Abstract
Since their first detection in 1988, penicillin-resistant Streptococcus pneumoniae isolates have rapidly spread in Iceland to account for close to 20% of all pneumococcal disease in that country by 1993. The major component (70%) of the resistant pneumococci identified from 1989 to 1992 was the progeny of a single multidrug-resistant clone (Icelandic clone) with a homogeneous chromosomal macrorestriction profile and identical multilocus enzyme type expressing serotype 6B and resistance to penicillin, tetracycline, chloramphenicol, erythromycin, and trimethoprim-sulfamethoxazole. The rest of the non-penicillin-susceptible isolates included bacteria with serotype 6A and serogroups 19 and 23. The unique geographic and epidemiological setting and the availability of a complete collection of all non-penicillin-susceptible isolates of S. pneumoniae in Iceland prompted us to carry out a molecular epidemiological study to monitor the fate of the Icelandic clone between 1989 and 1996; in addition, we wished to extend the characterization to representative groups of all non-penicillin-susceptible serotype 6B pneumococci which showed variations in antibiotype and which were recovered in Iceland between late 1989 and the end of 1996. Also included in the study were non-penicillin-susceptible isolates of serogroup 23. Pulsed-field gel electrophoresis of SmaI-restricted chromosomal DNA and Southern hybridization with the lytA DNA probe and probes specific for antibiotic resistance genes were used to characterize pneumococcal isolates. The results show that (i) the Icelandic clone remained the predominant type among penicillin-resistant S. pneumoniae through 1996; (ii) the emergence of variants of the Icelandic clone which had lost one or more of the antibiotic resistance phenotypes and/or resistant genes, singly or in combination, was documented during the surveillance period; and (iii) isolates belonging to the internationally spread multidrug-resistant serotype 23F clone were present in the Icelandic collection since late 1989 but did not increase in number during the subsequent years.
The rapid spread of penicillin-resistant and multidrug-resistant Streptococcus pneumoniae across the world has been well documented (1). The import and spread of international multidrug-resistant clones to various countries in South America (6, 7, 8, 12, 21, 28) and the United States (9) and the introduction of a single multidrug-resistant clone that was first identified in Iceland in 1989 (25) are prime examples. The ability of pneumococci to acquire resistance genes through natural transformation, even from other species, is also well known (10, 11, 16). The excessive use of antimicrobials agents applies the selective pressure that maintains strains harboring these resistance genes once introduced into communities (2, 3, 4). In the wake of this global spread, measures to reduce the use of antimicrobial agents have been cited as an important factor in fighting increasing resistance. Recent reports described the success of such measures in reducing the number of resistant strains among disease-causing isolates (23; K. G. Kristinsson, M. A. Hjalmarsdottir, and T. Gudnason, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-22, p. 74, 1998). Still, the fact remains that as long as resistant clones are present in the population, the resistance genes are available, and the threat of a resurgence of resistance through the spread of strains harboring them or horizontal transfer to previously susceptible strains is real. Actual loss of resistance through deletion or inactivation of resistance genes and subsequent stabilization of resulting susceptible clones is a process that actually removes the resistance genes from the gene pool, making them unavailable to future generations of susceptible bacteria. This process, in a natural setting, has not been observed until now, however. Information on how frequent this process is and how stable and how “successful” the resulting susceptible clones are in competition with the original multidrug-resistant clone is invaluable in assessing the effectiveness of reduced antimicrobial agent usage as a weapon in the fight against drug-resistant bacteria.
An extensive surveillance system for monitoring the antibiotic resistance of S. pneumoniae has been in place in Iceland since the early 1980s. This system has made it possible to closely monitor penicillin-resistant pneumococci since their first appearance in the country in December 1988 (15). Molecular typing established that the rapid increase in the frequency of penicillin-resistant pneumococci was mainly due to the introduction and spread of an international multidrug-resistant clone (Icelandic clone) of serotype 6B (25) that, by 1992, accounted for over 70% of all non-penicillin-susceptible isolates of S. pneumoniae in Iceland (13). The phenotype of the clone includes resistance to penicillin, tetracycline, chloramphenicol, erythromycin, and trimethoprim-sulfamethoxazole (SXT). The remaining 30% of non-penicillin-susceptible pneumococci identified in Iceland consisted primarily of isolates of serogroups 19 and 23 and serotype 6A. In addition, a number of strains of serotype 6B with antibiotic resistance patterns different from that of the imported multidrug-resistant clone have also been identified. The purpose of this study was to characterize representative groups of these isolates with molecular fingerprinting techniques in order to clarify their relationship to one another and to other genetic lineages of non-penicillin-susceptible pneumococci identified in surveillance studies in other countries.
MATERIALS AND METHODS
Geography and population.
Iceland is a small island, about 104,000 km2, in the North Atlantic ocean. Its population of only about 270,000 people makes it possible to screen all bacterial isolates from patients for antimicrobial susceptibility. The Department of Microbiology at the National University Hospital in Reykjavik, Iceland, collects, retests, and stores all isolates with reduced susceptibility.
Bacterial isolates.
All pneumococci isolated from patients in Iceland are routinely tested for susceptibility to various antimicrobial agents using the Kirby-Bauer method and National Committee for Clinical Laboratory Standards criteria (29). Resistance to penicillin is screened by disk diffusion tests with oxacillin disks. Non-oxacillin-susceptible isolates are referred to the Department of Microbiology, National University Hospital, where the susceptibility pattern is confirmed and the strains are serotyped and then stored at −70°C. In this study, isolates from the same individual were considered repeat isolates if they were identical and recovered with an interval of less than 30 days and were excluded. A complete collection of all non-penicillin-susceptible isolates of S. pneumoniae in Iceland is available for study. In addition, penicillin-susceptible isolates found to be resistant to three or more antimicrobial agents, i.e., multidrug resistant, were collected. A total of 1,311 isolates were collected from 1989 to 1996, and of those, 1,017 were of serotype 6B.
