Abstract
Sexual reproduction in flowering plants takes place without an aqueous environment. Sperm are carried by pollen through air to reach the female gametophyte, though the molecular basis underlying the protective strategy of the male gametophyte is poorly understood. Here we compared the published transcriptomes of Arabidopsis thaliana pollen, and of heat-responsive genes, and uncovered insights into how mature pollen (MP) tolerates desiccation, while developing and germinating pollen are vulnerable to heat stress. Germinating pollen expresses molecular chaperones or “heat shock proteins” in the absence of heat stress. Furthermore, pollen tubes that grew through pistils at basal temperature showed induction of the endoplasmic reticulum (ER) stress response, which is a characteristic of stressed vegetative tissues. Recent studies show MP contains mRNA–protein (mRNP) aggregates that resemble “stress” granules triggered by heat or other stresses to protect cells. Based on these observations, we postulate that mRNP particles are formed in maturing pollen in response to developmentally programmed dehydration. Dry pollen can withstand harsh conditions as it is dispersed in air. We propose that, when pollen lands on a compatible pistil and hydrates, mRNAs stored in particles are released, aided by molecular chaperones, to become translationally active. Pollen responds to osmotic, mechanical, oxidative, and peptide cues that promote ER-mediated proteostasis and membrane trafficking for tube growth and sperm discharge. Unlike vegetative tissues, pollen depends on stress-protection strategies for its normal development and function. Thus, heat stress during reproduction likely triggers changes that interfere with the normal pollen responses, thereby compromising male fertility. This holistic perspective provides a framework to understand the basis of heat-tolerant strains in the reproduction of crops.
Pollen normally dehydrates to promote desiccation tolerance in pollen grains and then rehydrates to initiate germination; thus, further demands on these responses by heat stress reduce male fertility.
Introduction
Sexual reproduction in flowering plants takes place on land without an aqueous environment. Immotile sperm cells are carried by pollen (male gametophyte) through air and the pistil to reach the egg in the female gametophyte. Plants have developed strategies to protect gametes from environmental stress. The female gametophyte is relatively well-protected as it is buried inside the pistil that remains attached to the vegetative parent plant. The 2- to 3-celled male gametophyte is relatively vulnerable as it is exposed at pollen dispersal, though the molecular basis of stress protection is poorly understood. Furthermore, sexual reproduction is particularly sensitive to temperature fluctuations (Hedhly, 2011) and to combination stress from heat and water deficit (Sinha et al., 2021). High temperatures cause reductions in fruit and seed set. Two developmental stages were identified as particularly heat sensitive, one before anthesis and one after anthesis (Bac-Molenaar et al., 2015). The weakest link in reproductive stress tolerance is often associated with the male gametophyte (Zinn et al., 2010) during pollen development and in pollen functions after anthesis (Rieu et al., 2017). As seeds and fruits provide a major part of the human diet, it is imperative to understand the mechanisms and processes associated with heat-induced male sterility. An outstanding question in the field is why pollen development and function are particularly sensitive to heat (Rieu et al., 2017). Heat sensitivity at meiosis of the pollen mother cell, microspore formation, pollen development, and tapetum development within the anther was reviewed before (De Storme and Geelen, 2014; Rieu et al., 2017; Chaturvedi et al., 2021). Here we primarily focus on pollen maturation and germination to reveal insights into pollen responses to developmental cues and environmental stress.
Development of the male gametophyte (pollen) takes place inside the anther chamber of the male floral organ. It starts with the diploid microspore mother cell undergoing meiosis to give four haploid microspores followed by microgametogenesis that ends with the formation of a mature pollen (MP) grain (Borg et al., 2009; Figure 1). Each microspore divides to give a bicellular pollen (BCP) containing a large vegetative cell and a small generative cell. Subsequently, the generative cell alone divides to produce two sperm cells. In some plants, as in Arabidopsis thaliana, tricellular pollen (TCP) grains are formed before they are released from the anther. In other plants, such as tobacco (Nicotiana tabacum), BCP grains are released from the anther and the generative cell divides later within the elongating pollen tube (PT) to produce two sperms. Pollen function refers to events that start when the mature grain lands on a compatible female pistil and terminates with discharge of the sperm cells for double fertilization in the female gametophyte (Johnson et al., 2019). After pollen grains are transferred to a compatible female floral organ either by direct contact or vectors (air or insect), grains germinate and the vegetative cell forms a PT. The tube elongates rapidly inside a style and navigates to a well-protected ovule in the ovary. At the entrance to the female gametophyte within the ovule, the tube bursts in the synergid cells to release two sperms that fertilize the egg cell and the central cell. Double fertilization is followed by embryo and endosperm development to produce fruit and seed.
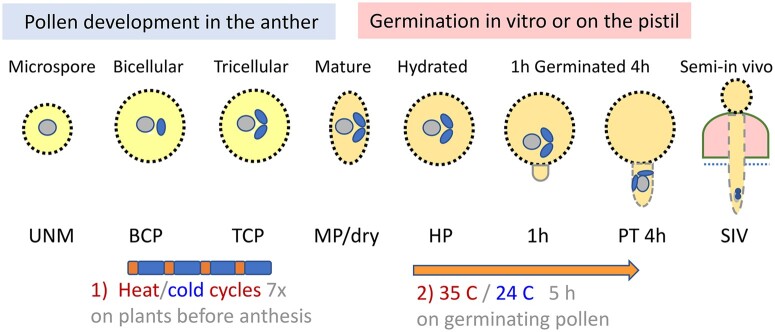
Figure 1.
Transcriptomes of pollen at different stages of development and germination in Arabidopsis. Inside the anther, meiosis of the microspore mother cell produces four uninucleate microspores (UNMs). Each microspore divides to give a BCP containing a large vegetative cell (nucleus in gray) and a small generative cell (blue). Division of the generative cell produces two sperm cells (blue) in the TCP. TCP develops to an MP (or dry). On germination medium, MP becomes HP, and a tube emerges in about 1 h and elongates in 4–5 h. SIV PTs refer to tubes grown through the pistil (pink) and emerged from the cut style onto a medium (scale reduced). Transcriptomes analyzed here are from three studies. Pollen stages UNM, BCP, TCP, and MP are from Honys and Twell (2004); MP, HP, and PT 4 h in vitro are from Wang et al. (2008), and dry, PT 0.5 h, PT 4 h, and SIV are from Qin et al. (2009). Transcriptome after heat stress is from two reports. In (1), whole plants were exposed to diurnal heat/cold (orange/blue bars) cycles for 7 d before anthesis. Each cycle was designed with 18 h at cool temperature (10°C) and a 6-h period in which temperatures were ramped up and down with 40°C mid-day peak for 1 h. MP RNA was analyzed (Rahmati Ishka et al., 2018). In (2), MP on germination medium was given 35°C (orange bar) or 24°C for 5 h before RNA was isolated (Poidevin et al., 2020).
Nearly all pollen functions are carried out by one vegetative cell, which ferries the relatively quiescent sperm cells to the female gametes (Zhang et al., 2017). Pollen functions include grain hydration, germination, tip growth, navigation to the ovule, and finally tube burst to release the two sperms for double fertilization. Thus the male gametophyte provides a powerful model to study the molecular and cellular changes in a single plant cell. Many transcriptome and proteome studies of developing and MP of Arabidopsis have been published (Holmes-Davis et al., 2005; Wang et al., 2008; Grobei et al., 2009; Qin et al., 2009; Loraine et al., 2013; Zhang et al., 2017). They provide a great resource to the research community, though much of the data remains unanalyzed. Although gene expression alone does not define a biochemical or biological role, the functions of many previously uncharacterized genes have been revealed at the molecular level and validated by subsequent mutant studies. Integrative analysis of single-cell data can discover relationships and show holistic views of a cell state (Stuart and Satija, 2019) and function. We have compared transcriptomes across different pollen experiments and unveiled holistic insights when combined with published experimental advances. This approach to integrate expression data of genes with functions across experiments is just beginning in pollen biology and serves as a template study for single cell-type investigations in plants.
Through data mining of genes responsive to heat stress on Arabidopsis pollen (Rahmati Ishka et al., 2018; Poidevin et al., 2020), we confirm and extend previous findings that conserved heat stress responses, like increase in molecular chaperone (HSP) transcripts (Frank et al., 2009) are observed in the male gametophyte; however, HSP and endoplasmic reticulum (ER) stress response genes are highly expressed in germinating pollen even in the absence of heat stress (Wang et al., 2008; Qin et al., 2009). Why? Recent studies have revealed that MP grains from Arabidopsis and tobacco contain mRNA–protein (mRNP) complexes/particles (Honys et al., 2009; Scarpin et al., 2017; Hafidh et al., 2018) that share similarity to stress granules (SGs) formed in eukaryotic cells (Nover et al., 1989; Wallace et al., 2015; Chantarachot and Bailey-Serres, 2018). SGs sequester mRNA and protein molecules and protect cells by arresting protein translation transiently (Protter and Parker, 2016). An attractive hypothesis emerging from these seemingly varied observations is that mRNA–protein (mRNP) particles/granules are formed in response to an intrinsic cue, such as dehydration, at pollen maturation, and when pollen hydrates after transfer to a compatible stigma, the granules disassemble releasing mRNAs for translation to support germination. Here we highlight findings from pollen and stress biology that led us to this model. Pollen germination and tube growth through the pistil apparently depend on responses to extracellular ligands as well as “non-heat” stress, including hydration, osmotic (Osm), and redox cues. As pollen survival and function rely on a series of intrinsic and extrinsic stress cues, an additional stress from heat, conceivably would disrupt conserved stress-protection machinery supported by HSP and ER unfolded protein response (UPR) for PT functions and sperm delivery. Any impairment or delay to critical pollen functions during a very short life span would reduce male fertility and decrease seed set. Understanding the cellular and molecular bases of pollen survival and function provides insights to finding strategies to improve male fertility in crops during heat spells and combination stress (Sinha et al., 2021).
Molecular chaperone and ER-stress response genes are upregulated in pollen by heat stress
To understand whether pollen and vegetative tissues respond to heat stress differentially, we compared some of the well-known heat-responsive genes from leaves (Sugio et al., 2009) and shoots (Schmid et al., 2005; Kilian et al., 2007) with those from pollen. Analysis and validation of the transcriptome results are described in the figure legends and Methods (see Supplemental Data). Quantitative variation between results may reflect the experimental conditions used by different laboratories. The overall response of molecular chaperone genes in pollen to different heat treatments shared striking similarities (Table 1; Supplemental Table S1). Rahmati Ishka et al. (2018) determined the transcriptome of MP grains collected from plants that were subjected to diurnal heat/cold cycles for 1 week before anthesis. The diurnal cycle was designed with 18 h at cool temperature (10°C) and a 6-h period in which temperatures were ramped up and down with a 40°C midday peak. This stress cycle subjected developing pollen to heat stress. Poidevin et al. (2020) collected MP from plants grown under normal conditions and determined the transcriptome of in vitro germinating pollen at 35°C (heat stress) or 24°C (basal) (Figure 1). As heat stress unfolds proteins and can promote aggregation, and interfere with cellular processes, the chaperone system is induced to protect cells (Mogk et al., 2018). Molecular chaperones are involved in processes, like folding of newly synthesized proteins, transport of proteins across membranes, refolding of proteins after stress, preventing aggregation of misfolded proteins, and degradation of unfolded proteins. To control protein homeostasis, Hsp70 works with cochaperones, like Hsp40/DnaJ and nucleotide-exchange factors. DnaJ proteins target substrates to Hsp70, stimulating its ATP hydrolysis and trapping the substrate (Mogk et al., 2018). Upon release from Hsp70, the substrate protein folds to its native state. As shown in Table 1 and Supplemental Table S1, heat-induced significant increases in transcript levels in 40 out of 52 HSP genes from the Hsp70, Hsp20, Hsp90, as well as Hsp40/DnaJ families. Furthermore, expression of Hop3, an Hsp70–Hsp90 organizing protein and peptidyl-prolyl cis–trans isomerases, ROF1 and ROF2, were enhanced. The foldases are essential components of the cytosolic protein response (Sugio et al., 2009). Overall, the upregulation of HSP genes by heat in pollen resembles that in vegetative tissues, though the average fold-change in pollen is less than that in shoots (Supplemental Table S1). Furthermore, results from the ribosome protection fragment (Poidevin et al., 2020) infer that most of the upregulated transcripts in pollen are translated.
Table 1.
Transcripts of many molecular chaperones are elevated by heat stress in pollen as in leaves
| Genes upregulated by heat |
||||||
|---|---|---|---|---|---|---|
| Total | Pol1 | Pol2 | Pol2 | Shoot | Leaf | |
| Protein Class/Family | Genes | RNA1 | RNA2 | RPF | RNA3 | RNA4 |
|
| ||||||
| No. | Number of Genes Induced | |||||
| HSP20 | 19 | 13 | 16 | 16 | 17 | 16 |
| HSP70, 90, 100 | 24 | 20 | 20 | 20 | 20 | 18 |
| HSP40/DnaJ | 9 | 7 | 5 | 6 | 6 | 6 |
| Foldase | 6 | 5 | 5 | 5 | 6 | 6 |
| ROS Scavenger/Other | 5 | 3 | 2 | 2 | 2 | 2 |
“Total genes” indicates the number (no.) of differentially expressed genes for each protein class from four studies. Number of genes induced refers to the members of the class that show a significant increase in FC (log2FC > 1) of RNA transcripts in response to heat stress.
