Abstract
Nucleotide sequences of 426 bp from the mitochondrial (mt) cytochrome b genes of six anamorph species and two species of Neosartorya teleomophs of Aspergillus section Fumigati were determined. These sequences were used to build nucleotide- and amino acid-based trees for phylogenetic analysis. Thirteen strains of A. fumigatus including 10 clinical isolates of A. fumigatus, 1 type culture of A. fumigatus var. fumigatus, 1 type culture of A. fumigatus var. ellipticus, and 1 strain of A. fumigatus var. albus, had the same nucleotide sequences. One strain of A. fumisynnematus, two strains labeled A. neoellipticus, two strains of A. viridinutans, and one strain of A. duricaulis had distinct nucleotide and amino acid sequences. Two strains of A. brevipes were divided into two types. One produced a 1,500-bp fragment that included an intron. The nucleotide sequences of its two exons were similar to those of the A. fumigatus, and the derived amino acid sequence was the same as that for A. fumigatus. The other produced a 426-bp fragment and had the same nucleotide and amino acid sequences as A. unilateralis. Neosartorya fischeri var. fischeri and N. stramenia had nucleotide sequences that differed from that of A. fumigatus. These species possessed their own characteristic nucleotide sequences that differed from each other. In comparisons of homologous sequences from four other pathogenic species of Aspergillus, regions specific to section Fumigati were found. The mt cytochrome b gene analysis was valuable for the identification, classification, and phylogenetic analysis of isolates of section Fumigati.
Aspergillus fumigatus is a widely distributed mold which has been isolated from natural and residential environments and which is thought to be the Aspergillus species most pathogenic for humans. It is capable of causing a wide spectrum of human diseases, ranging from allergic bronchopulmonary aspergillosis and aspergilloma to invasive aspergillosis and systemic infection due to hematogenous dissemination. Infection in the immunocompromised host is often fatal (2, 3, 11, 14, 35).
A. fumigatus infections are usually detected by standard cultural and/or histological methods. The organism is identified on the basis of its morphological features. This species, however, is morphologically more variable than the species description of Raper and Fennell (31) would indicate. Clinical isolates can be remarkably different from food- or soilborne isolates, showing, for example, more floccose growth with fewer conidia (15, 24, 33). Species closely related to A. fumigatus, such as A. neoellipticus (A. fumigatus var. ellipticus), Neosartorya pseudofischeri, and N. fischeri, rarely cause infectious diseases (16, 18, 29, 30, 34). It is medically and mycologically important to identify these species and to understand their phylogenic relationships. At present, however, the relationships among taxa in section Fumigati remain unclear. For example, taxonomic questions about the relationship of A. fumigatus to A. neoellipticus remain to be solved, as do questions about the taxonomic states of A. brevipes, A. duricaulis, and A. unilateralis.
The mitochondrial (mt) cytochrome b gene has been used to study the evolution and phylogenetic relationships of many animals, such as birds, mammals, and fish (1, 5, 7, 8, 12, 13, 20, 21, 23), although the sequence of this gene has been determined for only six species of fungi before our study (36). We have previously demonstrated that the mt cytochrome b gene region is very powerful and useful for the identification, classification, and phylogenetic analysis of pathogenic Aspergillus species (36).
The purpose of this study was to determine the sequences of the mt cytochrome b genes of A. fumigatus strains from clinical and nonclinical sources and to compare them with sequences from related species in order to verify the identification of these species and to clarify their phylogenetic relationships.
MATERIALS AND METHODS
Strains and DNA extraction.
Twenty-seven strains were used in this study (Table 1). mt DNA extraction was carried out as reported previously (36).
TABLE 1.
