Abstract
Initiation of stomatal closure by various stimuli requires activation of guard cell plasma membrane anion channels, which are defined as rapid (R)- and slow (S)-type. The single-gene loss-of-function mutants of these proteins are well characterized. However, the impact of suppressing both the S- and R-type channels has not been studied. Here, by generating and studying double and triple Arabidopsis thaliana mutants of SLOW ANION CHANNEL1 (SLAC1), SLAC1 HOMOLOG3 (SLAH3), and ALUMINUM-ACTIVATED MALATE TRANSPORTER 12/QUICK-ACTIVATING ANION CHANNEL 1 (QUAC1), we show that impairment of R- and S-type channels gradually increased whole-plant steady-state stomatal conductance. Ozone-induced cell death also increased gradually in higher-order mutants with the highest levels observed in the quac1 slac1 slah3 triple mutant. Strikingly, while single mutants retained stomatal responsiveness to abscisic acid, darkness, reduced air humidity, and elevated CO2, the double mutant lacking SLAC1 and QUAC1 was nearly insensitive to these stimuli, indicating the need for coordinated activation of both R- and S-type anion channels in stomatal closure.
Combined impairment of guard cell slow and rapid anion channels results in increased stomatal conductance and complete stomatal insensitivity to abscisic acid, darkness, and elevated CO2.
Introduction
Gas exchange through stomatal pores maintains the balance between transpirational water loss and photosynthetic CO2 uptake. Opening and closure of stomatal pores are regulated by the accumulation and release of osmotically active ions across guard cell membranes. Membrane depolarization and activation of anion channels are among the first steps to initiate stomatal closure. This leads to the activation of voltage-dependent potassium channels, followed by water efflux. Thus, for stomata to close, multiple channels need to be activated/inactivated in a coordinated manner (Kollist et al., 2014; Hedrich and Geiger, 2017; Jezek and Blatt, 2017).
Long before identification of the respective proteins, patch-clamp studies identified two types of anion channels in guard cells. Slow (S)-type anion channels activate in seconds, conduct primarily chloride and nitrate, and are weakly dependent on membrane potential, whereas rapid (R)-type anion channels activate in milliseconds, conduct primarily malate but also sulfate, and their activation is dependent on membrane voltage (Schroeder and Hagiwara, 1989; Hedrich et al., 1990). By now, the molecular nature of main guard cell S-type and R-type anion channels has been identified and their functionality verified using the corresponding mutants in Arabidopsis thaliana (Negi et al., 2008; Vahisalu et al., 2008; Meyer et al., 2010). Apart from modeling work, which indicated the need for coordinating the R- and S-type anion channels to prime the guard cells into the ion efflux cycle (Jezek and Blatt, 2017), these proteins have typically been studied in isolation. Therefore, information about their coordinated function in the regulation of stomatal aperture and plant responsiveness to the environment is lacking.
Mutations in the anion channel protein SLOW ANION CHANNEL1 (SLAC1) lead to more open stomata and severely impaired stomatal responses to almost all environmental and endogenous stimuli that result in stomatal closure (Negi et al., 2008; Vahisalu et al., 2008; Merilo et al., 2013; Guzel Deger et al., 2015). SLAC1 HOMOLOG3 (SLAH3) conducts predominantly nitrate and compared with SLAC1 shows higher voltage-dependence (Geiger et al., 2011). Pathogenic bacteria can use stomatal pores to infect host plants and guard cells have mechanisms to counteract bacterial invasion by stomatal closure. Recent studies indicated that the SLAH3 anion channel is involved in controlling stomatal closure triggered by microbial elicitors (Guzel Deger et al., 2015; Liu et al., 2019).
The R-type anion channel ALUMINUM-ACTIVATED MALATE TRANSPORTER 12 (ALMT12) was identified by screening ALMTs expressed in Arabidopsis guard cells (Meyer et al., 2010). Stomatal responses of almt12 mutants were also impaired, but these phenotypes were weaker than those observed in slac1 mutants. This could be a result of genetic redundancy, as the ALMT gene family consists of 13 members (Dreyer et al., 2012) and R-type anion currents were only 40% reduced in the almt12 guard cells (Meyer et al., 2010). As ALMT12 is not aluminum-activated, it is often referred to as QUICK-ACTIVATING ANION CHANNEL 1 (QUAC1; Malcheska et al., 2017) and hereafter we will follow this nomenclature.
Despite the importance of S-type and R-type anion channels in guard cell function, no study used plants that carry impairment in both of these channels. Here, we generated multiple double and triple mutants impaired in the major S-type channels SLAC1 and SLAH3, and the R-type channel QUAC1, and characterized the stomatal function of these plants in response to environmental factors and abscisic acid (ABA). We show that while the single mutants slac1 and quac1 retain partial stomatal sensitivity, the double mutant quac1 slac1 is almost nonresponsive to ABA, darkness, reduced air humidity, and elevated concentration of CO2. Hence, the combined action of S- and R-type channels is essential for proper stomatal function.
