Abstract
Recent research on the regulation of cellular phosphate (Pi) homeostasis in eukaryotes has collectively made substantial advances in elucidating inositol pyrophosphates (PP-InsP) as Pi signaling molecules that are perceived by the SPX (Syg1, Pho81, and Xpr1) domains residing in multiple proteins involved in Pi transport and signaling. The PP-InsP-SPX signaling module is evolutionarily conserved across eukaryotes and has been elaborately adopted in plant Pi transport and signaling systems. In this review, we have integrated these advances with prior established knowledge of Pi and PP-InsP metabolism, intracellular Pi sensing, and transcriptional responses according to the dynamics of cellular Pi status in plants. Anticipated challenges and pending questions as well as prospects are also discussed.
This review article summarizes recent advances in the dynamics of intracellular phosphate metabolism and discusses how it transmits signals to coordinate the cellular phosphate transport systems for cellular Pi homeostasis control.
Introduction
Phosphorus (P) is one of the building blocks for nucleic acids, lipid membranes, and ATP in cells and is a macronutrient essential for plant growth and development. It is also a key determinant of agricultural yield. Plants take up P in the form of orthophosphate (Pi, H2PO4−/−) which is often fixed in soils, resulting in a low level of available Pi (<10 µM) in most soils (Holford, 1997). To secure the food supply, excessive application of P fertilizer is usually practiced in agriculture. Unlike nitrogen (N) fertilizer that can be chemically reproduced, P fertilizer is mainly sourced from finite and geographically restricted natural P rock reserves, which face rapid depletion due to intensive excavation to meet the agricultural demand over the past decades. Such a situation has resulted in the increasing market price of P fertilizer, and a threat to global food security and agricultural sustainability (Obersteiner et al., 2013). A sustainable measure to circumvent this issue would be to increase the P usage efficiency of crops through breeding (van de Wiel et al., 2016; Heuer et al., 2017), which will rely on knowledge of how plants sense and respond to environmental P levels.
Plants sense the available Pi level in the soil and respond accordingly to maintain cellular Pi homeostasis through coordination of Pi acquisition, translocation, storage, metabolism, and remobilization. In response to a low Pi environment, plants initiate multifaceted adaptive mechanisms to increase external Pi acquisition and enhance internal Pi remobilization and utilization, the so-called Pi starvation response (PSR). The PSR involves a series of metabolic, morphological, and transcriptomic changes mediated through signal transduction, protein modifications, and transcriptional reprogramming, which are suppressed upon Pi replenishment (Vance et al., 2003; Chiou and Lin, 2011; Secco et al., 2013). Recently, considerable advances have been made in identifying the intracellular Pi sensing signal(s), including Pi per se and inositol phosphates (InsP). In this review, we summarize current knowledge on the dynamics of intracellular Pi metabolism and discuss how it transmits signals to coordinate the cellular Pi transport systems for cellular Pi homeostasis control.
Dynamics of Pi metabolism in response to external Pi supply
About 0.2% of plant dry mass is made up of P, which comprises inorganic forms, such as Pi and pyrophosphate (PPi), and organic forms, such as nucleic acids, phospholipids (P-lipids), and low molecular mass P-esters (Veneklaas et al., 2012). The level of Pi generally reflects P fertilizer applied to plants (White and Hammond, 2008). Depending on the external Pi supply and using different measurement methods, the cytosolic Pi concentration has been estimated to be in the micromolar range (60–80 μM) by in vivo 31P-NMR analyses of Arabidopsis thaliana suspension cells (Pratt et al., 2009) or up to the millimolar range (1–10 mM) by live imaging of a Pi biosensor (Versaw and Garcia, 2017; Sahu et al., 2020). The cytosolic Pi concentration can be maintained at the beginning of Pi fluctuation due to buffering capacity from other subcellular pools (vacuoles in particular) and adjustment of Pi acquisition but ultimately declines when the Pi from those internal pools and external resources is exhausted after long-term Pi starvation (Mukherjee et al., 2015). On the other hand, the level of PPi, which is a byproduct in the final steps of the synthesis of macromolecules such as proteins, nucleic acids, and cellulose, etc., is relatively insensitive to Pi starvation when compared with cellular ATP pools (Heinonen, 2001; Plaxton and Podestá, 2007; Ferjani et al., 2012). At the early stage of Pi deprivation, PPi, relative to ATP, is favored as a phosphoryl donor for cytosolic glycolysis (Davies et al., 1993; Plaxton and Podestá, 2007).
When the cellular P level is in the optimal range (<4 mg/g dry weight), approximately 50% of total P is in the organic form (Veneklaas et al., 2012). In the organic P pool, ribosomal RNAs have the largest proportion (∼50%), followed by P-lipids, P-esters, DNA and other RNAs, and phosphoproteins (Veneklaas et al., 2012; Busche et al., 2021). Under severe P starvation conditions, when the supply of Pi cannot meet the demand to maintain optimal growth, recycling of Pi from the organic P pools appears to be crucial. Up to 90% of RNA (Hewitt et al., 2005) and DNA from mitochondria and chloroplasts or plastids (Takami et al., 2018) were reported to be mobilized after long-term Pi deficiency. Moreover, the replacement of phospholipids by other lipids such as sulfoquinovosyl diacylglycerol and digalactosyldiacylglycerol in response to Pi starvation has been reported in many plant species (Härtel et al., 2000; Kelly and Dörmann, 2002; Nakamura, 2013). Under normal growth conditions, cytosolic ATP was measured to be 0.8–1.2 mM using a FRET-based ATP sensor (Voon et al., 2018). When the cytoplasmic Pi level declines, large reductions (up to 80%) in intracellular levels of ATP, ADP, and related nucleotides also occur (Duff et al., 1989; Plaxton and Podestá, 2007; Zhu et al., 2019).
In seeds, P is predominantly stored as organic P in the form of InsP, which is a group of metabolites with various numbers of Pi covalently bound to a myo-inositol molecule (Irvine, 2005). Inositol hexakisphosphate (InsP6; also known as phytic acid, PA) interacts with cations, such as Ca2+, Fe3+, K+, Mg2+, and Zn2+, to form phytates that commonly represent up to 75% of total P in seeds (Raboy, 1997). InsPs are present in the plant vegetative tissues at a much lower concentration (<200 µM depending on tissues or species analyzed; Phillippy et al., 2015). While the level of InsP6 is irresponsive to changes of cellular Pi (Kuo et al., 2018; Dong et al., 2019), its diphosphorylated products, that is 5-diphosphoinositol pentakisphosphate 5PP-InsP5 (InsP7) and bis-diphosphoinositol tetrakisphosphate 1,5(PP)2-InsP4 (InsP8) (collectively termed PP-InsP), are subjected to change (Dong et al., 2019), a phenomenon also observed in yeast and human cells (Lee et al., 2007; Lonetti et al., 2011; Wild et al., 2016; Gu et al., 2017). As discussed in the following section, PP-InsPs are considered as energy-dependent metabolic messengers that reflect the cellular Pi status and are perceived by the SPX (named after the Saccharomyces cerevisiae Syg1 and Pho81 proteins and the mammalian Xpr1) sensor domain for cellular Pi homeostasis control (Wild et al., 2016; Azevedo and Saiardi, 2017; Gerasimaite et al., 2017; Jung et al., 2018; Shears, 2018; Lorenzo‐Orts et al., 2020; Ried et al., 2021).
Pi transport systems and their regulation in response to external Pi supply
The initial acquisition of Pi is mainly achieved by PHOSPHATE TRANSPORTER 1 (PHT1) located in the plasma membranes of root cells (Shin et al., 2004). The activity of PHT1 is modulated by external Pi availability. Under Pi-limited conditions, transcription of PHT1s is upregulated by PHOSPHATE STARVATION RESPONSE 1 (PHR1) and its close homolog PHR1-like 1 (PHL1), a class of MYB transcription factors that play a major role in controlling the PSR in Arabidopsis (Rubio et al., 2001; Bustos et al., 2010). Other than PHR1, NITRATE-INDUCIBLE, GARP-TYPE TRANSCRIPTIOANL REPRESSOR1.2 (NIGT1.2), and OsWRKY21 or OsWRKY108 transcription factors were shown to directly regulate the transcription of PHT1s (Wang et al., 2020b; Zhang et al., 2021a). Moreover, phyB-mediated activation of PHT1s via phytochrome interacting factors PIF4/PIF5 and HY5 transcription factors in response to Pi deficiency was reported, which highlights the importance of optimizing plant growth under different light and nutrient conditions (Sakuraba et al., 2018).
The exit of PHT1 proteins from the endoplasmic reticulum (ER) is facilitated by PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR 1 (PHF1), which regulates the targeting of PHT1 proteins to plasma membranes (Gonzalez et al., 2005). In response to Pi replenishment, transcription of PHT1s and exiting of PHT1 proteins from the ER are suppressed (Bayle et al., 2011; Chen et al., 2015). The abundance of PHT1 proteins is controlled through ubiquitin-mediated degradation by NITROGEN LIMITATION ADAPTATION (NLA), a ubiquitin E3 ligase, in the plasma membrane, and PHOSPHATE 2 (PHO2)/UBC24, a ubiquitin E2 conjugase in the ER (Huang et al., 2013; Lin et al., 2013; Park et al., 2014). Ubiquitinated PHT1 proteins are delivered to vacuoles for degradation via ENDOSOMAL COMPLEX REQUIRED FOR TRANSPORT (ESCRT)-III-associated protein ALG2-interacting protein X (ALIX) (Cardona-López et al., 2015). Arabidopsis AtNLA and AtPHO2 are regulated post-transcriptionally, cleaved by miR827 and miR399, respectively, particularly under low P (Aung et al., 2006; Bari et al., 2006; Chiou et al., 2006; Hsieh et al., 2009; Lin et al., 2013). PHT1 proteins can be phosphorylated depending on the external P supply. A high level of Pi promotes phosphorylation of Arabidopsis AtPHT1;1 at Ser-514 (Bayle et al., 2011), or of rice (Oryza sativa) OsPT8 at Ser-517 mediated by casein kinase 2 (Chen et al., 2015), resulting in retention of Pi transporters in the ER to prevent excess Pi accumulation. Conversely, low Pi leads to dephosphorylation of OsPT8 at Ser-517 via OsPP95, a type 2C protein phosphatase, to facilitate its ER exit (Yang et al., 2020b). In addition, phosphorylation at Ser-520 of AtPHT1;1 was reported to enhance its transport activity (Wang et al., 2020a).
