Abstract
Virus-induced gene silencing (VIGS) is a versatile and attractive approach for functional gene characterization in plants. Although several VIGS vectors for maize (Zea mays) have been previously developed, their utilities are limited due to low viral infection efficiency, insert instability, short maintenance of silencing, inadequate inoculation method, or abnormal requirement of growth temperature. Here, we established a Cucumber mosaic virus (CMV)-based VIGS system for efficient maize gene silencing that overcomes many limitations of VIGS currently available for maize. Using two distinct strains, CMV-ZMBJ and CMV-Fny, we generated a pseudorecombinant-chimeric (Pr) CMV. Pr CMV showed high infection efficacy but mild viral symptoms in maize. We then constructed Pr CMV-based vectors for VIGS, dubbed Pr CMV VIGS. Pr CMV VIGS is simply performed by mechanical inoculation of young maize leaves with saps of Pr CMV-infected Nicotiana benthamiana under normal growth conditions. Indeed, suppression of isopentenyl/dimethylallyl diphosphate synthase (ZmIspH) expression by Pr CMV VIGS resulted in non-inoculated leaf bleaching as early as 5 d post-inoculation (dpi) and exhibited constant and efficient systemic silencing over the whole maize growth period up to 105 dpi. Furthermore, utilizing a ligation-independent cloning (LIC) strategy, we developed a modified Pr CMV-LIC VIGS vector, allowing easy gene cloning for high-throughput silencing in maize. Thus, our Pr CMV VIGS system provides a much-improved toolbox to facilitate efficient and long-duration gene silencing for large-scale functional genomics in maize, and our pseudorecombination-chimera combination strategy provides an approach to construct efficient VIGS systems in plants.
A pseudorecombinant-chimeric Cucumber mosaic virus-based virus-induced gene silencing system rapidly and efficiently triggers persistent gene silencing in maize.
Introduction
Virus-induced gene silencing (VIGS) exploits an RNA-mediated antiviral defense mechanism, known as RNA interference (RNAi) and post-transcriptional gene silencing (PTGS), to achieve downregulation of gene expression and often “loss-of-function” phenotypes in plants (Vance and Vaucheret, 2001; Becker and Lange, 2010; Duan et al., 2012). Compared with other loss-of-function approaches, VIGS owns several advantages described as following. (1) VIGS can be simply performed to rapidly and efficiently cause gene knockdown, and does not need stable plant transformation. (2) VIGS can be used in both forward and reverse genetics to bridge the gap between genotype and phenotype. (3) VIGS can simultaneously silence multiple members of a gene family. (4) VIGS allows rapid comparisons of gene function in different species or different backgrounds in a single species (Burch-Smith et al., 2004; Senthil-Kumar and Mysore, 2011). With these advantages, VIGS has been extensively employed for gene function elucidation through phenotypic screening in plants (Burch-Smith et al., 2004; Dong et al., 2007; Purkayastha and Dasgupta, 2009; Unver and Budak, 2009; Becker and Lange, 2010; Bernacki et al., 2010; Senthil-Kumar et al., 2013; Buhrow et al., 2016; Gunupuru et al., 2019; Kant and Dasgupta, 2019).
Up to date, more than 40 viruses have been successfully engineered into VIGS vectors, 10 of which have been reported for VIGS in monocots. Bamboo mosaic virus and its satellite RNA (BaMV) was modified for VIGS in Brachypodium distachyon (Liou et al., 2014); Brome mosaic virus (BMV) in barley (Hordeum vulgare), maize (Zea mays), rice (Oryza sativa), and sorghum (Sorghum bicolor; Ding et al., 2006, 2018; Zhu et al., 2014; Singh et al., 2018); Barley stripe mosaic virus (BSMV) in many monocot species including Aegilops Tauschii, barley, B. distachyon, ginger (Zingiber officinale), Hedychium coronarium, maize, oat (Avena sativa), rye (Secale cereale), wheat (Triticum aestivum and Haynaldia villosa), and Zingiber zerumbet (Holzberg et al., 2002; Scofield et al., 2005; Renner et al., 2009; Pacak et al., 2010; Wang et al., 2010; Yuan et al., 2011; Mahadevan et al., 2014; Groszyk et al., 2017; Jarugula et al., 2018; Tavakol, 2018; Chen et al., 2019); Chinese wheat mosaic virus (CWMV) in wheat (Yang et al., 2018); Cucumber mosaic virus (CMV) in banana (Musa acuminata), lily (Lilium leichtlinii), and maize (Wang et al., 2016; Tzean et al., 2019; Tasaki et al., 2020); Cymbidium mosaic virus (CymMV) in Phalaenopsis spp. (Lu et al., 2007; Hsieh et al., 2013); Foxtail mosaic virus (FoMV) in barley, foxtail millet (Setaria italica), wheat and maize (Liu et al., 2016; Mei et al., 2016); Maize rayado fino virus (MRFV) in maize (Mlotshwa et al., 2020); Rice tungro bacilliform virus (RTBV) in rice (Purkayastha et al., 2010); and Tobacco rattle virus (TRV) in wheat and maize (Zhang et al., 2017). These VIGS vectors were all derived from natural monocot hosts except that TRV isolated from dicots (Liu et al., 2002; Zhang et al., 2017).
Maize has great importance to economy. As a model monocot species, research into maize functional genomics urgently demands powerful tools such as VIGS. Of the six VIGS vectors for maize, BMV was the first being used to silence maize genes. Modification of the BMV RNA3 sequence offers better stability of inserts to enhance silencing efficacy; however, insert instability of the BMV-based VIGS remains a constraint for durable gene repression (Ding et al., 2006, 2018). VIGS-vector based on the naturally maize-infecting CMV-ZMBJ strain induces gene silencing by vascular puncture inoculation (VPI). However, VPI often causes plant death and this VIGS system works at a relatively low temperature (18°C–20°C), which is not optimal for maize growth (Wang et al., 2016). VIGS mediated by FoMV requires cost-ineffective biolistic bombardment (Mei et al., 2016). TRV-induced gene silencing is achieved by vacuum agroinfiltration of maize seeds, but efficiency of such silencing needs to be further verified (Zhang et al., 2017). VIGS via BSMV or MRFV has been also recently adapted to maize by VPI, but silencing was observed in only 8.2%–28% plants for BSMV and 18%–40% for MRFV, which might be attributed to the low infection rates of these viruses in maize (Jarugula et al., 2018; Mlotshwa et al., 2020).
CMV belongs to the genus Cucumovirus in the family Bromoviridae, consisting of tripartite linear, positive sense single-stranded (ss) RNA genome of RNA1, RNA2, and RNA3 (Palukaitis et al., 1992; Palukaitis and García-Arenal, 2003). CMV has been well-characterized by an extremely broad host range and high variability (Carrère et al., 1999). Several CMV strains have been successfully developed as VIGS vectors in dicots such as Antirrhinum majus, chili pepper (Capsicum annuumsuch), Nicotiana benthamiana, soybean (Glycine max), spinach (Spinacia oleracea), and tomato (Solanum lycopersicum; Otagaki et al., 2006; Nagamatsu et al., 2007; Kim et al., 2011, 2020; Hong et al., 2012; Du et al., 2014; Cheng et al., 2015), as well as monocots including banana, lily, and maize (Wang et al., 2016; Liu et al., 2019; Tzean et al., 2019; Tasaki et al., 2020). Plant genes of interest were introduced into CMV genome through an insertion site mostly located on genomic RNA2, with one exception that on CMV RNA3 (Tasaki et al., 2020).
In this study, we developed a very efficient VIGS system based on a pseudorecombinant-chimeric (Pr) CMV between CMV-Fny and CMV-ZMBJ. CMV-Fny highly infects maize with severe stunting symptoms (Wahyuni et al., 1992), whereas CMV-ZMBJ only induces mild mosaic symptoms (Wang et al., 2016). Pr CMV-based VIGS, dubbed Pr CMV VIGS, is very effective (almost 100%) and occurs throughout whole maize plant within the entire growth period. Further, via further modification with ligation independent cloning (LIC), we have made the VIGS vectors suitable for high-throughput functional genomics in maize.