Results
Whole-plant steady-state stomatal conductance of higher-order anion channel mutants
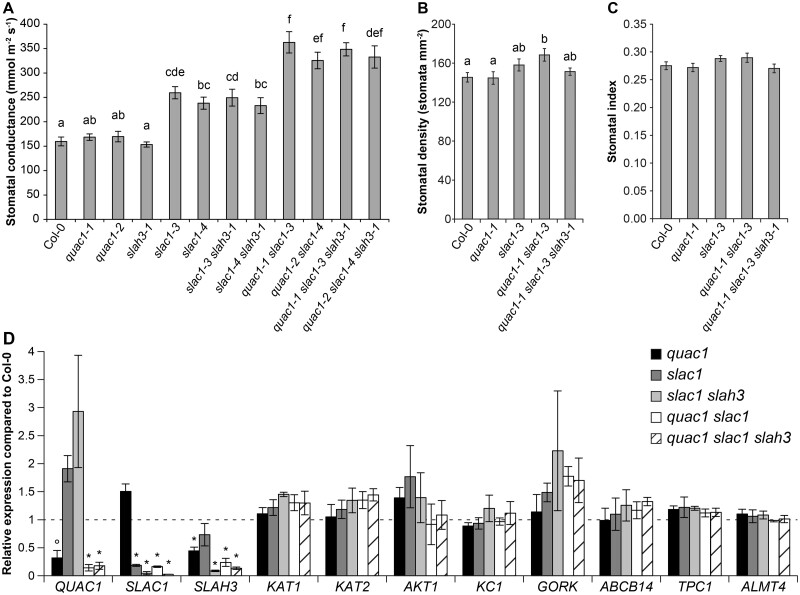
To study the combined effect of R- and S-type anion channel activity, we generated several double and triple mutant combinations between quac1, slac1 and slah3 (Supplemental Figure S1). We measured their steady-state stomatal conductances (gs) and stomatal responses to various treatments with a custom-built multichamber gas-exchange system for intact plants (Kollist et al., 2007). The absence of QUAC1 or SLAH3 alone had no effect on gs. Impairment of R- and S-type anion channel functions in quac1-1 slac1-3 and quac1-2 slac1-4 double mutants resulted in increased stomatal conductance compared with slac1 mutants (Figure 1A). Removal of SLAH3 in the double and triple mutants did not lead to an additional increase in stomatal conductance (Figure 1A).
Figure 1.
Stomatal conductance, stomatal density and index, and gene expression in guard cell-enriched samples of Col-0 and anion channel mutants. A, Whole-plant steady-state stomatal conductance (gs) of 3- to 4-week-old plants. Letters denote statistically significant differences between lines (ANOVA with Tukey unequal N honestly significant difference (HSD) post hoc test, P < 0.05; average ± se; n = 7–12). B, Stomatal density of 5-week-old plants. Letters denote statistically significant differences between lines (ANOVA with Tukey post hoc test, P < 0.05; average ± se; n = 19). C, Stomatal index of 5-week-old plants (bars indicate average ± se, n = 19). D, Relative expression of selected marker genes from Col-0 wild-type, quac1, slac1, slac1 slah3, quac1 slac1, and quac1 slac1 slah3 guard cell-enriched samples determined via RT-qPCR and depicted relative to Col-0. The mean of three biological replicates is shown; error bars depict ±se. Statistically significant differences from Col-0 (*P < 0.05; °P < 0.10; ANOVA with Dunnett’s test).
We measured the stomatal density and stomatal index of generated mutants to check whether their higher gs is associated with changes in stomatal development. Higher stomatal density was detected in the quac1-1 slac1-3 mutant, but not in the quac1-1 slac1-3 slah3-1 mutant and single mutants of S- and R-type channels (Figure 1B). There were no differences in stomatal index (Figure 1C). Thus, increased gs was likely caused by more open stomata rather than by altered stomatal density.
Removal of important anion channels could lead to changed expression of other ion channel- and transporter-encoding genes. We measured the transcripts of POTASSIUM CHANNEL IN ARABIDOPSIS THALIANA 1 (KAT1), KAT2, ARABIDOPSIS THALIANA K+ TRANSPORTER 1 (AKT1), ARABIDOPSIS THALIANA K+ RECTIFYING CHANNEL 1 (KC1), GATED OUTWARDLY-RECTIFYING K+ CHANNEL (GORK1), ATP-BINDING CASSETTE B14 (ABCB14), TWO-PORE CHANNEL 1 (TPC1), and ALMT4 and also anion channel genes SLAC1, SLAH3, and QUAC1 in guard cell-enriched RNA with reverse transcription quantitative PCR (RT-qPCR). The expression of SLAC1, SLAH3, and QUAC1 was significantly reduced in the respective mutants as expected (Figure 1D). We observed a somewhat higher transcript level of QUAC1 in slac1 and slac1 slah3 mutants, and similarly SLAC1 expression was somewhat higher in the quac1 mutant, but these differences were not statistically significant. We did not observe significantly altered transcript levels of other tested ion-channel genes and thus feedback regulation at the transcriptional level is unlikely.