Pollen1 (Pol1), Pol2, and Shoot and Leaf refer to transcriptome results of Rahmati Ishka et al. (2018), Poidevin et al. (2020), Kilian et al. (2007), and Sugio et al. (2009) from A. thaliana, respectively. Heat stress regimes for Pol1 and Pol2 are described in Figure 1; transcript levels were determined using RNA-seq. Shoot or Leaf indicates transcriptome changes of heat-treated vegetative plant (37–38°C) determined using ATH1 microarray (Kilian et al., 2007; Sugio et al., 2009). RPF refers to a ribosomal protection fragment increased significantly by heat, a measure of mRNA undergoing translation. Gene identification number and heat-induced FC (log2FC) are shown in Supplemental Table S1.
Genes encoding ER-localized chaperones and foldases were also induced by heat consistent with the notion that the UPR (Deng et al., 2013) is activated in pollen after heat stress. Notable examples include the ER membrane-bound calnexin (CNX1), ER lumen Hsp70 protein (BiP1 and BiP3), calreticulin (CRT1a and CRT1b), Hsp40/DnaJ co-chaperones, like ERDj3A/thermo-sensitive male sterile 1 (TMS1), ERDj3B, and P58IPK, as well as several protein disulfide isomerases (PDIs; PDIL) and ER–Golgi trafficking regulators (p24; Table 2; Supplemental Table S2, A). Transcripts of plant-specific TIN1 (tunicamycin-induced) are very abundant in MP but are weakly increased by heat in pollen in contrast to a 60-fold increase in leaves (Sugio et al., 2009). According to the current model, BiP is recruited by an Hsp40/DnaJ to aid the insertion of newly synthesized polypeptides into the ER lumen. Glycoproteins in the lumen are bound by calnexin or calreticulin to be further modified and folded. During the folding process, disulfide bonds are formed by PDIs of which several members are expressed in pollen (Supplemental Table S2A). ERO1, an oxidoreductin, then oxidizes PDI homologs by transferring electrons from the ER to oxygen in the cytosol. UTR1 and UTR3 transport nucleotide-sugars (UDP-Glu) into the ER lumen for glycosylation (Reyes et al., 2010), whereas UTR2 moves UDP-Gal into the Golgi (Norambuena et al., 2005). Proteins are delivered to their destination or secreted, but misfolded proteins are guided to the ER-associated protein degradation (ERAD) system. Genes encoding components of the ERAD and upregulated by heat, included Derlin1, Derlin2-1, and Hrd1B (Supplemental Table S2A). Hrd1 is part of the ubiquitin ligase complex that works with an AAA-ATPase (CDC48) to remove misfolded proteins out of the ER and then ubiquinates them for degradation by the 26s proteasome (Liu and Howell, 2016).
Table 2.
ER-stress response genes are induced by heat in pollen (A) and in PTs grown at basal temperature (B)
| A. Genes Up by Heat |
B. Genes Up in Pol Tube |
C. Heat | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Genes | Pol1 | Pol2 | Pol2 | Wang | Qin | Qin | Leaf and Shoot | |
| Function/Protein Class | RNA1 | RNA2 | RPF2 | PT/MP | PT/dry | SIV/dry | ||
|
| ||||||||
| Number of Genes Induced | ||||||||
| ER Chaperone | ||||||||
| BIP, CNX, CRT, and ERDJ | 11 | 10 | 11 | 11 | 7 | 0 | 10 | 8 |
| ER/Golgi Traffick | ||||||||
| p24delta#, ERD2 | 10 | 6 | 7 | 7 | 4 | 0 | 7 | 1 |
| ER Isomerase | ||||||||
| PDIL, ERO1 | 6 | 3 | 5 | 5 | 3 | 0 | 5 | 2 |
| ER-associated degradation | ||||||||
| DER, Hrd1B, and CDC48B | 5 | 5 | 5 | 4 | 2 | 1 | 4 | 3 |
| Transporter UDP-Gal UTr1 to UTr3 | 3 | 2 | 2 | 2 | 2 | 0 | 2 | 2 |
| Glycosylation-related GPT, UGGT, and TIN1 | 3 | 2 | 2 | 2 | 0 | 0 | 1 | 1 |
“Total genes” indicates the number of differentially expressed genes in each protein class from six experiments. Number of genes induced refers to members of each class that show a significant increase (log2FC > 1) of RNA transcripts in response to heat stress (A and C) or PT germination (B). Examples of genes for each class are abbreviated. Gene identification numbers and log2FC values are provided in Supplemental Table S2.
(A) Genes upregulated by heat. Pol1 and Pol2 refer to transcriptome data of Rahmati Ishka et al. (2018) and Poidevin et al. (2020) from A. thaliana, respectively. Heat stress regimes for Pol1 and Pol2 pollen are described in Figure 1, and transcript levels determined using RNA-seq. “RPF” refers to ribosomal protection fragment, a measure of mRNA undergoing translation. See Supplemental Table S2 for gene descriptions and the fold increase in transcript (log2FC) for each gene.
(B) Induction of transcripts during PT germination and growth at basal temperature. Wang et al. (2008) tested expression in PTs (4 h) grown in vitro relative to MP. Qin et al. (2009) tested expression in PTs grown in vitro (PT, 4 h) or SIV relative to dry (ungerminated) pollen. Variation in transcript accumulation in PT may be caused by different experimental conditions and dehydration state of MP and dry pollen.
(C) Induction by heat stress in leaves and shoots. Average value from two experiments is shown (Kilian et al., 2007; Sugio et al., 2009).
The comparisons show that Arabidopsis pollen at different stages is competent to sense and respond to heat stress by upregulating genes encoding molecular chaperones and the ER UPRs just like in vegetative tissues (Kilian et al., 2007; Sugio et al., 2009). These results extend previous findings on pollen responses to heat from crop plants (Frank et al., 2009).
Transcripts encoding molecular chaperones and ER-stress response proteins are elevated in PTs growing through the pistil at basal temperature
Surprisingly, a comparison of transcriptome results of Arabidopsis pollen germinated in vitro (Wang et al., 2008) or under semi-in vivo (SIV) conditions (Qin et al., 2009), revealed upregulation of many molecular chaperones relative to that in ungerminated pollen in the absence of any heat stress (Table 3; Supplemental Table S3). An SIV protocol to study PT growth in Arabidopsis was established, as in vitro tube growth is several-fold slower than growth through the pistil (Palanivelu and Johnson, 2010). Moreover, PTs grown through the pistil target unfertilized ovules efficiently compared to in vitro grown tubes, indicating tubes sense and respond to signals from the pistil (Palanivelu and Preuss, 2006). To identify genes that prime the tubes growing in vivo, PTs grown under a “semi-in vivo” (SIV) condition were collected for transcriptome analysis (Qin et al., 2009). The SIV condition was accomplished by first pollinating pistils, and then cutting the style transversely and placing it horizontally on a solid medium, so elongating PTs exit from the cut end and grow onto the medium (Figure 1). After 3-h growth, SIV-grown tubes were excised for RNA extraction. As experimental conditions used in Wang (Wang et al., 2008) and Qin (Qin et al., 2009) may differ, we have compared changes in transcript level as log2 fold-change (FC) in pollen germinated in vitro or SIV relative to ungerminated pollen of each study.
Table 3.
Genes encoding molecular chaperones are upregulated in germinating pollen and in tubes at basal temperature
| Genes upregulated in PT |
|||||
|---|---|---|---|---|---|
| Protein Class | Total | Wang | Wang | Qin | Qin |
| Genes No. | HP/MP | PT/MP | PT/dry | SIV/dry | |
|
| |||||
| Number of Genes Induced | |||||
| All Classes | 62 | 33 | 36 | 24 | 54 |
| HSP20 | 15 | 9 | 9 | 11 | 14 |
| HSP70, 90, and 100 | 20 | 12 | 14 | 8 | 20 |
| HSP40/DnaJ | 17 | 7 | 8 | 2 | 15 |
| Foldase | 5 | 3 | 3 | 3 | 4 |
| Other | 5 | 2 | 2 | 0 | 1 |
“Total genes” indicates the number of differentially expressed genes in each protein class from two studies. Number of genes induced refers to the members of each protein class that show a significant increase in FC (log2FC > 1) of RNA transcripts in response to pollen germination of tube growth. Wang et al. (2008) tested HP and PT grown in vitro for 4 h. Qin et al. (2009) tested PTs grown in vitro (PT) and SIV. Gene identification numbers and fold-change (log2FC) values are provided in Supplemental Table S3.
After pollen hydration and tube growth in vitro, at least nine members of the Hsp20 family were significantly upregulated (from 4- to 30-fold) relative to dry pollen in both studies (Table 3; Supplemental Table S3). Interestingly, transcript levels of small Hsp (sHSP) were consistently higher in SIV-grown PTs (Supplemental Table S3). Similar upregulation of Hsp70, Hsp90, and Hsp101 chaperones by at least four-fold, was observed in PTs grown in vitro, and particularly in SIV-grown PTs (Supplemental Table S3). Upregulation of most Hsp40/DnaJ cochaperone transcripts was low or not significant, unless tubes were grown through the style (Table 3; Supplemental Table S3). Hsp40 cochaperones, include DjB12, DjB16, DjB20, and DjC32, as well as ER-associated ERDj3A, ERDj3B, and P58IPK (Supplemental Table S3; Yamamoto et al., 2008). In summary, 49 out of 52 HSP genes are upregulated in PTs grown through the pistil (SIV), relative to 31 HSP genes in PTs germinated in vitro.
Strikingly, the ER-stress response (or the UPR) is clearly activated in PTs germinated especially under SIV conditions without heat stress (Supplemental Table S2C). Notable genes, considered as molecular signatures of the UPR, include BiP1, BiP3, CNX1, CRT1a, CRT1b, Derlin 1 and 2, PDIL1-2, ERO1, and UTr3 (Table 2 (B); Supplemental Table S2B). Curiously, transcript levels of a tunicamycin-induced protein (TIN1) are already high in MP before germination. Its transcript accumulates at the TCP stage through tube growth with those encoding ER DnaJ chaperone (ERDJ3A), and sugar-nucleotide transporter (UTr1), suggesting that MP is primed for the UPR. Combined with upregulation of foldases and other components of the UPR, these results suggest that many molecular chaperones have roles in supporting germination and tube formation. Furthermore, our comprehensive analyses confirm and extend the idea that ER-stress response is activated in growing tubes (Fragkostefanakis et al., 2016; Liu and Howell, 2016) independent of thermoprotection. Mutants of numerous ER-stress response genes (Supplemental Table S2) are either male gametophyte lethal, defective in male transmission, pollen germination, or tube elongation (Fragkostefanakis et al., 2016; Singh et al., 2021). The ER-stress response is regulated by ER sensors and ER-associated transcription factors (TFs; see Section “TFs responsive to PT growth are also responsive to other stresses”), though what cues activate it in germinating pollen is unclear.
Signals sensed by pollen germinating in vitro or in vivo: Osm and oxidative
What signal(s) are sensed by pollen on the stigma and in a pistil to boost activation of transcription in PTs? Aside from Osm, calcium (Ca2+), arabinogalactan proteins, and yet undefined pistil factors, one strong candidate is reactive oxygen species (ROS) based on several observations. First, stigmas of various angiosperms produce ROS constitutively, predominantly as H2O2 (McInnis et al., 2006). Second, the NADPH oxidase RbohD, as well as peroxidases, are highly expressed in the stigma based on transcriptome results of Arabidopsis (Swanson et al., 2005; Klepikova et al., 2016). Recently, ROS production was shown to be stimulated by RALF23/33 peptide binding to a receptor kinase complex ANJEA-FERONIA (ANJ-FER) in unpollinated stigma cells, while pollination reduces ROS allowing pollen hydration. Pollen coat protein peptides from a compatible pollen compete and interact with the stigma receptor kinase complex ANJ-FER to promote pollen hydration and germination (Liu et al., 2021). Thus, recognition of compatible pollen by the stigma is a prerequisite for water movement from papilla cells to pollen grains.
Hydration of pollen produces a mechanical stress which could lead to ROS signaling in pollen (Hamilton et al., 2015; Basu and Haswell, 2020). Rehydration of the pollen grain imposes a hypoosmotic shock as cells swell and membranes are stretched. MSL8, a pollen-specific, membrane tension-gated ion channel was shown to sense and respond to changes in membrane tension. Mutant msl8 pollen grains showed reduced viability after hydration and germinating tubes burst more frequently than wild-type tubes (Hamilton et al., 2015). Recently, the msl10 null mutant showed decreased response to hypoosmotic shock-induced cytoplasmic Ca2+ transient and ROS production in seedlings (Basu and Haswell, 2020), indicating that MSL10 is a membrane sensor that links the perception of cell swelling to downstream signaling, including ROS production.
PT growth depends on ROS signaling as shown by double mutants of two pollen-specific NADPH oxidases localized at the plasma membrane (Boisson-Dernier et al., 2013; Kaya et al., 2014; Lassig et al., 2014). Both AtRbohH and AtRbohJ activities are activated by Ca2+ via their EF hands and by phosphorylation, and ROS are detected in tubes germinating on the stigma papilla (Kaya et al., 2014). One role of apoplastic ROS is to alter wall properties and balance cell wall loosening with stiffening as cells grow (Swanson and Gilroy, 2010; Karkonen and Kuchitsu, 2015). PTs from null mutants of rbohH and rbohJ burst more frequently, have short tubes and reduced seed set (Boisson-Dernier et al., 2013; Kaya et al., 2014). Thus, pollen hydration on the stigma is likely perceived by membrane sensor(s) which are connected to Ca2+-activated ROS production that in turn modulates pollen wall integrity and tube tip growth. Perhaps ROS plus secreted factors from pollen–pistil interaction (Doucet et al., 2016) upregulate the transcription of >900 genes in SIV tubes (Qin et al., 2009) relative to those expressed in PTs grown in vitro (Supplemental Table S3).