Fungal strains used in the studya
| Species | IFM no. | Source |
|---|---|---|
| A. brevipes | IFM 47028 | IFO 5821 (ATCC 16899, CBS 118.53, NRRL 2439, ex type) |
| A. brevipes | IFM 46976 | NHMIC FD-085 |
| A. duricaulis | IFM 47047 | CBS 481.65 (ATCC 16900, ex type) |
| A. fumigatus | IFM 5355 | MTU 06002 |
| A. fumigatus | IFM 40804 | NHL, patient |
| A. fumigatus | IFM 40806 | NHL, patient |
| A. fumigatus | IFM 40807 | NHL, lung |
| A. fumigatus | IFM 40819 | NHL, patient |
| A. fumigatus | IFM 41206 | VIF, cattle mastitis |
| A. fumigatus | IFM 41392 | CUCM, sputum |
| A. fumigatus | IFM 45916 | Chiba Cancer Center Hospital |
| A. fumigatus | IFM 45917 | Chiba Cancer Center Hospital |
| A. fumigatus | IFM 46980 | NHMIC, patient |
| A. fumigatus var. fumigatus | IFM 47042 | CBS 110.46 (ATCC 16907, ex type) |
| A. fumigatus var. ellipticus | IFM 47043 | CBS 487.65 (ATCC 16903, ex type) |
| A. fumigatus var. albus | IFM 46980 | NHMIC, FD-144 |
| A. fumisynnematus | IFM 42277 | NHMIC, soil |
| A. neoellipticus | IFM 46892 | NHMIC FD-136, patient |
| A. neoellipticus | IFM 46893 | NHMIC FD-143, patient |
| A. unilateralis | IFM 41405 | IFO 8136 (CBS 126.56, ATCC 16902, ex type) |
| A. unilateralis | IFM 47044 | IFO 8008 (CBS 283.66) |
| A. viridinutans | IFM 47045 | CBS 127.56 (ATCC 16901, NRRL 4365, ex type) |
| A. viridinutans | IFM 47046 | CBS 594.91 |
| N. fischeri var. fischeri | IFM 47022 | IFO 31844 |
| N. fischeri | IFM 46945 | NHMIC, FA-023 |
| N. fischeri | IFM 46946 | NHMIC, FA-141 |
| N. stramenia | IFM 42226 | IFO 31358 |
| N. stramenia | IFM 47027 | IFO 9611 |
Abbreviations: ATCC, American Type Culture Collection, Rockville, Md.; CBS, Centraalbureau voor Schimmelcultures, Baarn, The Netherlands; CUCM, Department of Chest Medicine, School of Medicine, Chiba University, Chiba, Japan; IFM, Institute for Food Microbiology (at present, the Research Center for Pathogenic Fungi and Microbial Toxicoses, Chiba University), Chiba, Japan; IFO, Institute for Fermentation, Osaka, Japan; MTU, Department of Bacteriology, Faculty of Medicine, the University of Tokyo, Tokyo, Japan; NHL, National Institute of Hygienic Sciences, Tokyo, Japan; NHMIC, National History Museum & Institute, Chiba, Japan; NRRL, Agricultural Research Service Culture Collection, Northern Regional Research Center, Peoria, Ill.; VIF, National Veterinary Institute, Helsinki, Finland.
Primers and PCR amplification.
The primer E1m (5′-TGAGGTGCTACAGTTATTAC-3′) was designed and was used as a forward primer, and primer E2 (5′-GGTATAGMTCTTAAWATAGC-3′) or rEME2 (5′-AAAATAGCATAGAAAGGTAA-3′) was used as the reverse primer (36). The PCR cycling protocol was as follows: each cycle consisted of denaturation for 1 min at 94°C, annealing for 1 min at 50°C, and extension for 2 min at 72°C for 30 cycles (36).
Sequencing.
Both strands of the fragments were sequenced with the Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems Division of Perkin-Elmer Japan Co., Ltd.) on an ABI Prism 377 DNA sequencer with forward primer E1m and reverse primer rEME2 or E2 (36).
Computer analysis.
DNA and amino acid sequences derived from the yeast mt genetic code were aligned and compared by using the GENETYX-MAC program in Genetic Information Processing Software (Software Development Co., Ltd., Tokyo, Japan), and phylogenetic trees were generated by the unweighted pair group method with arithmetric mean (UPGMA). Estimation of phylogenetic relationships was done by using standard errors for each branching point. Standard errors were calculated by the method of Nei (27). The PAUP program (version 4.0; beta version) was used for the neighbor joining (NJ), maximum likelihood (ML), and maximum parsimony (MP) methods.
Nucleotide sequence accession numbers.
The nucleotide sequences of the cytochrome b genes determined in this study appear in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession nos. AB000566 and AB000567, AB000586 to AB000593, AB025434 to AB025445, and AB026120.
RESULTS
By using the primer pairs designed for the study, the 426-bp (E1m-rEME2) or 437-bp (E1m-E2) fragments were amplified from all strains tested except one strain of A. brevipes, strain IFO 5821, which showed an approximately 1,500-bp fragment. The 402-bp nucleotide sequences excluding the uncertain parts of the primer sequence and the 132-residue derived amino acid sequences were aligned (Fig. 1 and 2).