Impairment of S- and R-type anion channels leads to severely impaired stomatal responses to environmental factors and ABA
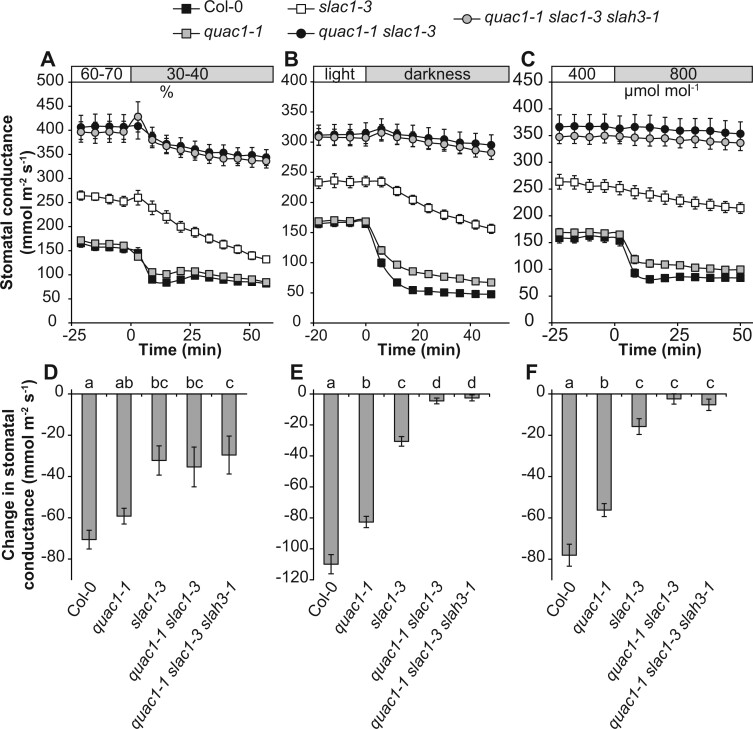
To explore the role of S- and R-anion channels in stomatal closure, we treated plants with reduced air humidity, darkness, and elevated CO2 concentration (Figure 2; see also Supplemental Figure S2 for additional mutant alleles). Consistent with previous findings, quac1 lines were weakly impaired in their response to these stimuli, whereas slac1 lines showed stronger phenotypes (Figure 2; Supplemental Figure S3). In Col-0 wild-type and quac1 plants, the stomatal response to reduced humidity was completed within the first 20 min, the slac1 mutant displayed slower but sustained stomatal closure during the 60-min experiment, and this response was further impaired in the quac1 slac1 double mutant (Figure 2A). In the quac1 slac1 mutant, the stomata were almost unresponsive to darkness and elevated CO2 and no closure was detected by the end of the treatments (Figure 2; Supplemental Figure S2). Further removal of SLAH3 function did not change the stomatal sensitivity of the quac1-1 slac1-3 slah3-1 triple mutant compared with the quac1 slac1 double mutants (see also Supplemental Figure S2 for the slah3-1 single mutant).
Figure 2.
Time-dependent changes in stomatal conductance. A–C, Time-course measurements of stomatal conductance in response to reduced air humidity (A), darkness (B), and elevated CO2 (C). D–F, Changes in stomatal conductance during the first 18 min. Letters denote statistically significant differences between lines (ANOVA with Tukey HSD post hoc test, P < 0.05; n = 12). The data in all figures are represented as average ± se.