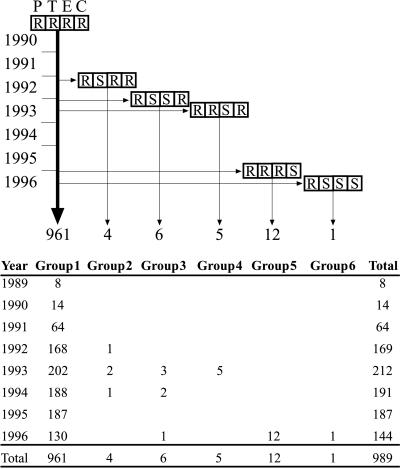
Sixty-nine isolates were selected from the collection of serotype 6B isolates (non-penicillin susceptible or multidrug resistant) for characterization by molecular fingerprinting methods. The first group of 13 isolates was picked at random from the large number of strains that exhibited the antibiotype typical of the Icelandic clone: penicillin MIC of 1.0 mg/liter and resistance to erythromycin, tetracycline, chloramphenicol, and SXT (15). Of the 1,311 non-penicillin-susceptible isolates recovered between 1989 and 1996 in Iceland, 1,017 expressed serotype 6B, and the vast majority of these (961 isolates) had the antibiotype characteristic of the Icelandic clone (see group 1 in Table 1). A group of 13 isolates was selected to confirm that these isolates also retained the characteristic molecular type of the Icelandic clone (25). The second group of isolates selected for molecular typing included all 52 serotype 6B isolates that were collected between 1990 and 1996 and that had the typical penicillin MIC of the Icelandic clone (1.0 mg/liter) but differed from the Icelandic clone in their pattern of resistance to the other antibacterial agents. The first such isolate showing an “incomplete” antibiotype of the Icelandic clone was detected in April 1992. The third group of isolates selected for molecular typing included four serotype 6B strains that were resistant to tetracycline, erythromycin, and SXT but susceptible to penicillin (see the last four strains in Table 1). Finally, as the fourth group of isolates to be typed by molecular methods, we selected all 54 non-penicillin-susceptible isolates of serogroup 23 which were recovered between 1989 and 1996 in Iceland. Ten of these showed the antibiotype characteristic of the Spanish/U.S. epidemic clone (resistance to penicillin, tetracycline, and chloramphenicol) (18, 19).
TABLE 1.
Phenotypic and genotypic characteristics of antimicrobial agent-resistant isolates of serotype 6B in Iceland
| Isolate | Patient
|
Isolate
|
Penicillin MIC (mg/liter) | Resistant pattern fore:
|
SmaI pattern | Group | Size (kb) of positive bands after Southern blotting with the following probef:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | Underlying diseasea | Placeb | Yr | Monc | Sourced | Pen | Tet | Erm | Cam | lytA | tetM | ermB | ||||
| 176 | 3 | NA | EF | 1993 | JUL | NA | 1 | R | R | R | R | A1 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 186 | 33 | PN | HF | 1993 | OCT | SP | 1 | R | R | R | R | A1 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 190 | <1 | OM | RE | 1993 | NOV | ME | 1 | R | R | R | R | A1 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 458 | <1 | OM | EF | 1996 | JAN | NA | 0.75 | I | R | R | R | A1 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 459 | 1 | SI | SO | 1996 | FEB | NP | 1 | R | R | R | R | A1 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 460 | 1 | SU | RE | 1996 | MAR | NP | 1 | R | R | R | R | A1 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 461 | <1 | NA | RE | 1996 | APR | NP | 1.5 | R | R | R | R | A1 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 462 | 1 | SK | RE | 1996 | MAY | NP | 1.5 | R | R | R | R | A1 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 463 | 1 | BR | SN | 1996 | JUN | NP | 1.5 | R | R | R | R | A1 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 464 | 2 | OM | RE | 1996 | JUL | NP | 1.5 | R | R | R | R | A1 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 465 | 1 | OM | EF | 1996 | JUL | ME | 0.75 | I | R | R | R | A1 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 467 | 1 | OM | RE | 1996 | SEP | NP | 1 | R | R | R | R | A1 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 207 | 1 | PH | NA | 1994 | MAR | NP | 2 | R | R | R | R | A4 | 1 | 341, 85, 80, 55 | 380 | 380 |
| 141 | 2 | OM | NA | 1992 | APR | NP | 1 | R | S | R | R | A1 | 2 | 341, 85, 80, 55 | 380 | 380 |
| 187 | 2 | FE | EF | 1993 | OCT | NP | 1 | R | S | R | R | A1 | 2 | 341, 85, 80, 55 | 380 | 380 |
| 192 | 1 | OM | EF | 1993 | NOV | ME | 2 | R | S | R | R | A1 | 2 | 341, 85, 80, 55 | 380 | 380 |
| 213 | 2 | NA | EF | 1994 | MAY | NP | 1 | R | S | R | R | A1 | 2 | 341, 85, 80, 55 | 380 | 380 |