Results
Infectivity of pseudorecombinant and chimeric CMV vectors
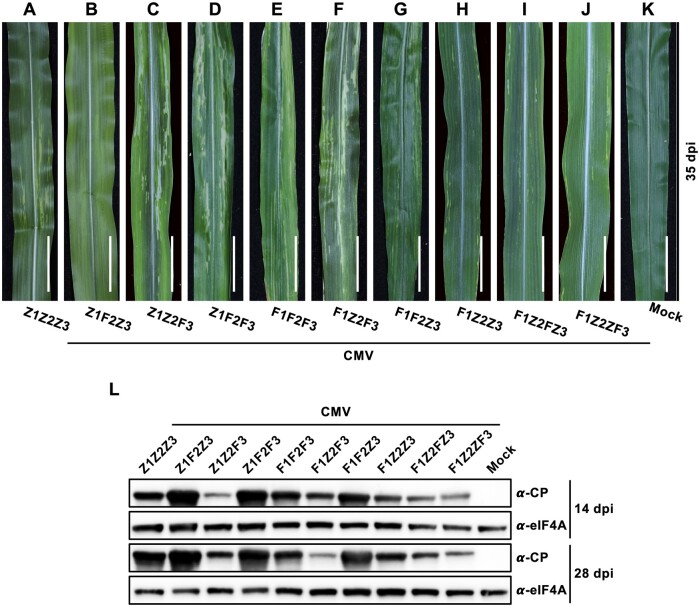
To confirm the infection in maize of two CMV strains Fny and ZMBJ which share amino acid sequence identities between 76.6% and 97.7% among all five viral proteins (Supplemental Table S1), we used the sap of N. benthamiana leaves agroinfiltrated with these two virus infectious clones to mechanically inoculate maize plants. Infiltration of N. benthamiana leaf with Agrobacterium tumefaciens harboring the infectious cDNA clones of CMV-ZMBJ (containing genomic RNA components 1, 2, and 3, called CMVZ1Z2Z3) or CMV-Fny (containing genomic RNA components 1, 2, and 3, also called CMVF1F2F3; Figure 1, A) resulted in efficient systemic CMV infection (Supplemental Figure S1). At 7 d post-agroinfiltration (dpai), CMVZ1Z2Z3 caused mild mosaic symptom in systemic leaves, whereas CMVF1F2F3 resulted in leaf distortion and stunting symptoms on systemic leaves (Supplemental Figure S1, A). However, both CMVZ1Z2Z3 and CMVF1F2F3 induced no obvious symptoms in infiltrated leaves at 7 dpai (Supplemental Figure S1, A). In maize, CMVZ1Z2Z3 and CMVF1F2F3 exhibited different viral symptom and infection efficiency. CMVZ1Z2Z3 induced yellowish spotting in upper non-inoculated maize leaves (Figure 2, A) and 43.8% maize plants were infected with CMVZ1Z2Z3 at 14 d post-inoculation (dpi) and about 50.0% at 28 dpi (Table 1), whereas CMVF1F2F3 caused severe virus symptoms in maize including leaf clearing, leaf distortion, stunting, and most infected plants become dead finally (Figure 2, E and Supplemental Figure S2), and the inoculated plants were infected with the efficiency of 93.8% at 14 dpi and 100% at 28 dpi (Table 1). CMV infection was confirmed in all agroinfiltrated N. benthamiana and inoculated maize plants by western blot analysis using an anti-CP antibody (Supplemental Figure S1, B and Figure 2, L). These results demonstrate that CMV-ZMBJ and CMV-Fny are distinct in pathogenicity and symptomatology in N. benthamiana and maize.
Figure 1.
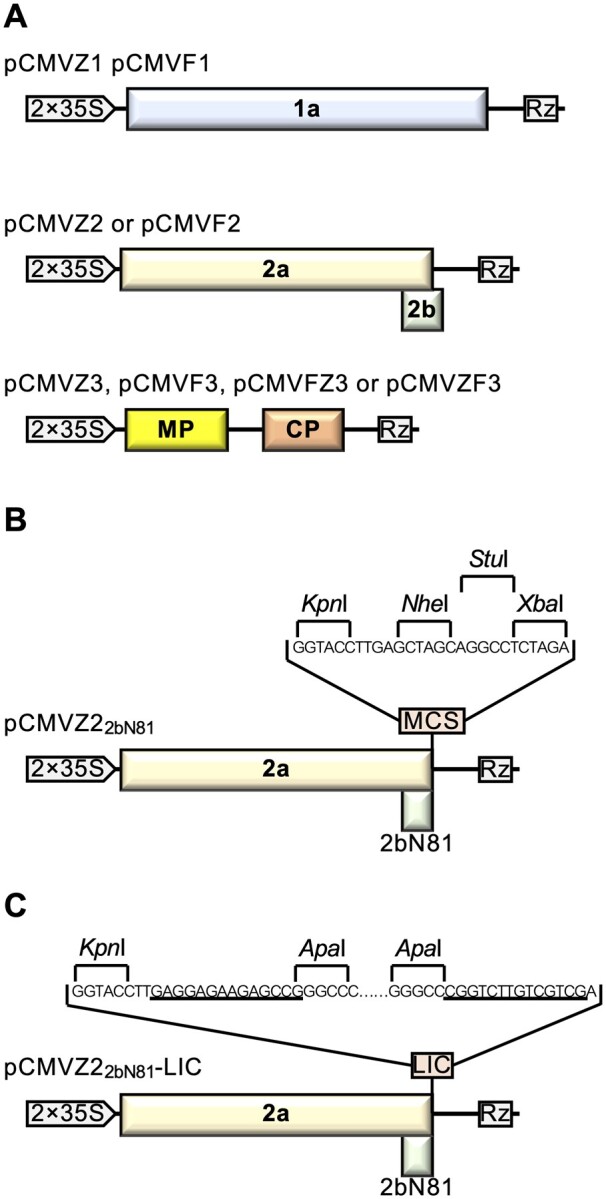
Schematic representation of viral constructs derived from CMV. A, Schematic representation of the full-length cDNA clones of CMV. pCMVZ1, pCMVZ2, and pCMVZ3 were constructed from CMV-ZMBJ, whereas pCMVF1, pCMVF2, and pCMVF3 were from CMV-Fny. pCMVFZ3 and pCMVZF3 were two hybrid constructs. CMV genome encodes four proteins: 1a, 2a, MP, and coat protein (CP). Each vector contains double Cauliflower mosaic virus (CaMV) 35S promoters (2 × 35S, indicated by arrow heads) and a ribozyme sequence (Rz) derived from Tobacco ring spot virus (TRSV). B, The organization of pCMVZ22bN81 (Wang et al., 2016) for use in Pr CMV VIGS system. The MCS was used for the insertion of target gene. C, The organization of pCMVZ22bN81-LIC for use in Pr CMV-LIC-based VIGS system. The LIC site was used for the insertion of target gene by LIC approach (Dong et al., 2007). Nucleotide sequences underlined represent the LIC adaptors.
Figure 2.
CMV infection in maize plants. A–K, Leaf phenotypes of maize infected with CMV. Plants at 2–3-leaf stage were inoculated with saps of N. benthamiana leaves agroinfiltrated with CMV. Plant leaves were photographed at 35 dpi for each pseudorecombinant and chimeric CMV. Scale bars are 2 cm. L, Western blot analysis of CP in maize plants infected with CMV at 14 and 28 dpi. The protein expression of eukaryotic translation initiation factor 4A (eIF4A) was used as the internal control. F, viral RNA from CMV-Fny; Z, viral RNA from CMV-ZMBJ; FZ, chimeric viral RNA with 5′-UTR and MP gene from Fny-RNA3 and CP gene and 3′-UTR from ZMBJ-RNA3; ZF, chimeric viral RNA with 5′-UTR and MP gene from ZMBJ-RNA3 and CP gene and 3′-UTR from Fny-RNA3.
Table 1.
Summary of systemic infectivity of CMV in maize
| CMVa |
Infection efficiencyb |
||||
|---|---|---|---|---|---|
| RNA1 | RNA2 | RNA3 | 14 dpi | 28 dpi | |
| Z1Z2Z3 | Z | Z | Z | 43.8% (7/16) | 50.0% (8/16) |
| Z1F2Z3 | Z | F | Z | 13.3% (2/15) | 26.7% (4/15) |
| Z1Z2F3 | Z | Z | F | 20.0% (3/15) | 86.7% (13/15) |
| Z1F2F3 | Z | F | F | 80.0% (12/15) | 93.3% (14/15) |
| F1F2F3 | F | F | F | 93.8% (15/16) | 100.0% (16/16) |
| F1Z2F3 | F | Z | F | 100.0% (16/16) | 100.0% (16/16) |
| F1F2Z3 | F | F | Z | 81.3% (13/16) | 87.5% (14/16) |
| F1Z2Z3 | F | Z | Z | 68.8% (11/16) | 93.8% (15/16) |
| F1Z2FZ3 | F | Z | FZ | 86.7% (13/15) | 93.3% (14/15) |
| F1Z2ZF3 | F | Z | ZF | 60.0% (9/15) | 93.3% (14/15) |
aF, viral RNA from CMV-Fny; Z, viral RNA from the CMV-ZMBJ; FZ, chimeric viral RNA with 5′UTR and MP gene from Fny-RNA3 and CP gene and 3′-UTR from ZMBJ-RNA3; and ZF, chimeric viral RNA with 5′-UTR and MP gene from ZMBJ-RNA3 and CP gene and 3′-UTR from Fny-RNA3.
bAll of the inoculated plants were analyzed by western blotting from three independent experiments. The number of plants infected/the number of plants inoculated is indicated in the parenthesis.