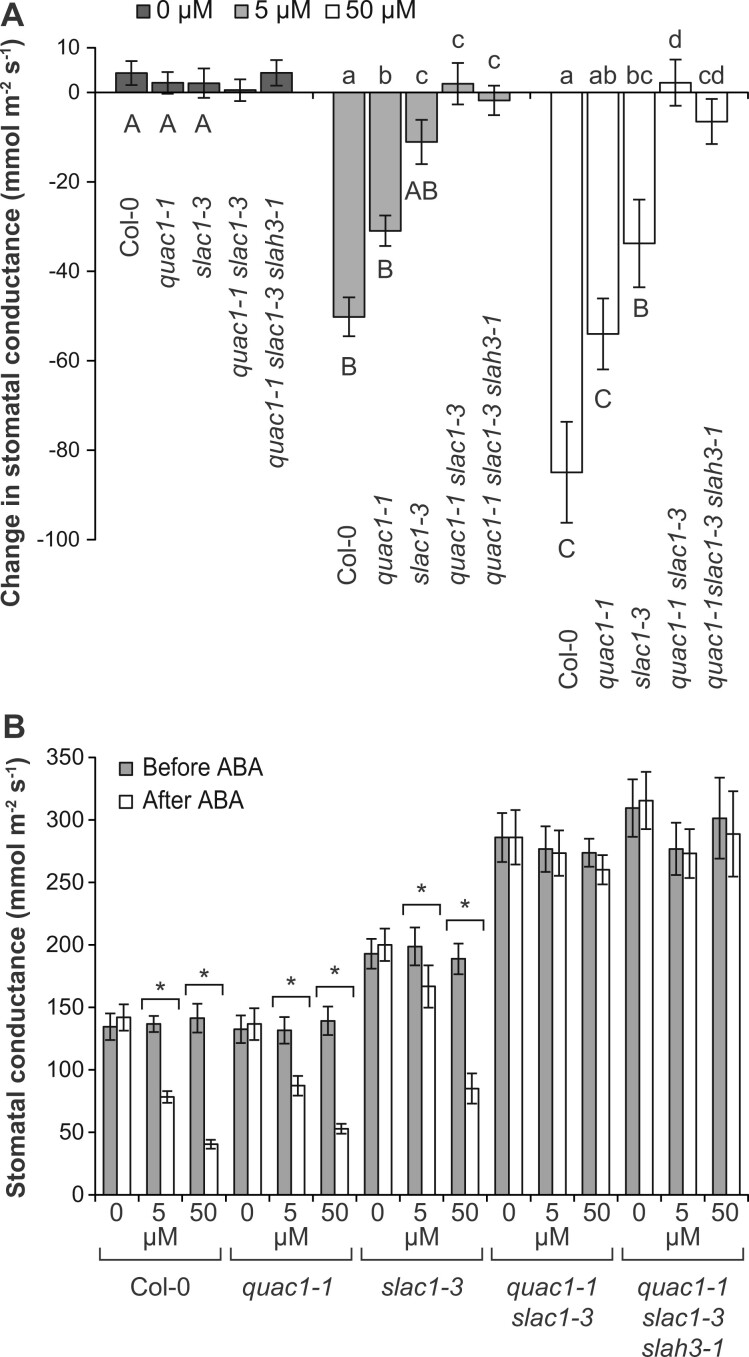
To test the S- and R-type channel function in stomatal ABA response we used two concentrations, as the slac1 mutant is severely impaired in stomatal closure at low ABA concentrations, but shows a partial response at higher ABA concentrations at 1 and 3 h (Laanemets et al., 2013). Here, we tested ABA (5 and 50 µM) responses at 16 and 56 min. Stomatal closure was only weakly reduced in quac1-1 plants, but more strongly impaired in slac1-3 plants (Figure 3). Strikingly, the quac1 slac1 mutant was completely insensitive to ABA, even at the very high 50-µM ABA concentration (Figure 3; see also Supplemental Figure S3 for additional alleles), indicating that both S- and R-type anion channels are required for stomatal response to ABA.
Figure 3.
Stomatal response to foliar ABA spraying (0, 5, or 50 µM). A, Changes in stomatal conductance during the first 16 min. Lowercase letters denote statistically significant differences between lines within the same treatment, capital letters denote statistically significant differences, and no capital letters point at no significant difference between treatments within the genotype (ANOVA with Tukey HSD post hoc test, P < 0.05; average ± se; n = 9). B, Average ± se (n = 9) stomatal conductance before and 56 min after treatment with ABA. Statistically significant differences are denoted by *P < 0.05 between pre- and post-treatment stomatal conductance values (repeated measures ANOVA with Tukey’s post hoc test).
Impairment of all three anion channels leads to the highest sensitivity to the air pollutant ozone
We assayed the extent of ozone (O3)-induced cell death in S- and R-type channel mutants with a quantifiable ion leakage assay (Figure 4). The slac1-3 mutant was sensitive to O3, but not the slah3-1 and quac1-1 mutants (Figure 4). The combined impairment of both S- and R-type channel functions resulted in increased cell death, which was further enhanced in the quac1-1 slac1-3 slah3-1 triple mutant, especially at the 8-h timepoint. We concluded that the higher the gs and the stronger the impairment of stomatal function in combined R- and S-type channel mutants, the higher the O3 uptake and the resulting cell death. Furthermore, the highest O3 sensitivity of the triple mutant suggests that even though the SLAH3 anion channel had no detectable role in steady-state and stress-induced stomatal regulation, it might have a role in the regulation of apoplastic reactive oxygen species-induced cell death.
Figure 4.