| 170 | 1 | OM | RE | 1993 | APR | ME | 1 | R | R | S | R | A1 | 4 | 341, 85, 80, 55 | 380 | 380 |
| 178 | 1 | OM | RE | 1993 | JUL | ME | 1 | R | R | S | R | A1 | 4 | 341, 85, 80, 55 | 380 | 380 |
| 179 | 1 | OM | RE | 1993 | AUG | ME | 1 | R | R | S | R | A1 | 4 | 341, 85, 80, 55 | 380 | 380 |
| 195 | <1 | UR | RE | 1993 | DEC | NP | 2 | R | R | S | R | A1 | 4 | 341, 85, 80, 55 | 380 | 380 |
| 197 | 2 | OM | RE | 1993 | DEC | ME | 1 | R | R | S | R | A1 | 4 | 341, 85, 80, 55 | 380 | 380 |
| 437 | <1 | OM | HF | 1996 | MAY | ME | 1.5 | R | I | I | S | A1 | 5 | 341, 85, 80, 55 | 380 | 380 |
| 418 | 7 | SU | RE | 1996 | JAN | NS | 0.25 | I | R | R | S | A1 | 5 | 341, 85, 80, 55 | 380 | 380 |
| 419 | 1 | SU | RE | 1996 | FEB | ME | 1.5 | R | R | R | S | A1 | 5 | 341, 85, 80, 55 | 380 | 380 |
| 420 | 1 | OM | RE | 1996 | FEB | ME | 1 | R | R | R | S | A1 | 5 | 341, 85, 80, 55 | 380 | 380 |
| 422 | 1 | OM | RE | 1996 | MAR | NP | 0.5 | I | R | R | S | A1 | 5 | 341, 85, 80, 55 | 380 | 380 |
| 425 | 1 | OM | RE | 1996 | MAR | ME | 1.5 | R | R | R | S | A1 | 5 | 341, 85, 80, 55 | 380 | 380 |
| 426 | 1 | ES | RE | 1996 | MAR | ME | 1 | R | R | R | S | A1 | 5 | 341, 85, 80, 55 | 380 | 380 |
| 427 | 1 | SU | RE | 1996 | MAR | ME | 0.75 | I | R | R | S | A1 | 5 | 341, 85, 80, 55 | 380 | 380 |
| 429 | 3 | SI | HF | 1996 | MAR | NP | 1 | R | R | R | S | A1 | 5 | 341, 85, 80, 55 | 380 | 380 |
| 438 | <1 | CS | RE | 1996 | MAY | NP | 1.5 | R | R | R | S | A1 | 5 | 341, 85, 80, 55 | 380 | 380 |
| 441 | 1 | OM | HF | 1996 | JUL | NP | 1 | R | R | R | S | A1 | 5 | 341, 85, 80, 55 | 380 | 380 |
| 444 | 78 | CS | RE | 1996 | SEPT | SP | 1.5 | R | R | R | S | A1 | 5 | 341, 85, 80, 55 | 380 | 380 |
| 432 | 1 | RH | RE | 1996 | APR | ME | 1 | R | S | S | S | A1 | 6 | 341, 85, 80, 55 | 380 | 380 |
| 164 | <1 | OM | RE | 1993 | FEB | NP | 1 | R | S | S | R | A2 | 3 | 341, 85, 80, 55 | NS | NS |
| 166 | 2 | SI | RE | 1993 | FEB | NP | 1 | R | S | S | R | A2 | 3 | 341, 85, 80, 55 | NS | NS |
| 169 | 1 | OM | RE | 1993 | MAR | NP | 1 | R | S | S | R | A2 | 3 | 341, 85, 80, 55 | NS | NS |
| 210 | 3 | SI | RE | 1994 | MAR | NP | 1 | R | S | S | R | A2 | 3 | 341, 85, 80, 55 | NS | NS |
| 434 | 1 | NA | RE | 1996 | MAY | NP | 1 | R | S | S | R | A2 | 3 | 341, 85, 80, 55 | NS | NS |
| 203 | 3 | PT | RE | 1994 | JAN | PG | 1 | R | S | S | R | A3 | 3 | 370, 85, 80, 55 | NS | NS |
| 120 | 1 | OM | RE | 1990 | DEC | ME | 0.25 | I | S | S | S | B1 | 7 | 90, 60 | NT | NT |
| 121 | 1 | UR | RE | 1990 | DEC | NP | 0.25 | I | S | S | S | B1 | 7 | 90, 60 | NT | NT |
| 122 | 40 | SI | RE | 1990 | DEC | NP | 0.125 | I | S | S | S | B1 | 7 | 90, 60 | NT | NT |
| 172 | 62 | CS | NA | 1993 | MAY | SP | 0.25 | I | S | S | S | B1 | 7 | 90, 60 | NT | NT |
| 206 | 1 | OM | RE | 1994 | MAR | ME | 0.25 | I | S | S | S | B1 | 7 | 90, 60 | NT | NT |
| 223 | 3 | PN | RE | 1994 | SEP | NP | 0.125 | I | S | S | S | B1 | 7 | 90, 60 | NT | NT |
| 148 | 30 | CS | NA | 1992 | OCT | SP | 0.25 | I | S | S | S | B2 | 7 | 125, 60 | NT | NT |
| 220 | 2 | SI | RE | 1994 | AUG | NP | 0.25 | I | S | S | S | B2 | 7 | 125, 60 | NT | NT |
| 229 | 2 | OM | NA | 1994 | DEC | ME | 0.125 | I | S | S | S | B2 | 7 | 125, 60 | NT | NT |
| 157 | 2 | OM | RE | 1992 | NOV | NP | 0.25 | I | S | S | S | B3 | 7 | 125, 90, 80, 60 | NT | NT |
| 158 | 2 | OM | RE | 1992 | DEC | NP | 0.125 | I | S | S | S | B3 | 7 | 125, 90, 80, 60 | NT | NT |
| 174 | 3 | CS | RE | 1993 | JUN | NP | 0.25 | I | S | S | S | B3 | 7 | 125, 90, 80, 60 | NT | NT |
| 194 | <1 | SE | US | 1993 | DEC | BL | 0.25 | I | S | S | S | B4 | 7 | 290, 90 | NT | NT |
| 139 | <1 | OM | RE | 1992 | FEB | ME | 1 | R | R | S | R | C1 | 7 | 341, 125, 55 | NT | NT |
| 145 | 2 | OM | NA | 1992 | MAY | ME | 1 | R | R | S | R | C1 | 7 | 341, 125, 55 | NT | NT |
| 100 | <1 | CO | RE | 1993 | MAY | EY | 2 | R | R | S | R | C1 | 7 | 341, 125, 55 | NT | NT |
| 175 | <1 | NA | NA | 1993 | JUN | NP | 1 | R | R | S | R | C1 | 7 | 341, 125, 55 | NT | NT |
| 102 | 1 | SI | NA | 1993 | DEC | NP | 1 | R | R | S | R | C1 | 7 | 341, 125, 55 | NT | NT |
| 104 | <1 | OM | NA | 1989 | APR | ME | 2 | R | R | S | R | C2 | 7 | 341, 125, 65, 55 | NT | NT |
| 116 | 3 | NA | AF | 1990 | JUN | NP | 1 | R | R | S | R | C3 | 7 | 341, 90, 85, 55 | NT | NT |