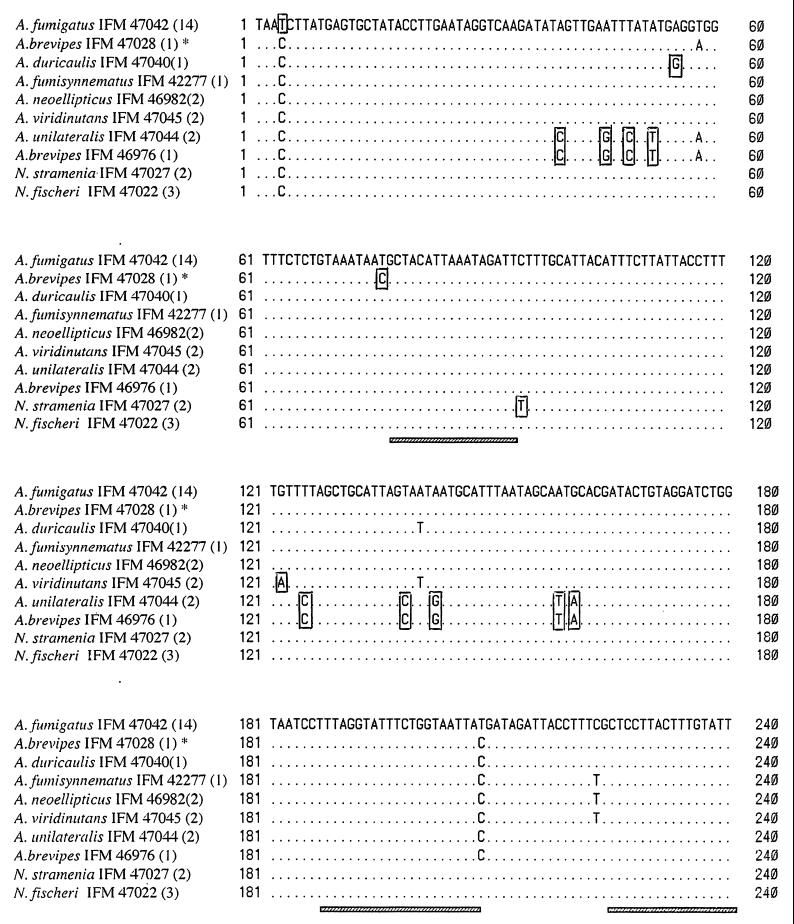
FIG. 1.
Partial sequences of the cytochrome b genes from species of Aspergillus section Fumigati and two species of Neosartorya. Dots indicate that the nucleotides are the same as those of A. fumigatus. Species-specific nucleotides are boxed. The hatched bars indicate the section Fumigati-specific regions. The numbers in parentheses indicate the numbers of strains with the same nucleotide sequences. ∗, the strain has an intron. The exons were used for alignment.
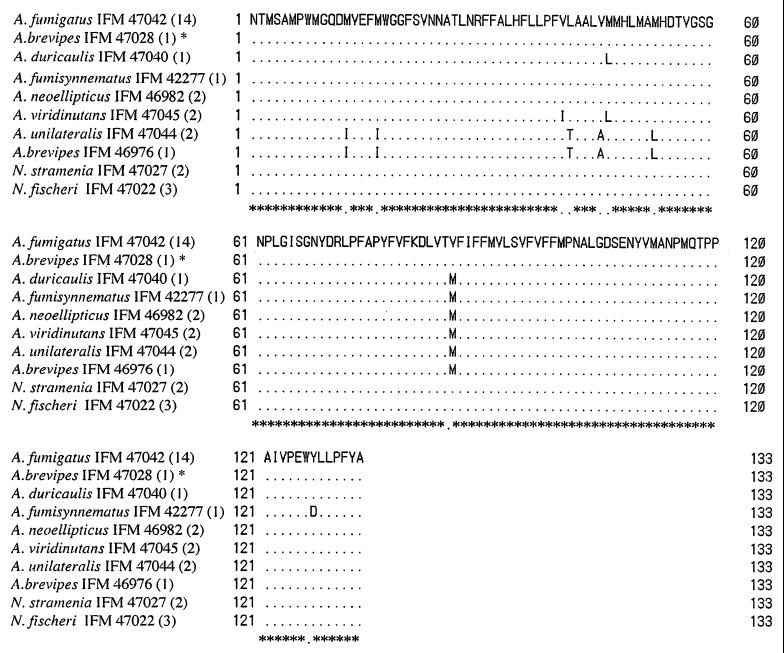
FIG. 2.
Multiple alignment of the amino acid sequences estimated from 402-bp nucleotide sequences of the cytochrome b genes of the species of Aspergillus section Fumigati and two species of Neosartorya. Dots indicate that the sequences of amino acid are the same as those of A. fumigatus. The numbers in parentheses indicate the numbers of strains with the same amino acid sequences. ∗, the strain has an intron. The exons were used for alignment.
The tree obtained by UPGMA was compared with the other three trees (those obtained by NJ, ML, and MP methods). In some cases the tree obtained by UPGMA and the trees obtained by other methods were different, and those obtained by the other methods were not as good as that obtained by UPGMA (the trees obtained by the other methods are not shown). The nucleotide- and amino acid-based trees were built by UPGMA (Fig. 3 and 4) by the use of sequences of the species of section Fumigati and other pathogenic species of Aspergillus (A. terreus, A. flavus, A. niger, and A. nidulans). Table 2 shows pairwise comparisons of the numbers of differences in the nucleotide and amino acid sequences between strains.