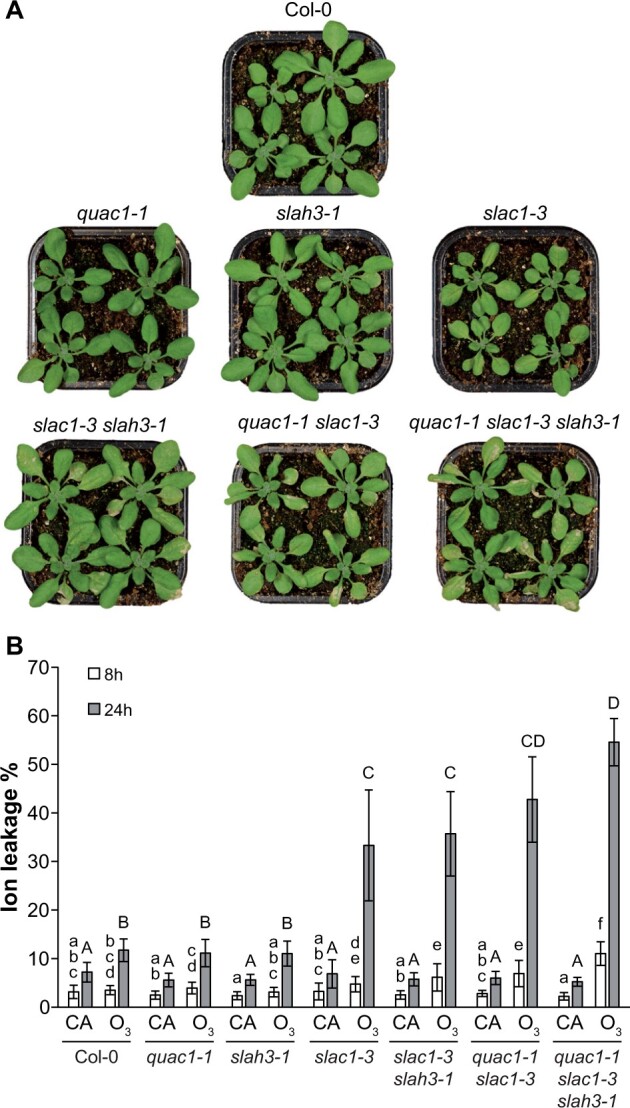
O3 sensitivity of Col-0 and anion channel mutants. Col-0 and anion channel mutants were exposed to 350 ppb O3 for 6 h. A, Representative photos of studied mutants taken 48 h after the onset of O3 exposure. B, Electrolyte leakage from O3-treated (350 ppb for 6 h) and control (clean air [CA]) plants was measured 8 and 24 h after the onset of exposure and plotted as percent of total ion content. Bars represent means of three biological replicates ±sd (n = 3). Lowercase letters denote statistically significant differences between lines 8 h after the onset of exposure and capital letters denote statistically significant differences between lines 24 h after the onset of exposure (two-way ANOVA genotype × treatment interaction with Tukey’s post hoc test, P < 0.05).
Discussion
In this work, we used a genetic approach to address the combined action of guard cell plasma membrane S- and R-type anion channels in stomatal closure. The quac1 slac1 plants were almost unresponsive to darkness, elevated CO2, reduced air humidity, and ABA (Figures 2 and 3; Supplemental Figure S3), indicating that both types of anion channels are required to launch the sequence of events that lead to stomatal closure. As guard cell anion channels differ in their ion selectivity (Geiger et al., 2009; Meyer et al., 2010), they are responsible for the efflux of different ions in stomatal closure. Furthermore, experimental and modeling approaches have demonstrated that removal of essential ion channels alters conditions in guard cells; slac1 guard cells display elevated cytosolic Ca2+ and pH that severely suppressed the activities of K+ channels, leading to slowed stomatal opening (Wang et al., 2012; Laanemets et al., 2013) and impaired stomatal closure was observed in response to reduced air humidity in slac1 plants (Wang et al., 2017). Thus, it is likely that more severe phenotypes of double and triple mutants of SLAC1, SLAH3, and QUAC1 are linked to altered homeostasis of signaling inputs for initiation of stomatal closure and impaired ion efflux.
We observed reduced, but clear stomatal closure in response to abiotic treatments and ABA in slac1 slah3 plants (Supplemental Figure S3). This indicates that full impairment of S-type anion channels alone (Guzel Deger et al., 2015) is not enough to remove guard cell responses to abiotic factors. R‐type anion currents were only 40% reduced in quac1 plants (Meyer et al., 2010). Malate, primarily transported by QUAC1, was shown to have a role in stomatal closure by elevated CO2 (Lee et al., 2008). This could be the reason why further removal of QUAC1 in double and triple mutants was enough to abolish stomatal responses to darkness and elevated CO2. Minor but detectable stomatal closure in the quac1 slac1 slah3 triple mutant by reduced air humidity (Figure 2A) could be explained by the residual R-type channel activity in the triple mutant or by the passive stomatal closure that does not involve active regulation of ion transport across guard cell membranes. The latter has been shown to be a part of air humidity-induced stomatal regulation (Wang et al., 2017; Merilo et al., 2018; Pantin and Blatt, 2018).