| 161 | <1 | OM | RE | 1992 | DEC | NP | 1 | R | R | S | S | C3 | 7 | 341, 90, 85, 55 | NT | NT |
| 132 | 1 | OM | NA | 1991 | DEC | ME | 0.5 | I | S | R | R | C4 | 7 | 341, 140, 125, 55, 50 | NT | NT |
| 165 | 75 | CS | SN | 1993 | FEB | SP | 0.5 | I | R | S | S | E1 | 7 | 295, 90, 55 | NT | NT |
| 133 | 94 | CS/LE | WE | 1992 | JAN | SP | 0.125 | I | R | R | S | R1 | 7 | 80, 30 | NT | NT |
| 183 | 1 | RH | NA | 1993 | SEP | NP | 0.015 | S | R | R | S | F1 | 7 | 290, 90, 50 | NT | NT |
| 208 | 1 | OM | RE | 1994 | MAR | ME | 0.015 | S | R | R | S | F1 | 7 | 290, 90, 50 | NT | NT |
| 214 | 1 | OM | RE | 1994 | MAY | ME | 0.03 | S | R | R | S | F1 | 7 | 290, 90, 50 | NT | NT |
| 202 | NA | NA | NA | 1994 | JAN | SU | 0.015 | S | R | R | S | G1 | 7 | 290, 90, 55 | NT | NT |
BL, blood; EY, eye; ME, middle ear; NP, nasopharynx; NS, nose secretion; PG, parotid gland; SP, sputum; NA, data not available.
AF, Austfirdir area, EF, Eyjafjordur area; HF, Hafnarfjordur; RE, Reykjavik area; SN, Sudurnes; SO, southern Iceland; US, U.S. Naval Hospital; WE, western Iceland; NA, data not available.
JAN, January; FEB, February; MAR, March; APR, April; MAY, May; JUN, June; JUL, July; AUG, August; SEP, September; OCT, October; NOV, November; DEC, December.
BR, bronchitis; CO, conjunctivitis; CS, cough or lung secretion; ES, ear secretion; FE, fever; LE leukemia; OM, otitis media; PH, pharyngitis; PN, pneumonia; PT, parotitis; RH, rhinitis; SE, sepsis; SI, sinusitis; SK, skin infections; SU, surveillance; UR, upper-respiratory tract infection; NA, data not available.
Pen, penicillin; Tet, tetracycline; Erm, erythromycin; Cam, chloramphenicol. R, resistant; I, intermediate; S, susceptible.
NS, no signal; NT, not tested.
PFGE.
Preparation of chromosomal DNA and pulsed-field gel electrophoresis (PFGE) were performed as previously described (25).
Analysis of PFGE patterns.
Analysis of PFGE patterns was done by visual inspection of photographs of ethidium bromide-stained gels. Isolates sharing identical PFGE patterns were considered to belong to the same PFGE type (clone) and were identified by an arbitrarily assigned uppercase letter. Isolates that differed from such a type in up to three bands were considered to be subtype variants and were identified by numerical subscripts to the same uppercase letter (27).
DNA hybridization.
The restriction fragments from the PFGE analysis were transferred to nylon membranes and probed using the ECL system (Amersham). Probes were labeled according to the manufacturer's guidelines and hybridized to the membrane-bound DNA, and chemiluminescence was detected by exposure on film. Probes for the tetracycline resistance gene tetM and the erythromycin resistance gene ermB were generated as described by Marchese et al. (17). A DNA probe for the lytA gene, recently identified as a useful epidemiological marker (20), was also used to further confirm strain identity.
RESULTS
A total of 69 serotype 6B S. pneumoniae isolates recovered from clinical samples in Iceland between 1989 and 1996 were analyzed by microbiological and molecular techniques (Table 1). Classifying isolates only by phenotype (i.e., serotype and antibiotype) proved inaccurate, since some bacteria sharing common serotype and susceptibility patterns were genetically heterogeneous, as indicated by their completely different PFGE patterns. On the other hand, genotyping by PFGE combined with the results of DNA hybridization, serotyping, and antibiotic susceptibility pattern testing provided a powerful method for tracking changes in individual clones.
Evolution of the S. pneumoniae Icelandic clone recovered from patients between 1989 and 1996.
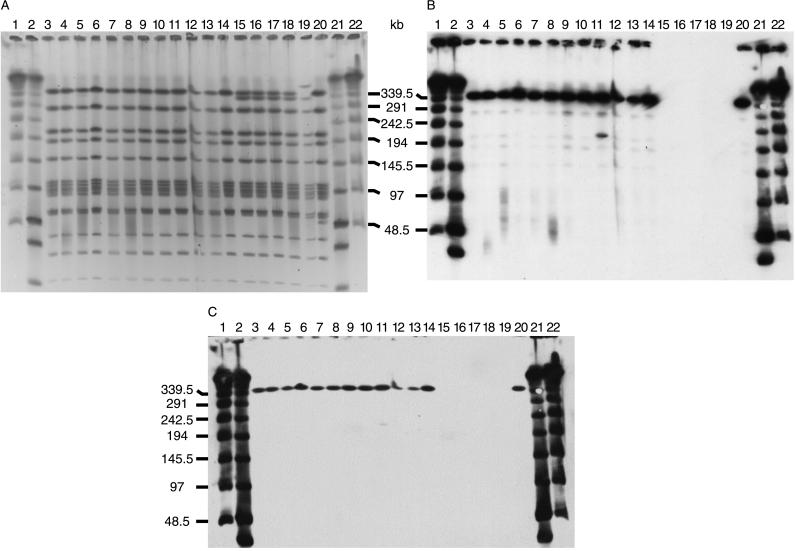
Among the 69 serotype 6B isolates examined, a total of seven PFGE types were identified on the basis of SmaI restriction patterns (see patterns A, B, C, E, F, G, and R in Table 1). The largest group, composed of 41 isolates, shared the common PFGE type A or its subtype variants characteristic of the original multidrug-resistant Icelandic clone. These isolates also showed a unique set of four lytA-hybridizing bands of 341, 85, 80, and 55 kb (Fig. 1A and Table 1), except for isolate 203, where a single band difference which resulted in a shift of the largest lytA-positive fragment to 370 kb was seen.