Cellular Pi homeostasis is also controlled by Pi allocation between the cytosol and organelles. About 70%–95% of the intracellular Pi is stored in vacuoles at micromolar to millimolar concentrations according to external Pi supply (Yang et al., 2017) as a result of Pi influx and efflux activities operated by Arabidopsis PHOSPHATE TRANSPORTER 5 (AtPHT5)/VACUOLAR PHOSPHATE TRANSPORTER (AtVPT) and rice VACUOLAR PHOSPHATE EFFLUX TRANSPORTER 1/2 (OsVPE1/2), respectively (Liu et al., 2015, 2016; Xu et al., 2019). When cytosolic Pi is high, excess Pi is stored in the vacuole by AtPHT5. Loss-of-function atpht5 mutants have a significant reduction of vacuolar Pi and increased cytosolic Pi levels as revealed by 31P-NMR studies or a genetically encoded FRET-based Pi sensor (Liu et al., 2016; Luan et al., 2019; Sahu et al., 2020). When the cytosolic Pi level is low, vacuolar Pi is released by the Pi efflux transporter OsVPE1/2 (Xu et al., 2019) to support the cytosolic demand of Pi. It is important to note that the change in AtPHT5 activity can affect the expression of AtPHT1s (Liu et al., 2016), suggesting intracellular coordination to maintain an optimal cytosolic Pi concentration.
Pi is also transported across the membranes of other organelles. PHT2 (Daram et al., 1999; Versaw and Harrison, 2002; Rausch et al., 2004; Guo et al., 2013; Liu et al., 2020) and some members of PHT4 (Ferro et al., 2002; Roth et al., 2004; Guo et al., 2008) mediate Pi transport across the envelop membranes of the plastid/chloroplast. They contribute to ATP synthesis or starch accumulation in chloroplasts or participate in stress responses, which affects plant growth (Irigoyen et al., 2011; Wang et al., 2011; Karlsson et al., 2015). Additionally, Pi can be exchanged with phosphorylated C3, C5, or C6 carbon compounds via plastidic phosphate translocators (pPTs) across the plastid envelope to ensure Pi balance between the stroma and cytosol (Weber and Linka, 2011; Poirier and Jung, 2015).
PHT3 is involved in Pi transport across mitochondrion inner membranes to support the generation of ATP (Hamel et al., 2004; Zhu et al., 2012) and PHT4;6 is responsible for Pi export from Golgi essential for sugar-nucleotide metabolism (Guo et al., 2008; Cubero et al., 2009). Nevertheless, very little is known about the regulation of these organellar Pi transporters.
The Pi allocation between organs in plants relies on the Pi efflux activities of the PHOSPHATE1 (PHO1) family of proteins (Poirier et al., 1991). PHO1 is located in the Arabidopsis root stellar cells and mediates unloading of Pi into the xylem apoplastic space for subsequent translocation up to the shoots (Poirier et al., 1991; Hamburger et al., 2002; Arpat et al., 2012; Liu et al., 2012). Its physiological role for root-to-shoot Pi allocation was demonstrated by the reduced Pi in the shoots and higher Pi accumulation in the roots of pho1 mutants in Arabidopsis and rice (Poirier et al., 1991; Secco et al., 2010; Che et al., 2020). AtPHO1 is also expressed in leaf guard cells where it is involved in the stomatal response to ABA (Zimmerli et al., 2012), and in the chalazal seed coat to transfer Pi from maternal tissues to embryos in developing seeds (Vogiatzaki et al., 2017). In rice, PHO1s are also expressed at the vasculature of the uppermost node connecting to the panicle and in developing seed tissues, where they are involved in the allocation of Pi during grain filling (Che et al., 2020; Ma et al., 2021). In Medicago (Medicago truncatula), PHO1 mediates the transfer of Pi from nodule infected cells to bacteroids (Nguyen et al., 2021). Negative regulations of Arabidopsis PHO1 either by WRKY6 at the transcriptional level (Chen et al., 2009) or by its upstream open-reading frame at the translational level (Reis et al., 2020) were reported. Conversely, the interaction between rice PHO1.2 mRNA and its cis-natural antisense transcript can enhance its translation (Jabnoune et al., 2013; Reis et al., 2021).
Taken together, the activities of cellular Pi transport systems in plant cells are modulated by the external Pi levels as well as internal demands at the cellular and organismal levels via multiple mechanisms, including transcriptional, post-transcriptional, and post-translational regulation. It is worth noting that several of these transporters or their regulators (i.e. NLA, PHT5, and PHO1) possess a common SPX domain, a protein domain that perceives cellular Pi levels by binding to InsPs/PP-InsPs as discussed below.
Intracellular P sensors: SPX domains and their roles in Pi signaling and transport
Plant genomes encode four groups of SPX domain-containing proteins: SPX single-domain proteins and three other types of proteins comprising an N-terminal SPX domain in conjunction with an additional domain including PHO1 (SPX-EXS (S. cerevisiae Erd1, mammalian Xpr1, and S. cerevisiae Syg1)) and NLA (SPX-RING (Really Interesting New Gene)) and PHT5/VPT (SPX-MFS (Major Facilitator Superfamily)) (Secco et al., 2012a). In yeast, SPX domain-containing proteins include a cyclin-dependent kinase inhibitor Phosphatase 81 (Pho81); Pi transporters of PHO87, PHO90, and PHO91; glycerophosphocholine phosphodiesterase GDE1; polyphosphate (polyP) synthesis complex vacuolar transporter chaperone 2–5 (VTC2-VTC5); and a plasma membrane protein with unknown function SYG1 (Secco et al., 2012b; Desfougeres et al., 2016). In contrast, the human genome encodes only one SPX domain-containing protein Xenotropic and Polytropic Retrovirus Receptor 1 (XPR1), a homolog of PHO1, which is involved in cellular Pi efflux (Giovannini et al., 2013; Legati et al., 2015). Diversification of SPX domain protein-coding genes in yeast and plant genomes may imply the requirement of more elaborate regulation of P sensing in sessile organisms.
Several lines of evidence suggest that SPX domains have a Pi sensing function. First, mutations of the SPX domain impair Pi transport activities of the transporters that bear it (Wege et al., 2016; Wild et al., 2016). Second, SPX domains mediate protein–protein interactions to regulate Pi transport (Wykoff et al., 2007; Hürlimann et al., 2009; Lin et al., 2013) or transcription of PSR genes (Lv et al., 2014; Puga et al., 2014; Wang et al., 2014; Desmarini et al., 2020) in various organisms from yeast to plants. Importantly, structural studies of the SPX domain and identification of Pi signaling molecules, InsP/PP-InsP as its binding ligands provide direct evidence for its role as an intracellular Pi sensor (Wild et al., 2016). Crystal structures demonstrated that the SPX domain comprising 135–380 amino acids is arranged in six alpha-helices with highly conserved and positively charged amino acid residues located in the alpha 2 and alpha 4 helices that are denoted by the Pi binding cluster (PBC) and lysine surface cluster (KSC). The PBC and KSC constitute the basic binding surface for negatively charged InsP/PP-InsP and are essential for the SPX protein functions (Wild et al., 2016). The SPX domains have a higher binding affinity to InsP/PP-InsP (at the sub-micromolar level for InsP6–7) than to Pi and PPi (at the millimolar and sub-millimolar level, respectively; Wild et al., 2016). The presence of SPX domains in a variety of proteins involved in Pi transport and transcriptional regulation of PSR accentuates the need for multifaceted sensing mechanisms for cellular Pi homeostasis coordination in plants. Current knowledge regarding the roles of SPX domains for the function of different groups of SPX-domain-containing proteins is discussed below.
SPX single-domain proteins
The function of SPX single-domain proteins has been studied extensively with regard to their interactions with AtPHR1 in Arabidopsis or OsPHR2 in rice (Lv et al., 2014; Puga et al., 2014; Wang et al., 2014). The Arabidopsis genome encodes four (AtSPX1-4) whereas rice contains six SPX (OsSPX1-6) single-domain proteins. The expression of all SPX genes except AtSPX4 and OsSPX4 is upregulated in response to Pi starvation (Duan et al., 2008; Wang et al., 2009). Under Pi-replete conditions, interactions between SPX1/2 and AtPHR1/OsPHR2 in the nucleus prevent AtPHR1/OsPHR2 from binding to the P1BS cis-element (GNATATNC) in the promoter of many PSR genes (Puga et al., 2014; Wang et al., 2014). Recently, it was reported that the KHR motif (AtPHR1K325, AtPHR1H328, and AtPHR1R335) at the surface of the coiled-coil domain of AtPHR1 is required for its interaction with AtSPX1 (Ried et al., 2021). Alternatively, cytosolic OsSPX4 interacts with OsPHR2 to sequester OsPHR2 from targeting to the nucleus (Lv et al., 2014). Under Pi starvation, OsSPX4 undergoes rapid turnover mediated by RING-finger SPX4 degradation E3 ligases (OsSDEL1/2) (Ruan et al., 2019). The interaction between AtSPX1/OsSPX1 and AtPHR1/OsPHR2 was originally shown to be facilitated by Pi and its analog phosphite (Phi) at an optimal concentration of 15 mM in vitro (Puga et al., 2014; Wang et al., 2014). While the dynamics of cytosolic Pi levels in response to external Pi changes remains debatable, InsP7 that is metabolically dependent on cytosolic Pi levels was later shown to trigger the OsSPX4–OsPHR2 interaction at sub-micromolar concentrations (Wild et al., 2016), suggesting a role for SPX as a sensor to transducing cellular Pi levels.
Interestingly, OsSPX4 also interacts with a master transcription regulator of the nitrate response NIN-LIKE PROTEIN 3 (OsNLP3), a rice homolog of AtNLP7 (Hu et al., 2019). Under low nitrate conditions, regardless of Pi level, OsSPX4 interacts with OsPHR2 and OsNLP3 and retains them in the cytosol to prevent their activation on PSR genes and nitrate responsive genes (Hu et al., 2019; Hu and Chu, 2020). Under high nitrate and low Pi conditions, the nitrate sensor NITRATE TRANSPORTER 1.1 (NRT1.1) (Ho et al., 2009) recruits a plasma membrane-localized E3 ubiquitin ligase NRT1.1B INTERACTING PROTEIN 1 (OsNBIP1), together with OsSDEL1/2, to ubiquitinate OsSPX4 (Hu et al., 2019). This leads to a low level of OsSPX4 in the cytosol and an increase in the nucleus translocation of OsPHR2 and OsNLP3 for gene activation (Hu et al., 2019), suggesting that OsSPX4 has a pivotal role in the N–P interactive regulation network. Further study showed that REGULATOR OF LEAF INCLINATION 1 (RLI1)/HIGHLY INDUCED BY NITRATE GENE 1 (HINGE1), a MYB-transcription factor closely related to PHR2, could compete with OsPHR2 for binding to OsSPX1/2/4 to enhance PSR (Zhang et al., 2021b). Moreover, the nitrate-inducible GARP-type transcriptional repressor 1 (NIGT1) was reported to act as a transcriptional repressor of SPX genes by directly binding to the SPX promoters in Arabidopsis (Ueda et al., 2020). AtSPX4 can also interact with AtPAP1 to regulate anthocyanin biosynthesis in an InsP-dependent manner (He et al., 2021b). In rice, a basic helix–loop–helix protein OsbHLH6 can competitively inhibit the interaction between OsSPX4 and OsPHR2 and thus regulate PSRs (He et al., 2021a). Therefore, sequestration of AtPHR1/OsPHR2 by SPX proteins can be modulated by repression of SPX transcription, degradation of SPX proteins, or competitive binding with the SPX domain by other proteins.