ABA controls the activity of the protein kinase OPEN STOMATA 1 (OST1) and there seems to be a common agreement for the crucial role of OST1 in reduced air humidity-induced stomatal closure as ost1 mutants were found to have very weak humidity responses in several studies (Xie et al., 2006; Merilo et al., 2018). OST1 can activate both SLAC1 and QUAC1 (Geiger et al., 2009; Imes et al., 2013) and thus ost1 mutants should be functionally very similar to S- and R-type anion channel double mutants. Indeed, reduced air humidity-induced stomatal responses of quac1 slac1 double mutants (Figure 2A) resemble those of the ost1 mutant (Merilo et al., 2018), further supporting the importance of coordinated activation of both S- and R-type anion channels for stomatal regulation in response to reduced air humidity.
In contrast, stomatal responses to darkness and elevated CO2 are only partially impaired in ost1 plants (Merilo et al., 2013; Sierla et al., 2018) and this is similar to the single mutants of S- and R-type anion channels, whereas respective double and triple mutants were unresponsive to darkness and elevated CO2 (Figure 2; Supplemental Figure S3). This indicates that for darkness and elevated CO2, additional kinases are required for the activation of these channels. The potential candidates are CALCIUM-DEPENDENT PROTEIN KINASEs (CPKs; Scherzer et al., 2012) and the receptor protein GUARD CELL HYDROGEN PEROXIDE-RESISTANT 1 (GHR1; Sierla et al., 2018). In CO2 responses, the protein kinase HIGH LEAF TEMPERATURE 1 (HT1; Hashimoto et al., 2006) and mitogen-activated protein kinases, MPK12 and MPK4 were found to be involved in controlling SLAC1 activation (Tõldsepp et al., 2018). Collectively, several kinases are involved to coordinate the regulation of S-type anion channel activity and further research is needed to address whether some of these regulators might also control the activation of R-type anion channels.
SLAH3 can only be activated by CPKs and, besides guard cells, SLAH3 is expressed in mesophyll cells as well (Geiger et al., 2011). In response to pathogens, SLAH3 regulates stomatal closure together with SLAC1 (Guzel Deger et al., 2015; Liu et al., 2019). In our experiments, the only apparent function of SLAH3 was seen in the O3 experiments, where the triple mutant quac1 slac1 slah3 had the highest cell death. S-type anion channel activity has been implicated as a regulator of cell death, independent of its role in stomatal regulation (Kurusu et al., 2013). Increased cell death in the quac1 slac1 slah3 mutant could reflect the role of SLAH3 in the regulation of reactive oxygen species-induced cell death also in mesophyll cells.
Here we showed that combined impairments in SLAC1 and QUAC1 anion channels had an additive effect on stomatal conductance and stomatal sensitivity to abiotic factors and ABA. The double mutant quac1-1 slac1-3 was insensitive to ABA, darkness and elevated CO2, and responded very little to reduced air humidity. Thus, coordinated activation of both R- and S-type anion channels is needed in stomatal stress signaling. Double and triple mutants made available in this study can be used in future experiments to test the activities of other ion channels and the Ca2+ and pH levels in them to gain mechanistic insight into the events during stomatal closure.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana Col-0, slac1-3 (SALK_099139), slac1-4 (SALK_137265), quac1-1 (SM_3_38592), quac1-2 (SM_3_1713), and slah3-1 (GK-371G03) plants were from the European Arabidopsis Stock Center (www.arabidopsis.info). Double mutants and other crosses were made through standard techniques and genotyped with PCR-based markers (Supplemental Table S1).
Plants for gas-exchange measurements were sown into a 2:1 (v:v) peat:vermiculite mixture and grown as described in Kollist et al. (2007). Plants were grown in growth chambers (AR-66LX, Percival Scientific, USA and Snijders Scientific, Belgia) with a 12-h photoperiod, 23/18°C day/night temperature, 150 μmol m−2 s−1 light and 70% relative humidity. Plants were 24–30 d old during gas-exchange experiments.