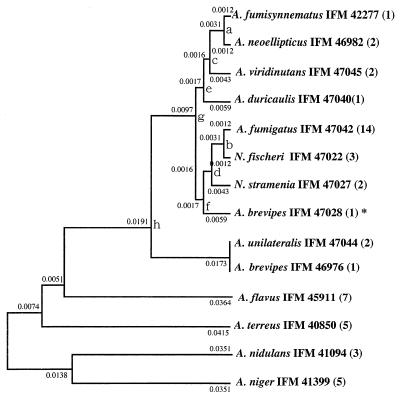
FIG. 3.
Phylogenetic tree of species of Aspergillus section Fumigati and other related species based on the mt cytochrome b gene sequence. UPGMA was used. The standard error of each branching point is as follows: a, ±0.00125; b, ±0.00125; c, ±0.00369; d, ±0.00369; e, ±0.00387; f, ±0.00387; g, ±0.00449; h, ±0.00747. The standard errors for points c, d, e, f, g, and h were calculated by use of data for three species (c, A. fumisynnematus IFM 42277, A. neoellipticus IFM 46982, and A. viridinutans IFM 47045; d, A. fumigatus IFM 47042; N. fischeri IFM 47022, and N. stramenia IFM 47027; e, A. duricaulis IFM 47040, A. viridinutans IFM 47045, and A. fumisynnematus IFM 42277; f, A. brevipes IFM 47028, N. stramenia IFM 47027, and A. fumigatus IFM 47042; g, A. fumigatus IFM 47042, A. duricaulis IFM 47040, and A. fumisynnematus IFM 42277; h, A. unilateralis IFM 47044, A. brevipes IFM 47028, and A. duricaulis IFM 47040). The numbers in parentheses indicate the numbers of strains with same nucleotide sequences. ∗, the strain has an intron, and the exons were used.
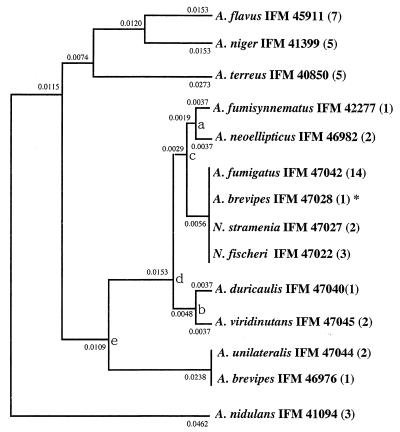
FIG. 4.
Phylogenetic tree obtained by use of the amino acid sequences estimated from the 402-bp nucleotide sequences of the mt cytochrome b genes. UPGMA was used. The standard error of each branching point is as follows: a, ±0.00377; b, ±0.00377; c, ±0.00377; d, ±0.00402; e, ±0.00794. The standard errors for points c, d, and e were calculated by use of data for three species (c, A. fumigatus IFM 47042, A. fumisynnematus IFM 42277, and A. neoellipticus IFM 46982; d, A. duricaulis IFM 47040, A. neoellipticus IFM 46982, and A. fumigatus IFM 47042; e, A. duricaulis IFM 47040, A. duricaulis IFM 47040, and A. fumigatus IFM 47042). The numbers in parentheses indicate the numbers of strains with same nucleotide sequences. ∗, the strain has an intron, and the exons were used.
TABLE 2.
Numbers of nucleotide and amino acid sequence between different speciesa
| Species | No. of nucleotide or amino acid sequence differencesb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | |
| A | 0 | 2 | 2 | 1 | 3 | 6 | 6 | 0 | 0 | |
| B | 5 | 2 | 2 | 1 | 3 | 6 | 6 | 0 | 0 | |
| C | 7 | 6 | 2 | 1 | 1 | 6 | 6 | 2 | 2 | |
| D | 5 | 6 | 6 | 1 | 3 | 6 | 6 | 2 | 2 | |
| E | 4 | 5 | 5 | 1 | 2 | 5 | 5 | 1 | 1 | |
| F | 7 | 6 | 4 | 4 | 3 | 7 | 7 | 3 | 3 | |
| G | 15 | 12 | 14 | 14 | 13 | 14 | 0 | 6 | 6 | |
| H | 15 | 12 | 14 | 14 | 13 | 14 | 0 | 6 | 6 | |
| I | 3 | 5 | 6 | 7 | 6 | 7 | 15 | 16 | 0 | |
| J | 1 | 4 | 6 | 4 | 3 | 6 | 14 | 14 | 3 | |
Abbreviations: A, A. fumigatus; B, A. brevipes IFM 47028; C, A. duricanlis; D, A. fumisynnematus; E, A. neoellipticus; F, A. viridinutans; G, A. unilateralis; H, A. brevipes IFM 46976; I, N. stramenia; J, N. fischeri.