FIG. 1.
PFGE (A) and Southern blotting (B and C) of isolates characteristic of the Spanish/Icelandic clone. SmaI digests were separated by PFGE and subsequently transferred to nylon membranes for hybridization. (A) Lanes 3 through 14 show the typical PFGE pattern of the Spanish/Icelandic clone (pattern A1). The two largest fragments cannot be separated under these conditions. Lanes 15 through 18 show isolates with a deletion in one of the two largest fragments which, subsequently, show up in the PFGE pattern (pattern A2). Lanes 19 and 20 show patterns A3 and A4, respectively. Lanes 1, 2, 21, and 22 are molecular size markers. (B) Southern blot of the gel in panel A with a tetM probe. Isolates with PFGE patterns A2 and A3 show no signal with the probe. (C) a Southern blot of the gel in panel A with an ermB probe. As in panel B, isolates with PFGE patterns A2 and A3 show no signal with the probe.
These 41 S. pneumoniae isolates could be further resolved into six groups, (summarized in Table 1 and Fig. 2) by adding to the characterization the variations in antibiotype and reactivity with the tetM and ermB probes.
FIG. 2.
Evolution of the Spanish/Icelandic clone in Iceland. The majority of the strains belonging to the Spanish/Icelandic clone appear unchanged and are represented by the bold vertical arrow. The appearance of variants that have lost the resistance phenotype is represented by the horizontal arrows, and the vertical arrows demonstrate how many isolates of each variant have been detected (from 1989 through September 1996) and to which group they belong. The boxes represent (from left to right) resistance (R) or susceptibility (S) to penicillin (P), tetracycline (T), erythromycin (E), and chloramphenicol (C).
(i) Group 1.
Analysis with the molecular typing methods confirmed that the 13 serotype 6B isolates chosen from the 1993, 1994, and 1996 collection and expressing resistance to penicillin, tetracycline, erythromycin, chloramphenicol, and SXT were indeed representatives of the original Icelandic clone already identified as the dominant multidrug-resistant S. pneumoniae clone in Iceland between 1990 and 1993 (13, 25). These isolates continued to be recovered from various parts of the country.
(ii) Group 2.
Four isolates recovered between 1992 and 1994 (the first isolates obtained in April 1992) had lost resistance to tetracycline. These isolates were identical to the original Icelandic clone in PFGE type and lytA, ermB, and tetM hybridization pattern but tested phenotypically susceptible to tetracycline, in spite of the strong hybridization signal they gave with the tetM DNA probe. All four isolates were recovered in northeastern Iceland.
(iii) Group 3.
Six isolates, recovered in 1993, 1994, and 1996, were susceptible to both tetracycline and erythromycin and showed no hybridization signal when tested with the tetM and ermB probes (Fig. 1B and C). These six isolates had a deletion involving the loss of 25 to 30 kb of material from the largest SmaI fragment (Fig. 1A). All six isolates were recovered in Reykjavik.
(iv) Group 4.
Five isolates recovered in 1993 with SmaI and lytA patterns identical to those of the original Icelandic clone appeared to have lost phenotypic resistance to erythromycin in spite of the fact that they gave a strong signal with the ermB probe. All five patients lived within 1.5 km of each other in downtown Reykjavik, including two sisters residing at the same address and two other girls living across the street from each other.
(v) Group 5.
A cluster of 12 isolates recovered in 1996 had the same SmaI and lytA patterns as the imported Icelandic clone but were susceptible to chloramphenicol. It remains to be determined if their susceptibility is due to the inactivation or loss of the cat (chloramphenicol acetyltransferase) gene. These isolates were all recovered in Reykjavik or surrounding towns.
(vi) Group 6.
A single isolate recovered in 1996 was found to have “lost” resistance to tetracycline, erythromycin, and chloramphenicol. However, it retained the original PFGE pattern (A1), and Southern blots showed positive signals for all three resistance genes. This isolate was recovered in Reykjavik.
Recovery of new clonal types of serotype 6B S. pneumoniae with reduced susceptibility to penicillin from clinical specimens in Iceland between 1989 and 1996.
Of the 69 serotype 6B S. pneumoniae isolates with reduced penicillin susceptibility analyzed, 41 isolates were relatives of the original Icelandic clone, while the remaining 28 isolates (see group 7 in Table 1) were represented by pneumococci with six distinct PFGE types (B, C, E, F, G, and R). Bacteria with PFGE type B were intermediately resistant to penicillin and susceptible to the other drugs and were recovered from 13 patients. This group was further divided into four subtypes based on single band differences in PFGE patterns. Of particular epidemiological interest are the three isolates in subgroup B3 and the single isolate in subgroup B4. Strains in subgroup B3 were isolated in late 1992 through the middle of 1993. Two were isolated from the same child residing at the University of Iceland student residences. The first of these was isolated in December 1992, and the second was isolated in June 1993. The third isolate was recovered from another child residing in the student residences at the same time as the first child. This finding indicates the presence of this particular clone at the university student day-care center over a period of at least 7 months. The single strain of subgroup B4 was isolated at the U.S. Naval Hospital on the Keflavik Navy Base, and although this subgroup is closely related to the group B isolates from Icelandic patients, it has not been identified outside the base.