TFs responsive to PT growth are also responsive to other stresses
The transcriptional response to heat stress has been extensively studied in vegetative tissues, but much less is known about the transcriptional regulation of heat responses in developing pollen (Fragkostefanakis et al., 2016). The rapid and strong transcriptional activation of genes, encoding chaperones and foldases in the vegetative plant, is controlled by the activity of heat shock TFs (Ohama et al., 2017). Heat stress is likely sensed in cells as an imbalance of unfolded proteins which leads to activation of master TFs, such as HsfA1 (Scharf et al., 2012; Ohama et al., 2017). Activation of HSF TFs is proposed to result from (1) the release of the master regulator from molecular chaperones (Hsp70/90), and (2) changes in Ca2+ and/or ROS levels (Ohama et al., 2017). Using heat-upregulated TF genes as a criterion (Rahmati Ishka et al., 2018), concluded that developing pollen responds to heat differently from vegetative tissues. To understand the responses of pollen to heat and to germination, we compared heat-responsive TF profiles in germinating pollen (Poidevin et al., 2020) with that in shoots (Sugio et al., 2009) and PTs germinated in vitro and SIV (Table 4; Supplemental Table S4).
Table 4.
TFs increased in germinating PTs at optimal temperatures overlap partially with those responsive to other stresses
| A1 | A2 | A3 | B | C1 | C2 | C3 | ||
|---|---|---|---|---|---|---|---|---|
| Pol PT/d | Pol SIV/d | Cont. Pol | Heat-Pol | Heat Sh1 | Heat Sh2 | Cont. Sh1 | ||
| Gene ID | Name | Qin | Qin | Qin | Poidevin | Kilian | Sugio | Kilian |
| log2FC | log2FC | log10 | log2FC | log2FC | log2FC | log10 | ||
|
| ||||||||
| At4G17750 | HSFA1A | 0.13 | 0.3 | 2.3 | 0.81 | −0.13 | x | 1.8 |
| At1G32330 | HSFA1D | 0.69 | 2 | 1.8 | 1.2 | 0.33 | 1.30 | 2.4 |
| At2G26150 | HSFA2 | 0.42 | 3.13 | 1.0 | x | 8.05 | 7.12 | 1.4 |
| At5G03720 | HSFA3 | 0.26 | −0.22 | 1.4 | x | 0.27 | 3.15 | 1.8 |
| At5G45710 | HSFA4C | −0.18 | −0.77 | 3.0 | −0.06 | −0.35 | x | 2.1 |
| At4G13980 | HSFA5 | −0.35 | −1.58 | 3.1 | −0.18 | 0.35 | x | 1.8 |
| At3G51910 | HSFA7A | 0.29 | −0.09 | 1.2 | x | 6.22 | 7.65 | 1.7 |
| At3G63350 | HSFA7B | 0.23 | −0.23 | 1.4 | x | 5.3 | 6.21 | 1.3 |
| At1g67970 | HSFA8 | 0.01 | 0.55 | 1.8 | x | 0.04 | −2.47 | 1.7 |
| At4G36990 | HSFB1 | 0.31 | 0.15 | 1.2 | 1.6 | 5.42 | 3.73 | 2.0 |
| At5G62020 | HSFB2A | 0.22 | 0.87 | 2.2 | 0.19 | 3.55 | 2.08 | 2.3 |
| At4G11660 | HSFB2B | 0.15 | 2.58 | 1.5 | 1.4 | 4.94 | 4.23 | 1.7 |
| At1G22985 | CRF7 | 0.6 | 1.06 | 1.9 | −0.41 | 0.13 | 3.14 | 2.0 |
| At2G38340 | DREB19 | 1.2 | 3.13 | 1.1 | −0.4 | 0.54 | 4.33 | 1.2 |
| At5G05410 | DREB2A | 2.23 | 3.72 | 1.6 | 0.75 | 4.69 | 5.7 | 1.9 |
| At3G11020 | DREB2B | 1.37 | 1.11 | 2.0 | −0.57 | 1.13 | 3.22 | 1.7 |
| At1g64380 | ERF061 | 1.42 | 3.36 | 1.2 | −2.4 | −0.84 | −3.16 | 1.6 |
| At3g10800 | bZIP28 | 0.04 | 0.2 | 2.8 | 0.4 | 0.21 | 2.6 | 1.2 |
| At1G42990 | bZIP60 | 0.07 | 1.74 | 2.6 | 0.2 | 1.6 | 1.48 | 2.6 |
| At5G24360 | IRE1-1 | 0.79 | 1.71 | 2.3 | −0.07 | −0.24 | x | 2.6 |
| At2g17520 | IRE1-2 | 0.38 | 1.52 | 2.1 | −0.25 | −0.66 | x | 2.2 |
| At1g60040 | AGL49 | 2.43 | 1.57 | 1.8 | x | −0.1 | x | 2.5 |
| At1g01720 | ANAC002 | 0.64 | 1.04 | 1.6 | 1 | 2.25 | 2 | 2.4 |
| At3g49530 | ANAC062 | 2.29 | 4.53 | 1.4 | −0.19 | 0.8 | x | 2.0 |
| At5g04760 | DIV2 | 2.5 | 2.75 | 1.7 | −1.55 | 0.57 | x | 2.2 |
| At5g10550 | GTE2 | −0.1 | 0.83 | 2.8 | 0.09 | 1.23 | 3.29 | 2.6 |
| At1G17790 | GTE5 | 1.13 | 1.93 | 1.6 | −0.83 | 0.22 | x | 2.1 |
| At5g65630 | GTE7 | 0.29 | 1.73 | 2.2 | 1.4 | −0.79 | 2.36 | 2.6 |
| At3g24500 | MBF1C | 3.06 | 4.82 | 1.6 | 2.77 | 6.48 | 5.55 | 2.3 |
| At1G56170 | NF-YC2 | 0.04 | −0.01 | 2.0 | 3.5 | 3.12 | 4.87 | 2.2 |
| At2G41900 | OXS2/TZF7 | −0.4 | −1.1 | 1.6 | 7.6 | −0.89 | −1.8 | 2.6 |
| At1G18330 | EPR1 | 0.12 | 0.09 | 1.1 | x | 2.79 | 4.61 | 2.6 |
All (FCs are provided as log2 values (A1, A2, B, C1, and C2). Transcript abundance for each gene in pollen (P, A3) and shoots grown under control conditions (Sh, C3) are given as log10 values. (A) Changes in transcript levels in response to PT growth in vitro (PT/d) or in SIV condition (SIV/d) relative to dry pollen grain (d) are shown as log2FC (Qin et al., 2009). (B and C) Heat-stress-induced changes in transcripts in pollen (Pol, Poidevin et al., 2020, B) and shoot (Sh1, Kilian et al., 2007; Sh2, Sugio et al., 2009). x, no data or not significant.
Heat-responsive TFs in germinating pollen partially overlapped with those in shoots. Of 13–17 TFs that increased in shoots (Table 4(C1) and (C2)), 6–7 TFs are also upregulated in heat-stressed pollen (Table 4(B)). If the thermo-protection machinery is conserved in PTs as in shoots according to HSP and ER-stress response proteins (Supplementary Tables S1 and S2), what is the basis of the differential TF response? In seedlings, HSFs are upregulated significantly by heat (log2FC of 3–7; Kilian et al., 2007; Sugio et al., 2009). However, in MP grains, transcripts of several HSFs (including HSFA1a, A4c, A5, and B2A) are already present at moderate to high levels. Heat stress did not significantly change their gene expression, though other HSFs (HSFA2, HSFA1d, HSFB1, and HSFB2b) were upregulated at least two-fold in one or both studies (Table 4). Heat-induced HSF2A expression in developing pollen (Rahmati Ishka et al., 2018) would be consistent with its role in priming for thermotolerance (Scharf et al., 2012; Fragkostefanakis et al., 2016). Furthermore, another master regulator, DREB2A was not significantly upregulated by heat in germinating pollen, though it was expressed in MP (Table 4). DREB2B and DREB19 (DREB2E) transcripts detected in pollen showed little to no response to heat. In contrast, MBF1C, a Multiprotein Bridging Factor1, is upregulated significantly by heat in both germinating pollen and seedlings, as are GTE7 (global TF) and NF-YC2. Thus, the transcriptional network of pollen in response to heat partially overlaps with that seen in heat-stressed vegetative tissues. However, several HSF and DREB genes in Arabidopsis plants are upregulated by drought stress alone (Rizhsky et al., 2004; Kilian et al., 2007), suggesting that some TFs in pollen grains respond to hyperosmotic stress from dehydration during maturation.
The primary sensors and transducers of ER stress are several ER membrane-associated proteins and TFs (Howell, 2013). In vegetative cells, heat causes accumulation in the ER lumen of unfolded proteins which are then recognized by BiP chaperones. The dissociation of BiP from the membrane spanning bZip28 causes the TF to transit to the Golgi where it is cleaved by protease (S-1-P) at the cytosolic side. The soluble TF then moves to the nucleus to regulate downstream genes (Howell, 2013). In another response to ER stress, bZip60 mRNA held at the ER–cytosolic interface is spliced by IRE1 (INOSITOL-REQUIRING ENZYME1, a protein kinase/ribonuclease) spanning the ER membrane, and the translation of the IRE-spliced form of bZip60 results in a protein that moves to the nucleus to activate heat-responsive genes. Although, heat induced little to no change in expression of bZIP28 or bZIP60 in germinating pollen (Table 4(B)), these transcripts are already present at moderate levels in MP suggesting the ER UPR is ready for activation.
Interestingly, 9 of 14 TFs induced by heat in seedlings are also elevated at basal temperatures in germinating pollen (in vitro or SIV) relative to MP (Table 4). MP contains moderate transcript levels of HSFA1, HSFA4, HSFA5, or HSFB2A, and their expression is not altered significantly after hydration and PT growth (Table 4). However, expression of HSFA2, HSFA1d, and HSFB2b is enhanced significantly especially in SIV PTs. The upregulation of additional TF genes, like those encoding DREB2A, DREB19, MBF1C, ANAC062, and DIV2, is observed in SIV PTs (Table 4). Furthermore, regulators of the ER UPR (bZip28, IRE1a, IRE1b, and bZip60) (Liu and Howell, 2016) are expressed in dry pollen, though only bZip60 and IRE1b showed significant upregulation in SIV PTs, supporting the enhanced expression of ER-stress response genes (Table 2(B)). In the absence of heat stress, transcript increases in the genes encoding HSFA2, HSFB2B, MBF1C, DREB2A, and other TFs (ERF061, ANAC062, and DIV2), especially in PTs growing through the style, are likely responding to signals from Osm and/or oxidative changes plus cues from pollen–pistil interactions, such as secreted ligands and perhaps hormones (see section “RNP aggregates in pollen grains may serve as sites of stored MRNA”). MBF1C is a transcription cofactor, and its transcript is elevated in response to heat, as well as oxidative, drought, and salt stress (Suzuki et al., 2005). Partial overlap of TFs expressed in PTs growing through the style with those induced by heat (Table 4) and other abiotic stresses (Swindell et al., 2007) suggests crosstalk of developmental and nonheat stress cues, and convergence of regulatory networks at common signaling intermediates, like ROS. Alternatively, pollen has a distinct transcriptional regulatory program that responds to nonheat stress cues to activate the ER UPR and promote germination and tube growth.
RNP aggregates in pollen grains may serve as sites of stored mRNA
The increased expression of small HSPs and other molecular chaperones in the absence of heat stress suggests they perform critical roles in pollen germination and tube growth. Based on recent advances in stress biology, we speculate below that some molecular chaperones in pollen are crucial for recovery from stress, such as in the disassembly of “stress granules” formed to protect cells. SGs are defined as aggregates composed of proteins and mRNA molecules, mostly stalled translation initiation complexes that usually form in a reversible manner upon cellular stress (Protter and Parker, 2016; Figure 2).
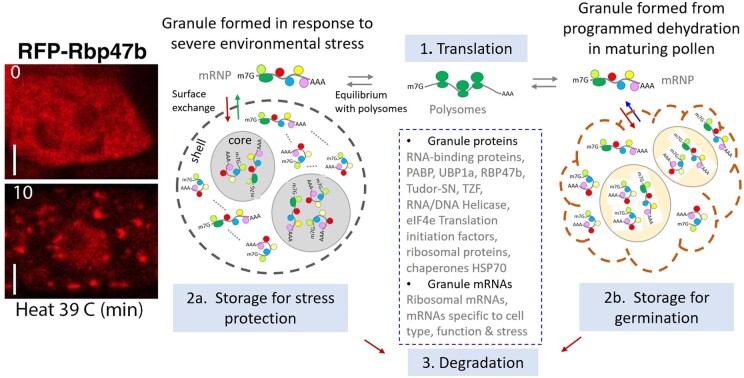
Figure 2.