A virus suitable for development of VIGS vector should have high infectivity but mild or none symptoms in plants. To achieve this, six CMV-ZMBJ and CMV-Fny pseudorecombinant strains including CMVZ1F2Z3, CMVZ1Z2F3, CMVZ1F2F3, CMVF1Z2F3, CMVF1F2Z3, and CMVF1Z2Z3 were investigated. In N. benthamiana, all pseudorecombinant CMVs were infectious (Supplemental Figure S1), suggesting compatibility of viral genomic RNAs between ZMBJ and Fny strains. We further performed the infection assays in maize using the above pseudorecombinant CMVs. A higher infection efficiency in maize was usually obtained from pseudorecombinant CMVs containing Fny-RNA1 than those with ZMBJ-RNA1 (Table 1). Their symptom severity in maize decreased in order of CMVF1F2F3>CMVF1Z2F3>CMVZ1F2F3>CMVZ1Z2F3 CMVF1F2Z3>CMVZ1Z2Z3>CMVF1Z2Z3>CMVZ1F2Z3 (Figure 2, A–J). Two pseudorecombinant CMVF1Z2Z3 and CMVZ1F2Z3 caused mosaic symptoms milder even than CMVZ1Z2Z3 (Figure 2, A, B, and H). Moreover, the infectivity of CMVF1Z2Z3 (68.8% at 14 dpi and 93.8% at 28 dpi, respectively) was higher than CMVZ1Z2Z3 in maize (Table 1), indicating that pseudorecombination of RNAs from the two CMV strains can result in the emergence of viruses with higher infection efficiency but milder symptoms such as CMVF1Z2Z3.
However, CMVF1Z2Z3 exhibited a slow development of virus infection in maize with 68.8% plants were infected at 14 dpi but 93.8% were infected at 28 dpi (Table 1), which might be attributed to slow virus movement involved in RNA3. To fix this, we generated two chimeric RNA3 constructs pCMVFZ3 and pCMVZF3 by exchanging movement protein (MP) genes between RNA3s from CMV-Fny and CMV-ZMBJ (Figure 1, A). The infection of CMVF1Z2FZ3, with the replacement of MP from ZMBJ-RNA3 with that from Fny-RNA3, was elevated at the early stage of 14 dpi with the efficiency of 86.7% (Table 1), although a delayed viral symptom of mild leaf clearing was observed in some infected plants (Figure 2, I). In addition, CMVF1Z2ZF3 exhibited a similar development of virus infection in maize to CMVF1Z2Z3, with 60.0% plants were infected at 14 dpi but 93.3% were infected at 28 dpi (Table 1). Therefore, CMVF1Z2FZ3 was selected for development of VIGS vectors applicable for silencing in maize.
Gene silencing of ZmIspH based on Pr CMV VIGS vectors
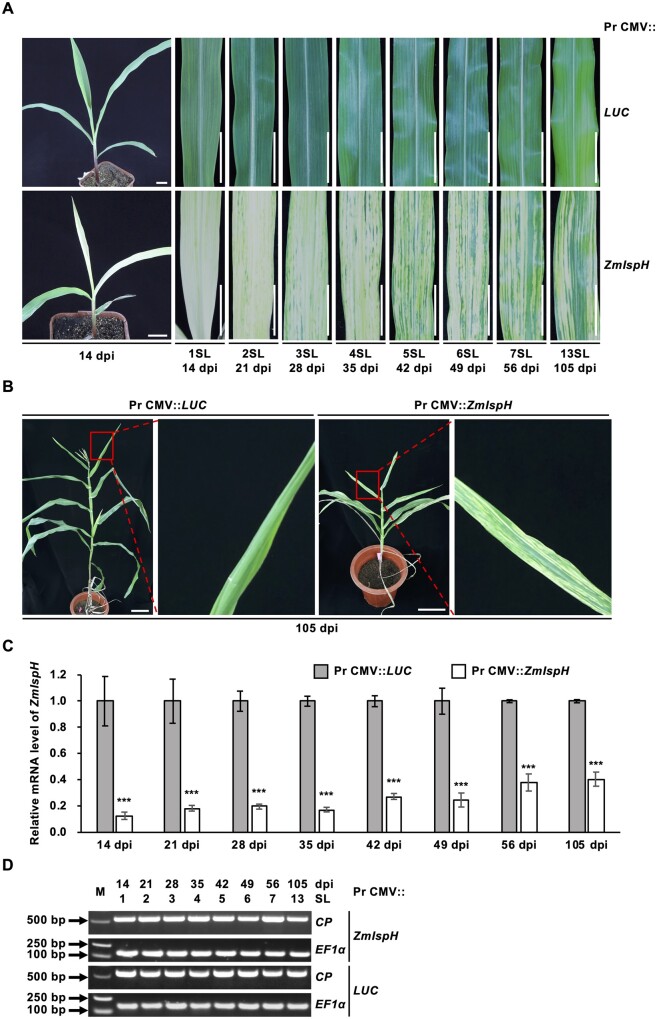
To exploit the pseudorecombinant-chimeric CMVF1Z2FZ3 as a VIGS vector, Pr CMV VIGS was performed as described previously (Wang et al., 2016), of which pCMVZ22bN81, a pCMVZ2 derivate by elimination of 82-116 coding sequences of 2b, is used for inserting the foreign gene sequence (Figure 1, B). To assess the ability of Pr CMV for VIGS in maize, Pr CMV::ZmIspH was generated via combination of pCMVZ22bN81::ZmIspH with a 215-bp insert of ZmIspH gene coding for an isopentenyl/dimethylallyl diphosphate synthase, with pCMVF1 and pCMVFZ3. Maize plants infected with Pr CMV::ZmIspH were daily monitored. Visible photobleaching or chlorosis induced by Pr CMV::ZmIspH silencing was observed on first systemic leaf (1SL, the second or third true leaf) as early as 5 dpi and mostly at 7–10 dpi, and spread on the expanded leaf at 14 dpi (Figure 3, A). Accompanied with the development of virus infection, chlorosis steaks or spots continuously appeared on the new emerging leaves and extended to the whole plant at 105 dpi, although the strength of VIGS attenuated over the time because of the loss of ZmIspH insert in viral RNA (Figure 3, A and B). Nevertheless, the ZmIspH silencing phenotype persisted for the entire observation period of 105 d in Pr CMV::ZmIspH-infected maize. It is worth noting that the same results were obtained in three independent experiments.
Figure 3.
Silencing of the ZmIspH gene in maize by Pr CMV VIGS. A and B, Phenotypes of maize plants infected with Pr CMV::ZmIspH or Pr CMV::LUC control. Photographs were taken at 14 dpi for the first systemic leaf (1SL), at 21 dpi for the 2SL, at 28 dpi for the 3SL, at 35 dpi for the 4SL, at 42 dpi for the 5SL, at 49 dpi for the 6SL, at 56 dpi for the 7SL and at 105 dpi for the 13SL, respectively. Scale bars are 2 cm in (A) and 10 cm in (B). C, RT-qPCR quantification of relative mRNA levels of ZmIspH gene in maize infected with Pr CMV::ZmIspH or Pr CMV::LUC control. Relative mRNA levels of ZmIspH were normalized against those of elongation factor 1-alpha (EF1α) as the reference gene. The bars represent the mean ± SE from at least three independent experiments. In all panels, black asterisks denote significant differences with respect to the control plants infected with Pr CMV::LUC determined by Student’s t test at ***P < 0.001. D, RT-PCR analysis of CP from leaves of maize infected with Pr CMV::ZmIspH or Pr CMV::LUC control. Plant EF1α mRNA was used as the internal control. Lane M is the 2 kb plus DNA marker.