The numbers of nucleotides differences are on the left of the diagonal, and the numbers of amino acids differences are on the right of the diagonal.
Alignment of the nucleotide sequences showed that individual species possessed characteristic nucleotide sequences (Fig. 1). On the other hand, regions specific to section Fumigati that were distinct from homologous sequences from four other pathogenic species of Aspergillus (36) were found (Fig. 1).
On the basis of the nucleotide sequences, the species of section Fumigati were placed in an independent cluster, and 27 strains were divided into nine types (Fig. 3). All strains of A. fumigatus, including 10 clinical isolates, reference strain A. fumigatus var. fumigatus CBS 110.46 (ex type), strain A. fumigatus var. ellipticus CBS 487.65 (ex type), and 1 strain of A. fumigatus var. albus had the same nucleotide sequences. Strains identified as A. fumigatus, A. unilateralis, A. brevipes IFO 5821, A. fumisynnematus, A. viridinutans, A. duricaulis, and A. neoellipticus all had nucleotide sequences that differed from each other. The other strain of A. brevipes, strain IFM 46976 (NHMIC FD-085), had a nucleotide sequence identical to that of A. unilateralis. N. fischeri var. fischeri and N. stramenia both had DNA sequences different from that of A. fumigatus.
On the basis of amino acid sequences, species of section Fumigati again formed a distinct cluster, in contradistinction to the species from other sections (Fig. 4). A. brevipes IFO 5821, N. stramenia, and N. fischeri var. fischeri had the same amino acid sequences as A. fumigatus. The amino acid sequence of A. brevipes IFM 46976 was identical to that of A. unilateralis.
DISCUSSION
Identification of A. fumigatus is important because it is one of the most important fungal pathogens. The identification of Aspergillus spp. isolated from clinical specimens depends primarily on morphological characteristics. However, morphology is insufficient for the identification of some clinical isolates because of the presence of pleomorphism and the poor development of conidial structure. Therefore, in recent years, some additional methods have been used in the study of A. fumigatus. Burnie et al. (6) used restriction fragment length polymorphism analysis to distinguish clinical isolates of A. fumigatus. They could classify 21 isolates into six types by using XbaI digestion of total cellular DNA (6). On the basis of secondary metabolite profiles, Frisvad and Samson (15) considered clinical A. fumigatus isolates to be very homogeneous and found that they could not be separated from the ex type isolate or other isolates from soil or foodstuffs. Our results obtained by DNA sequencing of the mt cytochrome b gene showed that clinical isolates of A. fumigatus from different sources have nucleotide sequences identical to each other and to that of the ex type isolate of A. fumigatus var. fumigatus. On the basis of the observed identity of nucleotide sequences, it can be concluded that clinical isolates of A. fumigatus are generally accurately identified. On the other hand, nucleotide sequences specific to other species in section Fumigati were found, as were distinct regions consistent for all members of section Fumigati. Use of these specific sequences and regions may facilitate the identification of section Fumigati strains at the species level, as well as direct diagnosis of aspergillosis with clinical specimens.
A. neoellipticus is known to be a human pathogen. A. fumigatus var. ellipticus (31) has been raised to the species rank as A. neoellipticus on the basis of its distinct smooth-walled and ellipsoidal conidia (22). Except for its conidial ornamentation, however, it closely resembles A. fumigatus var. fumigatus (15, 33). Our results showed that the ex type isolate of A. fumigatus var. ellipticus (A. neoellipticus Kozakiewicz) could not be distinguished from A. fumigatus var. fumigatus (Fig. 1 and 3). On the basis of morphology, secondary metabolite profiles (10, 15), DNA complementarity (30), and isozyme analysis (9), A. neoellipticus is not distinct from A. fumigatus (9, 10, 15, 30) and the species should be considered a variety of A. fumigatus. Our results also strongly support this view, even though two strains labeled A. neoellipticus were different from A. fumigatus var. fumigatus and from A. fumigatus var. ellipticus CBS 487.65 (ex type). Because the two anomalous isolates had nucleotide and amino acid sequences different from those of CBS 487.65, they appear to represent a new species in the section Fumigati. The mt cytochrome b sequence of A. fumigatus var. albus was also identical to that of A. fumigatus. Kozakiewicz (22) had previously concluded that A. fumigatus var. albus appears to be identical to A. fumigatus var. fumigatus in all respects except for its buff color.