A model of granule assembly and dynamics during stress and development. [Left] Heat stress induces granule formation. Transgenic Arabidopsis seedlings expressing RFP-labeled RBP47b were treated at 39°C for 0 or 10 min. Fluorescent foci appear in root tip cell in minutes. Bar = 5 um. (Gutierrez-Beltran et al., 2015, with permission). (1) Translation. Under optimal conditions, polyA+-RNAs loaded with ribosomes (green) (polysomal mRNA) are actively translated in the cytoplasm. (2a) Environmental stress-induced granule formation. In response to stress, like heat, hypoxia or dehydration, polysomes lose ribosomes, and the mRNAs are bound by various RNA-binding proteins (colored circles) forming mRNP (messenger ribonuclear protein). The mRNP complexes assemble into condensates forming (brown arrow) granules (circular dashed line) in the cytoplasm. Granules may have a stable dense core and a dynamic phase-separated shell and contain mRNAs that are translationally silent. Granules differ in mRNA content depending on the cell type and stress. During stress recovery (green arrow), the granules disassemble and the released mRNAs form polysomes and become translationally active (1). (2b) Programmed dehydration-induced granule formation. In developing pollen, many mRNA transcripts required for tube growth are pre-synthesized. The onset of dehydration is proposed to stimulate mRNA and RNA-binding protein (colored circles) complexes (mRNP) to coalesce forming (brown arrow) condensates or granules (brown dashed line) in maturing pollen. Upon hydration on a compatible pistil, the granules disaggregate, releasing mRNAs (blue arrow) to form polysomes (1) that become translationally active to support pollen germination. (3) Degradation. Some mRNAs in the cytoplasm or released from SGs are processed for degradation. Model is modified from SG dynamics in nonplant (Protter and Parker, 2016) and plants (Chantarachot and Bailey-Serres, 2018). Box shows examples of mRNA and protein components common to SGs (Jain et al., 2016, Kosmacz et al., 2019). SG proteins include those detected in granule fraction of tobacco pollen (Honys et al., 2009; Hafidh et al., 2018).
Pollen grains from many plants are competent to germinate and initiate tube extensions without de novo transcription; however, protein translation is essential for germination indicating the presence of stored messages. Shortly after germination, sustained tube growth requires de novo transcription and the translation of new as well as stored mRNAs. Although it was long known that MP grains contain stored or masked mRNAs (Linskens et al., 1970; Mascarenhas, 1993); how mRNA is stored in the cell was not determined until recently. Honys et al. (2009) found mRNA and proteins associated with aggregates (or particles) from MP of tobacco, N. tabacum. They separated ribonucleoproteins with a sucrose gradient and showed monosomes dominate (90%) in MP grains, whereas polysomes dominate (>90%) in 4-h-old PTs, indicating an increase in translation in the early stage of tube growth (Hafidh et al., 2018). To identify a candidate stored mRNA, Honys et al. (2000) tracked the distribution of a pollen-specific NTP303 mRNA which is transcribed late in developing pollen but is translated only after germination. They found that NTP303 mRNA is preferentially associated with a pellet fraction resistant to polysome-destabilizing conditions (Honys et al., 2000) suggesting that nontranslating mRNAs are held in EDTA-puromycin-resistant particles (EPPs). In addition to mRNA, EPPs contained ribosomal proteins, polyA-binding proteins, chaperones, and cytoskeleton proteins based on proteomic analysis (Honys et al., 2009; Hafidh et al., 2018). On the bases of these findings, pollen EPPs may be biochemically similar to SGs (Protter and Parker, 2016; Chantarachot and Bailey-Serres, 2018) and serve as sites for stored mRNAs (Figure 2).
Onset of dehydration as a developmental cue?
Less clear is what change(s) induced formation of “aggregates” in developing pollen. In plants, SGs are formed in response to acute environmental stress, such as heat, salt, or hypoxia (Chantarachot and Bailey-Serres, 2018). SGs protect the nontranslating mRNA from degradation, while processing bodies (PBs) containing decapping enzymes promote RNA degradation (Chantarachot and Bailey-Serres, 2018). Although, it is unclear what cellular change(s) is(are) sensed inside anthers, pollen reaches maturity when it is partially desiccated (Figure 1). After haploid microspores divide to form a BCP as in tobacco or TCP as in Arabidopsis, pollen loses water to enter a “quiescent” state as anthers begin to desiccate, causing anther dehiscence and release of MP (Firon et al., 2012). The partially dehydrated pollen (MP; ranging from <30% to 40% water) represents a “quiescent” state that can withstand the harsh conditions (Franchi et al., 2011) as pollen is exposed to the environment. In self-pollinated flowers like Arabidopsis, this period is short; however, pollen grains from insect- or wind-pollinated plants endure relatively long periods of desiccation and heat.
Pollen undergoes partial dehydration at the end of its development and after anther dehiscence based on several observations. These include change of grain shape from spherical to oblong and reduced water content (<30%), decrease in small vacuoles in the vegetative cell, increase in osmolytes such as sucrose, trehalose, and proline (Firon et al., 2012), and accumulation of dehydrins and late embryogenesis abundant (LEA) proteins (Grobei et al., 2009). LEA proteins, named for their abundance in developing seeds, are thought to protect membranes and proteins from aggregation due to desiccation or Osm stress (Goyal et al., 2005). Transcriptome data show microspores or BCP have low to no LEA transcripts; however, 8 out of 11 LEA transcripts peaked (up to 30- to 100-fold) in TCP of Arabidopsis and then decreased in germinating PTs (Supplemental Figure S1A). Moreover, 7 out of 11 AtLEA proteins are detected in MP based on proteomic analysis (Grobei et al., 2009). From this result, we infer that dehydration begins at the TCP phase in Arabidopsis, and in the BCP phase in tobacco (Supplemental Figure S1B).
The importance of anther dehydration on male fertility is supported by genetic studies in Arabidopsis. A mutant of DDE1 gene, encoding an enzyme (OPR3) for biosynthesis of jasmonic acid, causes delayed anther dehiscence resulting in male sterility (Sanders et al., 2000). The regulation and basis of dehydration are unclear, and are probably due to a combination of factors, including water flow out of the anther, blockage of water into the anther, and loss of water by evaporation. In a mutant lacking ICE1, an MYB TF, anthers dehisce too late to pollinate pistils that have surpassed anther height (Wei et al., 2018). As in leaves, ICE1 regulates stomatal development in anthers. One interpretation is reduced stomata in ice1 mutant retard water loss and anther dehiscence (Wei et al., 2018). The desiccation of anther locules promotes pollen maturation and viability, based on the dad1 mutant (Ishiguro et al., 2001). DAD1 encodes a stamen filament lipase that catalyzes the first step of jasmonic acid synthesis. Perhaps, the onset of desiccation at the late BCP (tobacco) or TCP (Arabidopsis) stage and the accompanying intracellular physico-chemical changes enhanced RNA- protein condensates in “stress” granules (Alberti and Hyman, 2021). This process is likely conserved in all flowering plants (Honys et al., 2009). The cue(s) and regulatory network that promote the formation of storage granules and the entry to a quiescent state are unexplored in pollen development. Understanding the basis for desiccation tolerance in pollen and developing seeds (Bai et al., 2020) could yield fundamental insights into drought tolerance in plants (Zhu, 2002, 2016).
SGs formed in response to heat or hypoxia share similarities with RNP aggregates in pollen
Cytoplasmic “stress” granules formed in response to environmental stress (Nover et al., 1989; Chantarachot and Bailey-Serres, 2018) share similarities with mRNP particles in pollen. First, plant cells exposed to heat, hypoxia, or dehydration, show ribosome run-off. This is evident as a decrease in polysomes and increase in monosomes (Kawaguchi et al., 2004; Branco-Price et al., 2008; Merret et al., 2017. The dominance of monosomes seen in MP grains (Hafidh et al., 2018) would suggest dehydration is a major cue for ribosome run-off in developing pollen. This idea is consistent with the finding that most stored mRNA are associated with monosomes in dry seeds (Bai et al., 2020). Second, heat or hypoxia stress triggered formation of cytoplasmic aggregates that resemble RNP particles in pollen. Heat shock granules (HSGs) first isolated by ultracentrifugation and fractionation are resistant to RNase, high salt, EDTA, and detergent, suggesting aggregate formed with tight structural binding (Nover et al., 1989). Cytoplasmic granules can be visualized in plant cells as fluorescent foci using markers (Chantarachot and Bailey-Serres, 2018). Core components of HSG like poly(A+) RNA, translation initiation factors (eIF4e), RNA binding proteins (RBP47, UBP1), and small HSP17 are useful markers. Unstressed cells showed disperse cytosolic fluorescence. After heat stress, fluorescent oligo-dT colocalized with RBP47 or eIF4e, and HSP17 colocalizes in part with GFP-UBP1 (Weber et al., 2008). Fluorescent foci of RFP-RBP47b appeared in seedlings after 10 min at 39°C (Gutierrez-Beltran et al., 2015; Figure 2). SGs differ from PBs as Hsp17 did not colocalize with foci containing a decapping enzyme (GFP- DCP1). Similar SGs are formed after 1 h hypoxia of A. thaliana seedlings (Sorenson and Bailey-Serres, 2014). UBPC1-GFP, which rescued Atubp1c mutant, colocalizes with C3-oligo dT or with Poly(A) binding protein (PABP2-RFP) after hypoxia. Thus poly(A+) mRNA, translation initiation factors, and RNA binding proteins (UBP1/RBP47 and PABP) are recruited together to cytoplasmic structures under stress conditions. The major components of SGs (Jain et al., 2016; Kosmacz et al., 2019) are also found in EPP particles from tobacco pollen (Honys et al., 2009; Hafidh et al., 2018 (see model Figure 2).
Granule disassembly at recovery from stress
After a stress period ends, cells gradually recover and resume translation. Recovery is evident when the fraction of polysomes to monosomes increases to levels seen in unstressed cells, whether the stress was from heat, hypoxia, or dehydration (Kawaguchi et al., 2004; Branco-Price et al., 2008; Merret et al., 2017). Recovery is accompanied by disassembly of heat SGs as seen by solubilization of HSP17 (Nover et al., 1989) or loss of cytoplasmic foci monitored by fluorescent SG markers in 1–2 h (Weber et al., 2008; Sorenson and Bailey-Serres, 2014).
Some molecular chaperones aid recovery from heat stress by promoting the release of mRNA from “stress granules.” hsp101 knock out mutants were defective in translation at recovery and in the dissociation of the SGs (Merret et al., 2017). Hsp101 has a role in binding mRNA encoding ribosomal proteins and also after release in translation. Another study showed that reduction of sHSP using RNA interference in the hsp101 background, delayed the recovery of complexed protein in the soluble fraction (McLoughlin et al., 2016), suggesting that molecular chaperones, like Hsp17, collaborate with HSP101 to dissociate the complex. The model is similar to that of bacteria and fungi where sHsp binding to native substrates enhanced the efficiency of the Hsp101 disaggregation machinery (Mogk et al., 2018). Moreover, Arabidopsis HSP101 interacts with proteasome subunits, suggesting a dynamic relationship between disaggregation and proteolysis of ubiquitylated proteins from the granules (McLoughlin et al., 2019).
Here, we postulate that complex disassembly is triggered by pollen hydration and pistil signals (Section “Transcripts encoding molecular chaperones and ER-stress response proteins are elevated in PTs growing through the pistil at basal temperature”) and that molecular chaperones participate in restoring the translation machinery. Several observations support this idea. First, polysomal RNA increased in PTs relative to low or no polysomes in MP, inferring activation of translation (Hafidh et al., 2018). Second, late pollen transcripts (e.g. SKU5-like Cu oxidases, pectin methylesterases [PMEs], and PM-H+ pump) initially enriched in EPP particles in MP shifted to the polysome fraction in PTs (Hafidh et al., 2018; Supplemental Figure S2). Third, the earliest transcripts induced significantly after pollen hydration included nine HSP20, four Hsp70s, three Hsp90s, and HSP101 which have a role in granule disassembly (Table 3; Wang et al., 2008). Furthermore, PTs germinated on a pistil (SIV) showed increased transcripts for additional Hsp70s and multiple Hsp40s. Activation of the ER-stress response in germinating pollen suggests a role of the ER in the dynamics and disassembly of SGs as proposed in animal cells where RNP-containing granules contact the ER and their changes are related to the ER translational capacity (Lee et al., 2020).
Transcripts unresponsive to heat are likely protected in mature and germinating pollen
The identification of pollen mRNAs stored in aggregates is just beginning. Here we applied two criteria to predict stored transcripts in developing pollen from Arabidopsis First, as dehydration occurs at pollen maturation, the transcript would accumulate at the TCP stage and persist through germination and tube elongation. Second, the transcript level would be resistant to heat stress applied to germinating pollen if the mRNA is protected in heat-stable particles as for SGs. Using both criteria, we found abundant transcripts that encode proteins involved in cell wall synthesis, wall modeling, signaling, and transport (Figures 3 and 4).
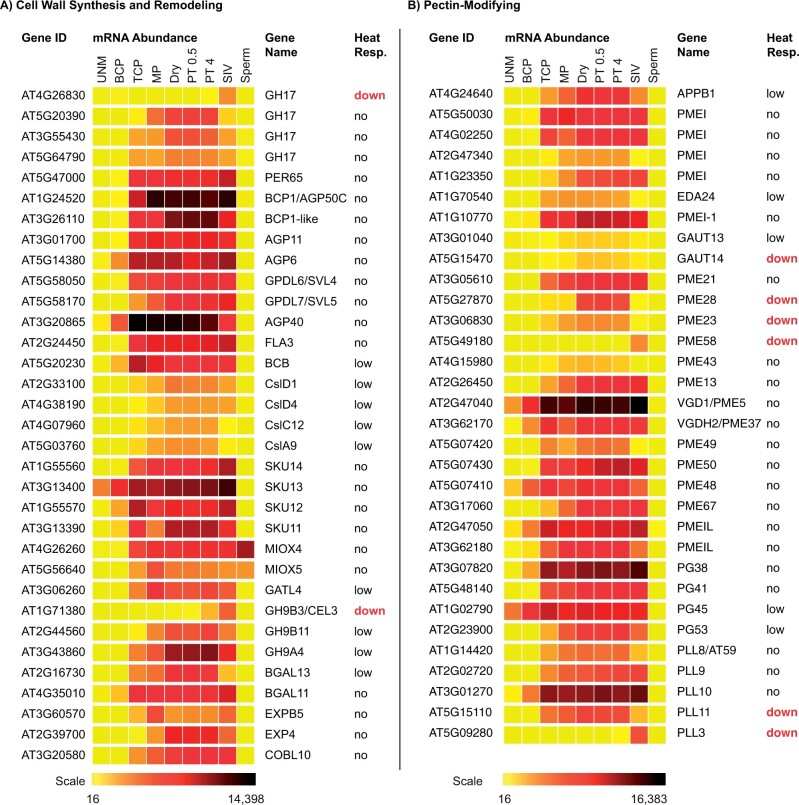
Figure 3.