We further analyzed the ZmIspH-silencing efficiency at various time points. The efficiency was evaluated by the percentage of ZmIspH mRNA reduction in Pr CMV::ZmIspH-infected plants relative to the control Pr CMV::LUC-infected plants via reverse transcription quantitative RT-PCR (RT-qPCR). Lower levels of endogenous ZmIspH transcripts, consistent with leaf photobleaching, were detected in Pr CMV::ZmIspH-infected plants compared with control plants. Reduction of ZmIspH mRNA level in photobleached leave ranged between 87.3% in the 1SL at 14 dpi and 59.4% in the 13SL at 105 dpi (Figure 3, C). The presence of Pr CMV vectors in maize plants was confirmed by RT-PCR (Figure 3, D). These results indicate that Pr CMV VIGS can efficiently cause gene silencing throughout the growth period of maize plants.
Genetic stability of Pr CMV VIGS system
VIGS vectors are normally unstable by occurrence of recombination during propagation, and insert stability is an important factor affecting silencing efficiency. In this study, we investigated the insert retention over time in N. benthamiana and maize plants infiltrated/inoculated with Pr CMV::ZmIspH, using RT-PCR with a pair of primers flanking the insert (Supplemental Table S2).
In N. benthamiana, no small-sized fragments was detected in the infiltrated leaves at 2–9 dpi (Supplemental Figure S3), indicating that Pr CMV::ZmIspH was intact in the infiltrated leaves. However, tiny shadows under the full-length fragment were weakly visible in two systemic leaf samples at 11 and 14 dpi (Supplemental Figure S3), suggesting a partial deletion of inserts occurred in systemic leaves after 11 dpi. Therefore, the infiltrated N. benthamiana leaves before 9-dpi can serve as a good inoculum source for Pr CMV VIGS in maize.
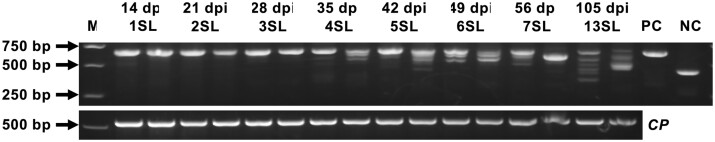
In maize, virus progenies containing the intact insert were present without detectable deletions in 1SL, 2SL, and 3SL. Low levels of small-sized inserts were only apparent in 4SL at 35 dpi, and subsequently the abundance of intact inserts and the size of inserts decreased with the post-inoculation time. The deletions of inserts occurred randomly and individually in infected plants, and the patterns of deleted inserts were not reproducible in different plants even inoculated with the same inocula. At the end of growth period (105 dpi), intact inserts were rarely detected but majority of viruses retained partial inserts containing various deletions in 13SL (Figure 4). No samples contain unique type of virus progenies with complete deletion of inserts during the whole observation.
Figure 4.
Genetic stability of gene insertion of ZmIspH in upper infected with Pr CMV::ZmIspH. Leaf samples were collected at 14 dpi for the first systemic leaf (1SL), at 21 dpi for the 2SL, at 28 dpi for the 3SL, at 35 dpi for the 4SL, at 42 dpi for the 5SL, at 49 dpi for the 6SL, at 56 dpi for the 7SL, and at 105 dpi for 13SL, respectively. Total RNA was isolated from the samples and subjected to RT-PCR amplification with primers flanking the insertion of ZmIspH (upper panel). The vector plasmid with the ZmIspH insert or no sequence insertion was amplified as the positive (lane PC) or negative (lane NC) control. CP was amplified and used as an internal control (lower panel). Lane M is the 2 kb plus DNA marker.
High-throughput Pr CMV-LIC VIGS system for gene silencing in maize
LIC strategy has been proved to be efficient for gene cloning (Dong et al., 2007; Yuan et al., 2011). Here, we adopted a similar LIC approach and generated Pr CMV-LIC VIGS vector for high-throughput cloning and gene silencing in maize (Figure 1, C). To test the validity of Pr CMV-LIC VIGS vector, the 215-bp ZmIspH fragment was cloned into this vector to generate Pr CMV-LIC::ZmIspH using LIC method. Maize plants infected with Pr CMV-LIC::ZmIspH developed photobleached phenotype in 1SL at 6–10 dpi, similar to the observations with Pr CMV::ZmIspH. At 21 dpi, the 2SL were almost entirely photobleached and ZmIspH transcripts were reduced by 78.0% in infected maize (Figure 5, A and B). Additionally, two more genes magnesium chelatase subunit H (ZmChlH) and phytoene desaturase (ZmPDS) were silenced using Pr CMV-LIC VIGS vector. In Pr CMV-LIC::ZmChlH-infected plants, a yellowing phenotype, typical for silencing of ChlH, was observed in systemic leaves and 66.6% of ZmChlH transcripts were silenced at 21 dpi. And in case of Pr CMV-LIC::ZmPDS, silencing of ZmPDS was also successfully achieved by the appearance of albino phenotype on the systemic leaves and reduction of ZmPDS transcripts was approximately 65.9% at 21 dpi (Figure 5, A and B). RT-PCR confirmed virus infection by Pr CMV-LIC vectors in maize plants (Figure 5, C). Taken together, these results suggested that maize genes could be efficiently silenced by the Pr CMV-LIC VIGS system.
Figure 5.
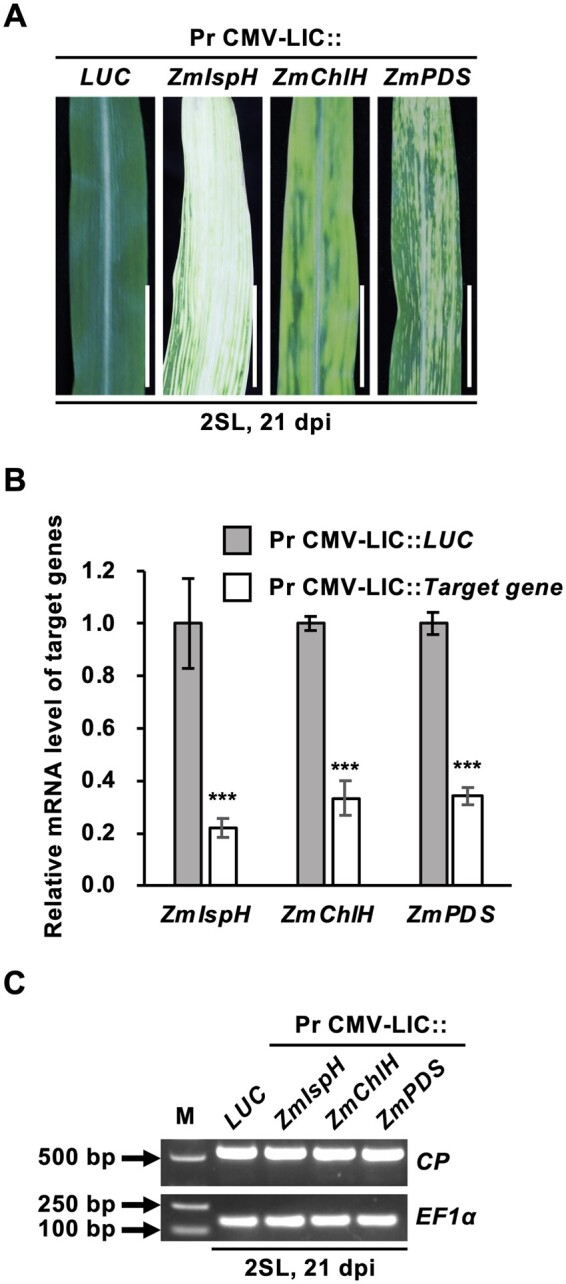
Silencing of the ZmIspH, ZmChlH, and ZmPDS genes in maize by Pr CMV-LIC VIGS vectors. A, Leaf phenotype of maize infected with Pr CMV-LIC::ZmIspH, Pr CMV-LIC::ZmChlH, Pr CMV-LIC::ZmPDS, or Pr CMV-LIC::LUC control. The second systemic leaf (2SL) was photographed 21 dpi for each construct. Scale bars are 2 cm. B, RT-qPCR quantification of relative mRNA levels of target genes in maize infected with Pr CMV-LIC VIGS vectors. Relative mRNA levels of target gene (ZmIspH, ZmChlH, or ZmPDS) were normalized against those of EF1α as the reference gene. The bars represent the mean ± SE from at least three independent experiments. In all panels, black asterisks denote significant differences with respect to the control plants infected with Pr CMV-LIC::LUC determined by Student’s t test at ***P < 0.001. C, RT-PCR analysis of CP (upper panel) from leaves of maize infected with Pr CMV-LIC VIGS vectors. Plant EF1α mRNA was used as the internal control. Lane M is the 2 kb plus DNA marker.
Discussion
In this article, we described a pseudorecombinant-chimeric CMV-based VIGS for efficient gene silencing in maize with advantages in silencing efficiency and effectivity, duration, adaptability in large-scale assay, and low-cost, providing an attractive tool in high-throughput maize functional genomics.