PHO1 (SPX-EXS)
PHO1 contains a cytosolic SPX domain at the N terminus, followed by four transmembrane motifs, and an EXS domain at the C-terminus (Wege et al., 2016). It has been shown that the SPX-spanning region of PHO1 interacts with PHO2 that mediates its degradation (Liu et al., 2012), and the truncation of the SPX domain did not affect the Pi efflux activity of PHO1 in a transient expression assay (Wege et al., 2016). However, a truncated version lacking the SPX domain or mutations at the PBC or KSC residues of PHO1 was unable to complement the impaired activity of root-to-shoot Pi translocation in pho1 mutants, suggesting that the SPX domain is essential and InsP/PP-InsP binding regulates its activity (Wege et al., 2016; Wild et al., 2016). In comparison, depletion of the cytosolic SPX domains of yeast PHO87 and PHO90 Pi transporters resulted in unrestricted Pi uptake and accumulation of cytosolic Pi to a toxic level, suggesting a repressive function of the SPX domain on these plasma-membrane Pi transporters (Hürlimann et al., 2009). While the Pi transport mechanism of PHO1 and its membrane localization remain enigmatic (Chiou, 2020), these confounding observations emphasize the necessity of structural analysis of PHO1 to dissect the role of SPX for its regulation. PHO1 has 10 other SPX-domain bearing homologs in Arabidopsis that have overlapping or non-overlapping expression and functions (Hamburger et al., 2002). It is therefore intriguing whether or how their SPX domains may differentially regulate their activity.
PHT5 (SPX-MFS)
As a major vacuolar Pi import transporter, PHT5 bears an N-terminal SPX domain predicted to face the cytosol and a MFS domain at the C-terminus. Unlike OsVPE1/2, the transcription level of AtPHT5 is irresponsive to Pi status (Liu et al., 2016) and therefore its activity may be regulated at the protein or posttranslational level, likely through its SPX domain. While the role of the SPX domain and its interaction with InsP/PP-InsP for AtPHT5 activity remains to be investigated, studies of the vacuolar P transport system in yeast demonstrated that PP-InsP and SPX control its activity. In yeast, Pi was stored in the vacuole in the form of inorganic polyP synthesized and transported by the VTC complex located on the vacuolar membranes, in which four of the five subunits (VTC2–VTC5) bear SPX domains. The polymerase activity of the VTC complex is dependent on the PP-InsP (InsP7) synthesis (Lonetti et al., 2011). Noticeably, while mutations of most PBC or KSC residues disrupt polyP polymerase activity of the VTC complex, substitutions at a particular residue (K126/129 in VTC3/4) result in constitutive activation of polyP synthesis in the absence of InsP7 (Wild et al., 2016), suggesting that PP-InsP binding may affect a structural change of the complex and hence its activity. InsP/PP-InsP can also stimulate the activity of the Pi exporter PHO91 in yeast vacuoles and Trypanosoma brucei acidocalcisomes (Potapenko et al., 2018), suggesting conservation in adoption of the SPX domain for vacuolar P-storage control in eukaryotes.
NLA (SPX-RING)
AtNLA controls the protein abundance of AtPHT1 Pi transporters at plasma membranes according to the availability of Pi (Lin et al., 2013; Park et al., 2014). The N-terminal SPX domain of AtNLA interacts with AtPHT1 and directs it for ubiquitination mediated by its C-terminal RING domain E3 ligase (Lin et al., 2013; Yang et al., 2020a). Under Pi-deficient conditions, AtNLA-mediated ubiquitination of AtPHT1 is attenuated by Pi starvation-induced miR827 (Hsieh et al., 2009; Lin et al., 2013). AtNLA also modulates the degradation of the nitrate transporter NRT1.7 through the binding of its SPX domain (Liu et al., 2017), providing a potential argument for N–P crosstalk. Moreover, AtNLA was shown to interact with ORESARA1 (ORE1) through its SPX domain in the nucleus to control leaf senescence (Park et al., 2018). Whether InsP/PP-InsP also mediates the interaction of AtNLA–AtPHT1, AtNLA–AtNRT1.7, or AtNLA–AtORE1 in response to cellular Pi levels awaits further studies.
Collectively, it is evident that the SPX domain is relevant to the functions of Pi transport proteins, yet its mechanistic effects on the transport activities in association with their InsP/PP-InsP ligands remain largely unknown, which emphasizes the need for structural dissection with functional studies in the future.
Metabolic messengers for intracellular Pi signaling: PP-InsPs and their metabolism
Prior to the discovery of InsP and PP-InsP as the SPX-domain binding ligands, genetic and physiological studies suggested a role for InsP biosynthesis in the regulation of Pi homeostasis in yeast, animals, and plants (Norbis et al., 1997; Schell et al., 1999; Stevenson-Paulik et al., 2005; Lee et al., 2007; Lonetti et al., 2011; Kuo et al., 2014, 2018). InsP and PP-InsP are synthesized through step-wise phosphorylation catalyzed by evolutionarily conserved kinases (Figure 1). In plants, mutations in the inositol-tetrakisphosphate 1-kinase (ITPK1) or inositol pentakisphosphate 2-kinase (IPK1) cause excessive Pi accumulation and activation of PSR genes under Pi-replete conditions (Stevenson-Paulik et al., 2005; Kuo et al., 2018). Mutations of ITPK1 and IPK1 disturb the stoichiometry of InsP intermediates, including accumulation of InsP4 or InsP5 and decreases in InsP6-7-8 levels (Stevenson-Paulik et al., 2005; Laha et al., 2015; Kuo et al., 2018; Riemer et al., 2020), confounding differentiation of the causal InsP species that control Pi homeostasis. Two recent studies collectively provided evidence that InsP8 is a key molecule in mediating Pi signaling. They showed that kinase-dead mutations of the DIPHOSPHOINOSITOL PENTAKISPHOSPHATE KINASES (PPIP5K) in Arabidopsis, Vip Homologs (VIH1 and VIH2) resulted in no detectable InsP8 and the vih1 vih2 mutant exhibited severe growth defects with constitutive activation of PSR genes and excessive cellular Pi accumulation (Dong et al., 2019; Zhu et al., 2019). These phenotypes were significantly suppressed by loss-of-function mutations of PHR1 and PHL1, indicating that VIH1/2 control Pi homeostasis predominantly through PHR1/PHL1 (Zhu et al., 2019). Also, InsP8 levels were positively corresponded to cellular Pi levels (Dong et al., 2019). Together with the observation that PP-InsPs facilitate the SPX–PHR1 interaction at a near sub-micromolar concentration (Wild et al., 2016), it is hypothesized that high concentrations of cellular Pi drive VIH to synthesize InsP8 that promotes the SPX–PHR1 interaction and thus sequesters PHR1 from transcriptional activation of PSR genes, whereas under low cellular Pi, InsP8 decreases and releases PHR1 from interacting with SPX to activate PSR gene expression (Dong et al., 2019; Zhu et al., 2019).
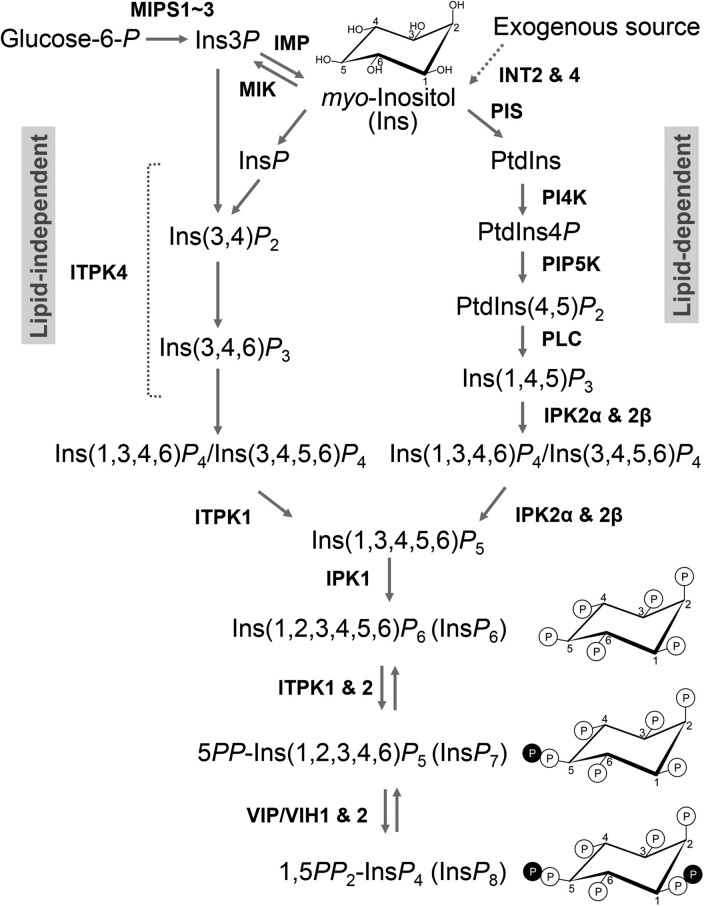
Figure 1.
InsP and PP-InsP biosynthesis pathways in plants. Schematic pathways for the synthesis of InsP6 and PP-InsP in plants. The biosynthesis of InsP consists of a “lipid-dependent pathway” based on biochemical activities of homologous enzymes in Arabidopsis and a “lipid-independent pathway” identified in duckweed (Spirodela polyrhiza) (Brearley and Hanke, 1996; Stevenson-Paulik et al., 2005; Kuo et al., 2018). There are two classes of kinases that synthesize the PP-InsP in eukaryotes: IP6K that phosphorylates InsP6 to generate 5PP-InsP5 (InsP7) and PPIP5K that can phosphorylate 5PP-InsP5 to produce 1,5(PP)2-InsP4 (InsP8) (Shears, 2018). Plant genomes do not encode IP6K homologs and the InsP6 kinase activities of ITPK1 (and ITPK2) have been postulated to generate 5PP-InsP5 (Adepoju et al., 2019; Laha et al., 2019; Whitfield et al., 2020). The plant PPIP5K homologs, VIP1/VIH2 and VIP2/VIH1 coordinately catalyze 5PP-InsP5 into 1,5(PP)2-InsP4 (Desai et al., 2014; Laha et al., 2015; Zhu et al., 2019). MIPS, inositol-3-phosphate synthases; IMP, inositol monophosphatase; MIK, myo-inositol kinase; INT2 and 4, inositol transporters; PtdIns, phosphatidylinositol; PIS, phosphatidylinositol synthase; PI4K, phosphatidylinositol 4-kinase; PIP5K, phosphatidylinositol-4-phosphate 5-kinase; PLC, phospholipase C; IPK2α and 2β, inositol polyphosphate 6-/3-kinases; ITPK1 and 4, inositol 1,3,4-trisphosphate 5-/6-kinase; IPK1, inositol pentakisphosphate 2-kinase; and VIP/VIH1 and 2, Vip1 homologs. The dashed bracket, unresolved catalytic steps mediated by ITPK4. The dashed arrow, an uptake process; reversible arrows, kinase/ADP phosphotransferase, and kinase/pyrophosphatase activities of ITPK1 and VIP/VIH1 and 2, respectively.