Many transcripts that accumulated in maturing pollen are unresponsive to heat stress in germinating pollen. A, Cell wall synthesis and remodeling and (B) Pectin modifiers. Diagram showing relative transcript levels in developing and germinating pollen. Pollen stages refer to: (1) UNM; (2) BCP; (3) TCP, and (4) MP (Honys and Twell (2004); (5) dry pollen (dry); (6) PT 0.5 h (PT 0.5); (7) PT 4 h (PT4); (8) SIV PT (Qin et al. 2009); and (9) sperm (Borges et al., 2008). Expression value scale ranges from yellow (low), red (medium) to black (high). Transcriptome profile generated using Arabidopsis Heat Tree Viewer (http://pheattreecit.services.brown.edu/, Johnson MA). Heat response of each gene (right) is designated as “no” or “low” response when no significant change (log2FC) in transcript level was observed in germinating pollen at 35°C versus at 24°C (Poidevin et al., 2020). “down” indicates genes that are significantly downregulated by heat stress. Genes are described in Supplemental Table S6.
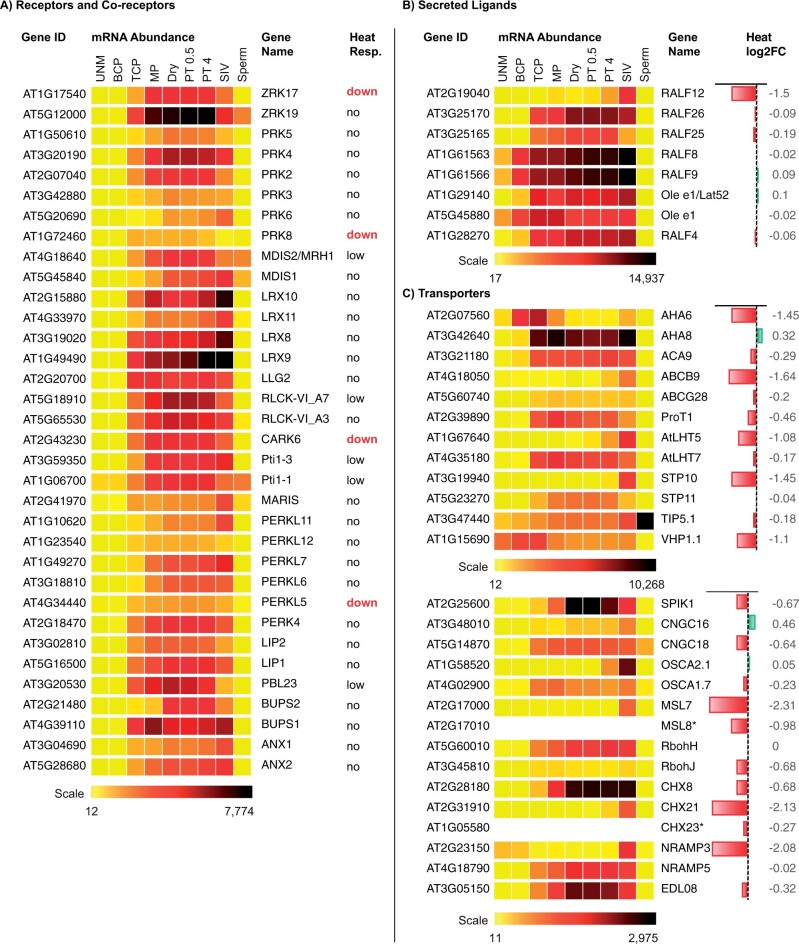
Figure 4.
Heat stress-unresponsive transcripts and heat-repressed transcripts in germinating pollen. Relative expression pattern of genes encoding signaling components (A and B) and transporters (C) during pollen development as described in Figure 3. Many genes highly expressed from 3-TCP to 8-SIV are also largely unresponsive to heat. SIV activated genes (A and C) are downregulated by heat. Note: MSL8 and CHX23 transcripts were detected by RNA-seq data from MP and PT (Rahmati Ishka et al., 2018; Poidevin et al. 2020). Heat response of each gene (right) is designated as yes (down), low or no change, or as log2 FC. “down” indicates a significant change when log2 FC is less than −1 based on Poidevin et al. (2020). Genes are described in Supplemental Table S6.
Many transcripts that are likely protected in aggregates (Figures 3 and 4) are not only specifically or preferentially expressed in pollen, but also are critical for pollen functions. The synthesis and remodeling of cell walls in pollen, and functional studies based on mutant analyses, have been reviewed (Mollet et al., 2013; Dehors et al., 2019), so only a few examples are highlighted. Cellulose is important for tube wall integrity. Pollen carrying a single mutant of cellulose synthase-like gene (csld1 or csld4) showed disorganized wall, increased tube burst at germination, and defective male transmission (Bernal et al., 2008; Wang et al., 2011). Pectin, a major polysaccharide of pollen wall, is synthesized in the Golgi apparatus and secreted to the tube tip in Golgi-derived vesicles. Methylesterified homogalaturonan (HG) secreted at the apical zone appears to have plasticity that can accommodate growth. HG can be modified or remodeled to change the mechanical properties of the wall by: (1) PMEs which remove methyl groups and expose carboxylic groups that bind Ca2+ to mediate cross-linking of adjacent polymers and (2) pectate lyases (PLLs) and polygalacturonase (PG) that cleave demethylated HG backbone. PMEs are critical for modulating wall plasticity, and PLLs or PG may cause wall loosening. HG is synthesized by several Golgi-associated galacturonosyl transferases (GAUT) in pollen, and loss of GAUT activity results in impaired pollen germination and tube elongation (Atmodjo et al., 2013). For example, GAUT1 interacts with either GAUT5, 6, or 7 to mediate activity, and male infertility is severe in triple mutants (gaut5,6,7; Lund et al., 2020). These four GAUT genes are expressed in developing pollen (uninucleate microspore [UNM], BCP, and TCP) and functionally distinct from two others, GAUT13 and GAUT14, which are preferentially expressed in MP and in PTs (Figure 3B). Double mutants of gaut13gaut14 produced short swollen tubes and disorganized walls (Wang et al., 2013). Curiously, single PME mutants, Vanguard-like PME (vgd1)/pme4 (Jiang et al., 2005) or pme48, resulted in premature tube burst, or delayed germination suggesting a strict balance between methylesterification/de-methylesterification of homogalacturan is needed to maintain the mechanics of the wall (Leroux et al., 2015). Pectin remodeling may also affect the adhesion and guidance of PT in the style (Mollet et al., 2013). At least 15 out of 66 PME members are expressed in pollen (Figure 3B). PME activity is likely tuned spatially and temporally in PTs, as shown by the targeted exocytosis of a PMEI at the tip, and endocytosis of a PMEI at the sub-apex (Rockel et al., 2008). PMEIs are proteins that bind and block PME activity. Like pollen PME, approximately 16 PMEI genes are highly expressed in pollen (Figure 3B; Honys and Twell, 2004; Qin et al., 2009; Klepikova et al., 2016) and two-thirds show expression pattern consistent with stored transcripts (based on Heat Tree Viewer). Their specific roles are largely not understood. Based on the differential pH dependence of PME activities and the pH sensitivity for PMEI–PME binding, PME could be finely modulated by local pH (Hocq et al., 2017; Senechal et al., 2017). Prime candidates for pH regulation include the collaboration of plasma membrane (AHA) or endomembrane (VHA) proton pumps, with cation/proton antiporters (CHX) that are preferentially expressed in pollen and associated with dynamic endomembranes (Sze and Chanroj, 2018). The roles of these transporters on wall remodeling, via PME and PMEI activities, and signaling in pollen behavior need to be determined.
Interestingly, many transcripts of receptor-like kinases (RLKs) that sense and transduce external signals at the tip to the elongating tube cell are synthesized in TCP and likely stored in granules (Figure 4A). For example, two CrRLK1L RLKs, ANX1/2 interact with BUPS1/2 receptor kinases to form a heterodimer that supports PT integrity (Ge et al., 2019). Double mutants of anx1/2 (Boisson-Dernier et al., 2013) or bups1/2 cause precocious tube rupture. ANX1/2 coordinate cell wall integrity through ROS production from NADPH oxidases (RbohH/J) (Boisson-Dernier et al., 2013). Recently, the ANX1/2 receptor complex was shown to interact with extracellular ligands, RALF4/19 (Ge et al., 2019) or in partnership with LRX Leucine-rich repeat extensins (Mecchia et al., 2017) to maintain proper tube growth, and disruption of this interaction by a female-secreted RAPID ALKALINIZATION FACTOR (RALF) is proposed to cause tube rupture and sperm discharge. RALF4/19 plus several other RALFs are cysteine-rich peptides specific to pollen and their transcripts may be stored (Figure 4B). PTs of double mutant ralf4/19 also rupture. Thus, cell wall-sensing receptors, like the ANX1/2 complex, regulate and coordinate wall status with the internal growth machinery (Cheung and Wu, 2011).
Transcriptome analysis of transporters showed some transcripts accumulate late during pollen development (Bock et al., 2006) and are relatively heat-stable in germinating pollen (Poidevin et al., 2020). Transporters active in PTs were reviewed before (Michard et al., 2017), we highlight a few genes essential for tube growth dependent on pH and Ca2+ homeostasis. ACA9 transcript is unresponsive to heat stress, and likely stored in granules. It is the only PM Ca2+ extrusion pump showing abundant transcripts from TCP to the PT stage (Figure 4C; Bock et al., 2006). Null aca9 mutants show 80% reduced seed set due to defective tube growth and fertilization (Schiott et al., 2004). High-affinity Ca2+ extrusion pumps generate and maintain a steep Ca2+ gradient across the PM (high outside), thus ACA9 could participate in Ca2+ signaling and pectin wall remodeling. PM AHAs generate the primary proton motive force in cells not only for transport of nutrients across the PM, but also for pH homeostasis. Although, three AHAs are preferentially expressed in pollen, only AHA8 transcript is predicted to be stored in granules. The expression pattern of AHA6 and AHA9 infers they are developmentally regulated (Figure 4C;Bock et al., 2006). Studies of a triple aha6/8/9 mutant indicate that PM H+-pumping ATPases are essential for tube growth and fertilization (Hoffmann et al., 2020). Other transcripts unresponsive to heat, include a Ca2+-permeable channel CNGC18, NADPH oxidase RbohH, sugar transporter STP11, amino acid transporter, LHT7, and K+/H+-cotransporters, CHX8 and CHX23. Yet other members of gene families are heat-sensitive and newly transcribed in PTs growing in the pistil, such as LHT5, AHA6, STP10, and CHX21 (Figure 4C). A cngc18 null mutant was male sterile, as PTs were short, kinky, and unable to grow into the transmitting tract (Frietsch et al., 2007). Intriguingly, PTs carrying a double mutant of chx21chx23 (cation/H+ transporters) failed to turn toward ovules, suggesting pH homeostasis in the endomembrane system is critical for sensing and responding to cues that guide directional growth at the pollen tip (Lu et al., 2011).
In summary, 75% of abundant transcripts that accumulated in the TCP and persisted in the PT, are unresponsive to heat stress (Supplemental Table S5) and possibly protected in RNP particles. These transcripts encode many cell wall synthesis proteins, pectin remodelers, receptors, and transporters that are critical for supporting rapid germination and tube growth in A. thaliana. Experimental support for stored transcripts is emerging in tobacco pollen, based on the first RNA-seq transcriptome of developing pollen and PTs, where nontranslating and translating mRNA fractions were separated (Hafidh et al., 2018). Transcripts abundant in RNP particles (EPP) of MP and growing PTs (PT4), included wall remodeling enzymes, such as an extracellular Cu oxidase (SKU5-13 homolog) and VGD1. In contrast, these transcripts were low in the polysome fraction of MP grains but increased in 4 h tubes (Supplemental Figure S2) indicating increased translation in growing tubes. Other examples of transcripts that become translationally active in tubes include pectin remodelers, receptors, and transporters are shown in Supplemental Figure S2 (data from Hafidh et al., 2018). Furthermore, two late pollen-expressed transcripts (Figure 3; SKU14 and PLL8) were detected using the viral MS2-GFP chimeric RNA system in cytoplasmic foci of MP from Arabidopsis (Scarpin et al., 2017). Verification of stored mRNAs in cytoplasmic granules in pollen and growing tubes is needed. Nevertheless, the predicted stored transcripts in Arabidopsis (Figures 3–4) are strikingly similar to EPP-associated transcripts from tobacco pollen (Supplemental Figure S2).
