression in poplar roots. J Exp Bot 64: 2283–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Cho SK, Son O, Xu Z, Hwang I, Kim WT (2009) Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell 21: 622–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Chung GC, Jang JY, Ahn SJ, Zwiazek JJ (2012) Overexpression of PIP2;5 aquaporin alleviates effects of low root temperature on cell hydraulic conductivity and growth in Arabidopsis. Plant Physiol 159: 479–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Zwiazek JJ (2019) Regulation of water transport in Arabidopsis by methyl jasmonate. Plant Physiol Biochem 139: 540–547 [DOI] [PubMed] [Google Scholar]
- Li G, Boudsocq M, Hem S, Vialaret J, Rossignol M, Maurel C, Santoni V (2015a) The calcium-dependent protein kinase CPK7 acts on root hydraulic conductivity. Plant Cell Environ 38: 1312–1320 [DOI] [PubMed] [Google Scholar]
- Li G, Chen T, Zhang Z, Li B, Tian S (2020a) Roles of Aquaporins in Plant-Pathogen Interaction. Plants 9: 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Tillard P, Gojon A, Maurel C (2016) Dual regulation of root hydraulic conductivity and plasma membrane aquaporins by plant nitrate accumulation and high-affinity nitrate transporter NRT2.1. Plant Cell Physiol 57: 733–742 [DOI] [PubMed] [Google Scholar]
- Li L, Wang H, Gago J, Cui H, Qian Z, Kodama N, Ji H, Tian S, Shen D, Chen Y, et al. (2015b) Harpin Hpa1 interacts with aquaporin PIP1;4 to promote the substrate transport and photosynthesis in Arabidopsis. Sci Rep 5: 17207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhang L, Mo X, Ji H, Bian H, Hu Y, Majid T, Long J, Pang H, Tao Y, et al. (2019) Rice aquaporin PIP1;3 and harpin Hpa1 of bacterial blight pathogen cooperate in a type III effector translocation. J Exp Bot 70: 3057–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu Q, Feng H, Deng J, Zhang R, Wen J, Dong J, Wang T (2020b) Dehydrin MtCAS31 promotes autophagic degradation under drought stress. Autophagy 16: 862–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang X, Yang Y, Li R, He Q, Fang X, Luu DT, Maurel C, Lin J (2011) Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell 23: 3780–3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Equiza MA, Navarro-Rodenas A, Lee SH, Zwiazek JJ (2014) Hydraulic adjustments in aspen (Populus tremuloides) seedlings following defoliation involve root and leaf aquaporins. Planta 240: 553–564 [DOI] [PubMed] [Google Scholar]
- Luu DT, Martinière A, Sorieul M, Runions J, Maurel C (2012) Fluorescence recovery after photobleaching reveals high cycling dynamics of plasma membrane aquaporins in Arabidopsis roots under salt stress. Plant J 69: 894–905 [DOI] [PubMed] [Google Scholar]
- Ma N, Xue J, Li Y, Liu X, Dai F, Jia W, Luo Y, Gao J (2008) Rh-PIP2;1, a rose aquaporin gene, is involved in ethylene-regulated petal expansion. Plant Physiol 148: 894–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Shatil-Cohen A, Ben-Dor S, Wigoda N, Perera IY, Im YJ, Diminshtein S, Yu L, Boss WF, Moshelion M, et al. (2015) Do phosphoinositides regulate membrane water permeability of tobacco protoplasts by enhancing the aquaporin pathway? Planta 241: 741–755 [DOI] [PubMed] [Google Scholar]
- Maathuis FJ, Filatov V, Herzyk P, Krijger GC, Axelsen KB, Chen S, Green BJ, Li Y, Madagan KL, Sanchez-Fernandez R, Forde BG, et al. (2003) Transcriptome analysis of root transporters reveals participation of multiple gene families in the response to cation stress. Plant J 35: 675–692 [DOI] [PubMed] [Google Scholar]