For the nine isolates belonging to PFGE type C, the penicillin MICs ranged from 0.5 to 2.0 mg/liter, and the isolates were resistant to tetracycline, chloramphenicol, and SXT.
The remaining six isolates were represented by four different PFGE patterns (E, F, G, and R). The penicillin MICs for these isolates ranged from susceptible to intermediately resistant values; all of the isolates were resistant to tetracycline, and all but one were resistant to erythromycin as well.
Multidrug-resistant serotype 23F Spanish/U.S. clone identified in Iceland.
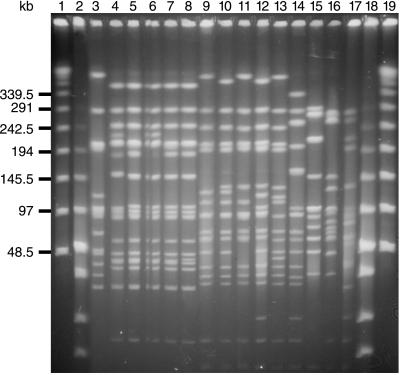
PFGE analysis of the 54 serogroup 23 isolates that were detected in Iceland between late 1989 and the end of 1996 showed that 10 of these, expressing serotype 23F, had PFGE profiles and lytA hybridization patterns identical to those of the international Spanish/U.S. clone (Fig. 3). The isolates were resistant to penicillin, tetracycline, and chloramphenicol and were recovered during the entire period, between late 1989 and the end of 1996, in the Reykjavik area; the first isolate was identified in November 1989, i.e., the same year when the multidrug-resistant serotype 6B clone was first detected in Iceland.
FIG. 3.
PFGE of isolates of serotype 23F. Lane 4 shows the pattern of a control strain of the Spanish/Cleveland clone of serotype 23F. Lanes 5 through 8 are Icelandic isolates of serotype 23F, identical to the Spanish/Cleveland clone. Lane 3 and lanes 9 through 13 show the dominant PFGE patterns of serotype 23F isolates in Iceland. Lanes 14 through 17 show the patterns of other isolates of serotype 23F in Iceland. Lanes 1, 2, 18, and 19 are molecular size markers.
DISCUSSION
Many factors combine to make Iceland a unique location for studying the evolution and spread of antibiotic resistance and resistant clones. Being an island in the North Atlantic, the country is geographically isolated. A high standard of living, an excellent health care system, and active surveillance systems make it possible to keep track of changes in frequencies and levels of antibiotic resistance in bacteria. A population of less than 300,000 people results in manageable collections of isolates, and the application of molecular and microbiological typing techniques, such as the ones used in this communication, provides a method for tracking the evolution and diversification of individual clones.
Between 1989 and 1996, 1,311 non-penicillin-susceptible and/or multidrug-resistant pneumococci were recovered. Of these, 1,017 were serotype 6B, and the great majority (961, or 94%) shared the typical resistance pattern of the multidrug-resistant Icelandic clone. A group of these isolates, picked randomly from 1993, 1994, and 1996, indeed shares the molecular characteristics of the Icelandic clone. This finding demonstrates that the multidrug-resistant Icelandic clone originally imported into Iceland in 1989 has remained the dominant drug-resistant clone throughout the 1990s. Following the rapid expansion of this clone in the early 1990s, non-penicillin-susceptible pneumococci with serotype 6A and serogroups 19 and 23 were also detected in Iceland. These pneumococci had PFGE patterns different from that of the dominant 6B clone (unpublished data), thus ruling out capsular transformation of the multidrug-resistant 6B clone.
Perhaps the most interesting observation registered by Icelandic surveillance is the emergence of variants of the Icelandic clone in which one or more of the antibiotic resistance phenotypes have been lost. Such isolates were first detected in 1992, and between that year and 1996 a total of 28 serotype 6B isolates of S. pneumoniae clearly belonging to the Icelandic clone on the basis of their chromosomal PFGE and lytA patterns showed differences in antibiotype from the Icelandic clone. Among these, four isolates became phenotypically tetracycline susceptible without losing reactivity to the tetM gene, as indicated by the strong hybridization signal. Another group of six isolates became susceptible to both tetracycline and erythromycin. They no longer hybridized with the ermB and tetM probes; this result appeared to be related to a deletion of 25 to 30 kb from the SmaI fragment characteristically hybridizing with the ermB and tetM DNA probes in the case of the original Icelandic clone. The size of the deletion indicates the possible loss of a transposon carrying the resistant determinants (10), and these isolates are currently under further investigation. Five isolates became phenotypically susceptible to erythromycin but still gave a strong signal with the ermB probe, a cluster of 12 isolates became susceptible to chloramphenicol, and 1 isolate lost resistance to tetracycline, chloramphenicol, and erythromycin. The mechanisms of loss and/or inactivation of resistance genes observed in these isolates are not yet understood.
One may consider these antibiotype variants of the Icelandic clone as products of an evolutionary process that has taken place in the in vivo environment of S. pneumoniae since the introduction of the multidrug-resistant clone to Iceland. The epidemiological features of these variants indicate that the loss of the antibiotic resistance phenotype did not interfere with the capacity of the strains to survive and even undergo a limited degree of spread, suggesting that these strains have retained at least some degree of competitiveness in spite of the loss of one or more antibiotic resistance traits. For instance, the six tetracycline- and erythromycin-susceptible isolates with the deleted ermB and tetM genes were isolated over a period of 4 years. Infection of several patients and/or carriers over intervals of several years was also documented for the four tetracycline-susceptible strains carrying the inactivated tetM gene. The five erythromycin-susceptible isolates with the inactivated ermB gene were also recovered from a number of patients over more than 6 months. The emergence and modest but significant spread of these variants of the Icelandic clone with reduced antibiotic resistance profiles may be a reflection of the reduction in antimicrobial agent consumption that was initiated by the publicity urging physicians and the general public to use antimicrobial agents prudently (14). The first isolate with such a reduced antibiotype (tetracycline susceptibility) was detected in April 1992, i.e., shortly after the campaign for a reduction in antibiotic prescriptions began. Methods to eradicate or silence antibiotic resistance genes are not currently available (22), but an environment with reduced amounts of antimicrobial agents should allow the survival of strains in which one or more of the antibiotic resistance genes are lost or inactivated through a spontaneous evolutionary process. The emergence of such less resistant variants among members of a multidrug-resistant clone may be an unexpected benefit of more prudent antimicrobial agent use.