Agroinfiltration is the most common method for VIGS in dicots, but not for monocots, which leaves are usually difficult to infiltrate. Several inoculation alternatives have been adapted for VIGS in maize, including VPI of seeds (Wang et al., 2016; Jarugula et al., 2018; Mlotshwa et al., 2020), mechanical inoculation with viral RNA transcripts generated by in vitro transcription or saps of agroinfiltrated N. benthamiana leaves (Ding et al., 2006, 2018; Zhu et al., 2014), biolistic bombardment (Mei et al., 2016), and vacuum agroinfiltration and co-cultivation (Zhang et al., 2017). However, VPI can damage maize seeds and inhibits germination. Biolistic bombardment is costly and relies on specific equipment, and the usefulness of vacuum agroinfiltration and co-cultivation is still unclear. Here, utilizing N. benthamiana agroinfiltration followed by mechanical inoculation of maize with N. benthamiana sap, similar to the approach used in the improved BMV-based VIGS (Zhu et al., 2014; Ding et al., 2018), we were able to induce efficient gene silencing in maize.
Silencing efficiency and duration alongside virus infectivity are the major factors which are often used to assess usefulness of a VIGS system. In previous CMV-ZMBJ-based VIGS, the viral infection rate was approximately 59% by VPI in the maize B73 line, but only about 60% of the infected plants showed different degrees of silencing. Moreover, the silenced efficiency in five systemic young leaves varies from 25% to 78% over 60 d among individual plants (Wang et al., 2016). The low virus infectivity by VPI is not unique to CMV, but similar results were obtained from other VIGS vectors BSMV and MRFV (Jarugula et al., 2018; Mlotshwa et al., 2020). The improved BMV-based VIGS displayed better insert stability, leading to duration of predominant silencing till 30 dpi (Ding et al., 2018). When VIGS by FoMV was applied to sweet corn, the reduction of target gene transcripts was between 25.4% and 27.8% in L4 and L6 (here we refer to 1SL and 3SL), and seven systemic leaves showed silencing phenotype photobleaching (Mei et al., 2016). The number of leaves with silencing phenotype and duration of silencing by TRV-mediated VIGS were not reported (Zhang et al., 2017). By contrast, Pr CMV VIGS system has a long maintenance of gene silencing in maize. Pr CMV:ZmIspH caused highly efficient and durable silencing of ZmIspH (59.4%–87.3%) for over 105 d, the longest duration of VIGS in maize ever reported. A total of 13 systemic leaves were ZmIspH-silenced. Moreover, almost 100% the tested plants were systemically infected, in which Pr CMV VIGS occurred.
Pr CMV VIGS possesses additional good features in insert stability and environmental requirements compared with CMV ZMBJ-based VIGS. It has been demonstrated that the 215-bp ZmIspH insert in CMV ZMBJ vector was stable in the second leaf without any deletion (Wang et al., 2016). However, the same ZmIspH insert was more stable in our Pr CMV vectors. No detectable loss of insert occurred in the infiltrated leaves of N. benthamiana, which ensure that viral inoculums from infiltrated N. benthamiana leaves were intact before performing VIGS in maize. Insert stability was improved in our Pr CMV maize VIGS system, and ZmIspH insert was stable in at least three leaves without detectable deletions (Figure 4). Moreover, it was worthwhile noting that the low temperature (18°C–20°C), for optimization of CMV ZMBJ-based VIGS (Wang et al., 2016), was no longer required in our experiments, and Pr CMV VIGS led to efficient gene silencing under room temperature (25°C) that is favorable for plant growth.
LIC strategy facilitates gene cloning and enables simultaneous production of vectors in a large scale (Aslanidis and De Jong, 1990; Dieckman et al., 2002). This method has been employed for high-throughput VIGS and subsequent rapid gene function analysis in plants (Dong et al., 2007; Yuan et al., 2011). We also developed a Pr CMV-LIC VIGS system, and ZmIspH, ZmChlH, and ZmPDS were efficiently silenced using this system, suggesting its potential use for large-scale gene function analysis in maize.
Pseudorecombination of different CMV strains produced viruses with various infectivity and symptom expression (Carrère et al., 1999; Nagamatsu et al., 2007; Phan et al., 2014; Cheng et al., 2015; Kim et al., 2020). Interestingly, pseudorecombinant CMVs containing Fny-RNA1 normally caused high infectivity in maize, suggesting a role of RNA1 in CMV infectivity in maize (Table 1). RNA1 of CMV encodes the viral replicase, 1a protein, that is required for synthesis of negative-strand RNA and also reported to be functional in symptom determination and virus movement (Roossinck and Palukaitis, 1990; Gal-On et al., 1994; Divéki et al., 2004). Moreover, replacement of ZMBJ-MP with Fny-MP enhances the rate of systemic infection, suggesting an essential role of MP in rapid development of CMV infection in maize, although both MP and CP have been demonstrated to be responsible for cell-to-cell movement and long-distance movement (Perry et al., 1994; Canto et al., 1997; Kaplan et al., 1997; Choi et al., 2005).
Symptom determinants of CMV have been previously investigated and mapped to RNA3 (Shintaku et al., 1992; Zhang et al., 1994; Suzuki et al., 1995; Carrère et al., 1999; Szilassy et al., 1999; Qiu et al., 2018; Sáray et al., 2021) and RNA2 (Ding et al., 1995; Shi et al., 2002; Soards et al., 2002; Du et al., 2007, 2008; Ziebell et al., 2007; Lewsey et al., 2009; Phan et al., 2014), but RNA1 with only one case (Divéki et al., 2004). Symptom determinants varied with CMV strains and the host species, and pseudorecombination of CMVs also altered the viral symptomatology. In our experiments, RNA2 of CMV-ZMBJ or CMV-Fny contributed to symptom development in N. benthamiana. All pseudorecombinant CMVs containing Fny-RNA2 caused severe symptoms of leaf distortion and stunting like CMV-Fny, whereas the others containing ZMBJ-RNA2 induced mosaic spotting symptom similar to wild-type CMV-ZMBJ (Supplemental Figure S1). However, CMV RNA3 also contributes to the symptom determinant in maize because infection with pseudorecombinant CMVs containing Fny-RNA3 always resulted in leaf clearing and distortion, that was different from the yellowish spotting caused by the other pseudorecombinant CMVs or wild-type CMV-ZMBJ (Figure 2). Consistent with our observation, the symptoms induced by R-CMV in local leaves of maize were determined by the 5′-part of RNA3 (Carrère et al., 1999).
CMV has an extremely broad host range of over 1,200 monocot and dicot species from 100 families including cereal, fruit, vegetable, and ornamental crops (Palukaitis et al., 1992; Palukaitis and García-Arenal, 2003; Scholthof et al., 2011). In this regard, our Pr CMV VIGS system may be also applicable to induce efficient and persistent gene silencing in other host species. Moreover, our work reveals pseudorecombination–chimera combination as a strategy to construct efficient and effective VIGS systems in plants.
Materials and methods
Plant materials and growth conditions
Maize (Z. mays, inbred line B73) and N. benthamiana were cultivated in controlled-environment growth room at 25°C with approximately 50% humidity under a 16-h d/8-h night photoperiod. N. benthamiana seedlings were used in this study for virus propagation via agroinfiltration.
Construction of CMV-derived vectors
Infectious cDNA clones of CMV genomic RNAs 1, 2, and 3 have been described previously (Cheng et al., 2015; Wang et al., 2016) and designated as pCMVZ1, pCMVZ2, and pCMVZ3 for CMV-ZMBJ, and pCMVF1, pCMVF2, and pCMVF3 for CMV-Fny, respectively (Figure 1, A). To generate chimeric RNA3 construct pCMVFZ3, 5′-UTR and MP regions were PCR amplified using pCMVF3 as template, whereas CP and 3′-UTR regions were amplified using pCMVZ3 as template. The chimeric FZ3 RNA3 was then obtained by overlap PCR. The resulting PCR product was digested with BamHI and subsequently ligated into StuI and BamHI digested pCB301 (Cheng et al., 2015). Another chimeric construct pCMVZF3 comprising 5′-UTR and MP region of ZMBJ-RNA3, CP and 3′-UTR region of Fny-RNA3 was similarly constructed as pCMVZF3. Primers used for the construction of these two mutants were listed in Supplemental Table S3.
pCMVZ22bN81 is a CMV RNA2 vector as described (Wang et al., 2016), which coding region of amino acids 82-111 of 2b protein is replaced by a multiple cloning site (MCS; Figure 1, B).