- Martinez F, Rodrigo G, Aragones V, Ruiz M, Lodewijk I, Fernandez U, Elena SF, Daros JA (2016) Interaction network of tobacco etch potyvirus NIa protein with the host proteome during infection. BMC Genomics 17: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiniere A, Fiche JB, Smokvarska M, Mari S, Alcon C, Dumont X, Hematy K, Jaillais Y, Nollmann M, Maurel C (2019) Osmotic stress activates two reactive oxygen species pathways with distinct effects on protein nanodomains and diffusion. Plant Physiol 179: 1581–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Boursiac Y, Luu DT, Santoni V, Shahzad Z, Verdoucq L (2015) Aquaporins in plants. Physiol Rev 95: 1321–1358 [DOI] [PubMed] [Google Scholar]
- Maurel C, Nacry P (2020) Root architecture and hydraulics converge for acclimation to changing water availability. Nat Plants 6: 744–749 [DOI] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Rodrigues O (2016) Aquaporins and plant transpiration. Plant Cell Environ 39: 2580–2587 [DOI] [PubMed] [Google Scholar]
- McLoughlin F, Arisz SA, Dekker HL, Kramer G, de Koster CG, Haring MA, Munnik T, Testerink C (2013) Identification of novel candidate phosphatidic acid-binding proteins involved in the salt-stress response of Arabidopsis thaliana roots. Biochem J 450: 573–581 [DOI] [PubMed] [Google Scholar]
- Nakayama T, Shinohara H, Tanaka M, Baba K, Ogawa-Ohnishi M, Matsubayashi Y (2017) A peptide hormone required for Casparian strip diffusion barrier formation in Arabidopsis roots. Science 355: 284–286 [DOI] [PubMed] [Google Scholar]
- Niittylä T, Fuglsang AT, Palmgren MG, Frommer WB, Schulze WX (2007) Temporal analysis of sucrose-induced phosphorylation changes in plasma membrane proteins of Arabidopsis. Mol Cell Proteomics 6: 1711–1726 [DOI] [PubMed] [Google Scholar]
- Péret B, Li GW, Zhao J, Band LR, Voß U, Postaire O, Luu DT, Da Ines O, Casimiro I, Lucas M, et al. (2012) Auxin regulates aquaporin function to facilitate lateral root emergence. Nature Cell Biol 14: 991–998 [DOI] [PubMed] [Google Scholar]
- Pertl-Obermeyer H, Wu XN, Schrodt J, Mudsam C, Obermeyer G, Schulze WX (2016) Identification of cargo for adaptor protein (AP) complexes 3 and 4 by sucrose gradient profiling. Mol Cell Proteomics 15: 2877–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister A, Barberon M, Alassimone J, Kalmbach L, Lee Y, Vermeer JEYamazaki M, Li G, Maurel C, Takano J, Kamiya T, Salt DE, Roppolo D, Geldner N (2014) A receptor-like kinase mutant with absent endodermal diffusion barrier displays selective nutrient homeostasis defects. Elife 3: e03115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou A, Jeanguenin L, Milhiet T, Batoko H, Chaumont F, Hachez C (2016) Salinity-mediated transcriptional and post-translational regulation of the Arabidopsis aquaporin PIP2;7. Plant Mol Biol 92: 731–744 [DOI] [PubMed] [Google Scholar]
- Pou A, Medrano H, Flexas J, Tyerman SD (2013) A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant Cell Environ 36: 828–8439 [DOI] [PubMed] [Google Scholar]
- Prado K, Boursiac Y, Tournaire-Roux C, Monneuse JM, Postaire O, Da Ines O, Schäffner AR, Hem S, Santoni V, Maurel C (2013) Regulation of Arabidopsis leaf hydraulics involves light-dependent phosphorylation of aquaporins in veins. Plant Cell 25: 1029–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado K, Cotelle V, Li G, Bellati J, Tang N, Tournaire-Roux C, Martiniere A, Santoni V, Maurel C (2019) Oscillating aquaporin phosphorylation and 14-3-3 proteins mediate the circadian regulation of leaf hydraulics. Plant Cell 31: 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prak S, Hem S, Boudet J, Viennois G, Sommerer N, Rossignol M, Maurel C, Santoni V (2008) Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins. Role in sub-cellular trafficking of AtPIP2;1 in response to salt stress. Mol Cell Proteomics 7: 1019–1030 [DOI] [PubMed] [Google Scholar]