The success of the Icelandic clone is particularly impressive considering that another multidrug-resistant S. pneumoniae clone, the serotype 23F Spanish/U.S. clone, which was detected in Iceland as early as 1989, has failed to spread, even though a few strains have continued to be recovered in each of the subsequent years (Table 2). Reasons for the failure of the Spanish/U.S. clone to expand in Iceland is surprising, since this clone represents the single most “successful” pneumococcal clone in terms of its geographic spread and high degree of representation among clinical isolates in many other countries (5, 9, 17, 24, 26, 28). The factors determining the success of particular clones could relate to particular antimicrobial drug usage patterns in the community or the relative immunity of the population to the various surface antigens of individual clones.
TABLE 2.
Phenotypic and genotypic characteristics of antimicrobial agent-resistant isolates of serotype 23F in Iceland
| Isolate | Patient
|
Isolate
|
Penicillin MIC (mg/liter) | Resistance pattern fore:
|
SmaI pattern | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | Underlying diseasea | Placeb | Yr | Moc | Sourced | Pen | Tet | Erm | Cam | |||
| 107 | <1 | OM | RE | 1989 | NOV | NP | 2 | R | R | S | R | I |
| 118 | 1 | UR | RE | 1990 | NOV | NP | 1 | R | R | S | R | I |
| 124 | 58 | PN | RE | 1991 | FEB | NA | 2 | R | R | S | R | I |
| 126 | 4 | UR | NA | 1991 | JUL | NP | 1 | R | R | S | R | I |
| 130 | <1 | UR | NA | 1991 | NOV | NP | 1 | R | R | S | R | I |
| 155 | 5 | BR | RE | 1992 | OCT | TS | 1 | R | I | S | R | I |
| 177 | 59 | CS | RE | 1993 | JUL | SP | 1 | R | I | S | R | I |
| 405 | 1 | SI | RE | 1996 | MAR | ME | 1 | R | R | S | R | I |
| 413 | 76 | CS | RE | 1996 | JUN | SP | 1 | R | R | S | R | I |
| 415 | 67 | PN | NA | 1996 | AUG | SP | 1 | R | R | R | R | I |
BR, bronchitis; CS, cough or lung secretion; OM, otitis media; PN, pneumonia; SI, sinusitis; UR, upper-respiratory-tract infection.
RE, Reykjavik area; NA, data not available.
See Table 1, footnote c.
ME, middle ear; NP, nasopharynx; SP, sputum; TS, throat swab; NA, data not available.
See Table 1, footnote e.
Despite the appearance of new variants, the Icelandic clone has remained remarkably stable during the 7 years of study. Its continued superiority over other resistant clones may provide clues as to what factors are important for the epidemicity of a pneumococcal genetic lineage.
ACKNOWLEDGMENTS
Molecular characterization of the S. pneumoniae isolates was performed at The Rockefeller University, supported by grants from the National Institutes of Health (grant NIH RO1 AI37275) and the Lounsbery Foundation. K.G.K. and S.E.V. received grants from the Icelandic Research Council, The Research Fund of the University of Iceland, and the Scandinavian Society for Antimicrobial Chemotherapy.
REFERENCES
- 1.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Arason V A, Kristinsson K G, Sigurdsson J A, Stefansdottir G, Molstad S, Gudmundsson S. Do antimicrobials increase the carriage rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. Br Med J. 1996;313:387–391. doi: 10.1136/bmj.313.7054.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold K E, Leggiadro R J, Breiman R F, Lipman H B, Schwartz B, Appleton M A, Cleveland K O, Szeto H C, Hill B C, Tenover F C, Elliott J A, Facklam R R. Risk factors for carriage of drug-resistant Streptococcus pneumoniae among children in Memphis, Tennessee. J Pediatr. 1996;128:757–764. doi: 10.1016/s0022-3476(96)70326-8. [DOI] [PubMed] [Google Scholar]
- 4.Austin D J, Kristinsson K G, Anderson R M. The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci USA. 1999;96:1152–1156. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baquero F, Martinez-Beltran J, Loza E. A review of antibiotic resistance patterns of Streptococcus pneumoniae in Europe. J Antimicrob Chemother. 1991;28(Suppl. C):31–38. doi: 10.1093/jac/28.suppl_c.31. [DOI] [PubMed] [Google Scholar]
- 6.Brandileone M C, Di Fabio J L, Vieira V S D, Zanella R C, Casagrande S T, Pignatari A C, Tomasz A. Geographic distribution of penicillin resistance of Streptococcus pneumoniae in Brazil: genetic relatedness. Microb Drug Resist. 1998;4:209–217. doi: 10.1089/mdr.1998.4.209. [DOI] [PubMed] [Google Scholar]
- 7.Camou T, Hortal M, Tomasz A. The apparent importation of penicillin-resistant capsular type 14 Spanish/French clone of Streptococcus pneumoniae into Uruguay in the early 1990s. Microb Drug Resist. 1998;4:219–224. doi: 10.1089/mdr.1998.4.219. [DOI] [PubMed] [Google Scholar]
- 8.Castañeda E, Peñuela I, Vela M C, Tomasz A The Colombian Pneumococcal Study Group. Penicillin-resistant Streptococcus pneumoniae in Colombia: presence of international epidemic clones. Microb Drug Resist. 1998;4:233–239. doi: 10.1089/mdr.1998.4.233. [DOI] [PubMed] [Google Scholar]
- 9.Corso A, Severina E P, Petruk V F, Mauriz Y R, Tomasz A. Molecular characterization of penicillin resistant Streptococcus pneumoniae isolates causing respiratory disease in the United States. Microb Drug Resist. 1998;4:325–337. doi: 10.1089/mdr.1998.4.325. [DOI] [PubMed] [Google Scholar]
