Abstract
A rapid enzyme immunoassay (EIA) was developed for the phenotypic detection of diphtheria toxin among clinical isolates of corynebacteria. The assay uses equine polyclonal antitoxin as the capture antibody and an alkaline phosphatase-labeled monoclonal antibody, specific for fragment A of the toxin molecule, as the detecting antibody. The assay is rapid, sensitive, and specific: a final result is available within 3 h of colony selection, and the limits of detection are 0.1 ng of pure diphtheria toxin/ml. Toxigenicity could be detected with isolates grown on a diverse range of culture media, including selective agars. Toxin detection using the EIA was compared to that with the Elek test and PCR detection of fragment A of the diphtheria toxin (tox) gene, using 245 isolates of corynebacteria. The results for the EIA were in complete concordance with those of the Elek test: 87 toxigenic and 158 nontoxigenic isolates. Ten of the phenotypically nontoxigenic strains were found to contain fragment A of the tox gene but did not express the toxin protein. These isolates were found to be nontoxigenic in the Vero cell tissue culture cytotoxicity assay and were therefore nontoxigenic for diagnostic purposes. The EIA is a simple rapid phenotypic test which provides a definitive result on toxigenicity within one working day.
The reemergence of epidemic diphtheria in Russia and the Newly Independent States of the former Soviet Union during the 1990s has highlighted the fact that whenever there is a decrease in immunization coverage rates, epidemic diphtheria can reemerge (12). Within western Europe clinical diphtheria is rare; however, sporadic cases still occur, the majority of which are in travelers from areas of endemicity or epidemicity, such as the former USSR, the Indian subcontinent, Southeast Asia, and South America (5, 28). There has also been a significant increase in the isolation of nontoxigenic Corynebacterium diphtheriae in the United Kingdom and other countries of western Europe (5, 9, 11). These isolates are predominantly associated with sore throat, but cases associated with endocarditis and other systemic diseases have also been reported (18, 29, 31). Reliable, specific, and accurate methods for the detection of diphtheria toxin are therefore essential to differentiate sporadic toxigenic isolates from circulating nontoxigenic isolates.
The ideal test for the detection of toxigenicity should be simple, rapid, reliable, and sensitive and should correlate well with the biological activity of diphtheria toxin. The disadvantages of current methodologies have been documented (7). Many of the phenotypic methods currently available are technically demanding or lacking in sensitivity (7, 10, 30). Although genotypic PCR-based methods for the detection of the toxin gene (21, 22, 24) offer some advantages over phenotypic tests, they do not provide information on the ability of the organism to express biologically active diphtheria toxin and therefore cannot provide a definitive result on toxigenicity (7, 25). Enzyme immunoassays (EIAs) are widely used for the detection of microbial antigens and markers (13, 23, 27). The sensitivity of two-site immunometric EIAs can be improved by the incorporation of signal amplification technology (2, 20). We have therefore developed, standardized, and evaluated an amplified EIA for the rapid phenotypic detection of diphtheria toxin.
MATERIALS AND METHODS
Preparation of microtiter plates and monoclonal antibody conjugate.
Protein G-purified equine polyclonal antitoxin (2.0 μg/ml; Pasteur Mérieux, Lyon, France) was used to coat Nunc Maxisorp microtiter plates (DAKO Ltd., Ely, United Kingdom). Monoclonal antibody, specific to fragment A of the diphtheria toxin molecule, was prepared as described previously (13). Protein G-purified monoclonal antibody was conjugated to alkaline phosphatase (16) and used in the assay at a final concentration of 2 μg/ml. The conjugate buffer formulation was optimized to reduce nonspecific binding and was composed of triethanolamine buffer (pH 8.0) containing ionic detergent (0.1% [vol/vol]), bovine serum albumin (2% [wt/vol]), porcine immunoglobulin G (5.0% [vol/vol]), zinc chloride (0.1 mM), and magnesium chloride (1.0 mM) (DAKO Ltd.).