- Qing D, Yang Z, Li M, Wong WS, Guo G, Liu S, Guo H, Li N (2016) Quantitative and functional phosphoproteomic analysis reveals that ethylene regulates water transport via the C-terminal phosphorylation of aquaporin PIP2;1 in Arabidopsis. Mol Plant 9: 158–174 [DOI] [PubMed] [Google Scholar]
- Qiu J, McGaughey SA, Groszmann M, Tyerman SD, Byrt CS (2020) Phosphorylation influences water and ion channel function of AtPIP2;1. Plant Cell Environ 43: 2428–2442 [DOI] [PubMed] [Google Scholar]
- Quiroga G, Erice G, Ding L, Chaumont F, Aroca R, Ruiz-Lozano JM (2019) The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant Cell Environ 42: 2274–2290 [DOI] [PubMed] [Google Scholar]
- Rae L, Lao NT, Kavanagh TA (2011) Regulation of multiple aquaporin genes in Arabidopsis by a pair of recently duplicated DREB transcription factors. Planta 234: 429–444 [DOI] [PubMed] [Google Scholar]
- Rahman A, Kawamura Y, Maeshima M, Rahman A, Uemura M (2020) Plasma membrane aquaporin members PIPs act in concert to regulate cold acclimation and freezing tolerance responses in Arabidopsis thaliana. Plant Cell Physiol 61: 787–802 [DOI] [PubMed] [Google Scholar]
- Ricardi MM, Gonzalez RM, Zhong S, Dominguez PG, Duffy T, Turjanski PG, Salgado Salter JD, Alleva K, Carrari F, Giovannoni JJ, et al. (2014) Genome-wide data (ChIP-seq) enabled identification of cell wall-related and aquaporin genes as targets of tomato ASR1, a drought stress-responsive transcription factor. BMC Plant Biol 14: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues O, Reshetnyak G, Grondin A, Saijo Y, Leonhardt N, Maurel C, Verdoucq L (2017) Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc Natl Acad Sci USA 114: 9200–9205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales MA, Maurel C, Nacry P (2019) Abscisic acid coordinates dose-dependent developmental and hydraulic responses of roots to water deficit. Plant Physiol 180: 2198–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruschhaupt M, Mergner J, Mucha S, Papacek M, Doch I, Tischer SV, Hemmler D, Chiasson D, Edel KH, Kudla J, et al. (2019) Rebuilding core abscisic acid signaling pathways of Arabidopsis in yeast. EMBO J 38: e101859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade N, Weng F, Tajima H, Zeron Y, Zhang L, Rubio Wilhelmi MDM, Day G, Peleg Z, Blumwald E (2020) A cytoplasmic receptor-like kinase contributes to salinity tolerance. Plants (Basel) 9: 1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Romera B, Ruiz-Lozano JM, Li G, Luu DT, Martinez-Ballesta Mdel C, Carvajal M, Zamarreno AM, Garcia-Mina JM, Maurel C, Aroca R (2014) Enhancement of root hydraulic conductivity by methyl jasmonate and the role of calcium and abscisic acid in this process. Plant Cell Environ 37: 995–1008 [DOI] [PubMed] [Google Scholar]
- Shahzad Z, Canut M, Tournaire-Roux C, Martinière A, Boursiac Y, Loudet O, Maurel C (2016) A potassium-dependent oxygen sensing pathway regulates plant root hydraulics. Cell 167: 87–98 [DOI] [PubMed] [Google Scholar]