To establish Pr CMV-LIC VIGS system, pCMVZ22bN81-LIC vector was generated by cloning LIC cassette containing two LIC adaptors, two ApaI sites, chloramphenicol resistance, and ccdB genes into pCMVZ22bN81 to generate pCMVZ22bN81-LIC (Figure 1, C), which was subsequently maintained in Escherichia coli DB3.1. Primers used for this construction were also listed in Supplemental Table S3.
Generation of gene silencing vectors
A 215-bp ZmIspH DNA fragment was cloned into pCMVZ22bN81 to generate pCMVZ22bN81::ZmIspH used for silencing of ZmIspH in maize (Wang et al., 2016).
To silence genes of interest via Pr CMV-LIC VIGS system, three maize genes ZmIspH (215 bp), ZmChlH (201 bp), and ZmPDS (234 bp) were RT-PCR amplified using primers containing LIC adaptors. The PCR products were purified and treated with T4 DNA polymerase (Thermo Scientific) in the presence of 5 mM dATP (Promega) at 37°C for 30 min followed by 20 min of inactivation of T4 DNA polymerase at 75°C. The pCMVZ22bN81-LIC vector was digested with ApaI and similarly treated with T4 DNA polymerase in the presence of 5 mM dTTP (Promega). T4 DNA polymerase treated PCR products and pCMVZ22bN81-LIC vector were mixed, incubated at 70°C for 10 min, and subsequently 22°C for 30 min. The mixture was then directly transformed into E. coli DH5α competent cells. A 202-bp fragment of firefly luciferase gene was cloned into pCMVZ22bN81-LIC to generate pCMVZ22bN81-LIC::LUC similarly. Primers used for construction of these derivatives pCMVZ22bN81-LIC::ZmIspH, pCMVZ22bN81-LIC::ZmChlH, pCMVZ22bN81-LIC::ZmPDS, and pCMVZ22bN81-LIC::LUC were list in Supplemental Table S4.
Agrobacterium infiltration of N. benthamiana
Infectious CMV constructs were transformed into A. tumefaciens strain GV3101. Agrobacterium tumefaciens cells were individually cultivated overnight at 28°C in LB medium containing 50 mg/L kanamycin and 50 mg/L rifampicin. Agrobacterium cells harboring CMV RNAs 1, 2, and 3 constructs or their derivatives were equally mixed, collected by centrifugation, resuspended to an optical density of OD600 = 1.0 in infiltration buffer (10 mM MgCl2, and 10 mM MES pH 5.6, and 200 mM acetosyringone), and incubated at room temperature for 3 h before agroinfiltration. Agrobacterium suspensions were syringe infiltrated into fully expanded leaves of N. benthamiana at the 5-week-old stage. Mock-treated plants were infiltrated with the infiltration buffer.
Viral inoculation of maize plants
Infiltrated leaves of N. benthamiana were used as viral inoculums for sap inoculation of maize plants. Leaf samples were harvested at 7 dpai, homogenized in inoculation buffer (20 mM Na2HPO4-NaH2PO4 buffer, pH 7.2) at a 1:4 w/v ratio, and directly rub-inoculated onto 2–3-leaf stage maize seedlings pre-dusted with 400-mesh carborundum powder (Sigma–Aldrich). Mock-treated maize plants were inoculated with saps from mock-treated N. benthamiana leaves. Inoculated plants were wrapped with plastic covers and then incubated under dark and humid conditions at 25°C for 48 h. After removing the plastic covers, the plants were kept in the growth room at 25°C with 16 h of illumination. All virus infection experiments were replicated at least three times independently.
RNA extraction, RT-PCR, and RT-qPCR analyses
Total RNA was obtained from leaves using TransZol Up reagent (TransGen) following manufacturer’s instructions and quantified using Epoch Microplate Spectrophotometer (BioTek). First strand cDNA was synthesized using TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix kit (TransGen) with oligo(dT)18 or gene-specific primer. PCR was used to amplify gene interest and test the insert stability using primers listed in Supplemental Tables S2, S4. For RT-qPCR experiments, cDNA was amplified in triplicate using 2× M5 HiPer SYBR Premix EsTaq (with Tli RNaseH, Mei5bio) on a BioRad CFX96 Real Time PCR System (BioRad). Relative expression of interest gene was normalized to maize elongation factor 1-alpha (ZmEF1α) and calculated with the ΔΔCT method. All of the primer pairs used for RT-qPCR were annealed outside the region targeted for silencing and listed in Supplemental Table S2.
Statistical analysis
All statistical analyses were performed with SPSS 25.0 statistical software based on data obtained from triplicate samples. Error bars in all the figures represent standard errors of the means. P values were calculated using student’s t test.
Western blot analysis
Leaf tissues of N. benthamiana and maize were ground and homogenized in 2× Laemmli buffer (120 mM Tris–HCl, pH 6.8, 4% [w/v] SDS, 0.02% [w/v] bromophenol blue, 20% [v/v] glycerol, and 2% [v/v] 2-mercaptoethanol). Extracted total proteins were electrophoretically separated on 12% precast polyacrylamide SDS-PAGE gels (GenScript) and transferred to polyvinylidene difluoride membranes (Merck Millipore). After blocking with 5% (w/v) nonfat milk, the membranes were incubated with anti-CP (ADI, 1:3,000) or anti-eIF4A (Agrisera, 1:10,000) primary antibody, rinsed, and incubated with Rabbit anti-Goat secondary antibody conjugated with horseradish peroxidase (Easybio). The blots were visualized by chemiluminescence using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific) and Amersham Imager 680 (GE Healthcare).
Accession numbers
The virus sequence data from this article can be found in GenBank under the following accession numbers: CMV-Fny RNA1 (NC_002034), CMV-Fny RNA2 (NC_002035), CMV-Fny RNA3 (NC_001440), CMV-ZMBJ RNA1 (KT350980), CMV-ZMBJ RNA2 (KT350981), and CMV-ZMBJ RNA3 (KC693009). Sequences of plant genes can be accessed through Gramene database under the following accession numbers: ZmIspH (Zm00001d033896), ZmChlH (Zm00001d026603), ZmPDS (Zm00001d044558), and ZmEF1α (Zm00001d046499).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Viral infection in N. benthamiana plants agroinfiltrated with CMV vectors.
Supplemental Figure S2. Plant phenotypes of maize infected with CMV-Fny.
Supplemental Figure S3. The stability of gene insertion in N. benthamiana infected with Pr CMV::ZmIspH.
Supplemental Table S1. The amino acid sequence identity between CMV-Fny and CMV-ZMBJ.
Supplemental Table S2. Primers used for RT-PCR and RT-qPCR.
Supplemental Table S3. Primers used for vector construction.
Supplemental Table S4. Primers used for gene amplification.
Supplementary Material
Acknowledgments
We thank professors Tao Zhou and Jiuran Zhao for providing CMV-ZMBJ infectious clone and seeds of the maize inbred line B73.
Funding
This work was supported by the National Natural Science Foundation of China to Y.L. (31920103013, 31530059) and Y.H. (31872636), the Ministry of Science & Technology of China to Y.L. (2017YFA0503401) and Y.H. (2017YFE0110900), and the Postdoctoral Fellowship of Tsinghua-Peking Center for Life Sciences to H.L. and D.Z.
Conflict of interest statement. None declared.
H.L. and Y.L. conceived the project. H.L., K.X., and Y.L. designed the research. H.L. and D.Z. carried out the experiments. H.L., D.Z., Y.W., Q.L., Y.H., and Y.L. analyzed the data. All authors discussed the results and contributed to the final manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/General-Instructions) is Yule Liu (yuleliu@mail.tsinghua.edu.cn).