Bacterial strains.
Corynebacteria were selected from clinical isolates referred to the Streptococcus and Diphtheria Reference Unit, Central Public Health Laboratory, Colindale, London, United Kingdom, between 1988 and 1998. Three control strains were used for the toxigenicity tests: NCTC 10648 (C. diphtheriae biotype gravis; a strong toxin producer), NCTC 3984 (C. diphtheriae biotype gravis; a weak toxin producer), and NCTC 10356 (C. diphtheriae biotype belfanti; nontoxigenic). Ten isolates of C. diphtheriae and Corynebacterium ulcerans were used for the standardization of the EIA. These included six isolates of C. diphtheriae, which produced various amounts of diphtheria toxin in the Vero cell bioassay (7), and four isolates of C. ulcerans, which produced very weak precipitin lines in the Elek immunoprecipitation test. The strains, previously stored at −20°C in 16% (vol/vol) glycerol broth, were inoculated onto Columbia agar (Oxoid, Basingstoke, United Kingdom) supplemented with 5% (vol/vol) horse blood (CBA) and incubated at 37°C in air for 16 to 20 h. Isolates were also cultivated on Hoyle's tellurite agar (Oxoid), Tinsdale agar (Becton Dickinson, Oxford, United Kingdom), or Loeffler's medium (Oxoid) instead of CBA prior to testing in the standardized EIA to determine any effects of diagnostic culture media on the assay.
EIA for the detection of diphtheria toxin. (i) Standardization of inoculum density and incubation time for the preparation of bacterial culture supernatants.
A single colony of each isolate grown on CBA was suspended in 10 ml of brain heart infusion broth (Oxoid) supplemented with 0.4% (vol/vol) yeast extract and 0.2% (vol/vol) Tween 80 and incubated at 37°C in air for 18 h. The overnight suspension was diluted 10-fold (10−1 to 10−7) in 0.5 ml of Elek broth (3) (Elek medium without the addition of agar) supplemented with 16.6% (vol/vol) newborn bovine serum (ICN Biomedicals, Thame, United Kingdom). A standard plate count was used to determine the final cell density. The cultures were incubated at 37°C in air for between 1 and 24 h, after which the bacterial cells were removed by filtration through a 0.22-μm-pore-size membrane (Ultrapure 0.22 μm; Millipore, Watford, United Kingdom). The culture supernatants were stored at −20 or 4°C prior to analysis in the EIA.
(ii) Methodology for the standardized EIA.
Colonies on CBA were suspended in 0.5 ml of Elek broth at a cell density corresponding to McFarland standard no. 1 (108 CFU/ml) and were incubated for 1 h at 37°C in air. The bacterial cells were removed by filtration through a 0.22-μm-pore-size membrane (Ultrapure 0.22 μm), and the culture supernatants were stored at −20 or 4°C until they were analyzed. Two hundred microliters of filtered culture supernatant was added to the wells of a microtiter plate followed by 50 μl of alkaline-phosphatase-labeled monoclonal antitoxin (10 μg/ml). The plates were sealed with a plate sealer (ICN Pharmaceuticals Ltd.) and incubated aerobically at 37°C for 1 h. The plates were washed, and AmpliQ reagent (K6245; DAKO Ltd.) was used for the detection of alkaline phosphatase, in accordance with the manufacturer's instructions. Following a 30-min incubation at 37°C, the reaction was stopped by the addition of 100 μl of 1 M phosphoric acid, and the optical density at 490 nm was measured using an MRX1.2 microtiter plate reader [Dynex Technologies (U.K.) Ltd., Billingshurst, United Kingdom].
Elek immunoprecipitation test.
All isolates were tested for the production of diphtheria toxin by using the modified Elek immunoprecipitation test as described previously (10).
PCR for the detection of the diphtheria toxin gene.