- Shatil-Cohen A, Attia Z, Moshelion M (2011) Bundle-sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: a target of xylem-borne ABA? Plant J 67: 72–80 [DOI] [PubMed] [Google Scholar]
- Smokvarska M, Francis C, Platre MP, Fiche JB, Alcon C, Dumont X, Nacry P, Bayle V, Nollmann M, Maurel C, et al. (2020) A plasma membrane nanodomain ensures signal specificity during osmotic signaling in plants. Curr Biol 30: 4654–4664 [DOI] [PubMed] [Google Scholar]
- Soma F, Mogami J, Yoshida T, Abekura M, Takahashi F, Kidokoro S, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2017) ABA-unresponsive SnRK2 protein kinases regulate mRNA decay under osmotic stress in plants. Nat Plants 3: 16204. [DOI] [PubMed] [Google Scholar]
- Stecker KE, Minkoff BB, Sussman MR (2014) Phosphoproteomic analyses reveal early signaling events in the osmotic stress response. Plant Physiol 165: 1171–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E (1992) The biophysics of plant water: compartmentation, coupling with metabolic processes, and water flow in plant roots. In: Somero GN, Osmond CB, Bolis CL (eds) Water and Life: Comparative Analysis of Water Relationships at the Organismic, Cellular, and Molecular Levels. Springer-Verlag, Berlin, Germany, pp 273–304 [Google Scholar]
- Takase T, Ishikawa H, Murakami H, Kikuchi J, Sato-Nara K, Suzuki H (2011) The circadian clock modulates water dynamics and aquaporin expression in Arabidopsis roots. Plant Cell Physiol 52:373–383 [DOI] [PubMed] [Google Scholar]
- Tan X, Xu H, Khan S, Equiza MA, Lee SH, Vaziriyeganeh M, Zwiazek JJ (2018) Plant water transport and aquaporins in oxygen-deprived environments. J Plant Physiol 227: 20–30 [DOI] [PubMed] [Google Scholar]
- Tardieu F, Simonneau T, Parent B (2015) Modelling the coordination of the controls of stomatal aperture, transpiration, leaf growth, and abscisic acid: update and extension of the Tardieu-Davies model. J Exp Bot 66: 2227–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Wang X, Li P, Wang H, Ji H, Xie J, Qiu Q, Shen D, Dong H (2016) Plant aquaporin AtPIP1;4 links apoplastic H2O2 induction to disease immunity pathways. Plant Physiol 171: 1635–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu DT, Bligny R, Maurel C (2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425: 393–397 [DOI] [PubMed] [Google Scholar]
- Tran STH, Imran S, Horie T, Qiu J, McGaughey S, Byrt CS, Tyerman SD, Katsuhara M (2020) A survey of barley PIP aquaporin ionic conductance reveals Ca2+-sensitive HvPIP2;8 Na+ and K+ conductance. Int J Mol Sci 21: 7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JA (1999) Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J Exp Bot 50: 1055–1071 [Google Scholar]
- Tyerman SD, McGaughey SA, Qiu J, Yool AJ, Byrt CS (2021) Adaptable and Multifunctional Ion-Conducting Aquaporins. Annu Rev Plant Biol 72: 703–736 [DOI] [PubMed] [Google Scholar]
- Ueda M, Tsutsumi N, Fujimoto M (2016) Salt stress induces internalization of plasma membrane aquaporin into the vacuole in Arabidopsis thaliana. Biochem Biophys Res Commun 474: 742–746 [DOI] [PubMed] [Google Scholar]
- Vandeleur RK, Sullivan W, Athman A, Jordans C, Gilliham M, Kaiser BN, Tyerman SD (2014) Rapid shoot-to-root signalling regulates root hydraulic conductance via aquaporins. Plant Cell Environ 37: 520–538 [DOI] [PubMed] [Google Scholar]