Emerging evidence suggests that InsP7 and InsP8 are evolutionarily conserved Pi signaling molecules that are metabolically linked to cellular Pi levels. In yeast and human cells, the levels of cellular InsP7 and InsP8 are also positively associated with cellular Pi levels (Lonetti et al., 2011; Wild et al., 2016; Gu et al., 2017). Disruption of the yeast pKC1 suppressor (Kcs1; an inositol hexakisphosphate and inositol heptakisphosphate kinase) inhibited vacuolar polyP synthesis (Lonetti et al., 2011). Human inositol hexakisphosphate kinases IP6K (InsP7 synthesis kinase) and PPIP5K knockout lines or pharmacological inhibition of IP6K enzymatic activity blocked XPR1-mediated Pi efflux (Wilson et al., 2019; Li et al., 2020). It is therefore relevant to understand how cellular Pi status relays InsP7 and InsP8 synthesis and their turnover.
Recent studies demonstrated that ITPK1 and VIH are bifunctional enzymes that are capable of catalyzing reversible steps to synthesize and hydrolyze InsP7 and InsP8, respectively, depending on the cellular ATP levels (Zhu et al., 2019; Riemer et al., 2020; Whitfield et al., 2020). The cellular ATP/ADP ratio changes in accordance with the cellular Pi level, being high when cells are supplied with sufficient Pi, and decreasing upon Pi starvation (Gu et al., 2017; Zhu et al., 2019). At a high cellular ATP/ADP ratio, the ITPK1 kinase phosphorylates InsP6 to generate InsP7, which is in turn phosphorylated into InsP8 by VIH. On the other hand, as the cellular Pi level decreases in response to Pi starvation, a decreased ATP/ADP ratio stimulates ADP-transferase activity of ITPK1 and the VIH phosphatase activity (possessed by the C-terminal domain of VIH) for InsP7 and InsP8 hydrolysis (Zhu et al., 2019; Riemer et al., 2020; Whitfield et al., 2020).
Because the human IP6K displays a Km of 1 mM for ATP that is comparable to the cellular ATP concentration (Wundenberg and Mayr, 2012; Voon et al., 2018), InsP7 has been considered to be a metabolic messenger, acting as a cellular energy sensor (Shears, 2009; Wundenberg and Mayr, 2012; Wilson et al., 2013). However, genetic studies in plants and human cell lines, in which loss-of-function mutations of VIH1/2 and PPIP5Ks exerted the strongest effects, respectively, on the PSR activation and attenuation of XPR1-mediated Pi efflux (phenocopied XPR1 knockout lines), argued that InsP8 is the cellular Pi-sensing signal (Zhu et al., 2019; Li et al., 2020). The fact that levels of InsP8 are more responsive to cellular ATP and Pi changes (Gu et al., 2017; Dong et al., 2019), and that Pi and Phi are both able to inhibit PPIP5K phosphatase activity or suppress PSR in planta (Gu et al., 2017; Zhu et al., 2019), further substantiate the claim that InsP8 is the cellular Pi signal despite that in vivo impacts of Phi on the cellular InsP8 level has yet to be demonstrated.
The earlier observations that the non-metabolizable Pi analog Phi could mimic Pi to suppress the PSR when Pi was depleted implied that Pi per se is a signal (Ticconi et al., 2001; Varadarajan et al., 2002). A more direct demonstration of Pi as a possible signal in triggering the PSR was that Pi or Phi was able to promote the interaction between PHR1 and SPX1 in vitro (Puga et al., 2014; Wang et al., 2014). The binding affinity of Pi to the SPX domain according to isothermal titration calorimetry was measured to be in the millimolar range, which despite being lower than InsP/PP-InsP by two–three orders, is compatible with the physiological concentrations (Wild et al., 2016; Versaw and Garcia, 2017; Sahu et al., 2020). In contrast, no binding between OsSPX4 and OsPHR2 was detected in the presence of Pi (Wild et al., 2016) arguing against Pi as a signaling molecule. Moreover, replenishment of Pi, but not Phi, to P-starved rice was able to stabilize OsSPX4 (Osorio et al., 2019), hinting that P metabolites rather than Pi per se mediate the OsSPX4–OsPHR2 interaction.
Unresolved issues regarding intracellular Pi signaling mediated by PP-InsPs and SPX domains
An overview based on our current knowledge of intracellular Pi signaling and the activities of Pi transport systems under different cellular Pi levels is shown in Figure 2. Considering the different roles of SPX-bearing proteins in modulating cellular Pi homeostasis, how they perceive Pi-signals to function in accordance will be the focus of future research. Whether InsP/PP-InsP act as ligands in mediating protein–protein interactions as a general mechanism (as exemplified by the PHR1–InsP8–SPX1 interaction) that applies to the regulation of other SPX-proteins awaits further investigation. To investigate this scenario, identifying the interacting components of other SPX proteins becomes a prerequisite. Nevertheless, it is possible that the binding of PP-InsP to the SPX domain per se may regulate the function of the protein it bears independent of interacting partners as a result of structural changes (Furkert et al., 2020; Lorenzo‐Orts et al., 2020).
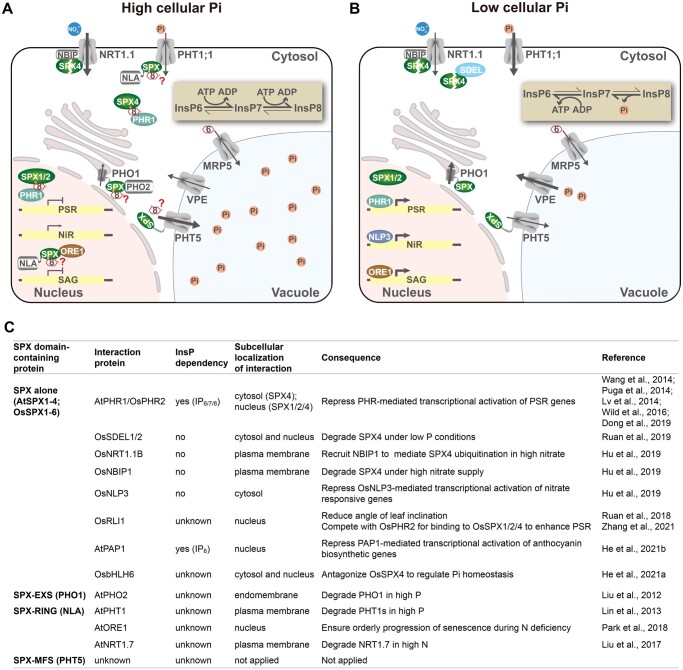
Figure 2.
A model for PP-InsP sensing by SPX domain proteins in plants. Under Pi-replete conditions (A), high cellular Pi and ATP levels drive InsP8 synthesis, which promotes the PHR–SPX interaction and thus suppresses the activation of PSR genes. Under Pi-depleted conditions (B), the decrease of cellular Pi and ATP results in a decline of InsP8 level. Accordingly, PHR1 is released to activate PSR genes. Expression of nitrate-responsive genes (NiR) and senescence-associated genes (SAG) is also activated by NLP3 and ORE1, respectively. It is likely that changes in the InsP8 level also regulates the activity of other SPX-domain proteins, including NLA, PHO1, and PHT5 involved in Pi acquisition, translocation, and storage, respectively. MRP5 is a vacuolar InsP6
synthesis, which promotes the PHR–SPX interaction and thus suppresses the activation of PSR genes. Under Pi-depleted conditions (B), the decrease of cellular Pi and ATP results in a decline of InsP8 level. Accordingly, PHR1 is released to activate PSR genes. Expression of nitrate-responsive genes (NiR) and senescence-associated genes (SAG) is also activated by NLP3 and ORE1, respectively. It is likely that changes in the InsP8 level also regulates the activity of other SPX-domain proteins, including NLA, PHO1, and PHT5 involved in Pi acquisition, translocation, and storage, respectively. MRP5 is a vacuolar InsP6 transporter. Thickness of arrows indicates the strength of activities. C, A summary of proteins interacting with SPX domain-containing proteins and their mediated physiological responses (see text for details).
transporter. Thickness of arrows indicates the strength of activities. C, A summary of proteins interacting with SPX domain-containing proteins and their mediated physiological responses (see text for details).
Another glaring question regards how InsP/PP-InsP as Pi-signaling molecules coordinate the activities of different Pi transport systems temporally and spatially to modulate cellular Pi homeostasis in response to changes in cellular Pi levels. Comparisons between the affinities of different InsPs and PP-InsPs to SPX domains and their relative cellular levels suggest the possibility of competitive bindings. For example, the ratio of cellular concentration of InsP6 to InsP7 (more than three orders) is greater than that of their binding affinity to SPX (two orders; Laha et al., 2015; Wild et al., 2016; Kuo et al., 2018), and InsP6 has been demonstrated to compete with InsP7 in protein pyrophosphorylation (Saiardi et al., 2004; Wu et al., 2016). Therefore, the competitive outcomes of binding of InsP/PP-InsP to SPX-domain proteins need to be assessed in the physiological context. Similarly, the discrepancy in the binding affinity of the SPX domains from different proteins for a given InsP/PP-InsP species (such as InsP8) has to be considered equally. SPX domains from different proteins (SPX, PHO1, NLA, and PHT5) may also sense the change in InsP or PP-InsP at different affinities, which allows them to function differently at particular cellular InsP/PP-InsP levels. Recently, the development of capillary electrophoresis coupled to electrospray ionization mass spectrometry (CE–ESI–MS) allowed differentiating PP-InsP isomers and has identified isomers of InsP7 in planta, that is 5-PP-InsP5 and 1,3-PP-InsP5 (Qiu et al., 2020). Implementation of this analytical approach should facilitate dissecting the biological relevance of PP-InsP isomers and their interacting proteins.