Transcripts sensitive to heat stress are newly synthesized in PTs growing on the pistil
Two protein classes whose transcripts were downregulated by heat are transporters (Poidevin et al., 2020) and metabolite interconversion enzymes (Supplemental Figure S3C(i)), as analyzed using Panther 16 (Mi et al., 2021). Transporters include MSL7, CHX21, AHA6, and STP10 (Figure 4C), and enzymes, include Gly-Asp-Ser-Leu (GDSL) esterase/lipases. Curiously, 75%–80% of genes downregulated by heat in germinating pollen, are upregulated in tubes growing under SIV conditions (Supplemental Figure S3C(ii)). One interpretation is that many SIV-induced transcripts (approximately 1,100; Qin et al., 2009) are not only transcriptionally regulated by factors or cues from hydration and pollen–pistil interactions, a large subset is also repressed by heat-responsive factors. Heat impairs pollen germination and tube growth (Poidevin et al., 2020) indicating the transcriptional regulation of many newly transcribed genes post pollination are crucial for optimal pollen functions.
ER-mediated proteostasis
Clearly, protein translation in germinating pollen relies heavily on ER-bound polyribosomes, based on the predicted stored transcripts (Figures 3 and 4) and newly synthesized mRNAs. In addition to membrane integral proteins (receptors and pumps), highly abundant transcripts encode secreted ligands, extracellular wall proteins, and remodeling enzymes. ER is the hub and gateway for the synthesis, modification, and sorting of materials to their destination by membrane trafficking and targeted secretion of materials to the wall. Furthermore, the Golgi apparatus is the hub for the synthesis of noncellulosic wall polysaccharides, like pectins (Atmodjo et al., 2013). Together, the activities place an extreme burden on ER and the endomembrane system, contributing to activation of the ER UPR (Section “Transcripts encoding molecular chaperones and ER-stress response proteins are elevated in PTs growing through the pistil at basal temperature”; Fragkostefanakis et al., 2016; Howell, 2017) and perhaps promoting ER dynamics and expansion to support rapid tube growth and sperm delivery in the female gametophyte. This model is consistent with findings that the UPR plays a central role in the normal development and function of eukaryotic cells specialized for secretion, as in pancreatic cells (Moore and Hollien, 2012). A future challenge is to understand how the UPR response is related to the dynamics, proliferation, and function of the ER network that makes contacts and communicates with organelles, like mitochondria, endosomes, Golgi, and plasma membrane (Wu et al., 2018).
Model: formation and disaggregation of complexes containing stored mRNAs rely on the stress-protection machinery
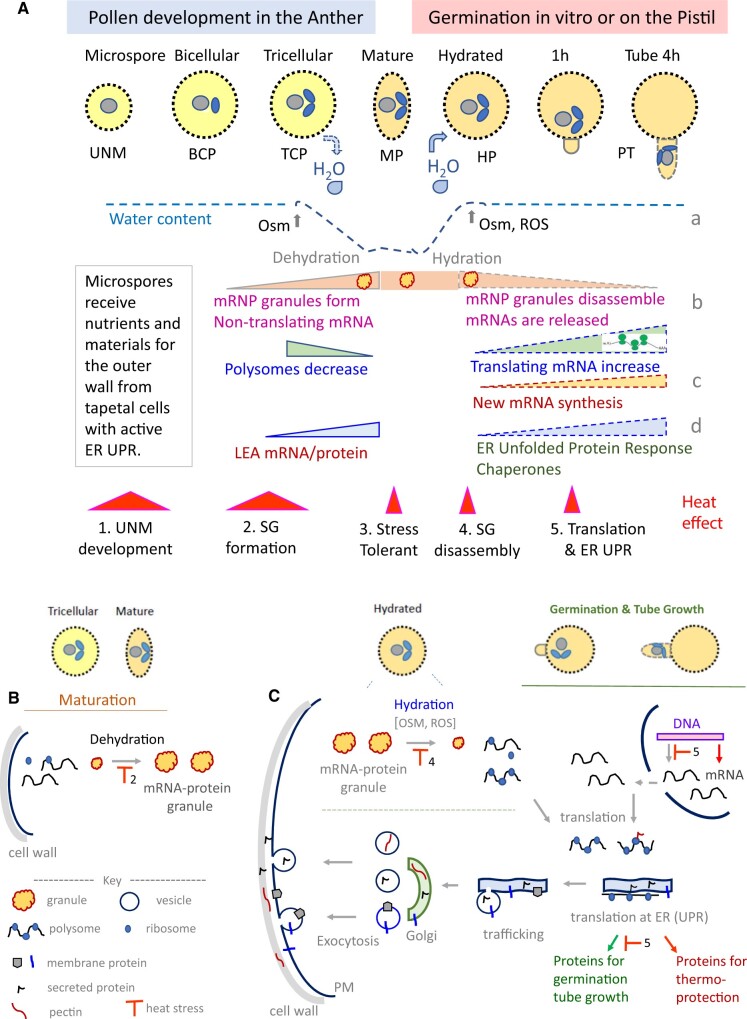
Here we present a model from myriad observations that show pollen responds to programmed dehydration at pollen maturation and then recovery from desiccation for germination. Pollen has adapted a conserved survival strategy where cells respond to short-term stress by sequestering mRNA and proteins in “stress” granules to stall translation for a limited period. We reason that the dependence on stress responses for pollen viability and function is an underlying basis for heat sensitivity of the male gametophyte. The proposed working model (Figure 5A) based on available information needs to be tested and refined (see Outstanding Questions box).
Figure 5.
Model showing the dynamics of mRNP granules and the cellular changes as pollen dehydrates at maturation and then rehydrates for germination. A, Fate of mRNA at pollen maturation and at germination. The haploid microspore divides to produce a TCP (as in Arabidopsis) or a BCP (tobacco) inside the anther. Pollen then undergoes partial dehydration (H2O removal) to reach maturity and the grains are exposed as anthers dehisce. After pollen lands on a compatible stigma, rehydration, Osm, ROS, and pistil factors stimulate pollen germination and tube growth. a, Relative hydration state of the pollen stages is shown as a dotted line. b, Dehydration is postulated to decrease polysome content (green) and enhance formation of mRNP granules (pink). Upon rehydration, the granules disassemble. Nontranslating mRNA decreases (pink dashed) as translating mRNA (green) increases in growing tubes. Upward or downward slope indicates an increase or decrease. c, New mRNAs are transcribed in pollen germinating in vitro and on the pistil. d, LEA transcripts increase in TCP inferring dehydration onset as pollen grains mature. Molecular chaperones and the ER UPR are activated in germinating pollen and particularly after tube growth through the pistil. Heat stress (orange triangles) sensed at different stages (1–5) would perturb pollen development, maturation, or germination (see Figure 5, B and C, and text). B and C, Cellular changes at pollen maturation, pollen hydration and tube growth at optimal temperature and effects of heat stress. Respective pollen stages are shown above. B, Maturation of TCP in the anther. Onset of dehydration promotes the packaging of mRNAs into mRNP granules in the cytoplasm of a maturing pollen grain. C, Germination and tube growth. As grain hydrates at the pistil, RNP granules disassemble and release mRNAs forming polysomes to begin translation. De novo transcription is activated in the nucleus producing new mRNAs. Released and newly synthesized transcripts encoding secreted and membrane proteins are translated at the ER, modified and sorted to their destination. The ER UPR is activated in germinating pollen. Heat stress (Orange) at 2 could inhibit granule formation at pollen maturation (B), granule disassembly at hydration (4), ER-mediated protein synthesis for tube growth (5), or new transcription in the nucleus (4 and 5). MP is desiccation- and heat-tolerant. Heat stress is postulated to reduce male fertility as it diverts heat stress-responsive genes and ER-mediated proteostasis for thermo-protection purposes instead of PT functions for sperm delivery.
After formation of the haploid microspore and mitosis, the BCP in tobacco or the TCP in A. thaliana undergoes partial desiccation. Dehydration, sensed as hyperosmotic stress (Zhu, 2016) by the vegetative cell, inhibits translation and triggers formation of more cytoplasmic “granules.” These granules hold mRNA, RNA-binding proteins, and translation initiation factors, and selected molecular chaperones in pollen (Hafidh et al., 2018) similar to heat-induced SGs (Chantarachot and Bailey-Serres, 2018). Pollen enters a quiescent state to tolerate the harsh environment as it is carried by wind or insect from the anther to the female pistil.
After landing on a compatible stigma, dry pollen grains hydrate, and granules disassemble releasing stored mRNP for translation. We propose that Hsp101 along with Hsp70, and small Hsp20 work together to disaggregate the complex of stored mRNA and promote formation of the translation machinery as seen in the disassembly of heat SGs (Liberek et al., 2008; McLoughlin et al., 2016; Merret et al., 2017; Mogk et al., 2018). Upregulation of molecular chaperones shortly after pollen hydration and germination (Table 3) is consistent with this idea.
Hydration and pistil-derived factors lead to Osm, mechanical, oxidative, hormonal, and/or secreted peptide cues that upregulate transcription of genes, including molecular chaperone, foldase, and the ER-stress response (Tables 2 and 3) to initiate germination and tube elongation. Stored transcripts as well as newly synthesized mRNAs encoding proteins for wall synthesis and remodeling, signaling, and transport (Figures 3 and 4) are translated, modified, and sorted to their destination via the endomembrane system (Figure 5B) for rapid tube growth, tip navigation and finally tube burst to discharge sperm at the ovule.
Heat stress could compromise pollen development and performance depending on the time and stage. We propose five major outcomes when heat stress is sensed at different stages (see Heat 1–5 in Figure 5, A–C). (1) Microspore development is vulnerable to heat spells in part because the ER UPR is activated in tapetal cells lining the anther chamber in unstressed plants (Iwata et al., 2008; Deng et al., 2016; Singh et al., 2021). Tapetal cells provide nutrients to the developing pollen, synthesize and secrete compounds for the outer pollen wall, thus disruption of the UPR by heat compromises pollen development and subsequent germination. (2) Heat stress before pollen maturation would induce thermotolerance responses that directly interfere or compete with formation of mRNP storage granules (Figure 2) in response to programmed dehydration. Although the protein components of SG are largely conserved for heat or drought stress, the composition, and type of mRNAs stored in pollen granules are specific for germination and tube growth (Figures 3 and 4). Granules are diverse, and their composition and properties are thought to differ depending on the cellular stress and the cell type (Protter and Parker, 2016). Consequently, granules with a deficient set of mRNAs could result in reduced pollen vigor. (3) After dehydration and maturation, pollen grains would be relatively desiccation- and heat-tolerant during pollen dispersal.
(4 and 5) Heat stress after pollen arrives at the stigma would (a) stimulate formation of heat SGs and inhibit release of stored mRNAs; (b) repress de novo transcription of pollen genes normally activated by pollen–pistil interactions, including RLKs, and transporters (Figures 3 and 4; Poidevin et al., 2020); and (c) induce the ER-stress response to synthesize molecular chaperones for thermo-protection purposes and promote ubiquitin-mediated degradation of proteins which are required for tube growth. Together, a delay in pollen germination and tube growth of several hours could severely compromise male fertility and reduce seed set. One study supporting this model is seen in a mutant of an ER-localized DnaJ chaperone, ERDj3A, expressed in pollen. Seed set in the mutant is severely reduced at 30°C, but not at 22°C (Yang et al., 2009). At 30°C, PT growth in vivo is strongly retarded in the mutant; however, plant growth was not affected. The gene was named TMS1. Thus except for stress tolerance of MP grains, multiple essential processes during pollen development and after germination are compromised by heat stress.
Conclusion and future perspectives
We have illuminated relationships and insights by comparing transcriptomes of developing, mature, and germinating pollen with or without heat stress. First, unlike most vegetative cells, a subset of pollen genes encoding cytoplasmic and ER molecular chaperones, and foldases are induced at germination in response to hydration and other pistil factors in the absence of heat stress. These chaperones are thought to aid release of stored transcripts for protein translation and ER-mediated proteostasis for germination. A similar set of molecular chaperones and UPR genes are upregulated by heat stress in pollen indicating thermo-protection strategies are conserved in pollen as observed in vegetative tissues. Second, a subset of transcripts is unresponsive to heat stress and predicted to be stored in granules at pollen maturation when dehydration sets in. These transcripts are induced late in developing pollen, and their levels remain high through pollen germination and tube growth. Estimated to be 21% of pollen-expressed genes (Hafidh et al., 2018), many stored transcripts are likely released and become translationally active after pollen landing on the stigma to initiate germination. Third, another subset of genes (10%–22%) is newly transcribed in pollen germinated in vitro and on the pistil at basal temperatures (Qin et al., 2009). These de novo synthesized transcripts include many transporters; however, their transcription is repressed by heat stress in germinating pollen. These transcripts are essential for sustained PT growth, signaling, and wall remodeling. Thus pollen survival and vigor depend on (1) protection of a subset of pre-synthesized transcripts in MP; (2) translational activation of stored transcripts after pollen lands on a compatible stigma, (3) de novo transcription of another subset of genes by hydration, ROS, and other factors from pollen–pistil interactions, and (4) a very active ER UPR that may participate in granule disassembly and mediate proteostasis for the synthesis, folding and trafficking of secreted and membrane proteins for germination, tip growth, navigation, and tube burst after reaching the female gametophyte.