Detection of fragment A of the diphtheria toxin gene (248 bp) was performed on all isolates as described previously (24). An artificial template was added to each reaction as an internal control. The control template contained an internal 58-bp deletion, which allowed it to be distinguished from the natural product by electrophoretic mobility. The presence of the 190-bp amplicon in the negative reaction showed that the PCR had been successful and prevented false negatives. Primers and the internal control oligonucleotide were obtained from Novocastra Laboratories, Newcastle, United Kingdom.
Tissue culture cytotoxicity assay.
The Vero cell cytotoxicity assay for the detection of diphtheria toxin was performed as described previously (7); cell death was determined by visual examination of the cultures using an inverted microscope. The specificity of the cytotoxic effect was confirmed by positive inhibition with equine diphtheria antitoxin (50 μl at 2.5 × 10−3 IU/ml; 66/153, Third British Standard, NIBSC).
RESULTS
Sensitivity of the EIA.
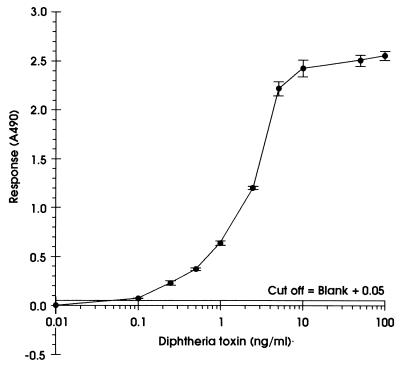
Purified diphtheria toxin [Calbiochem-Novobiochem (U.K.) Ltd., Nottingham, United Kingdom] was used to determine the sensitivity of the EIA. A titration curve is shown in Fig. 1; the limits of detection were found to be 0.1 ng/ml. Microtiter plates containing the culture supernatant and alkaline phosphatase labeled monoclonal antibody could be incubated at either room temperature (approximately 20°C) or 37°C, and either statically or with shaking, without a loss in sensitivity of detection of diphtheria toxin (data not shown). The plates were, therefore, routinely incubated statically at 37°C in air. Detection of alkaline phosphatase activity was always performed statically at 37°C in air.
FIG. 1.
Titration curve for the detection of pure diphtheria toxin using the EIA. Each point is the average (± standard deviation) of three replicate wells.
Effects of inoculum density and incubation time on the detection of toxigenicity.
The effects of inoculum density and incubation time on the detection of toxigenicity in the EIA were initially determined using two strains of C. diphtheriae, NCTC 10648 and NCTC 3954. Tenfold serial dilutions (10−1 to 10−7) of an overnight suspension were made in Elek broth and incubated for 1, 2, 4, 8, 16, and 24 h at 37°C prior to testing in the EIA. Using both a 1- and a 2-h incubation period, the minimum number of cells required for the detection of toxigenicity in the EIA was approximately 106 CFU/ml. An increase in the incubation time permitted detection of toxigenicity at lower inoculum densities. Using a 4- and an 8-h incubation, toxigenicity could be detected with approximately 105 and 103 CFU/ml, respectively, and for incubations of 16 and 24 h, toxigenicity could be detected from the lowest inoculum density examined (1 to 10 CFU/ml).
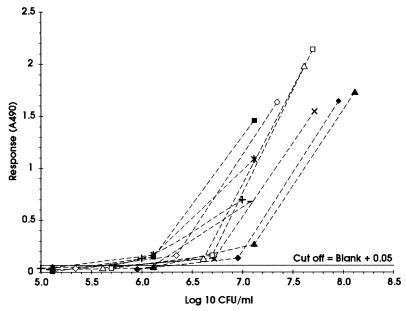
For the EIA to be a potential routine test for toxigenicity, it was desirable that the results be available within one working day. Therefore, the effect of inoculum density on the detection of toxigenicity with a 1-h incubation in Elek broth prior to testing in the EIA was determined using a panel of 10 isolates known to produce various amounts of diphtheria toxin. The results are shown in Fig. 2. The minimum inoculum density which enabled toxigenicity to be detected for all 10 isolates was found to vary (1 × 106 to 1.2 × 107 CFU/ml) according to the isolate being tested. To prevent the occurrence of false negatives due to an insufficient inoculum and to simplify inoculum preparation, an inoculum density corresponding to McFarland standard no. 1 (approximately 108 CFU/ml) was used in the standardized EIA.