- 10.Courvalin P, Carlier C. Transposable multiple antibiotic resistance in Streptococcus pneumoniae. Mol Gen Genet. 1986;205:291–297. doi: 10.1007/BF00430441. [DOI] [PubMed] [Google Scholar]
- 11.Dowson C G, Hutchison A, Brannigan J A, George R C, Hansman D, Linares J, Tomasz A, Maynard Smith J, Spratt B G. Horizontal transfer of penicillin-binding protein genes in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1989;86:8842–8846. doi: 10.1073/pnas.86.22.8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Echániz-Aviles G, Velázquez-Meza M E, Carnalla-Barajas M N, Soto-Noguerón A, di Fabio J L, Solórzano-Santos F, Jiménez-Tapia Y, Tomasz A. Predominance of the multiresistant 23F international clone of Streptococcus pneumoniae among isolates from Mexico. Microb Drug Resist. 1998;4:241–246. doi: 10.1089/mdr.1998.4.241. [DOI] [PubMed] [Google Scholar]
- 13.Kristinsson K G. Epidemiology of penicillin resistant pneumococci in Iceland. Microb Drug Resist. 1995;1:121–125. doi: 10.1089/mdr.1995.1.121. [DOI] [PubMed] [Google Scholar]
- 14.Kristinsson K G. Effect of antimicrobial use and other risk factors on antimicrobial resistance in pneumococci. Microb Drug Resist. 1997;3:117–123. doi: 10.1089/mdr.1997.3.117. [DOI] [PubMed] [Google Scholar]
- 15.Kristinsson K G, Hjalmarsdottir M A, Steingrimsson O. Increasing penicillin resistance in pneumococci in Iceland. Lancet. 1992;339:1606–1607. doi: 10.1016/0140-6736(92)91868-9. [DOI] [PubMed] [Google Scholar]
- 16.Laible G, Spratt B G, Hakenbeck R. Interspecies recombinational events during the evolution of altered PBP2X genes in penicillin-resistant clinical isolates of S. pneumoniae. Mol Microbiol. 1991;5:1993–2002. doi: 10.1111/j.1365-2958.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 17.Marchese A, Ramirez M, Schito G C, Tomasz A. Molecular epidemiology of penicillin-resistant Streptococcus pneumoniae isolates recovered in Italy from 1993 to 1996. J Clin Microbiol. 1998;36:2944–2949. doi: 10.1128/jcm.36.10.2944-2949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muñoz R, Musser J M, Crain M, Briles D E, Marton A, Parkinson A J, Sorensen U, Tomasz A. Geographic distribution of penicillin resistant clones of Streptococcus pneumoniae: characterization by penicillin binding protein profile, surface protein A typing and multilocus enzyme analysis. Clin Infect Dis. 1992;15:112–118. doi: 10.1093/clinids/15.1.112. [DOI] [PubMed] [Google Scholar]
- 19.Muñoz R, Coffey T J, Daniels M, Dowson C G, Laible G, Casal J, Hakenbeck R, Jacobs M, Musser J M, Spratt B G, Tomasz A. Intercontinental spread of a multiresistant clone of serotype 23F Streptococcus pneumoniae. J Infect Dis. 1991;164:302–306. doi: 10.1093/infdis/164.2.302. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez M, Severina E, Tomasz A. A high incidence of prophage carriage among natural isolates of Streptococcus pneumoniae. J Bacteriol. 1999;181:3618–3625. doi: 10.1128/jb.181.12.3618-3625.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi A, Corso A, Pace J, Regueira M, Tomasz A. Penicillin resistant Streptococcus pneumoniae in Argentina: frequent occurrence of an internationally spread serotype 14 clone. Microb Drug Resist. 1998;4:225–231. doi: 10.1089/mdr.1998.4.225. [DOI] [PubMed] [Google Scholar]
- 22.Salyers A A, Amabile-Cuevas C F. Why are antibiotic resistance genes so resistant to elimination? Antimicrob Agents Chemother. 1997;41:2321–2325. doi: 10.1128/aac.41.11.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seppala H, Klaukka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, Huovinen P. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 24.Setchanova L, Tomasz A. Molecular characterization of penicillin-resistant Streptococcus pneumoniae isolates from Bulgaria. J Clin Microbiol. 1999;37:638–648. doi: 10.1128/jcm.37.3.638-648.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soares S, Kristinsson K G, Musser J M, Tomasz A. Evidence for the introduction of a multiresistant clone of serotype 6B Streptococcus pneumoniae from Spain to Iceland in the late 1980s. J Infect Dis. 1993;168:158–163. doi: 10.1093/infdis/168.1.158. [DOI] [PubMed] [Google Scholar]
- 26.Tarasi A, Chong Y, Lee K, Tomasz A. Spread of the serotype 23F multidrug-resistant Streptococcus pneumoniae clone to South Korea. Microb Drug Resist. 1997;3:105–109. doi: 10.1089/mdr.1997.3.105. [DOI] [PubMed] [Google Scholar]
- 27.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasz A, Corso A, Severina E P, Echániz-Aviles G, Brandileone M C, Camou T, Castañeda E, Figueroa O, Rossi A, Di Fabio J L. Molecular epidemiologic characterization of penicillin-resistant Streptococcus pneumoniae invasive pediatric isolates recovered in six Latin-American countries: an overview. PAHO/Rockefeller University Workshop. Pan American Health Organization. Microb Drug Resist. 1998;4:195–207. doi: 10.1089/mdr.1998.4.195. [DOI] [PubMed] [Google Scholar]
- 29.Woods G L, Washington J A. Antimicrobial susceptibility tests: dilution and disk diffusion methods. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 1327–1341. [Google Scholar]