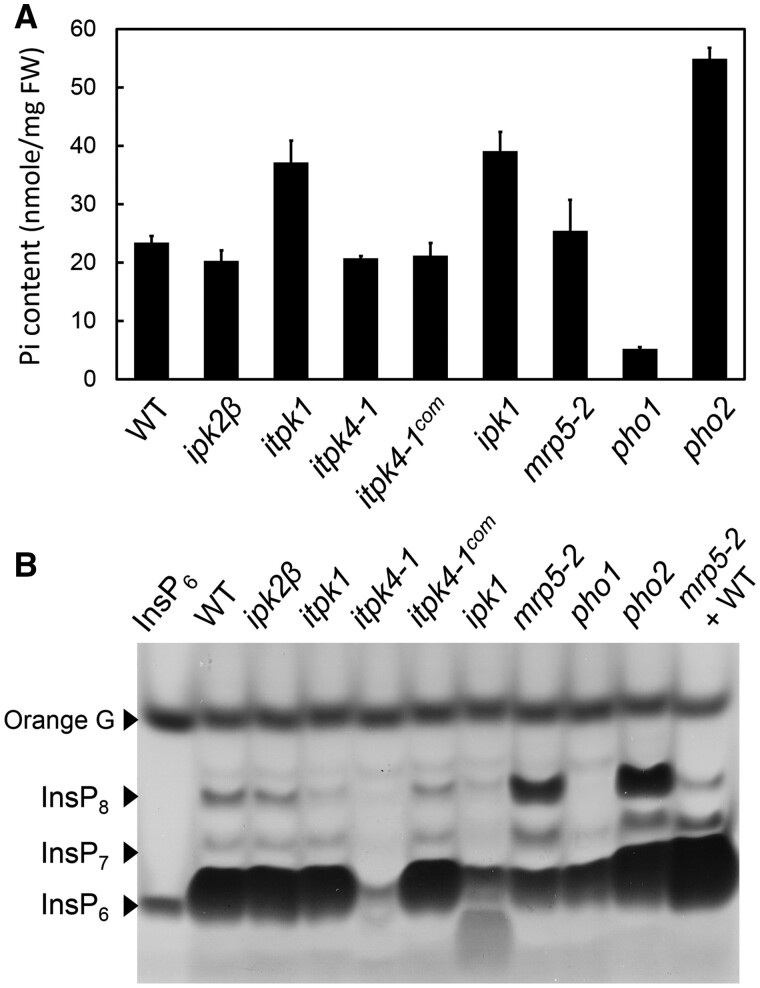
Furthermore, the subcellular compartmentalization of the InsP/PP-InsP should be considered. We and others have observed that changes in overall cellular InsP7 and InsP8 levels did not necessarily alter Pi homeostasis. The Arabidopsis itpk4 mutation resulted in substantial decreases of InsP6-7-8 as ipk1 and itpk1 without affecting cellular Pi content nor constitutively activating PSR genes under Pi-replete conditions (Figure 3; Kuo et al., 2018). On the other hand, the multidrug resistance‐associated protein 5 (mrp5) mutants detective in storage of InsP6 into vacuoles exhibited overall elevated InsP7 and InsP8 in vegetative tissues (Figure 3; Riemer et al., 2020), similar to the pho2 mutants (Dong et al., 2019), yet they displayed wild-type levels of cellular Pi (Figure 3; Kuo et al., 2018). One conceivable explanation is that the PP-InsPs have to be generated and/or present at a definite subcellular location to serve as “effective” Pi-signals. The development of subcellular PP-InsP tracing markers will facilitate addressing this question.
Figure 3.
Cellular InsP7-8 levels are not necessarily positively associated with cellular Pi levels. A, The shoot Pi contents of various mutants (14-d-old seedlings grown on half-strength Murashige and Skoog medium) involved in InsP/PP-InsP and Pi metabolism as described by Kuo et al. (2018). itpk4-1com denotes the itpk4-1 complementary line expressing the YFP-tagged ITPK4 CDS (Kuo et al., 2018). Error bars, SE of three independent experiments. B, InsP/PP-InsP profiles in the shoots of the corresponding genotypes shown in (A). Commercially purchased InsP6 (Sigma) and the mixture of mrp5-2 and Col-0 wild-type seed extract (mrp5-2 +WT) were used as markers for InsP6-7-8 (Desai et al., 2014). InsP/PP-InsP were extracted from equal mass of 14-d-old seedlings (1 g) and enriched by TiO2 beads followed by polyacrylamide gel electrophoresis analysis as described (Wilson et al., 2015).
By using synthetic InsP7 as bait to search for its interacting proteins, a recent study identified a myriad of proteins absent of SPX domains, among which are the ribose-phosphate pyrophosphokinases 1 and 2 (PRPS1 and PRPS2) and their associated proteins PRPSAP1 and PRPSAP2 that are involved in nucleotide biosynthesis (Furkert et al., 2020). This finding suggests a potential correlation between the Pi-signaling and nucleotide biosynthesis and inspires speculation about whether SPX-independent proteins can perceive PP-InsPs to modulate cellular Pi homeostasis. On the other hand, InsP7 has been shown to mediate enzyme-independent pyrophosphorylation of proteins involved in biological processes that are tightly linked to the cellular energy status such as glycolysis and ribosome biogenesis (Bhandari et al., 2007; Szijgyarto et al., 2011; Thota et al., 2015; Wu et al., 2016). Protein pyrophosphorylation has not been reported in plants and whether PP-InsPs can modulate the activity of plant Pi transport systems via this mechanism remains to be explored.
Conclusions
Over recent years, active research into InsP metabolism driven by the study of Pi signaling in plants has not only elucidated key kinases responsible for InsP/PP-InsP biosynthesis but also extended our knowledge of InsP metabolism in plants. This advance is also expected to foster the investigation of previously unsolved biological processes that intersect with InsPs/PP-InsPs metabolism. With the identification of SPX domains as intracellular sensors that perceive InsP/PP-InsP as cellular Pi signaling molecules, we expect accelerated progress toward a comprehensive understanding of the regulation of Pi transport systems at the organismal level.
Advances box
Structural and functional analyses of the SPX domains establish their role as intracellular P sensors through the binding with PP-InsPs rather than Pi.
Characterizations of the enzymes and their corresponding mutants involved in InsP and PP-InsP biosynthesis pathways identify PP-InsPs as metabolic messengers for intracellular Pi signaling in response to extracellular P availability.
InsP7 and InsP8 are evolutionarily conserved Pi signaling molecules that are metabolically linked to cellular Pi levels.
InsP8 as an intracellular ligand modulates the interaction between SPX and PHR proteins, which in turn controls the transcriptional activation of PSR genes.
SPX proteins are engaged in the regulation of the crosstalk between N and P signaling networks.
Outstanding questions box
What is the molecular basis underlying PP-InsP-mediated regulation of Pi transport proteins? Do PP-InsPs generally act as ligands in mediating protein–protein interactions and/or directly modulate the activities of their receptor proteins through structural or chemical modifications?
How are the subcellular levels of InsP/PP-InsP controlled in accordance with the dynamics of cellular Pi levels?
Are the activities of SPX-bearing Pi transporters modulated by competitive bindings of different InsP/PP-InsP species according to their differential affinities and physiological levels?
Funding
Work in the laboratory of T.-J.C. is supported by an Academia Sinica Investigator Award (AS-IA-106-L02) and grants from the Ministry of Science and Technology (MOST 107-2311-B-001-038-MY3, MOST 107-2311-B-001-005-MY3) in Taiwan. We thank Ching-Mei Sun for graphic illustrations.
Conflict of interest statement. None declared.
T.-J.C., Z.W., and H.-F.K. conceived the outline of the manuscript. Z.W. and H.-F.K. summarized and interpreted the information from literature, in which Z.W. contributed to the Pi transport system and H.-F.K. to the PP-InsPs signaling. H.-F.K. conceived and performed the experiment presented in Figure 3. Z.W. and H.-F.K. wrote the manuscript under the supervision of T.-J.C. All authors contributed to the critical revision of this article and its final approval.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is Tzyy-Jen Chiou (tjchiou@gate.sinica.edu.tw).
References
- Adepoju O, Williams SP, Craige B, Cridland CA, Sharp AK, Brown AM, Land E, Perera IY, Mena D, Sobrado P, Gillaspy GE (2019) Inositol trisphosphate kinase and diphosphoinositol pentakisphosphate kinase enzymes constitute the inositol pyrophosphate synthesis pathway in plants. bioRxiv:724914 [Google Scholar]
- Arpat AB, Magliano P, Wege S, Rouached H, Stefanovic A, Poirier Y (2012) Functional expression of PHO1 to the Golgi and trans-Golgi network and its role in export of inorganic phosphate. Plant J 71:479–491 [DOI] [PubMed] [Google Scholar]
- Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ (2006) pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol 141:1000–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo C, Saiardi A (2017) Eukaryotic phosphate homeostasis: the inositol pyrophosphate perspective. Trends Biochem Sci 42:219–231 [DOI] [PubMed] [Google Scholar]
- Bari R, Datt Pant B, Stitt M, Scheible WR (2006) PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol 141:988–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle V, Arrighi JF, Creff A, Nespoulous C, Vialaret J, Rossignol M, Gonzalez E, Paz-Ares J, Nussaume L (2011) Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation. Plant Cell 23:1523–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari R, Saiardi A, Ahmadibeni Y, Snowman AM, Resnick AC, Kristiansen TZ, Molina H, Pandey A, Werner JK Jr, Juluri KR, et al. (2007) Protein pyrophosphorylation by inositol pyrophosphates is a posttranslational event. Proc Natl Acad Sci USA 104:15305–15310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brearley CA, Hanke DE (1996) Metabolic evidence for the order of addition of individual phosphate esters in the myo-inositol moiety of inositol hexakisphosphate in the duckweed Spirodela polyrhiza L. Biochem J 314:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche M, Scarpin MR, Hnasko R, Brunkard JO (2021) TOR coordinates nucleotide availability with ribosome biogenesis in plants. Plant Cell 33 :1615–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos R, Castrillo G, Linhares F, Puga MI, Rubio V, Perez-Perez J, Solano R, Leyva A, Paz-Ares J (2010) A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genet 6:e1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona-López X, Cuyas L, Marín E, Rajulu C, Irigoyen ML, Gil E, Puga MI, Bligny R, Nussaume L, Geldner N, et al. (2015) ESCRT-III-associated protein ALIX mediates high-affinity phosphate transporter trafficking to maintain phosphate homeostasis in Arabidopsis. Plant Cell 27:2560–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che J, Yamaji N, Miyaji T, Mitani-Ueno N, Kato Y, Shen RF, Ma JF (2020) Node-localized transporters of phosphorus essential for seed development in rice. Plant Cell Physiol 61:1387–1398 [DOI] [PubMed] [Google Scholar]
- Chen J, Wang Y, Wang F, Yang J, Gao M, Li C, Liu Y, Liu Y, Yamaji N, Ma JF, et al. (2015) The rice CK2 kinase regulates trafficking of phosphate transporters in response to phosphate levels. Plant Cell 27:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-F, Li L-Q, Xu Q, Kong Y-H, Wang H, Wu W-H (2009) The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 21:3554–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Lin SI (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62:185–206 [DOI] [PubMed] [Google Scholar]
- Chiou T-J (2020) The diverse roles of rice PHO1 in phosphate transport: from root to node to grain. Plant Cell Physiol 61:1384–1386 [DOI] [PubMed] [Google Scholar]
- Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL (2006) Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell 18:412–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubero B, Nakagawa Y, Jiang XY, Miura KJ, Li F, Raghothama KG, Bressan RA, Hasegawa PM, Pardo JM (2009) The phosphate transporter PHT4;6 is a determinant of salt tolerance that is localized to the Golgi apparatus of Arabidopsis. Mol Plant 2:535–552 [DOI] [PubMed] [Google Scholar]