FIG. 2.
Effect of variations in inoculum density on the detection of toxigenicity using a 1-h incubation for the preparation of culture supernatants from six isolates of C. diphtheriae (□, ■, ▵, ▴, ◊, and ⧫) that produced various amounts of toxin and four isolates of C. ulcerans (×, ∗, +, and −) that produced very low levels of toxin.
Effects of culture media on the detection of toxigenicity.
The effects of culture media commonly used in the laboratory diagnosis of diphtheria on the detection of toxigenicity using the EIA was determined with 10 isolates of C. diphtheriae and C. ulcerans. The isolates were grown on Hoyle's Tellurite agar, Tinsdale agar, or Loeffler's agar prior to testing in the EIA under the standardized conditions described above (inoculum density corresponding to McFarland standard no. 1 and 1 h of incubation in Elek broth). A positive reaction occurred for all toxigenic isolates tested, irrespective of the medium on which they were grown prior to inoculation into Elek broth (data not shown).
Evaluation of the EIA.
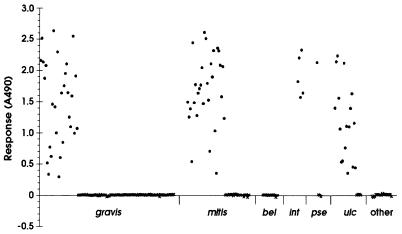
The optimized EIA was evaluated using a selection of 245 isolates of corynebacteria referred to the Streptococcus and Diphtheria Reference Unit (SDRU) between 1988 and 1998. The species and biotypes of the isolates are shown in Table 1 and included representatives of the potentially toxigenic species (C. diphtheriae, C. ulcerans, and Corynebacterium pseudotuberculosis) as well as representatives of other Corynebacterium spp. which are most commonly referred to the SDRU for identification and toxigenicity testing. The results of the determination of toxigenicity using the EIA are shown in Fig. 3. An optical density of 0.05 was used as the cutoff value for the determination of toxigenicity; using this cutoff value, 87 isolates were found to be toxigenic and 158 isolates were found to be nontoxigenic. Interestingly, isolates of C. ulcerans appeared to be the weakest toxin producers, and many of these isolates produced less diphtheria toxin (lower absorbance values) than NCTC 3984, the weakly toxigenic control strain for the Elek test. This finding confirms our previous, unpublished observations that C. ulcerans isolates often produce very weak precipitin lines in both modified and conventional Elek tests for the detection of toxigenicity.
TABLE 1.
Comparison of detection of toxigenicity using EIA, modified Elek test, and PCR detection of fragment A of diphtheria toxin gene
| Species and/or biotype (no. of isolates) | Toxigenicitya | No. of isolates determined by:
|
||
|---|---|---|---|---|
| EIA (3 hb) | Modified Elek (24 h) | PCR for toxA (6 h) | ||
| C. diphtheriae var. gravis (115) | + | 34 | 34 | 34 |
| − | 81 | 81 | 81 | |
| C. diphtheriae var. mitis (54) | + | 29 | 29 | 39 |
| − | 25 | 25 | 15 | |
| C. diphtheriae var. belfanti (12) | + | 0 | 0 | 0 |
| − | 12 | 12 | 12 | |
| C. diphtheriae var. intermedius (5) | + | 5 | 5 | 5 |
| − | 0 | 0 | 0 | |
| C. ulcerans (27) | + | 18 | 18 | 18 |
| − | 9 | 9 | 9 | |
| S. pseudotuberculosis (4) | + | 1 | 1 | 1 |
| − | 3 | 3 | 3 | |
| C. argentoratense (3) | − | 3 | 3 | 3 |
| C. imitans (3) | − | 3 | 3 | 3 |
| C. pseudodiphtheriticum (12) | − | 12 | 12 | 12 |
| C. amycolatum (4) | − | 4 | 4 | 4 |
| C. striatum (6) | − | 6 | 6 | 6 |
| Total (245) | + | 87 | 87 | 97 |
| − | 158 | 158 | 148 | |
+, toxigenic; −, nontoxigenic.