Occasional reports have described Neosartorya species as human pathogens. We also determined the nucleotide sequences of Neosartorya, the sole teleomorphic genus known to have anamorphs in Aspergillus section Fumigati. The three species selected for study included N. fischeri var. fischeri, N. stramenia, and four strains of N. pseudofischeri. N. fischeri and N. stramenia had the same amino acid sequences as A. fumigatus. N. pseudofischeri had an intron. The amino acid sequences of the exons were identical to the exons of labeled A. neoellipticus. Nonetheless, these species of Neosartorya had nucleotide sequences different from each other and also from those of the pathogens A. fumigatus and A. neoellipticus. Therefore, they could be distinguished from A. fumigatus and labeled A. neoellipticus (Table 2). N. pseudofischeri was different from A. fumigatus at five base pair positions and from A. neoellipticus at three base pair positions (data not shown).
Concerning A. brevipes, we found that one strain, strain IFO 5821, had an intron. The exon sequences of this strain were very similar to the 402-bp sequence of A. fumigatus (Fig. 3 and 4), although they contained different base pairs at five positions (Fig. 1). The derived amino acid sequence from this strain was identical to that of A. fumigatus. On the other hand, the other strain of A. brevipes, strain IFM 46976, had the same nucleotide and amino acid sequences as A. unilateralis. The two strains of A. brevipes were very distant from each other (Fig. 3 and 4). On the basis of scanning electron micrographs, Kozakiewicz (22) retained A. brevipes var. brevipes but reduced A. duricaulis to synonymy with A. brevipes and recombined A. brevipes var. unilateralis with A. unilateralis. Our results show that the purported strain A. brevipes IFM 46976 is the same as A. unilateralis, but neither of the strains was identical to the ex type isolate of A. duricaulis. Therefore, we do not consider A. duricaulis to be a synonym for A. brevipes. Comparing the morphology and secondary metabolites, Frisvad and Samson (15) also concluded that they do not belong to the same species. A. brevipes, A. duricaulis, and A. unilateralis produce viriditoxin (5, 38), cyclopaldic acid (4, 15), and mycophenolic acid (15), respectively. Results obtained by enzyme-linked immunosorbent assay also indicated that A. brevipes CBS 118.53, A. duricaulis CBS 481.65, and A. unilateralis CBS 283.65 and CBS 126.56 differ from A. fumigatus (10). On the basis of partial β-tubulin and hydrophobin sequences, Geiser et al. (17) studied the evolutionary relationships of the species in section Fumigati. Their results also showed that A. brevipe, A. unilateralis, and A. duricaulis were independent species.
A. fumisynnematus IFM 46981 (from Venezuelan soil) was reported by Horie et al. (19) to be a new species of section Fumigati because it was described as differing from A. fumigatus in having small conidial heads, short conidiophores borne on bundles of aerial hyphae or synnemata, and verruculose conidia. Our results support it as a distinct species.
We compared trees obtained by UPGMA and other methods (NJ, ML, and MP methods) assuming no constancy of the evolutionary rate. We preferred the tree obtained by UPGMA for two reasons. First, mt DNA is a favored molecule to be used as a molecular clock for molecular phylogenetic studies (32, 37). The rate of substitution of bases in cytochrome b genes is in proportion to evolutionary time. If the distance measure used is exactly linear with evolutionary time, that is, the evolutionary rate is constant or mt is used as a molecular clock, UPGMA gives the correct topology and correct branch lengths. Therefore, in this case, UPGMA was advocated for use in reconstructing phylogenetic trees (25, 26, 28). Second, by UPGMA, section Fumigati constituted a distinct cluster from A. flavus, A. niger, A. terreus, and A. nidulans. A. flavus and A. niger were very closely related in the tree obtained by UPGMA. Samson (33) suggested that these two species are also morphologically closely related and that they could be placed in the subgenus Circumdati. On the other hand, by other methods (e.g., the NJ, ML, and MP methods), section Fumigati did not constitute a distinct cluster or A. flavus and A. niger were very distant from each other.
In conclusion, the mt cytochrome b gene is important and useful for the identification and classification of the pathogenic species A. fumigatus and for clarification of the phylogenetic relationships among the pathogenic species A. fumigatus and related taxa.
ACKNOWLEDGMENTS
We thank the Institute for Fermentation, Osaka, Japan (IFO), for generously providing some of the strains used in this study. We also thank David Wood for assistance with the text.
We thank the Honor Scholarship (Association of International Education, Tokyo, Japan), the Sumitomo Scholarship (Social Welfares Business Group of Sumitomo Life Assurance Company, Osaka, Japan), Okamoto Scholarship Foundation, Chiba, Japan; and Yonnmaru Scholarship (Yonnmaru Alumni Association, Chiba University, School of Medicine, Chiba, Japan) for providing scholarships to L. Wang.