- Daram P, Brunner S, Rausch C, Steiner C, Amrhein N, Bucher M (1999) Pht2;1 encodes a low-affinity phosphate transporter from Arabidopsis. Plant Cell 11:2153–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JM, Poole RJ, Sanders D (1993) The computed free energy change of hydrolysis of inorganic pyrophosphate and ATP: apparent significance for inorganic-pyrophosphate-driven reactions of intermediary metabolism. Biochim Biophys Acta Bioenerg 1141:29–36 [Google Scholar]
- Desai M, Rangarajan P, Donahue JL, Williams SP, Land ES, Mandal MK, Phillippy BQ, Perera IY, Raboy V, Gillaspy GE (2014) Two inositol hexakisphosphate kinases drive inositol pyrophosphate synthesis in plants. Plant J 80:642–653 [DOI] [PubMed] [Google Scholar]
- Desfougeres Y, Gerasimaite RU, Jessen HJ, Mayer A (2016) Vtc5, a novel subunit of the vacuolar transporter chaperone complex, regulates polyphosphate synthesis and phosphate homeostasis in Yeast. J Biol Chem 291:22262–22275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarini D, Lev S, Furkert D, Crossett B, Saiardi A, Kaufman-Francis K, Li C, Sorrell TC, Wilkinson-White L, Matthews J, et al. (2020) IP7-SPX domain interaction controls fungal virulence by stabilizing phosphate signaling machinery. mBio 11:e01920–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Ma G, Sui L, Wei M, Satheesh V, Zhang R, Ge S, Li J, Zhang T-E, Wittwer C, et al. (2019) Inositol pyrophosphate InsP8 acts as an intracellular phosphate signal in Arabidopsis. Mol Plant 12:1463–1473 [DOI] [PubMed] [Google Scholar]
- Duan K, Yi K, Dang L, Huang H, Wu W, Wu P (2008) Characterization of a sub-family of Arabidopsis genes with the SPX domain reveals their diverse functions in plant tolerance to phosphorus starvation. Plant J 54:965–975 [DOI] [PubMed] [Google Scholar]
- Duff SM, Moorhead GB, Lefebvre DD, Plaxton WC (1989) Phosphate starvation inducible ‘bypasses’ of adenylate and phosphate dependent glycolytic enzymes in Brassica nigra suspension cells. Plant Physiol 90:1275–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferjani A, Segami S, Horiguchi G, Sakata A, Maeshima M, Tsukaya H (2012) Regulation of pyrophosphate levels by H+-PPase is central for proper resumption of early plant development. Plant Signal Behav 7:38–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro M, Salvi D, Rivière-Rolland H, Vermat T, Seigneurin-Berny D, Grunwald D, Garin J, Joyard J, Rolland N (2002) Integral membrane proteins of the chloroplast envelope: Identification and subcellular localization of new transporters. Proc Natl Acad Sci USA 99:11487–11492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furkert D, Hostachy S, Nadler-Holly M, Fiedler D (2020) Triplexed affinity reagents to sample the mammalian inositol pyrophosphate interactome. Cell Chem Biol 27:1097–1108 [DOI] [PubMed] [Google Scholar]
- Gerasimaite R, Pavlovic I, Capolicchio S, Hofer A, Schmidt A, Jessen HJ, Mayer A (2017) Inositol pyrophosphate specificity of the SPX-dependent polyphosphate polymerase VTC. ACS Chem Biol 12:648–653 [DOI] [PubMed] [Google Scholar]
- Giovannini D, Touhami J, Charnet P, Sitbon M, Battini JL (2013) Inorganic phosphate export by the retrovirus receptor XPR1 in metazoans. Cell Rep 3:1866–1873 [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Solano R, Rubio V, Leyva A, Paz-Ares J (2005) PHOSPHATE TRANSPORTER TRAFFIC FACILITATOR1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis. Plant Cell 17:3500–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Nguyen H-N, Hofer A, Jessen HJ, Dai X, Wang H, Shears SB (2017) The significance of the bifunctional kinase/phosphatase activities of diphosphoinositol pentakisphosphate kinases (PPIP5Ks) for coupling inositol pyrophosphate cell signaling to cellular phosphate homeostasis. J Biol Chem 292:4544–4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Jin Y, Wussler C, Blancaflor EB, Motes CM, Versaw WK (2008) Functional analysis of the Arabidopsis PHT4 family of intracellular phosphate transporters. New Phytol 177:889–898 [DOI] [PubMed] [Google Scholar]
- Guo C, Zhao X, Liu X, Zhang L, Gu J, Li X, Lu W, Xiao K (2013) Function of wheat phosphate transporter gene TaPHT2;1 in Pi translocation and plant growth regulation under replete and limited Pi supply conditions. Planta 237:1163–1178 [DOI] [PubMed] [Google Scholar]
- Hürlimann HC, Pinson B, Stadler-Waibel M, Zeeman SC, Freimoser FM (2009) The SPX domain of the yeast low-affinity phosphate transporter Pho90 regulates transport activity. EMBO Rep 10:1003–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger D, Rezzonico E, MacDonald-Comber Petetot J, Somerville C, Poirier Y (2002) Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell 14:889–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel P, Saint-Georges Y, de Pinto B, Lachacinski N, Altamura N, Dujardin G (2004) Redundancy in the function of mitochondrial phosphate transport in Saccharomyces cerevisiae and Arabidopsis thaliana. Mol Microbiol 51:307–317 [DOI] [PubMed] [Google Scholar]
- Härtel H, Dörmann P, Benning C (2000) DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc Natl Acad Sci USA 97:10649–10654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Lu H, Guo H, Wang Y, Zhao P, Li Y, Wang F, Xu J, Mo X, Mao C (2021a) OsbHLH6 interacts with OsSPX4 and regulates the phosphate starvation response in rice. Plant J 105:649–667 [DOI] [PubMed] [Google Scholar]
- He Y, Zhang X, Li L, Sun Z, Li J, Chen X, Hong G (2021b) SPX4 interacts with both PHR1 and PAP1 to regulate critical steps in phosphorus-status-dependent anthocyanin biosynthesis. New Phytol 230:205–217 [DOI] [PubMed] [Google Scholar]
- Heinonen JK (2001) Biological Role of Inorganic Pyrophosphate, Springer, Boston, MA [Google Scholar]
- Heuer S, Gaxiola R, Schilling R, Herrera-Estrella L, López-Arredondo D, Wissuwa M, Delhaize E, Rouached H (2017) Improving phosphorus use efficiency: a complex trait with emerging opportunities. Plant J 90:868–885 [DOI] [PubMed] [Google Scholar]
- Hewitt MM, Carr JM, Williamson CL, Slocum RD (2005) Effects of phosphate limitation on expression of genes involved in pyrimidine synthesis and salvaging in Arabidopsis. Plant Physiol Biochem 43:91–99 [DOI] [PubMed] [Google Scholar]
- Ho C-H, Lin S-H, Hu H-C, Tsay Y-F (2009) CHL1 functions as a nitrate sensor in plants. Cell 138:1184–1194 [DOI] [PubMed] [Google Scholar]
- Holford ICR (1997) Soil phosphorus: its measurement, and its uptake by plants. Soil Res 35:227–240 [Google Scholar]
- Hsieh LC, Lin SI, Shih AC, Chen JW, Lin WY, Tseng CY, Li WH, Chiou TJ (2009) Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol 151:2120–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Chu C (2020) Nitrogen–phosphorus interplay: old story with molecular tale. New Phytol 225:1455–1460 [DOI] [PubMed] [Google Scholar]
- Hu B, Jiang Z, Wang W, Qiu Y, Zhang Z, Liu Y, Li A, Gao X, Liu L, Qian Y, et al. (2019) Nitrate-NRT1.1B-SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nat Plants 5:401–413 [DOI] [PubMed] [Google Scholar]
- Huang TK, Han CL, Lin SI, Chen YJ, Tsai YC, Chen YR, Chen JW, Lin WY, Chen PM, Liu TY, et al. (2013) Identification of downstream components of ubiquitin-conjugating enzyme PHOSPHATE2 by quantitative membrane proteomics in Arabidopsis roots. Plant Cell 25:4044–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irigoyen S, Karlsson PM, Kuruvilla J, Spetea C, Versaw WK (2011) The sink-specific plastidic phosphate transporter PHT4;2 influences starch accumulation and leaf size in Arabidopsis. Plant Physiol 157:1765–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine RF (2005) Inositide evolution—towards turtle domination? J Physiol 566:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabnoune M, Secco D, Lecampion C, Robaglia C, Shu Q, Poirier Y (2013) A rice cis-natural antisense RNA acts as a translational enhancer for its cognate mRNA and contributes to phosphate homeostasis and plant fitness. Plant Cell 25:4166–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J-Y, Ried MK, Hothorn M, Poirier Y (2018) Control of plant phosphate homeostasis by inositol pyrophosphates and the SPX domain. Curr Opin Biotechnol 49:156–162 [DOI] [PubMed] [Google Scholar]
- Karlsson PM, Herdean A, Adolfsson L, Beebo A, Nziengui H, Irigoyen S, Ünnep R, Zsiros O, Nagy G, Garab G, et al. (2015) The Arabidopsis thylakoid transporter PHT4;1 influences phosphate availability for ATP synthesis and plant growth. Plant J 84:99–110 [DOI] [PubMed] [Google Scholar]
- Kelly AA, Dörmann P (2002) DGD2, an Arabidopsis gene encoding a UDP-galactose-dependent gigalactosyldiacylglycerol synthase is expressed during growth under phosphate-limiting conditions. J Biol Chem 277:1166–1173 [DOI] [PubMed] [Google Scholar]
- Kuo H-F, Hsu Y-Y, Lin W-C, Chen K-Y, Munnik T, Brearley CA, Chiou T-J (2018) Arabidopsis inositol phosphate kinases IPK1 and ITPK1 constitute a metabolic pathway in maintaining phosphate homeostasis. Plant J 95:613–630 [DOI] [PubMed] [Google Scholar]
- Kuo HF, Chang TY, Chiang SF, Wang WD, Charng YY, Chiou TJ (2014) Arabidopsis inositol pentakisphosphate 2-kinase, AtIPK1, is required for growth and modulates phosphate homeostasis at the transcriptional level. Plant J 80:503–515 [DOI] [PubMed] [Google Scholar]
- Laha D, Johnen P, Azevedo C, Dynowski M, Weiss M, Capolicchio S, Mao H, Iven T, Steenbergen M, Freyer M, et al. (2015) VIH2 regulates the synthesis of inositol pyrophosphate InsP8 and jasmonate-dependent defenses in Arabidopsis. Plant Cell 27:1082–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha D, Parvin N, Hofer A, Giehl RF, Fernandez-Rebollo N, von Wirén N, Saiardi A, Jessen HJ, Schaaf G (2019) Arabidopsis ITPK1 and ITPK2 have an evolutionarily conserved phytic acid kinase activity. ACS Chem Biol 14:2127–2133 [DOI] [PubMed] [Google Scholar]