Gas-exchange measurements
Stomatal conductance of intact plants was measured using a rapid-response gas-exchange measurement device similar to the one described by Kollist et al. (2007), consisting of eight thermostated flow-through whole-rosette cuvettes. First, plants were photographed for leaf area determination and then inserted into the measurement chambers. When stomatal conductance had stabilized, the following stimuli were applied: reduction in air humidity (decrease from 60%–70% to 30%–40%), darkness (decrease from 150 to 0 μmol m−2 s−1 light), CO2 (increase from 400 to 800 ppm), and spraying plants with 0, 5, or 50-µM ABA solution with 0.012% (v/v) Silwet L-77 (Duchefa) and 0.05% (v/v) ethanol. ABA-induced stomatal closure experiments were carried out as described previously (Merilo et al., 2018). Initial changes in stomatal conductance were calculated as gs18 − gs0, where gs0 is the pretreatment stomatal conductance and gs18 is the value of stomatal conductance 18 min after factor application; 16 min in the case of ABA spraying.
Stomatal density
Stomatal density and stomatal index measurements were carried out as described in Merilo et al. (2018) with leaves of 5-week-old plants.
RNA isolation and RT-PCR
Samples enriched with guard cells were isolated from 5-week-old plants by the ice-blender method. RNA was extracted with the E.Z.N.A. Plant RNA Kit (Omega Bio-tek). Total RNA was DNAseI treated, and cDNA was synthesized with Maxima H Minus reverse Transcriptase (Thermo Fischer Scientific). qPCR was performed in triplicate with 5× HOT FIREPol EvaGreen qPCR Mix Plus ROX (Soils Biodyne) on an Applied Biosystems 7900HT Fast real-time PCR system. Primer sequences and primer efficiencies are listed in Supplemental Table S1. Analysis of the quantitative PCR data was performed with qBase+ (Biogazelle). The reference genes used for normalization were PROTEIN PHOSPHATASE 2A SUBUNIT A3 (PP2AA3) and TAP42 INTERACTING PROTEIN OF 41 KDA (TIP4). Statistical analysis was performed on log2-transformed data.
O3 experiments
Seeds were sown on 1:1 peat/vermiculite, stratified for 3 d, and then grown at 22°C/19°C for a week. Seedlings were transplanted into fresh 1:1 peat/vermiculite. All plants were grown in a controlled chamber (Weiss Bio1300; Weiss Gallenkamp) at 22°C/19°C, in a relative humidity of 70%/90%, under a 12-h light/12-h dark cycle. O3 experiments (6 h of 350 nl L−1) were performed with 3-week-old plants. Ion leakage was measured as previously described (Sierla et al., 2018).
Statistical analysis
Statistical analyses were performed with Statistica, version 7.1 (StatSoft Inc., USA).
Accession numbers
Accession numbers can be found in Supplemental Table S1.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Representative photos of Col-0 and mutants used for whole-plant gas exchange experiments.
Supplemental Figure S2. Time-dependent changes in stomatal conductance.
Supplemental Figure S3. Initial closure rate of Col-0 and quac1 in response to low humidity, darkness, and elevated CO2.
Supplemental Table S1. Primers used for genotyping and qPCR.
Funding
This work was funded by the Estonian Ministry of Science and Education (PRG719 and PRG433) and European Regional Development Fund (Center of Excellence in Molecular Cell Engineering CEMCE).
Conflict of interest statement. The authors declare no conflict of interest.
Supplementary Material
H.K. and M.B. conceived the research plan with the help from E.M. M.B. generated new double, triple, and quadruple mutants. Gas exchange experiments were performed by P.J., M.N., and E.M. Ozone sensitivity measurements by T.V. H.K., M.B., and E.M. supervised the experiments. P.J., M.N., E.M., T.V., H.K., and M.B. analyzed the data. H.K. and M.B. wrote the article with input from all authors. H.K. agrees to serve as the author responsible for contact and ensures communication.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Hannes Kollist (hannes.kollist@ut.ee).