We propose that the male gametophyte is particularly vulnerable to heat, because its developmental program depends on severe stress-induced responses, unlike that of vegetative tissues. During vegetative growth, a multicellular, photosynthetic plant develops and grows normally under mild abiotic stress over a period of weeks or more. Most vegetative cells are not highly secretory, so energy, resources, and the stress-protection machinery, like the ER UPR, are not fully engaged as in the extending PT. A heat wave during vegetative growth will induce a full heat-protection response which could retard growth transiently without a substantial impact on reproduction. During reproductive growth at optimal temperature, our analyses suggest that developed pollen responds to programmed dehydration to mature, and then dry pollen recovers at rehydration to begin germination (Figure 5A). Both desiccation- and rehydration-induced responses are parts of a stress-protection strategy. First, to tolerate desiccation during pollen dispersal in a dry environment, mRNAs and proteins are protected in granules transiently (Figure 2). Second, to recover from desiccation, the granules containing nontranslating mRNP are disassembled and mRNAs are released for translation to support germination (Figure 5A). A bicellular or TCP has limited energy and resources to complete its functions in a short life span. Heat stress would induce conserved thermo-protection strategies that overwhelm the same machinery required for formation and disassembly of storage granules, and subsequent ER-mediated translation and delivery of secretory and membrane proteins (Figure 5B). The machinery required to mount desiccation tolerance in maturing pollen or the recovery at rehydration would be commandeered by any additional stress, thus heat stress would compromise male fertility. The holistic perspective based largely on A. thaliana and N. tabacum provides a useful framework to decipher the basis of crop varieties with heat-tolerant reproduction (Hedhly, 2011; Bac-Molenaar et al., 2015). An important challenge is to understand what regulates programmed dehydration and its timing, and the crosstalk between the pollen developmental program and abiotic stress signaling.
Outstanding Questions Box
What are the regulatory controls and the physiological basis of programmed dehydration that initiates pollen grain maturation?
Does programmed dehydration in maturing pollen induce similar responses as those triggered by extrinsic dehydration?
What responses are triggered by rehydration to promote recovery from desiccation at germination?
Is heat sensitivity of early pollen development largely due to disruption of a developmentally programmed ER UPR of tapetal cells?
What regulatory factors activate the ER UPR in germinating pollen in the absence of heat stress? What is the role of the ER network in the assembly and disassembly mRNP granules?
How do the stress-protective strategies essential for pollen development and function crosstalk with environmental stress cues?
Advances
The male gametophyte of flowering plants uses abiotic stress pathways to manage its normal development. Male gametophyte development and pollen function are particularly heat sensitive.
Protein translation is essential to initiate pollen germination, indicating pre-synthesized mRNA is stored in pollen grains as shown recently in tobacco.
In response to cellular stress, cytoplasmic granules composed of mRNA and proteins are formed to protect eukaryotic cells by arresting protein translation transiently.
Transcriptome profiles of developing and germinating pollen with or without heat stress from Arabidopsis thaliana reveal pollen responses to developmental, pistil and stress cues.
By integrating omics data and published studies, we propose that pollen forms mRNA-protein granules during desiccation as pollen matures and then releases mRNAs from granules for translation during hydration and germination.
As pollen depends on stress-protection strategies for its normal development and function, heat stress would compete for the molecular chaperones and ER-mediated proteostasis machinery required for desiccation tolerance and pollen function, and thus compromise male fertility.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Table S1. Genes encoding molecular chaperones are induced by heat in pollen as in leaves.
Supplemental Table S2. ER-stress response genes induced by heat in pollen are induced in PTs grown at basal temperature.
Supplemental Table S3. Genes encoding molecular chaperones are upregulated in germinating pollen and in tubes at basal temperature.
Supplemental Table S4. TFs expressed in developing and germinating pollen are also induced by other stresses.
Supplemental Table S5. Transcripts abundant in maturing pollen to PTs are largely unresponsive to heat stress.
Supplemental Table S6. Arabidopsis thaliana genes shown in Figures 3 and 4 and Supplemental Figure S1.
Supplemental Table S7. Accession no. of N. tabacum genes from Supplemental Figures S1 and S2.
Supplemental Figure S1 . LEA Transcript and protein expression infer the onset of dehydration in developing pollen of Arabidopsis and tobacco (N. tabacum).
Supplemental Figure S2 . Examples of mRNAs distributed in storage granules and in the polysome fraction in MP and PTs of tobacco.
Supplemental Figure S3 . Panther analysis shows heat-responsive genes have roles in pollen germination and tube growth at basal temperature.
Supplementary Material
Acknowledgments
We thank Warren Zhang, Alejandro Mendez, Junie Senthilkumar, Tony Pham, and Dr. Ashton T. Belew at the U. Maryland (College Park) for organizing pollen transcriptome data according to gene families. Discussions with L. Poidevin, A. Ferrando (U. Politècnica de València), E. Vierling (U. Mass), and T.J. Cooke (U. Maryland) are gratefully acknowledged.
Funding
This project was partially supported by National Science Foundation Arabidopsis 2010 IBN0209788 to H.S., National Science Foundation (NSF) Research Coordination Network grant on Integrative Pollen Biology # 0955910 to A.Y. Cheung, National Science Foundation grant IOS 1656774 to JFH, and National Science Foundation grant IOS-1939255 to M.A.J. and R.P.
Conflict of Interest Statement. None declared.
H.S. conceived the project and drafted the manuscript with contributions from all the authors. H.S. analyzed the omic data with R.P., M.A.J., and J.F.H. All authors revised the manuscript and approved the final version. H.S. agrees to serve as the author responsible for contact and ensures communication.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is Heven Sze (hsze@umd.edu).
References
- Alberti S, Hyman AA (2021) Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat Rev Mol Cell Biol 22: 196–213 [DOI] [PubMed] [Google Scholar]
- Atmodjo MA, Hao Z, Mohnen D (2013) Evolving views of pectin biosynthesis. Annu Rev Plant Biol 64: 747–779 [DOI] [PubMed] [Google Scholar]
- Bac-Molenaar JA, Fradin EF, Becker FF, Rienstra JA, van der Schoot J, Vreugdenhil D, Keurentjes JJ (2015) Genome-wide association mapping of fertility reduction upon heat stress reveals developmental stage-specific QTLs in Arabidopsis thaliana. Plant Cell 27: 1857–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai B, van der Horst S, Cordewener JHG, America T, Hanson J, Bentsink L (2020) Seed-stored mRNAs that are specifically associated to monosomes are translationally regulated during germination. Plant Physiol 182: 378–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D, Haswell ES (2020) The mechanosensitive ion channel MSL10 potentiates responses to cell swelling in Arabidopsis seedlings. Curr Biol 30: 2716–2728 e2716 [DOI] [PubMed] [Google Scholar]
- Bernal AJ, Yoo CM, Mutwil M, Jensen JK, Hou G, Blaukopf C, Sorensen I, Blancaflor EB, Scheller HV, Willats WG (2008) Functional analysis of the cellulose synthase-like genes CSLD1, CSLD2, and CSLD4 in tip-growing Arabidopsis cells. Plant Physiol 148: 1238–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock KW, Honys D, Ward JM, Padmanaban S, Nawrocki EP, Hirschi KD, Twell D, Sze H (2006) Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiol 140: 1151–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A, Lituiev DS, Nestorova A, Franck CM, Thirugnanarajah S, Grossniklaus U (2013) ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol 11: e1001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges F, Gomes G, Gardner R, Moreno N, McCormick S, Feijó JA, Becker JD (2008) Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiol 148: 1168–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg M, Brownfield L, Twell D (2009) Male gametophyte development: a molecular perspective. J Exp Bot 60: 1465–1478 [DOI] [PubMed] [Google Scholar]
- Branco-Price C, Kaiser KA, Jang CJ, Larive CK, Bailey-Serres J (2008) Selective mRNA translation coordinates energetic and metabolic adjustments to cellular oxygen deprivation and reoxygenation in Arabidopsis thaliana. Plant J 56: 743–755 [DOI] [PubMed] [Google Scholar]
- Chantarachot T, Bailey-Serres J (2018) Polysomes, stress granules, and processing bodies: a dynamic triumvirate controlling cytoplasmic mRNA fate and function. Plant Physiol 176: 254–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P, Wiese AJ, Ghatak A, Zaveska Drabkova L, Weckwerth W, Honys D (2021) Heat stress response mechanisms in pollen development. New Phytol 231: 571–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2011) THESEUS 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Curr Opin Plant Biol 14: 632–641 [DOI] [PubMed] [Google Scholar]
- De Storme N, Geelen D (2014) The impact of environmental stress on male reproductive development in plants: biological processes and molecular mechanisms. Plant Cell Environ 37: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehors J, Mareck A, Kiefer-Meyer MC, Menu-Bouaouiche L, Lehner A, Mollet JC (2019) Evolution of cell wall polymers in tip-growing land plant gametophytes: composition, distribution, functional aspects and their remodeling. Front Plant Sci 10: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Srivastava R, Howell SH (2013) Endoplasmic reticulum (ER) stress response and its physiological roles in plants. Int J Mol Sci 14: 8188–8212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Srivastava R, Quilichini TD, Dong H, Bao Y, Horner HT, Howell SH (2016) IRE1, a component of the unfolded protein response signaling pathway, protects pollen development in Arabidopsis from heat stress. Plant J 88: 193–204 [DOI] [PubMed] [Google Scholar]
- Doucet J, Lee HK, Goring DR (2016) Pollen acceptance or rejection: a tale of two pathways. Trends Plant Sci 21: 1058–1067 [DOI] [PubMed] [Google Scholar]
- Firon N, Nepi M, Pacini E (2012) Water status and associated processes mark critical stages in pollen development and functioning. Ann Bot 109: 1201–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragkostefanakis S, Mesihovic A, Hu Y, Schleiff E (2016) Unfolded protein response in pollen development and heat stress tolerance. Plant Reprod 29: 81–91 [DOI] [PubMed] [Google Scholar]
- Franchi GG, Piotto B, Nepi M, Baskin CC, Baskin JM, Pacini E (2011) Pollen and seed desiccation tolerance in relation to degree of developmental arrest, dispersal, and survival. J Exp Bot 62: 5267–5281 [DOI] [PubMed] [Google Scholar]
- Frank G, Pressman E, Ophir R, Althan L, Shaked R, Freedman M, Shen S, Firon N (2009) Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. J Exp Bot 60: 3891–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF (2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA 104: 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z, Zhao Y, Liu MC, Zhou LZ, Wang L, Zhong S, Hou S, Jiang J, Liu T, Huang Q, et al. (2019) LLG2/3 are co-receptors in BUPS/ANX-RALF signaling to regulate Arabidopsis pollen tube integrity. Curr Biol 29: 3256–3265 e3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal K, Walton LJ, Tunnacliffe A (2005) LEA proteins prevent protein aggregation due to water stress. Biochem J 388: 151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobei MA, Qeli E, Brunner E, Rehrauer H, Zhang R, Roschitzki B, Basler K, Ahrens CH, Grossniklaus U (2009) Deterministic protein inference for shotgun proteomics data provides new insights into Arabidopsis pollen development and function. Genome Res 19: 1786–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Beltran E, Moschou PN, Smertenko AP, Bozhkov PV (2015) Tudor staphylococcal nuclease links formation of stress granules and processing bodies with mRNA catabolism in Arabidopsis. Plant Cell 27: 926–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafidh S, Potesil D, Muller K, Fila J, Michailidis C, Herrmannova A, Fecikova J, Ischebeck T, Valasek LS, Zdrahal Z, et al. (2018) Dynamics of the pollen sequestrome defined by subcellular coupled omics. Plant Physiol 178: 258–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton ES, Jensen GS, Maksaev G, Katims A, Sherp AM, Haswell ES (2015) Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 350: 438–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedhly A (2011) Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ Exp Bot 74: 9–16 [Google Scholar]
- Hocq L, Pelloux J, Lefebvre V (2017) Connecting homogalacturonan-type pectin remodeling to acid growth. Trends Plant Sci 22: 20–29 [DOI] [PubMed] [Google Scholar]
- Hoffmann RD, Portes MT, Olsen LI, Damineli DSC, Hayashi M, Nunes CO, Pedersen JT, Lima PT, Campos C, Feijo JA, et al. (2020) Plasma membrane H(+)-ATPases sustain pollen tube growth and fertilization. Nat Commun 11: 2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes-Davis R, Tanaka CK, Vensel WH, Hurkman WJ, McCormick S (2005) Proteome mapping of mature pollen of Arabidopsis thaliana. Proteomics 5: 4864–4884 [DOI] [PubMed] [Google Scholar]
- Honys D, Combe JP, Twell D, Capková V (2000) The translationally repressed pollen-specific ntp303 mRNA is stored in non-polysomal mRNPs during pollen maturation. Sexual Plant Reprod 13: 135–144 [Google Scholar]
- Honys D, Renak D, Fecikova J, Jedelsky PL, Nebesarova J, Dobrev P, Capkova V (2009) Cytoskeleton-associated large RNP complexes in tobacco male gametophyte (EPPs) are associated with ribosomes and are involved in protein synthesis, processing, and localization. J Proteome Res 8: 2015–2031 [DOI] [PubMed] [Google Scholar]
- Honys D, Twell D (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 5: R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell SH (2013) Endoplasmic reticulum stress responses in plants. Annu Rev Plant Biol 64: 477–499 [DOI] [PubMed] [Google Scholar]