- Lee YS, Mulugu S, York JD, O’Shea EK (2007) Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science 316:109–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legati A, Giovannini D, Nicolas G, López-Sánchez U, Quintáns B, Oliveira JRM, Sears RL, Ramos EM, Spiteri E, Sobrido M-J, et al. (2015) Mutations in XPR1 cause primary familial brain calcification associated with altered phosphate export. Nat Genet 47:579–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Gu C, Hostachy S, Sahu S, Wittwer C, Jessen HJ, Fiedler D, Wang H, Shears SB (2020) Control of XPR1-dependent cellular phosphate efflux by InsP8 is an exemplar for functionally-exclusive inositol pyrophosphate signaling. Proc Natl Acad Sci USA 117:3568–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WY, Huang TK, Chiou TJ (2013) Nitrogen limitation adaptation, a target of microRNA827, mediates degradation of plasma membrane-localized phosphate transporters to maintain phosphate homeostasis in Arabidopsis. Plant Cell 25:4061–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang L, Luan M, Wang Y, Zhang C, Zhang B, Shi J, Zhao FG, Lan W, Luan S (2015) A vacuolar phosphate transporter essential for phosphate homeostasis in Arabidopsis. Proc Natl Acad Sci USA 112:6571–6578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TY, Huang TK, Tseng CY, Lai YS, Lin SI, Lin WY, Chen JW, Chiou TJ (2012) PHO2-dependent degradation of PHO1 modulates phosphate homeostasis in Arabidopsis. Plant Cell 24:2168–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TY, Huang TK, Yang SY, Hong YT, Huang SM, Wang FN, Chiang SF, Tsai SY, Lu WC, Chiou TJ (2016) Identification of plant vacuolar transporters mediating phosphate storage. Nat Commun 7:11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Sun Q, Wang K, Du Q, Li W-X (2017) Nitrogen limitation adaptation (NLA) is involved in source-to-sink remobilization of nitrate by mediating the degradation of NRT1.7 in Arabidopsis. New Phytol 214:734–744 [DOI] [PubMed] [Google Scholar]
- Liu XL, Wang L, Wang XW, Yan Y, Yang XL, Xie MY, Hu Z, Shen X, Ai H, Lin HH, et al. (2020) Mutation of the chloroplast-localized phosphate transporter OsPHT2;1 reduces flavonoid accumulation and UV tolerance in rice. Plant J 102:53–67 [DOI] [PubMed] [Google Scholar]
- Lonetti A, Szijgyarto Z, Bosch D, Loss O, Azevedo C, Saiardi A (2011) Identification of an evolutionarily conserved family of inorganic polyphosphate endopolyphosphatases. J Biol Chem 286:31966–31974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo‐Orts L, Couto D, Hothorn M (2020) Identity and functions of inorganic and inositol polyphosphates in plants. New Phytol 225:637–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan M, Zhao F, Han X, Sun G, Yang Y, Liu J, Shi J, Fu A, Lan W, Luan S (2019) Vacuolar phosphate transporters contribute to systemic phosphate homeostasis vital for reproductive development in Arabidopsis. Plant Physiol 179:640–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Q, Zhong Y, Wang Y, Wang Z, Zhang L, Shi J, Wu Z, Liu Y, Mao C, Yi K, et al. (2014) SPX4 negatively regulates phosphate signaling and homeostasis through its interaction with PHR2 in rice. Plant Cell 26:1586–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Zhang L, Gao Q, Wang J, Li X, Wang H, Liu Y, Lin H, Liu J, Wang X, et al. (2021) A plasma membrane transporter coordinates phosphate reallocation and grain filling in cereals. Nat Genet. 10.1038/s41588-021-00855-6 [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Banerjee S, Wheeler A, Ratliff LA, Irigoyen S, Garcia LR, Lockless SW, Versaw WK (2015) Live imaging of inorganic phosphate in plants with cellular and subcellular resolution. Plant Physiol 167:628–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y (2013) Phosphate starvation and membrane lipid remodeling in seed plants. Prog Lipid Res 52:43–50 [DOI] [PubMed] [Google Scholar]
- Nguyen NNT, Clua J, Vetal PV, Vuarambon DJ, De Bellis D, Pervent M, Lepetit M, Udvardi M, Valentine AJ, Poirier Y (2021) PHO1 family members transport phosphate from infected nodule cells to bacteroids in Medicago truncatula. Plant Physiol 185:196–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbis F, Boll M, Stange G, Markovich D, Verrey F, Biber J, Murer H (1997) Identification of a cDNA/protein leading to an increased Pi-uptake in Xenopus laevis Oocytes. J Membr Biol 156:19–24 [DOI] [PubMed] [Google Scholar]
- Obersteiner M, Peñuelas J, Ciais P, van der Velde M, Janssens IA (2013) The phosphorus trilemma. Nat Geosci 6:897–898 [Google Scholar]
- Osorio MB, Ng S, Berkowitz O, De Clercq I, Mao C, Shou H, Whelan J, Jost R (2019) SPX4 acts on PHR1-dependent and -independent regulation of shoot phosphorus status in Arabidopsis. Plant Physiol 181:332–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BS, Seo JS, Chua NH (2014) NITROGEN LIMITATION ADAPTATION recruits PHOSPHATE2 to target the phosphate transporter PT2 for degradation during the regulation of Arabidopsis phosphate homeostasis. Plant Cell 26:454–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BS, Yao T, Seo JS, Wong ECC, Mitsuda N, Huang CH, Chua NH (2018) Arabidopsis NITROGEN LIMITATION ADAPTATION regulates ORE1 homeostasis during senescence induced by nitrogen deficiency. Nat Plants 4:898–903 [DOI] [PubMed] [Google Scholar]
- Phillippy B, Perera I, Donahue J, Gillaspy G (2015) Certain malvaceae plants have a unique accumulation of myo-inositol 1,2,4,5,6-pentakisphosphate. Plants 4:267–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton WC, Podestá FE (2007) The functional organization and control of plant respiration. CRC Crit Rev Plant Sci 25:159–198 [Google Scholar]
- Poirier Y, Jung J-Y (2018) Phosphate transporters. In WC Plaxton, H Lambers, eds, Annual Plant Reviews, Vol. 48, Phosphorus Metabolism in Plants, Wiley-Blackwell, Oxford, UK, pp 125–158
- Poirier Y, Thoma S, Somerville C, Schiefelbein J (1991) Mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol 97:1087–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapenko E, Cordeiro CD, Huang G, Storey M, Wittwer C, Dutta AK, Jessen HJ, Starai VJ, Docampo R (2018) 5-Diphosphoinositol pentakisphosphate (5-IP7) regulates phosphate release from acidocalcisomes and yeast vacuoles. J Biol Chem 293:19101–19112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt J, Boisson AM, Gout E, Bligny R, Douce R, Aubert S (2009) Phosphate (Pi) starvation effect on the cytosolic Pi concentration and Pi exchanges across the tonoplast in plant cells: an in vivo 31P-nuclear magnetic resonance study using methylphosphonate as a Pi analog. Plant Physiol 151:1646–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga MI, Mateos I, Charukesi R, Wang Z, Franco-Zorrilla JM, de Lorenzo L, Irigoyen ML, Masiero S, Bustos R, Rodriguez J, et al. (2014) SPX1 is a phosphate-dependent inhibitor of phosphate starvation response 1 in Arabidopsis. Proc Natl Acad Sci USA 111:14947–14952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu D, Wilson MS, Eisenbeis VB, Harmel RK, Riemer E, Haas TM, Wittwer C, Jork N, Gu C, Shears SB, et al. (2020) Analysis of inositol phosphate metabolism by capillary electrophoresis electrospray ionization mass spectrometry. Nat Commun 11:6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raboy V (1997) Accumulation and storage of phosphate and mineralsInLarkins BA, Vasil IK, eds, Cellular and Molecular Biology of Plant Seed Development. Springer, Netherlands, Dordrecht, pp 441–477 [Google Scholar]
- Rausch C, Zimmermann P, Amrhein N, Bucher M (2004) Expression analysis suggests novel roles for the plastidic phosphate transporter Pht2;1 in auto- and heterotrophic tissues in potato and Arabidopsis. Plant J 39:13–28 [DOI] [PubMed] [Google Scholar]
- Reis RS, Deforges J, Schmidt RR, Schippers JHM, Poirier Y (2021) An antisense noncoding RNA enhances translation via localised structural rearrangements of its cognate mRNA. Plant Cell koab010. 10.1093/plcell/koab010 [DOI] [PubMed] [Google Scholar]
- Reis RS, Deforges J, Sokoloff T, Poirier Y (2020) Modulation of shoot phosphate level and growth by PHOSPHATE1 upstream open reading frame. Plant Physiol 183:1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried MK, Wild R, Zhu J, Pipercevic J, Sturm K, Broger L, Harmel RK, Abriata LA, Hothorn LA, Fiedler D, et al. (2021) Inositol pyrophosphates promote the interaction of SPX domains with the coiled-coil motif of PHR transcription factors to regulate plant phosphate homeostasis. Nat Commun 12:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemer E, Laha D, Harmel RK, Gaugler P, Pries V, Frei M, Hajirezaei M-R, Laha NP, Krusenbaum L, Schneider R, et al. (2020) ITPK1 is an InsP6/ADP phosphotransferase that controls systemic phosphate homeostasis in Arabidopsis. bioRxiv:100297
- Roth C, Menzel G, Petétot JM-C, Rochat-Hacker S, Poirier Y (2004) Characterization of a protein of the plastid inner envelope having homology to animal inorganic phosphate, chloride and organic-anion transporters. Planta 218:406–416 [DOI] [PubMed] [Google Scholar]
- Ruan W, Guo M, Wang X, Guo Z, Xu Z, Xu L, Zhao H, Sun H, Yan C, Yi K (2019) Two RING‐finger ubiquitin E3 ligases regulate the degradation of SPX4, the internal phosphate sensor, for phosphate homeostasis and signaling in rice. Mol Plant 12:1060–1074 [DOI] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, Martin AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev 15:2122–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A, Banerjee S, Raju AS, Chiou T-J, Garcia LR, Versaw WK (2020) Spatial profiles of phosphate in roots indicate developmental control of uptake, recycling, and sequestration. Plant Physiol 184 :2064–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiardi A, Bhandari R, Resnick AC, Snowman AM, Snyder SH (2004) Phosphorylation of proteins by inositol pyrophosphates. Science 306:2101–2105 [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Kanno S, Mabuchi A, Monda K, Iba K, Yanagisawaet S (2018) A phytochrome-B-mediated regulatory mechanism of phosphorus acquisition. Nat Plants 4:1089–1101 [DOI] [PubMed] [Google Scholar]
- Schell MJ, Letcher AJ, Brearley CA, Biber J, Murer H, Irvine RF (1999) PiUS (Pi uptake stimulator) is an inositol hexakisphosphate kinase. FEBS Lett 461:169–172 [DOI] [PubMed] [Google Scholar]