Time taken to obtain result.
FIG. 3.
Determination of toxigenicity among 245 isolates of corynebacteria by using the EIA. ●, toxigenic isolates; ×, nontoxigenic isolates; gravis, C. diphtheriae biotype gravis; mitis, C. diphtheriae biotype mitis; bel, C. diphtheriae biotype belfanti; int, C. diphtheriae biotype intermedius; pse, C. pseudotuberculosis; ulc, C. ulcerans; other, other corynebacteria (Corynebacterium argentoratense, Corynebacterium imitans, Corynebacterium pseudodiphtheriticum, Corynebacterium amycolatum, and Corynebacterium striatum).
The detection of toxigenicity using the EIA was compared to that with two other methods frequently used for the detection of diphtheria toxin—the Elek immunoprecipitation test (10) and PCR for the detection of fragment A of the toxin gene (24). The results are shown in Table 1. The EIA showed 100% correlation with the modified Elek test but provided a result within 3 h of colony selection in comparison to 24 h for the modified Elek test and 48 h for the conventional Elek test. In addition to its speed, the EIA was more sensitive than the Elek test, and interpretation of the results was simpler. Isolates that produced very weak precipitin lines in the Elek test produced a strong color reaction in the EIA and could easily be distinguished from nontoxigenic isolates on the basis of both microtiter plate readings and visual interpretation. Ten of 245 isolates (5%) were negative in the EIA and Elek test but gave a positive result for the PCR detection of the toxin gene. These strains were also tested in the Vero cell cytotoxicity assay and were found to be nontoxigenic.
The sensitivity of the EIA was determined using 55 of the 245 isolates that previously had been tested in the Vero cell cytotoxicity assay and the subcutaneous virulence test in guinea pigs (7). The results for the EIA showed 100% correlation with both assays that detected the biological activity of diphtheria toxin. Twenty-six isolates were identified as toxigenic, and 29 isolates were identified as nontoxigenic. The sensitivity of the EIA was 100% (95% confidence interval, 83.2 to 100%).
DISCUSSION
The detection of toxigenicity is the most important test in the laboratory diagnosis of diphtheria and should be initiated without delay following the isolation of any suspicious colonies. The phenotypic tests currently available for the detection of toxigenicity tend to be technically demanding or lacking in sensitivity (7) or have limited evaluations (30). In general, these methods require at least 16 to 24 h from selection of colonies to a final result. The delay between isolation of a suspicious organism and the results of toxigenicity testing can provoke great anxiety among laboratory staff, clinicians, and public health officials.
PCR detection of the diphtheria toxin gene is the most rapid method for the detection of toxigenicity; using pure cultures, a result is available within 4 to 5 h of colony selection, and detection of the toxin gene directly from clinical specimens has also been described (22). Although some studies have shown good correlation between genotypic (PCR) and phenotypic methods for the detection of toxigenicity (1, 14, 21, 22), other studies have identified isolates which possess the toxin gene but do not express a biologically and/or immunologically active form of the toxin molecule (7, 25). In this study, 10 of 245 isolates (5%) were negative in the phenotypic and biological assays used (EIA, Elek test, and tissue culture cytotoxicity assay) but gave a positive result for PCR detection of the toxin gene. These strains included six isolates from outbreaks of pharyngitis in the northern United States and Canada, described previously (7), and a further four isolates from the recent diphtheria epidemic in the former USSR. Such isolates appear to be relatively rare and have previously been reported from specific geographic locations; however, as the diphtheria epidemic diminishes in the former USSR, these isolates are being isolated in increasing numbers in many countries within eastern Europe (8). Current recommendations are that PCR should be used only in conjunction with a phenotypic test (6, 8). Although an accurate negative PCR result may be useful in the rapid exclusion of toxigenicity, a positive PCR result will require confirmation with a phenotypic test, which will consequently lead to a delay in the final result.