- Howell SH (2017) When is the unfolded protein response not the unfolded protein response? Plant Sci 260: 139–143 [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001) The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13: 2191–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Fedoroff NV, Koizumi N (2008) Arabidopsis bZIP60 is a proteolysis-activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell 20: 3107–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Wheeler JR, Walters RW, Agrawal A, Barsic A, Parker R (2016) ATPase-modulated stress granules contain a diverse proteome and substructure. Cell 164:487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, Sundaresan V, Ye D (2005) VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17: 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Harper JF, Palanivelu R (2019) A fruitful journey: pollen tube navigation from germination to fertilization. Annu Rev Plant Biol 70: 809–837 [DOI] [PubMed] [Google Scholar]
- Karkonen A, Kuchitsu K (2015) Reactive oxygen species in cell wall metabolism and development in plants. Phytochemistry 112: 22–32 [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Girke T, Bray EA, Bailey-Serres J (2004) Differential mRNA translation contributes to gene regulation under non-stress and dehydration stress conditions in Arabidopsis thaliana. Plant J 38: 823–839 [DOI] [PubMed] [Google Scholar]
- Kaya H, Nakajima R, Iwano M, Kanaoka MM, Kimura S, Takeda S, Kawarazaki T, Senzaki E, Hamamura Y, Higashiyama T, et al. (2014) Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 26: 1069–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D'Angelo C, Bornberg-Bauer E, Kudla J, Harter K (2007) The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50: 347–363 [DOI] [PubMed] [Google Scholar]
- Klepikova AV, Kasianov AS, Gerasimov ES, Logacheva MD, Penin AA (2016) A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J 88: 1058–1070 [DOI] [PubMed] [Google Scholar]
- Kosmacz M, Gorka M, Schmidt S, Luzarowski M, Moreno JC, Szlachetko J, Leniak E, Sokolowska EM, Sofroni K, Schnittger A, et al. (2019) Protein and metabolite composition of Arabidopsis stress granules. New Phytol 222: 1420–1433 [DOI] [PubMed] [Google Scholar]
- Lassig R, Gutermuth T, Bey TD, Konrad KR, Romeis T (2014) Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J 78: 94–106 [DOI] [PubMed] [Google Scholar]
- Lee JE, Cathey PI, Wu H, Parker R, Voeltz GK (2020) Endoplasmic reticulum contact sites regulate the dynamics of membraneless organelles. Science 367: eaay7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux C, Bouton S, Kiefer-Meyer MC, Fabrice TN, Mareck A, Guenin S, Fournet F, Ringli C, Pelloux J, Driouich A, et al. (2015) PECTIN METHYLESTERASE48 is involved in Arabidopsis pollen grain germination. Plant Physiol 167: 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberek K, Lewandowska A, Zietkiewicz S (2008) Chaperones in control of protein disaggregation. EMBO J 27: 328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linskens HF, Schrauwen JA, Konings RN (1970) Cell-free protein synthesis with polysomes from germinating Petunia pollen grains. Planta 90: 153–162 [DOI] [PubMed] [Google Scholar]
- Liu C, Shen L, Xiao Y, Vyshedsky D, Peng C, Sun X, Liu Z, Cheng L, Zhang H, Han Z, et al. (2021) Pollen PCP-B peptides unlock a stigma peptide-receptor kinase gating mechanism for pollination. Science 372: 171–175 [DOI] [PubMed] [Google Scholar]
- Liu JX, Howell SH (2016) Managing the protein folding demands in the endoplasmic reticulum of plants. New Phytol 211: 418–428 [DOI] [PubMed] [Google Scholar]
- Loraine AE, McCormick S, Estrada A, Patel K, Qin P (2013) RNA-seq of Arabidopsis pollen uncovers novel transcription and alternative splicing. Plant Physiol 162: 1092–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chanroj S, Zulkifli L, Johnson MA, Uozumi N, Cheung A, Sze H (2011) Pollen tubes lacking a pair of K+ transporters fail to target ovules in Arabidopsis. Plant Cell 23: 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund CH, Stenbaek A, Atmodjo MA, Rasmussen RE, Moller IE, Erstad SM, Biswal AK, Mohnen D, Mravec J, Sakuragi Y (2020) Pectin synthesis and pollen tube growth in Arabidopsis involves Three GAUT1 Golgi-anchoring proteins: GAUT5, GAUT6, and GAUT7. Front Plant Sci 11: 585774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas JP (1993) Molecular mechanisms of pollen tube growth and differentiation. Plant Cell 5: 1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnis SM, Desikan R, Hancock JT, Hiscock SJ (2006) Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: potential signalling crosstalk? New Phytol 172: 221–228 [DOI] [PubMed] [Google Scholar]
- McLoughlin F, Basha E, Fowler ME, Kim M, Bordowitz J, Katiyar-Agarwal S, Vierling E (2016) Class I and II small heat shock proteins together with HSP101 protect protein translation factors during heat stress. Plant Physiol 172: 1221–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin F, Kim M, Marshall RS, Vierstra RD, Vierling E (2019) HSP101 interacts with the proteasome and promotes the clearance of ubiquitylated protein aggregates. Plant Physiol 180: 1829–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecchia MA, Santos-Fernandez G, Duss NN, Somoza SC, Boisson-Dernier A, Gagliardini V, Martinez-Bernardini A, Fabrice TN, Ringli C, Muschietti JP, et al. (2017) RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 358: 1600–1603 [DOI] [PubMed] [Google Scholar]
- Merret R, Carpentier MC, Favory JJ, Picart C, Descombin J, Bousquet-Antonelli C, Tillard P, Lejay L, Deragon JM, Charng YY (2017) Heat shock protein HSP101 affects the release of ribosomal protein mRNAs for recovery after heat shock. Plant Physiol 174: 1216–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Ebert D, Muruganujan A, Mills C, Albou LP, Mushayamaha T, Thomas PD (2021) PANTHER version 16: a revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res 49: D394–D403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard E, Simon AA, Tavares B, Wudick MM, Feijo JA (2017) Signaling with ions: the keystone for apical cell growth and morphogenesis in pollen tubes. Plant Physiol 173: 91–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Bukau B, Kampinga HH (2018) Cellular handling of protein aggregates by disaggregation machines. Mol Cell 69: 214–226 [DOI] [PubMed] [Google Scholar]
- Mollet JC, Leroux C, Dardelle F, Lehner A (2013) Cell wall composition, biosynthesis and remodeling during pollen tube growth. Plants (Basel) 2: 107–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Hollien J (2012) The unfolded protein response in secretory cell function. Annu Rev Genet 46: 165–183 [DOI] [PubMed] [Google Scholar]
- Norambuena L, Nilo R, Handford M, Reyes F, Marchant L, Meisel L, Orellana A (2005) AtUTr2 is an Arabidopsis thaliana nucleotide sugar transporter located in the Golgi apparatus capable of transporting UDP-galactose. Planta 222: 521–529 [DOI] [PubMed] [Google Scholar]
- Nover L, Scharf KD, Neumann D (1989) Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol 9: 1298–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K (2017) Transcriptional regulatory network of plant heat stress response. Trends Plant Sci 22: 53–65 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Johnson MA (2010) Functional genomics of pollen tube-pistil interactions in Arabidopsis. Biochem Soc Trans 38: 593–597 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Preuss D (2006) Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biol 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poidevin L, Forment J, Unal D, Ferrando A (2020) Transcriptome and translatome changes in germinated pollen under heat stress uncover roles of transporter genes involved in pollen tube growth. Plant Cell Environ 44: 2167–2184 [DOI] [PubMed] [Google Scholar]
- Protter DSW, Parker R (2016) Principles and properties of stress granules. Trends Cell Biol 26: 668–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R (2009) Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet 5: e1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmati Ishka M, Brown E, Weigand C, Tillett RL, Schlauch KA, Miller G, Harper JF (2018) A comparison of heat-stress transcriptome changes between wild-type Arabidopsis pollen and a heat-sensitive mutant harboring a knockout of cyclic nucleotide-gated cation channel 16 (cngc16). BMC Genomics 19: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes F, Leon G, Donoso M, Brandizzi F, Weber AP, Orellana A (2010) The nucleotide sugar transporters AtUTr1 and AtUTr3 are required for the incorporation of UDP-glucose into the endoplasmic reticulum, are essential for pollen development and are needed for embryo sac progress in Arabidopsis thaliana. Plant J 61: 423–435 [DOI] [PubMed] [Google Scholar]
- Rieu I, Twell D, Firon N (2017) Pollen development at high temperature: from acclimation to collapse. Plant Physiol 173: 1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134: 1683–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockel N, Wolf S, Kost B, Rausch T, Greiner S (2008) Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J 53: 133–143 [DOI] [PubMed] [Google Scholar]
- Sanders PM, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler EW, Goldberg RB (2000) The arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell 12: 1041–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpin MR, Sigaut L, Temprana SG, Boccaccio GL, Pietrasanta LI, Muschietti JP (2017) Two Arabidopsis late pollen transcripts are detected in cytoplasmic granules. Plant Direct 1: e00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf KD, Berberich T, Ebersberger I, Nover L (2012) The plant heat stress transcription factor (Hsf) family: structure, function and evolution. Biochim Biophys Acta 1819: 104–119 [DOI] [PubMed] [Google Scholar]
- Schiott M, Romanowsky SM, Baekgaard L, Jakobsen MK, Palmgren MG, Harper JF (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101: 9502–9507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37: 501–506 [DOI] [PubMed] [Google Scholar]
- Senechal F, Habrylo O, Hocq L, Domon JM, Marcelo P, Lefebvre V, Pelloux J, Mercadante D (2017) Structural and dynamical characterization of the pH-dependence of the pectin methylesterase-pectin methylesterase inhibitor complex. J Biol Chem 292: 21538–21547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MB, Lohani N, Bhalla PL (2021) The role of endoplasmic reticulum stress response in pollen development and heat stress tolerance. Front Plant Sci 12: 661062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fritschi FB, Zandalinas SI, Mittler R (2021) The impact of stress combination on reproductive processes in crops. Plant Sci 311: 111007. [DOI] [PubMed] [Google Scholar]
- Sorenson R, Bailey-Serres J (2014) Selective mRNA sequestration by OLIGOURIDYLATE-BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc Natl Acad Sci USA 111: 2373–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart T, Satija R (2019) Integrative single-cell analysis. Nat Rev Genet 20: 257–272 [DOI] [PubMed] [Google Scholar]
- Sugio A, Dreos R, Aparicio F, Maule AJ (2009) The cytosolic protein response as a subcomponent of the wider heat shock response in Arabidopsis. Plant Cell 21: 642–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Rizhsky L, Liang H, Shuman J, Shulaev V, Mittler R (2005) Enhanced tolerance to environmental stress in transgenic plants expressing the transcriptional coactivator multiprotein bridging factor 1c. Plant Physiol 139: 1313–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R, Clark T, Preuss D (2005) Expression profiling of Arabidopsis stigma tissue identifies stigma-specific genes. Sexual Plant Reprod 18: 163–171 [Google Scholar]
- Swanson S, Gilroy S (2010) ROS in plant development. Physiol Plant 138: 384–392 [DOI] [PubMed] [Google Scholar]
- Swindell WR, Huebner M, Weber AP (2007) Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics 8: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H, Chanroj S (2018) Plant endomembrane dynamics: studies of K(+)/H(+) antiporters provide insights on the effects of pH and ion homeostasis. Plant Physiol 177: 875–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace EW, Kear-Scott JL, Pilipenko EV, Schwartz MH, Laskowski PR, Rojek AE, Katanski CD, Riback JA, Dion MF, Franks AM, et al. (2015) Reversible, specific, active aggregates of endogenous proteins assemble upon heat stress. Cell 162: 1286–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang W, Wang YQ, Liu YY, Wang JX, Zhang XQ, Ye D, Chen LQ (2013) Arabidopsis galacturonosyltransferase (GAUT) 13 and GAUT14 have redundant functions in pollen tube growth. Mol Plant 6: 1131–1148 [DOI] [PubMed] [Google Scholar]
- Wang W, Wang L, Chen C, Xiong G, Tan XY, Yang KZ, Wang ZC, Zhou Y, Ye D, Chen LQ (2011) Arabidopsis CSLD1 and CSLD4 are required for cellulose deposition and normal growth of pollen tubes. J Exp Bot 62: 5161–5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang WZ, Song LF, Zou JJ, Su Z, Wu WH (2008) Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiol 148: 1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Nover L, Fauth M (2008) Plant stress granules and mRNA processing bodies are distinct from heat stress granules. Plant J 56: 517–530 [DOI] [PubMed] [Google Scholar]
- Wei D, Liu M, Chen H, Zheng Y, Liu Y, Wang X, Yang S, Zhou M, Lin J (2018) INDUCER OF CBF EXPRESSION 1 is a male fertility regulator impacting anther dehydration in Arabidopsis. PLoS Genet 14: e1007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Carvalho P, Voeltz GK (2018) Here, there, and everywhere: the importance of ER membrane contact sites. Science 361: eaan5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Maruyama D, Endo T, Nishikawa S (2008) Arabidopsis thaliana has a set of J proteins in the endoplasmic reticulum that are conserved from yeast to animals and plants. Plant Cell Physiol 49: 1547–1562 [DOI] [PubMed] [Google Scholar]
- Yang KZ, Xia C, Liu XL, Dou XY, Wang W, Chen LQ, Zhang XQ, Xie LF, He L, Ma X, et al. (2009) A mutation in Thermosensitive Male Sterile 1, encoding a heat shock protein with DnaJ and PDI domains, leads to thermosensitive gametophytic male sterility in Arabidopsis. Plant J 57: 870–882 [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu W, Nagae TT, Takeuchi H, Zhang H, Han Z, Higashiyama T, Chai J (2017) Structural basis for receptor recognition of pollen tube attraction peptides. Nat Commun 8: 1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167: 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn KE, Tunc-Ozdemir M, Harper JF (2010) Temperature stress and plant sexual reproduction: uncovering the weakest links. J Exp Bot 61: 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.