References
- Aslanidis C, De Jong PJ (1990) Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res 18:6069–6074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Lange M (2010) VIGS—genomics goes functional. Trends Plant Sci 15:1–4 [DOI] [PubMed] [Google Scholar]
- Bernacki S, Karimi M, Hilson P, Robertson N (2010) Virus-induced gene silencing as a reverse genetics tool to study gene function. Methods Mol Biol 655:27–45 [DOI] [PubMed] [Google Scholar]
- Buhrow LM, Clark SM, Loewen MC (2016) Identification of an attenuated barley stripe mosaic virus for the virus-induced gene silencing of pathogenesis-related wheat genes. Plant Methods 12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Anderson JC, Martin GB, Dinesh-Kumar SP (2004) Applications and advantages of virus-induced gene silencing for gene function studies in plants. Plant J 39:734–746 [DOI] [PubMed] [Google Scholar]
- Canto T, Prior DA, Hellwald KH, Oparka KJ, Palukaitis P (1997) Characterization of cucumber mosaic virus. IV. Movement protein and coat protein are both essential for cell-to-cell movement of cucumber mosaic virus. Virology 237:237–248 [DOI] [PubMed] [Google Scholar]
- Carrère I, Tepfer M, Jacquemond M (1999) Recombinants of cucumber mosaic virus (CMV): determinants of host range and symptomatology. Arch Virol 144:365–379 [DOI] [PubMed] [Google Scholar]
- Chen H, Yue Y, Yu R, Fan Y (2019) A Hedychium coronarium short chain alcohol dehydrogenase is a player in allo-ocimene biosynthesis. Plant Mol Biol 101:297–313 [DOI] [PubMed] [Google Scholar]
- Cheng X, Shi W, Du Z, Liao Q (2015) Construction of virus-induced gene silencing vector based on Cucumber mosaic virus (CMV). J Agric Biotechnol 23:1550–1558 [Google Scholar]
- Choi SK, Palukaitis P, Min BE, Lee MY, Choi JK, Ryu KH (2005) Cucumber mosaic virus 2a polymerase and 3a movement proteins independently affect both virus movement and the timing of symptom development in zucchini squash. J Gen Virol 86:1213–1222 [DOI] [PubMed] [Google Scholar]
- Dieckman L, Gu M, Stols L, Donnelly MI, Collart FR (2002) High throughput methods for gene cloning and expression. Protein Expr Purif 25:1–7 [DOI] [PubMed] [Google Scholar]
- Ding SW, Li WX, Symons RH (1995) A novel naturally occurring hybrid gene encoded by a plant RNA virus facilitates long distance virus movement. EMBO J 14:5762–5772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XS, Mannas SW, Bishop BA, Rao X, Lecoultre M, Kwon S, Nelson RS (2018) An improved Brome mosaic virus silencing vector: greater insert stability and more extensive VIGS. Plant Physiol 176:496–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XS, Schneider WL, Chaluvadi SR, Mian MA, Nelson RS (2006) Characterization of a Brome mosaic virus strain and its use as a vector for gene silencing in monocotyledonous hosts. Mol Plant Microbe Interact 19:1229–1239 [DOI] [PubMed] [Google Scholar]
- Divéki Z, Salánki K, Balázs E (2004) The necrotic pathotype of the cucumber mosaic virus (CMV) ns strain is solely determined by amino acid 461 of the 1a protein. Mol Plant Microbe Interact 17:837–845 [DOI] [PubMed] [Google Scholar]
- Dong Y, Burch-Smith TM, Liu Y, Mamillapalli P, Dinesh-Kumar SP (2007) A ligation-independent cloning tobacco rattle virus vector for high-throughput virus-induced gene silencing identifies roles for NbMADS4-1 and -2 in floral development. Plant Physiol 145:1161–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Chen A, Chen W, Westwood JH, Baulcombe DC, Carr JP (2014) Using a viral vector to reveal the role of microRNA159 in disease symptom induction by a severe strain of cucumber mosaic virus. Plant Physiol 164:1378–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Chen F, Zhao Z, Liao Q, Palukaitis P, Chen J (2008) The 2b protein and the C-terminus of the 2a protein of cucumber mosaic virus subgroup I strains both play a role in viral RNA accumulation and induction of symptoms. Virology 380:363–370 [DOI] [PubMed] [Google Scholar]
- Du ZY, Chen FF, Liao QS, Zhang HR, Chen YF, Chen JS (2007) 2b ORFs encoded by subgroup IB strains of cucumber mosaic virus induce differential virulence on Nicotiana species. J Gen Virol 88:2596–2604 [DOI] [PubMed] [Google Scholar]
- Duan CG, Wang CH, Guo HS (2012) Application of RNA silencing to plant disease resistance. Silence 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal-On A, Kaplan I, Roossinck MJ, Palukaitis P (1994) The kinetics of infection of zucchini squash by cucumber mosaic virus indicate a function for RNA 1 in virus movement. Virology 205:280–289 [DOI] [PubMed] [Google Scholar]
- Groszyk J, Kowalczyk M, Yanushevska Y, Stochmal A, Rakoczy-Trojanowska M, Orczyk W (2017) Identification and VIGS-based characterization of Bx1 ortholog in rye (Secale cereale L.). PLoS ONE 12:e0171506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunupuru LR, Perochon A, Ali SS, Scofield SR, Doohan FM (2019) Virus-induced gene silencing (VIGS) for functional characterization of disease resistance genes in barley seedlings. Methods Mol Biol 1900:95–114 [DOI] [PubMed] [Google Scholar]
- Holzberg S, Brosio P, Gross C, Pogue GP (2002) Barley stripe mosaic virus-induced gene silencing in a monocot plant. Plant J 30:315–327 [DOI] [PubMed] [Google Scholar]
- Hong JS, Rhee S-J, Kim E-J, Kim T-S, Ryu KH, Masuta C, Lee GP (2012) Application of a reassortant cucumber mosaic virus vector for gene silencing in tomato and chili pepper plants. Plant Pathol J 28:81–86 [Google Scholar]
- Hsieh MH, Lu HC, Pan ZJ, Yeh HH, Wang SS, Chen WH, Chen HH (2013) Optimizing virus-induced gene silencing efficiency with Cymbidium mosaic virus in Phalaenopsis flower. Plant Sci 201–202:25–41 [DOI] [PubMed] [Google Scholar]
- Jarugula S, Willie K, Stewart LR (2018) Barley stripe mosaic virus (BSMV) as a virus-induced gene silencing vector in maize seedlings. Virus Genes 54:616–620 [DOI] [PubMed] [Google Scholar]
- Kant R, Dasgupta I (2019) Gene silencing approaches through virus-based vectors: speeding up functional genomics in monocots. Plant Mol Biol 100:3–18 [DOI] [PubMed] [Google Scholar]
- Kaplan IB, Gal-On A, Palukaitis P (1997) Characterization of cucumber mosaic virus. III. Localization of sequences in the movement protein controlling systemic infection in cucurbits. Virology 230:343–349 [DOI] [PubMed] [Google Scholar]
- Kim BM, Inaba J-I, Masuta C (2011) Virus induced gene silencing in Antirrhinum majus using the Cucumber mosaic virus vector: Functional analysis of the AINTEGUMENTA (Am-ANT) gene of A. majus. Hort Environ Biotechnol 52:176–182 [Google Scholar]
- Kim H, Onodera Y, Masuta C (2020) Application of cucumber mosaic virus to efficient induction and long-term maintenance of virus-induced gene silencing in spinach. Plant Biotechnol (Tokyo) 37:83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewsey M, Surette M, Robertson FC, Ziebell H, Choi SH, Ryu KH, Canto T, Palukaitis P, Payne T, Walsh JA, Carr JP (2009) The role of the Cucumber mosaic virus 2b protein in viral movement and symptom induction. Mol Plant Microbe Interact 22:642–654 [DOI] [PubMed] [Google Scholar]
- Liou MR, Huang YW, Hu CC, Lin NS, Hsu YH (2014) A dual gene-silencing vector system for monocot and dicot plants. Plant Biotechnol J 12:330–343 [DOI] [PubMed] [Google Scholar]
- Liu N, Xie K, Jia Q, Zhao J, Chen T, Li H, Wei X, Diao X, Hong Y, Liu Y (2016) Foxtail mosaic virus-induced gene silencing in monocot plants. Plant Physiol 171:1801–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Liu S, Wang R, Chen X, Fan Z, Wu B, Zhou T (2019) Analyses of miRNA functions in maize using a newly developed ZMBJ-CMV-2b(N81)-STTM vector. Front Plant Sci 10:1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Marathe R, Dinesh-Kumar SP (2002) Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J 30:415–429 [DOI] [PubMed] [Google Scholar]
- Lu HC, Chen HH, Tsai WC, Chen WH, Su HJ, Chang DC, Yeh HH (2007) Strategies for functional validation of genes involved in reproductive stages of orchids. Plant Physiol 143:558–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan C, Jaleel A, Deb L, Thomas G, Sakuntala M (2014) Development of an efficient virus induced gene silencing strategy in the non-model wild ginger-Zingiber zerumbet and investigation of associated proteome changes. PLoS ONE 10:e0124518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y, Zhang C, Kernodle BM, Hill JH, Whitham SA (2016) A foxtail mosaic virus vector for virus-induced gene silencing in maize. Plant Physiol 171:760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlotshwa S, Xu J, Willie K, Khatri N, Marty D, Stewart LR (2020) Engineering Maize rayado fino virus for virus-induced gene silencing. Plant Direct 4:e00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu A, Masuta C, Senda M, Matsuura H, Kasai A, Hong JS, Kitamura K, Abe J, Kanazawa A (2007) Functional analysis of soybean genes involved in flavonoid biosynthesis by virus-induced gene silencing. Plant Biotechnol J 5:778–790 [DOI] [PubMed] [Google Scholar]