- Wang C, Hu H, Qin X, Zeise B, Xu D, Rappel WJ, Boron WF, Schroeder JI (2016) Reconstitution of CO2 regulation of SLAC1 anion channel and function of CO2-permeable PIP2;1 aquaporin as CARBONIC ANHYDRASE4 interactor. Plant Cell 28: 568–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Calvo-Polanco M, Reyt G, Barberon M, Champeyroux C, Santoni V, Maurel C, Franke RB, Ljung K, Novak O, et al. (2019) Surveillance of cell wall diffusion barrier integrity modulates water and solute transport in plants. Sci Rep 9: 4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Wang M, Chen K, Wang S, Mur LAJ, Guo S (2018) Exploring the roles of aquaporins in plant-microbe interactions. Cells 7: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Sheng P, Tan J, Chen X, Lu G, Ma W, Heng Y, Lin Q, Zhu S, Wang J, et al. (2015) Plasma membrane receptor-like kinase leaf panicle 2 acts downstream of the DROUGHT AND SALT TOLERANCE transcription factor to regulate drought sensitivity in rice. J Exp Bot 66: 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Sklodowski K, Encke B, Schulze WX (2014) A kinase-phosphatase signaling module with BSK8 and BSL2 involved in regulation of sucrose-phosphate synthase. J Proteome Res 13: 3397–3409 [DOI] [PubMed] [Google Scholar]
- Wu XN, Chu L, Xi L, Pertl-Obermeyer H, Li Z, Sklodowski K, Sanchez-Rodriguez C, Obermeyer G, Schulze WX (2019) Sucrose-induced receptor kinase 1 is modulated by an interacting kinase with short extracellular domain. Mol Cell Proteomics 18: 1556–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XN, Sanchez Rodriguez C, Pertl-Obermeyer H, Obermeyer G, Schulze WX (2013) Sucrose-induced receptor kinase SIRK1 regulates a plasma membrane aquaporin in Arabidopsis. Mol Cell Proteomics 12: 2856–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wudick MM, Li X, Valentini V, Geldner N, Chory J, Lin J, Maurel C, Luu DT (2015) Subcellular redistribution of root aquaporins induced by hydrogen peroxide. Mol Plant 8: 1103–1114 [DOI] [PubMed] [Google Scholar]
- Xin XF, Nomura K, Aung K, Velasquez AC, Yao J, Boutrot F, Chang JH, Zipfel C, He SY (2016) Bacteria establish an aqueous living space in plants crucial for virulence. Nature 539: 524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Guo G, Zhang M, Liu CY, Hu Q, Lam H, Cheng H, Xue Y, Li J, Li N (2013) Stable isotope metabolic labeling-based quantitative phosphoproteomic analysis of Arabidopsis mutants reveals ethylene-regulated time-dependent phosphoproteins and putative substrates of constitutive triple response 1 kinase. Mol Cell Proteomics 12: 3559–3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip Delormel T, Boudsocq M (2019) Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana. New Phytol 224: 585–604 [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen L, Dong H. ( 2019a) Plant aquaporins in infection by and immunity against pathogens - A critical review. Front Plant Sci 10: 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Feng M, Chen W, Zhou X, Lu J, Wang Y, Li Y, Jiang CZ, Gan SS, Ma N, Gao J (2019b) In rose, transcription factor PTM balances growth and drought survival via PIP2;1 aquaporin. Nat Plants 5: 290–299 [DOI] [PubMed] [Google Scholar]
- Zhu D, Wu Z, Cao G, Li J, Wei J, Tsuge T, Gu H, Aoyama T, Qu LJ (2014) TRANSLUCENT GREEN, an ERF family transcription factor, controls water balance in Arabidopsis by activating the expression of aquaporin genes. Mol Plant 7: 601–615 [DOI] [PubMed] [Google Scholar]
- Zwiewka M, Nodzynski T, Robert S, Vanneste S, Friml J (2015) Osmotic stress modulates the balance between exocytosis and clathrin-mediated endocytosis in Arabidopsis thaliana. Mol Plant 8: 1175–1187 [DOI] [PubMed] [Google Scholar]