REFERENCES
- 1.Aquadro C F, Greenberg B D. Human mitochondrial DNA variation and evolution: analysis of nucleotide sequences from seven individuals. Genetics. 1983;103:287–312. doi: 10.1093/genetics/103.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baedana E J. The clinical specturum of aspergillosis. Part 1. Epidemiology, pathogenicity, infection in animals and immunology of Aspergillus. Crit Rev Lab Sci. 1980;13:21–83. doi: 10.3109/10408368009106444. [DOI] [PubMed] [Google Scholar]
- 3.Barnes A J, Denning D W. Aspergillus—significance as a pathogen. Rev Med Microbiol. 1993;4:176–180. [Google Scholar]
- 4.Brillinger G U, Heberle W, Weber B, Achenbach H. Metabolic products of microorganisms. 167. Cyclopaldic acid from Aspergillus duricaulis. 1. Production, isolation and biological properties. Arch Microbiol. 1978;116:245–252. doi: 10.1007/BF00417847. [DOI] [PubMed] [Google Scholar]
- 5.Brown W M, George M, Jr, Wilson A C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci USA. 1979;76:1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnie J P, Coke A, Matthews R C. Restriction endonuclease analysis of Aspergillus fumigatus DNA. J Clin Pathol. 1992;45:324–327. doi: 10.1136/jcp.45.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chikuni K, Minaka N, Ikenaga H. Molecular phylogeny of some Passeriformes, based on cytochrome b sequences. J Yamashina Inst Ornithol. 1996;28:1–8. [Google Scholar]
- 8.Chikuni K, Mori Y, Tabata T, Saito M, Monma M, Kosugiyama M. Molecular phylogeny based on the κ-casein and cytochrome b sequences in the mammalian soborder Ruminsntia. J Mol Evol. 1995;41:859–866. doi: 10.1007/BF00173165. [DOI] [PubMed] [Google Scholar]
- 9.Croft J H, Varga J. Application of RFLPs in systematics and population genetics of Aspergilli. In: Powell K A, et al., editors. The genus Aspergillus. New York, N.Y: Plenum Press; 1994. pp. 277–289. [Google Scholar]
- 10.Debeaupuis J P, Sarfati P, Goris A, Stynen D, Diaquin M, Latge J P. Exocellular polysaccharides of Aspergillus fumigatus and related taxa. In: Samson R A, Pitt J I, editors. Modern concepts in Penicillium and Aspergillus classification. New York, N.Y: Plenum Press; 1990. pp. 309–320. [Google Scholar]
- 11.Denning D W, Follansbee S, Scolaro M, Norris S, Edelstein D, Stevens D A. Pulmonary aspergillosis in AIDS. N Engl J Med. 1991;324:654–662. doi: 10.1056/NEJM199103073241003. [DOI] [PubMed] [Google Scholar]
- 12.Edwards S V, Wilson A C. Phylogenetically informative length polymorphism and sequence variability in mitochondrial DNA of Australian songbirds (Pomatostomus) Genetics. 1990;126:695–711. doi: 10.1093/genetics/126.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards S V, Arctander P, Wilson A C. Mitochondrial resolution of a deep branch in the genealogical tree for perching birds. Proc R Soc London Ser B. 1991;243:99–107. doi: 10.1098/rspb.1991.0017. [DOI] [PubMed] [Google Scholar]
- 14.Fanti F, Conti S, Campani L, Morace G, Dettori G, Polonelli L. Studies on the epidemiology of Aspergillus fumigatus infections in a university hospital. Eur J Epidemiol. 1989;5:8–14. doi: 10.1007/BF00145038. [DOI] [PubMed] [Google Scholar]
- 15.Frisvad J C, Samson R A. Chemotaxonomy and morphology of Aspergillus fumigatus and related taxa. In: Samson R A, Pitt J I, editors. Modern concepts in Penicillium and Aspergillus classification. New York, N.Y: Plenum Press; 1990. pp. 201–208. [Google Scholar]
- 16.Geber J, Chomicki J, Brandsberg J W, Jones R, Hammerman K J. Pulmonary aspergillosis caused by A. fischeri var. spinosus. Am J Clin Pathol. 1973;60:861–866. doi: 10.1093/ajcp/60.6.861. [DOI] [PubMed] [Google Scholar]
- 17.Geiser D M, Frisvad J C, Taylor J W. Evolutionary relationships in Aspergillus section Fumigati inferred from partial β-tubulin and hydrophobin DNA sequences. Mycologia. 1998;90:831–845. [Google Scholar]
- 18.Goriglione G, Stella G, Gafa L, Spata G, Oliveri S, Padhye A A, Ajello L. Neosartorya fischeri var. fischeri (Wehmer) Malloch and Cain 1972 (anamorph Aspergillus fischerianus Samson and Gams 1985) as a cause of mycotic keratitis. Eur J Epidemiol. 1990;6:382–385. doi: 10.1007/BF00151712. [DOI] [PubMed] [Google Scholar]