- Otagaki S, Arai M, Takahashi A, Goto K, Hong J-S, Masuta C, Kanazawa A (2006) Rapid induction of transcriptional and post-transcriptional gene silencing using a novel Cucumber mosaic virus vector. Plant Biotechnol 23:259–265 [Google Scholar]
- Pacak A, Geisler K, Jørgensen B, Barciszewska-Pacak M, Nilsson L, Nielsen TH, Johansen E, Grønlund M, Jakobsen I, Albrechtsen M (2010) Investigations of barley stripe mosaic virus as a gene silencing vector in barley roots and in Brachypodium distachyon and oat. Plant Methods 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palukaitis P, García-Arenal F (2003) Cucumoviruses. Adv Virus Res 62:241–323 [DOI] [PubMed] [Google Scholar]
- Palukaitis P, Roossinck MJ, Dietzgen RG, Francki RI (1992) Cucumber mosaic virus. Adv Virus Res 41:281–348 [DOI] [PubMed] [Google Scholar]
- Perry KL, Zhang L, Shintaku MH, Palukaitis P (1994) Mapping determinants in cucumber mosaic virus for transmission by Aphis gossypii. Virology 205:591–595 [DOI] [PubMed] [Google Scholar]
- Phan MS, Seo JK, Choi HS, Lee SH, Kim KH (2014) Pseudorecombination between two distinct strains of cucumber mosaic virus results in enhancement of symptom severity. Plant Pathol J 30:316–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha A, Dasgupta I (2009) Virus-induced gene silencing: a versatile tool for discovery of gene functions in plants. Plant Physiol Biochem 47:967–976 [DOI] [PubMed] [Google Scholar]
- Purkayastha A, Mathur S, Verma V, Sharma S, Dasgupta I (2010) Virus-induced gene silencing in rice using a vector derived from a DNA virus. Planta 232:1531–1540 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Zhang Y, Wang C, Lei R, Wu Y, Li X, Zhu S (2018) Cucumber mosaic virus coat protein induces the development of chlorotic symptoms through interacting with the chloroplast ferredoxin I protein. Sci Rep 8:1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner T, Bragg J, Driscoll HE, Cho J, Jackson AO, Specht CD (2009) Virus-induced gene silencing in the culinary ginger (Zingiber officinale): an effective mechanism for down-regulating gene expression in tropical monocots. Mol Plant 2:1084–1094 [DOI] [PubMed] [Google Scholar]
- Roossinck MJ, Palukaitis P (1990) Rapid induction and severity of symptoms in zucchini squash (Cucurbita pepo) map to RNA 1 of cucumber mosaic virus. Mol Plant Microbe Interact 3:188–192 [Google Scholar]
- Sáray R, Fábián A, Palkovics L, Salánki K (2021) The 28 Ser amino acid of cucumber mosaic virus movement protein has a role in symptom formation and plasmodesmata localization. Viruses 13:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholthof KB, Adkins S, Czosnek H, Palukaitis P, Jacquot E, Hohn T, Hohn B, Saunders K, Candresse T, Ahlquist P, Hemenway C, Foster GD (2011) Top 10 plant viruses in molecular plant pathology. Mol Plant Pathol 12:938–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield SR, Huang L, Brandt AS, Gill BS (2005) Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21-mediated leaf rust resistance pathway. Plant Physiol 138:2165–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthil-Kumar M, Lee HK, Mysore KS (2013) VIGS-mediated forward genetics screening for identification of genes involved in nonhost resistance. J Vis Exp :e51033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthil-Kumar M, Mysore KS (2011) New dimensions for VIGS in plant functional genomics. Trends Plant Sci 16:656–665 [DOI] [PubMed] [Google Scholar]
- Shi BJ, Palukaitis P, Symons RH (2002) Differential virulence by strains of Cucumber mosaic virus is mediated by the 2b gene. Mol Plant Microbe Interact 15:947–955 [DOI] [PubMed] [Google Scholar]
- Shintaku MH, Zhang L, Palukaitis P (1992) A single amino acid substitution in the coat protein of cucumber mosaic virus induces chlorosis in tobacco. Plant Cell 4:751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DK, Lee H-K, Dweikat I, Mysore KS (2018) An efficient and improved method for virus-induced gene silencing in sorghum. BMC Plant Biol 18:123–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soards AJ, Murphy AM, Palukaitis P, Carr JP (2002) Virulence and differential local and systemic spread of cucumber mosaic virus in tobacco are affected by the CMV 2b protein. Mol Plant Microbe Interact 15:647–653 [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kuwata S, Masuta C, Takanami Y (1995) Point mutations in the coat protein of cucumber mosaic virus affect symptom expression and virion accumulation in tobacco. J Gen Virol 76:1791–1799 [DOI] [PubMed] [Google Scholar]
- Szilassy D, Salánki K, Balázs E (1999) Stunting induced by cucumber mosaic cucumovirus-infected Nicotiana glutinosa is determined by a single amino acid residue in the coat protein. Mol Plant Microbe Interact 12:1105–1113 [DOI] [PubMed] [Google Scholar]
- Tasaki K, Yamagishi M, Masuta C (2020) Virus-induced gene silencing in lilies using cucumber mosaic virus vectors. Methods Mol Biol 2172:1–13 [DOI] [PubMed] [Google Scholar]
- Tavakol E (2018) Virus-induced gene silencing (VIGS) in Aegilops tauschii and its use in functional analysis of AetDREB2. Mol Biotechnol 60:41–48 [DOI] [PubMed] [Google Scholar]
- Tzean Y, Lee MC, Jan HH, Chiu YS, Tu TC, Hou BH, Chen HM, Chou CN, Yeh HH (2019) Cucumber mosaic virus-induced gene silencing in banana. Sci Rep 9:11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unver T, Budak H (2009) Virus-induced gene silencing, a post transcriptional gene silencing method. Int J Plant Genomics 2009:198680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance V, Vaucheret H (2001) RNA silencing in plants-defense and counterdefense. Science 292:2277–2280 [DOI] [PubMed] [Google Scholar]
- Wahyuni WS, Dietzgen RG, Hanada K, Francki RIB (1992) Serological and biological variation between and within subgroup I and II strains of cucumber mosaic virus. Plant Pathol 41:282–297 [Google Scholar]
- Wang R, Yang X, Wang N, Liu X, Nelson RS, Li W, Fan Z, Zhou T (2016) An efficient virus-induced gene silencing vector for maize functional genomics research. Plant J 86:102–115 [DOI] [PubMed] [Google Scholar]
- Wang X, Cao A, Yu C, Wang D, Wang X, Chen P (2010) Establishment of an effective virus induced gene silencing system with BSMV in Haynaldia villosa. Mol Biol Rep 37:967–972 [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang TY, Liao QS, He L, Li J, Zhang HM, Chen X, Li J, Yang J, Li JB, Chen JP (2018) Chinese wheat mosaic virus-induced gene silencing in monocots and dicots at low temperature. Front Plant Sci 9:1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Li C, Yan L, Jackson AO, Liu Z, Han C, Yu J, Li D (2011) A high throughput barley stripe mosaic virus vector for virus induced gene silencing in monocots and dicots. PLoS ONE 6:e26468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yu D, Zhang Y, Liu K, Xu K, Zhang F, Wang J, Tan G, Nie X, Ji Q, Zhao L, Li C (2017) Vacuum and co-cultivation agroinfiltration of (germinated) seeds results in tobacco rattle virus (TRV) mediated whole-plant virus-induced gene silencing (VIGS) in wheat and maize. Front Plant Sci 8:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Handa K, Palukaitis P (1994) Mapping local and systemic symptom determinants of cucumber mosaic cucumovirus in tobacco. J Gen Virol 75:3185–3191 [DOI] [PubMed] [Google Scholar]
- Zhu M, Chen Y, Ding XS, Webb SL, Zhou T, Nelson RS, Fan Z (2014) Maize elongin C interacts with the viral genome-linked protein, VPg, of sugarcane mosaic virus and facilitates virus infection. New Phytol 203:1291–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell H, Payne T, Berry JO, Walsh JA, Carr JP (2007) A cucumber mosaic virus mutant lacking the 2b counter-defence protein gene provides protection against wild-type strains. J Gen Virol 88:2862–2871 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.