- Secco D, Baumann A, Poirier Y (2010) Characterization of the rice PHO1 gene family reveals a key role for OsPHO1;2 in phosphate homeostasis and the evolution of a distinct clade in dicotyledons. Plant Physiol 152:1693–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secco D, Jabnoune M, Walker H, Shou H, Wu P, Poirier Y, Whelan J (2013) Spatio-temporal transcript profiling of rice roots and shoots in response to phosphate starvation and recovery. Plant Cell 25:4285–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secco D, Wang C, Arpat BA, Wang Z, Poirier Y, Tyerman SD, Wu P, Shou H, Whelan J (2012a) The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol 193:842–851 [DOI] [PubMed] [Google Scholar]
- Secco D, Wang C, Shou H, Whelan J (2012b) Phosphate homeostasis in the yeast Saccharomyces cerevisiae, the key role of the SPX domain-containing proteins. FEBS Lett 586:289–295 [DOI] [PubMed] [Google Scholar]
- Shears SB (2009) Diphosphoinositol polyphosphates: metabolic messengers? Mol Pharmacol 76:236–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears SB (2018) Intimate connections: Inositol pyrophosphates at the interface of metabolic regulation and cell signaling. J Cell Physiol 233:1897–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Shin HS, Dewbre GR, Harrison MJ (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39:629–642 [DOI] [PubMed] [Google Scholar]
- Stevenson-Paulik J, Bastidas RJ, Chiou ST, Frye RA, York JD (2005) Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proc Natl Acad Sci USA 102:12612–12617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szijgyarto Z, Garedew A, Azevedo C, Saiardi A (2011) Influence of inositol pyrophosphates on cellular energy dynamics. Science 334:802–805 [DOI] [PubMed] [Google Scholar]
- Takami T, Ohnishi N, Kurita Y, Iwamura S, Ohnishi M, Kusaba M, Mimura T, Sakamoto W (2018) Organelle DNA degradation contributes to the efficient use of phosphate in seed plants. Nat Plants 4:1044–1055 [DOI] [PubMed] [Google Scholar]
- Thota SG, Unnikannan CP, Thampatty SR, Manorama R, Bhandari R (2015) Inositol pyrophosphates regulate RNA polymerase I-mediated rRNA transcription in Saccharomyces cerevisiae. Biochem J 466:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticconi CA, Delatorre CA, Abel S (2001) Attenuation of phosphate starvation responses by phosphate in Arabidopsis. Plant Physiol 127:963–972 [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kiba T, Yanagisawa S (2020) Nitrate-inducible NIGT1 proteins modulate phosphate uptake and starvation signalling via transcriptional regulation of SPX genes. Plant J 102:448–466 [DOI] [PubMed] [Google Scholar]
- van de Wiel CCM, van der Linden CG, Scholten OE (2016) Improving phosphorus use efficiency in agriculture: opportunities for breeding. Euphytica 207:1–22 [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447 [DOI] [PubMed] [Google Scholar]
- Varadarajan DK, Karthikeyan AS, Matilda PD, Raghothama KG (2002) Phosphite, an analog of phosphate, suppresses the coordinated expression of genes under phosphate starvation. Plant Physiol 129:1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneklaas EJ, Lambers H, Bragg J, Finnegan PM, Lovelock CE, Plaxton WC, Price CA, Scheible WR, Shane MW, White PJ, et al. (2012) Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol 195:306–320 [DOI] [PubMed] [Google Scholar]
- Versaw WK, Garcia LR (2017) Intracellular transport and compartmentation of phosphate in plants. Curr Opin Plant Biol 39:25–30 [DOI] [PubMed] [Google Scholar]
- Versaw WK, Harrison MJ (2002) A chloroplast phosphate transporter, PHT2;1, influences allocation of phosphate within the plant and phosphate-starvation responses. Plant Cell 14:1751–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzaki E, Baroux C, Jung J-Y, Poirier Y (2017) PHO1 exports phosphate from the chalazal seed coat to the embryo in developing Arabidopsis seeds. Curr Biol 27:2893–2900 [DOI] [PubMed] [Google Scholar]
- Voon CP, Guan X, Sun Y, Sahu A, Chan MN, Gardestrom P, Wagner S, Fuchs P, Nietzel T, Versaw WK, et al. (2018) ATP compartmentation in plastids and cytosol of Arabidopsis thaliana revealed by fluorescent protein sensing. Proc Natl Acad Sci USA 115:10778–10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ying S, Huang H, Li K, Wu P, Shou H (2009) Involvement of OsSPX1 in phosphate homeostasis in rice. Plant J 57:895–904 [DOI] [PubMed] [Google Scholar]
- Wang F, Cui PJ, Tian Y, Huang Y, Wang HF, Liu F, Chen YF (2020a) Maize ZmPT7 regulates Pi uptake and redistribution which is modulated by phosphorylation. Plant Biotechnol J 18:2406–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GY, Shi JL, Ng G, Battle SL, Zhang C, Lu H (2011) Circadian clock-regulated phosphate transporter PHT4;1 plays an important role in Arabidopsis defense. Mol Plant 4:516–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang H-F, Chen Y, Sun M-M, Wang Y, Chen Y-F (2020b) The transcription factor NIGT1.2 modulates both phosphate uptake and nitrate influx during phosphate starvation in Arabidopsis and Maize. Plant Cell 32:3519–3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ruan W, Shi J, Zhang L, Xiang D, Yang C, Li C, Wu Z, Liu Y, Yu Y, et al. (2014) Rice SPX1 and SPX2 inhibit phosphate starvation responses through interacting with PHR2 in a phosphate-dependent manner. Proc Natl Acad Sci USA 111:14953–14958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber APM, Linka N (2011) Connecting the plastid: transporters of the plastid envelope and their role in linking plastidial with cytosolic metabolism. Annu Rev Plant Biol 62:53–77 [DOI] [PubMed] [Google Scholar]
- Wege S, Khan GA, Jung JY, Vogiatzaki E, Pradervand S, Aller I, Meyer AJ, Poirier Y (2016) The EXS domain of PHO1 participates in the response of shoots to phosphate deficiency via a root-to-shoot signal. Plant Physiol 170:385–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Hammond JP (2008) Phosphorus nutrition of terrestrial plants. InWhite PJ, Hammond JP, eds, The Ecophysiology of Plant–Phosphorus Interactions. Springer, Netherlands, Dordrecht, pp 51–81 [Google Scholar]
- Whitfield H, White G, Sprigg C, Riley AM, Potter BVL, Hemmings AM, Brearley CA (2020) An ATP-responsive metabolic cassette comprised of inositol tris/tetrakisphosphate kinase 1 (ITPK1) and inositol pentakisphosphate 2-kinase (IPK1) buffers diphosphosphoinositol phosphate levels. Biochem J 477:2621–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild R, Gerasimaite R, Jung JY, Truffault V, Pavlovic I, Schmidt A, Saiardi A, Jessen HJ, Poirier Y, Hothorn M, et al. (2016) Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science 352:986–990 [DOI] [PubMed] [Google Scholar]
- Wilson MS, Bulley SJ, Pisani F, Irvine RF, Saiardi A (2015) A novel method for the purification of inositol phosphates from biological samples reveals that no phytate is present in human plasma or urine. Open Biol 5:150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Jessen HJ, Saiardi A (2019) The inositol hexakisphosphate kinases IP6K1 and -2 regulate human cellular phosphate homeostasis, including XPR1-mediated phosphate export. J Biol Chem 294:11597–11608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Livermore TM, Saiardi A (2013) Inositol pyrophosphates: between signalling and metabolism. Biochem J 452:369–379 [DOI] [PubMed] [Google Scholar]
- Wu M, Chong LS, Perlman DH, Resnick AC, Fiedler D (2016) Inositol polyphosphates intersect with signaling and metabolic networks via two distinct mechanisms. Proc Natl Acad Sci USA 113:6757–6765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wundenberg T, Mayr G (2012) Synthesis and biological actions of diphosphoinositol phosphates (inositol pyrophosphates), regulators of cell homeostasis. Biol Chem 393:979–998 [DOI] [PubMed] [Google Scholar]
- Wykoff DD, Rizvi AH, Raser JM, Margolin B, O’Shea EK (2007) Positive feedback regulates switching of phosphate transporters in S. cerevisiae. Mol Cell 27:1005–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhao H, Wan R, Liu Y, Xu Z, Tian W, Ruan W, Wang F, Deng M, Wang J, et al. (2019) Identification of vacuolar phosphate efflux transporters in land plants. Nat Plants 5:84–94 [DOI] [PubMed] [Google Scholar]
- Yang S-Y, Lu W-C, Ko S-S, Sun C-M, Hung J-C, Chiou T-J (2020a) Upstream open reading frame and phosphate-regulated expression of rice OsNLA1 controls phosphate transport and reproduction. Plant Physiol 182:393–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Huang TK, Kuo HF, Chiou TJ (2017) Role of vacuoles in phosphorus storage and remobilization. J Exp Bot 68:3045–3055 [DOI] [PubMed] [Google Scholar]
- Yang Z, Yang J, Wang Y, Wang F, Mao W, He Q, Xu J, Wu Z, Mao C (2020b) PROTEIN PHOSPHATASE95 regulates phosphate homeostasis by affecting phosphate transporter trafficking in rice. Plant Cell 32:740–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Gu M, Liang R, Shi X, Chen L, Hu X, Wang S, Dai X, Qu H, Li H, et al. (2021a) OsWRKY21 and OsWRKY108 function redundantly to promote phosphate accumulation through maintaining the constitutive expression of OsPHT1;1 under phosphate-replete conditions. New Phytol 229:1598–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li Z, Wang W, Jiang Z, Guo L, Wang X, Qian Y, Huang X, Liu Y, Liu X, et al. (2021b) Modulation of nitrate-induced phosphate response by the MYB transcription factor RLI1/HINGE1 in the nucleus. Mol Plant 14:517–529 [DOI] [PubMed] [Google Scholar]
- Zhu J, Lau K, Puschmann R, Harmel RK, Zhang Y, Pries V, Gaugler P, Broger L, Dutta AK, Jessen HJ, et al. (2019) Two bifunctional inositol pyrophosphate kinases/phosphatases control plant phosphate homeostasis. eLife 8:e43582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Miao Q, Sun D, Yang G, Wu C, Huang J, Zheng C (2012) The mitochondrial phosphate transporters modulate plant responses to salt stress via affecting ATP and gibberellin metabolism in Arabidopsis thaliana. PLoS ONE 7:e43530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli C, Ribot C, Vavasseur A, Bauer H, Hedrich R, Poirier Y (2012) PHO1 expression in guard cells mediates the stomatal response to abscisic acid in Arabidopsis. Plant J 72:199–211 [DOI] [PubMed] [Google Scholar]