- 19.Horie Y, Miyaji M, Nishimura K, Taguchi H, Udagawa S. Aspergillus fumisynnematus, a new species from Venezuelan soil. Trans Mycol Soc Jpn. 1993;34:3–7. [Google Scholar]
- 20.Irwin D M, Kocher T D, Wilson A C. Evolution of the cytochrome b gene of mammals. J Mol Evol. 1991;32:128–144. doi: 10.1007/BF02515385. [DOI] [PubMed] [Google Scholar]
- 21.Kocher T D, Thomas W K, Meyer A, Edwards S V, Paabo S, Villablanca F X, Wilson A C. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci USA. 1989;86:6196–6200. doi: 10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozakiewicz Z. Aspergillus species on the stored products. Mycol Pap. 1989;161:1–188. [Google Scholar]
- 23.Krajewski C, Driskell A C, Baverstock P R, Braun M J. Phylogenetic relationships of the thylacine (Mammalia: Thylacinidae) among dasyuroid marsupials: evidence from cytochrome b DNA sequences. Proc R Soc London Ser B. 1992;250:19–27. doi: 10.1098/rspb.1992.0125. [DOI] [PubMed] [Google Scholar]
- 24.Leslie L E, Flannigan B, Milner L J R. Morphological studies on clinical isolates of Aspergillus fumigatus. J Med Vet Mycol. 1988;26:335–341. [PubMed] [Google Scholar]
- 25.Naruya S. Statistical methods for phylogenetic tree reconstruction. In: Rao C R, Chakraborty R, editors. Handbook of statistics. Vol. 8. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1991. pp. 317–346. [Google Scholar]
- 26.Naruya S. Reconstruction of gene trees from sequence data. Methods Enzymol. 1996;266:427–449. doi: 10.1016/s0076-6879(96)66027-3. [DOI] [PubMed] [Google Scholar]
- 27.Nei M. Human evolution at the molecular level. In: Ohta T, Aoki K, editors. Population genetics and molecular evolution. Berlin, Germany: Japan Scientific Society Press/Springer-Verlag; 1985. pp. 41–64. [Google Scholar]
- 28.Nei M. Phylogenetic trees. In: Nei M, editor. Molecular evolutionary genetics. New York, N.Y: Columbia University Press; 1987. pp. 287–326. [Google Scholar]
- 29.Padhye A A, Godfrey J H, Chandler F W, Peterson S W. Osteomyelitis caused by Neosartorya pseudofischeri. J Clin Microbiol. 1994;32:2832–2836. doi: 10.1128/jcm.32.11.2832-2836.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson S W. Neosartorya pseudofischeri sp. nov. and its relationship to other species in Aspergillus section Fumigati. Mycol Res. 1992;96:547–554. [Google Scholar]
- 31.Raper K B, Fennell D I. The Aspergillus fumigatus group. In: Raper K B, Fennell D I, editors. The genus of Aspergillus. Baltimore, Md: The Williams & Wilkins Co.; 1965. pp. 238–268. [Google Scholar]
- 32.Ruvolo M, Disotell T R, Allard M W, Brown W M, Honeycutt R L. Resolution of the African hominoid trichotomy by use of a mitochondrial gene sequence. Proc Natl Acad Sci USA. 1991;88:1570–1574. doi: 10.1073/pnas.88.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samson R A. Current systematics of the genus Aspergillus. In: Powell K A, et al., editors. The genus Aspergillus. New York, N.Y: Plenum Press; 1994. pp. 261–276. [Google Scholar]
- 34.Udagawa S, Tsubouchi H, Toyazaki N. Isolation and identification of Neosartorya species from house dust as hazardous indoor pollutants. Mycoscience. 1996;37:217–222. [Google Scholar]
- 35.Urata T, Kobayashi M, Imamura J, Tanaka Y, Muneishi H, Iwahara Y, Uemura Y, Taguchi H, Miyoshi I. Polymerase chain reaction amplification of AspfI and alkaline protease genes from fungus balls: clinical application in pulmonary aspergillosis. Int Med. 1997;36:19–27. doi: 10.2169/internalmedicine.36.19. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Yokoyama K, Miyaji M, Nishimura K. The identification and phylogenetic relationship of pathogenic species of Aspergillus based on the mitochondrial cytochrome b gene. Med Mycol. 1998;36:153–164. [PubMed] [Google Scholar]
- 37.Watson J D, Gilman M, Witkowski J, Zoller M. Mitochondrial DNA as a molecular clock. In: Watson J D, Gilman M, Witkowski J, Zoller M, editors. Recombinant DNA. 2nd ed. New York, N.Y: W. H. Freeman & Co.; 1991. pp. 446–447. [Google Scholar]
- 38.Weisleder D, Lillehoj E B. Structure of viriditoxin, a toxic metabolite of Aspergillus viridinutans. Tetrahedron Lett. 1971;48:4705–4706. [Google Scholar]