References
- Dreyer I, Gomez-Porras JL, Riaño-Pachón DM, Hedrich R, Geiger D (2012) Molecular evolution of slow and quick anion channels (SLACs and QUACs/ALMTs). Front Plant Sci 3: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Maierhofer T, Al-Rasheid KAS, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E, et al. (2011) Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal 4: ra32. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KAS, et al. (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci U S A 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzel Deger A, Scherzer S, Nuhkat M, Kedzierska J, Kollist H, Brosché M, Unyayar S, Boudsocq M, Hedrich R, Roelfsema MRG (2015) Guard cell SLAC1-type anion channels mediate flagellin-induced stomatal closure. New Phytol 208: 162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Negi J, Young J, Israelsson M, Schroeder JI, Iba K (2006) Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat Cell Biol 8: 391–397 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Busch H, Raschke K (1990) Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J 9: 3889–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Geiger D (2017) Biology of SLAC1-type anion channels—from nutrient uptake to stomatal closure. New Phytol 216: 46–61 [DOI] [PubMed] [Google Scholar]
- Imes D, Mumm P, Böhm J, Al-Rasheid KAS, Marten I, Geiger D, Hedrich R (2013) Open stomata 1 (OST1) kinase controls R–type anion channel QUAC1 in Arabidopsis guard cells. Plant J 74: 372–382 [DOI] [PubMed] [Google Scholar]
- Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Nuhkat M, Roelfsema MRG (2014) Closing gaps: linking elements that control stomatal movement. New Phytol 203: 44–62 [DOI] [PubMed] [Google Scholar]
- Kollist T, Moldau H, Rasulov B, Oja V, Rämma H, Hüve K, Jaspers P, Kangasjärvi J, Kollist H (2007) A novel device detects a rapid ozone-induced transient stomatal closure in intact Arabidopsis and its absence in abi2 mutant. Physiol Plant 129: 796–803 [Google Scholar]
- Kurusu T, Saito K, Horikoshi S, Hanamata S, Negi J, Yagi C, Kitahata N, Iba K, Kuchitsu K (2013) An S-type anion channel SLAC1 is involved in cryptogein-induced ion fluxes and modulates hypersensitive responses in tobacco BY-2 cells. PLoS One 8: e70623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanemets K, Wang Y-F, Lindgren O, Wu J, Nishimura N, Lee S, Caddell D, Merilo E, Brosche M, Kilk K, et al. (2013) Mutations in the SLAC1 anion channel slow stomatal opening and severely reduce K+ uptake channel activity via enhanced cytosolic [Ca2+] and increased Ca2+ sensitivity of K+ uptake channels. New Phytol 197: 88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Choi Y, Burla B, Kim Y-Y, Jeon B, Maeshima M, Yoo J-Y, Martinoia E, Lee Y (2008) The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nat Cell Biol 10: 1217–1223 [DOI] [PubMed] [Google Scholar]
- Liu Y, Maierhofer T, Rybak K, Sklenar J, Breakspear A, Johnston MG, Fliegmann J, Huang S, Roelfsema MRG, Felix G, et al. (2019) Anion channel SLAH3 is a regulatory target of chitin receptor-associated kinase PBL27 in microbial stomatal closure. eLife 8: e44474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcheska F, Ahmad A, Batool S, Müller HM, Ludwig-Müller J, Kreuzwieser J, Randewig D, Hänsch R, Mendel RR, Hell R, et al. (2017) Drought-enhanced xylem sap sulfate closes stomata by affecting ALMT12 and guard cell ABA synthesis. Plant Physiol 174: 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilo E, Laanemets K, Hu H, Xue S, Jakobson L, Tulva I, Gonzalez-Guzman M, Rodriguez PL, Schroeder JI, Broschè M, et al. (2013) PYR/RCAR receptors contribute to ozone-, reduced air humidity-, darkness-, and CO2-induced stomatal regulation. Plant Physiol 162: 1652–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilo E, Yarmolinsky D, Jalakas P, Parik H, Tulva I, Rasulov B, Kilk K, Kollist H (2018) Stomatal VPD response: there is more to the story than ABA. Plant Physiol 176: 851–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KAS, Geiger D, Marten I, Martinoia E, Hedrich R (2010) AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J 63: 1054–1062 [DOI] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486 [DOI] [PubMed] [Google Scholar]
- Pantin F, Blatt MR (2018) Stomatal response to humidity: blurring the boundary between active and passive movement. Plant Physiol 176: 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer S, Maierhofer T, Al-Rasheid KAS, Geiger D, Hedrich R (2012) Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Mol Plant 5: 1409–1412 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S (1989) Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338: 427 [Google Scholar]
- Sierla M, Horak H, Overmyer K, Waszczak C, Yarmolinsky D, Maierhofer T, Vainonen JP, Salojarvi J, Denessiouk K, Laanemets K, et al. (2018) The receptor-like pseudokinase GHR1 is required for stomatal closure. Plant Cell 30: 2813–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tõldsepp K, Zhang J, Takahashi Y, Sindarovska Y, Hõrak H, Ceciliato PHO, Koolmeister K, Wang Y-S, Vaahtera L, Jakobson L, et al. (2018) Mitogen-activated protein kinases MPK4 and MPK12 are key components mediating CO2-induced stomatal movements. Plant J 96: 1018–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang Y-F, Nishimura N, Chan W-Y, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, et al. (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hills A, Vialet-Chabrand S, Papanatsiou M, Griffiths H, Rogers S, Lawson T, Lew VL, Blatt MR (2017) Unexpected connections between humidity and ion transport discovered using a model to bridge guard cell-to-leaf scales. Plant Cell 29: 2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Papanatsiou M, Eisenach C, Karnik R, Williams M, Hills A, Lew VL, Blatt MR (2012) Systems dynamic modeling of a guard cell Cl− channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiol 160: 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wang Y, Williamson L, Holroyd GH, Tagliavia C, Murchie E, Theobald J, Knight MR, Davies WJ, Leyser HMO, et al. (2006) The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Curr Biol 16: 882–887 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.