The EIA described is a rapid, sensitive, and simple method for the detection of diphtheria toxin. The limits of detection are 0.1 ng/ml, and a result is available within 3 h of colony selection. Toxigenicity can be determined from isolates grown on a variety of media, including selective agars, such as Hoyle's Tellurite agar and Tinsdale agar, used for the isolation and screening of potentially toxigenic corynebacteria. Standardization of inoculum density and incubation time in Elek broth was essential to ensure the accurate detection of toxigenicity, particularly among weak toxin-producing isolates. These two factors affected the amount of diphtheria toxin released into the culture supernatant. We found that a short, 1-h incubation in Elek broth could be used, provided an inoculum density of greater than approximately 107 CFU/ml was used. A slightly higher inoculum density of 108 CFU/ml (McFarland standard no. 1) was used in the standardized EIA to eliminate false negatives due to the use of a low inoculum of a toxigenic strain. The inoculum density of 108 CFU/ml was easily achieved from both agar plate and slope cultures.
For inoculum densities of less than 107 CFU/ml, longer incubation times in Elek broth were required to ensure the production of adequate toxin for detection in the EIA. Using a 16-h incubation, toxigenicity could be detected from the lowest inoculum density tested, which corresponded to approximately 1 to 10 CFU/ml. This indicated that the EIA could be used for the detection of toxigenicity directly from clinical specimens. However, the effect of inhibitors and of the presence of other organisms in the clinical specimen should be fully evaluated before the assay undergoes a field evaluation in an area of endemicity or epidemicity.
EIAs for the detection of diphtheria toxin have been previously documented (13, 23, 27). The majority of these assays used similar designs (horse polyclonal antitoxin as the capture antibody and a mouse monoclonal antibody as the detecting antibody), with the exception of that of Hallas et al. (13), who used two monoclonal antibodies specific to fragment A as both the detecting and capture antibodies. The capture enzyme-linked immunosorbent assay method described by Nielsen et al. (23) and the sandwich dot immunobinding method of Peitrzak et al. (27) both reported limits of detection of approximately 10 ng/ml, and incubation times of 18 and 24 h, respectively, were required to ensure no false negatives. The assay described by Hallas et al. (13) showed good sensitivity, with limits of detection of 88 pg/ml; however, the final result was not available until 2 days after the selection of colonies and false-positive results were obtained for 4 of the 78 isolates tested. The amplified EIA we have described offers a number of advantages in comparison with these methods: the limits of detection are 0.1 ng/ml, a final result is available within 3 h of colony selection, and no false positives or negatives were detected among the 245 isolates tested. The incorporation of the DAKO signal amplification technology offered increased assay sensitivity compared to conventional EIAs. Amplified immunoassays of this format have demonstrated good clinical sensitivity (4, 15), providing sensitivity equivalent to that of molecular amplification methods (26).
The amplified EIA developed in this study is a rapid, simple, and specific method with which a definitive toxigenicity result can be determined within one working day. As such, the EIA should contribute significantly to the laboratory diagnosis of diphtheria on a global basis. The assay can be used for the rapid testing of sporadic isolates or for batch testing a larger number of isolates within areas of the world where diphtheria is endemic or epidemic.
ACKNOWLEDGMENTS
This work was supported by a Research Grant from the U.K. Home Office.
We thank Robert C. George for his support and encouragement throughout this study and critical review of the manuscript. We are also grateful to Andrei Malik and Mark Dunn for their assistance, in particular in the provision of coated plates, conjugates, and amplification reagents and critical review of the manuscript.
REFERENCES
- 1.Aravena-Roman M, Bowman R, O'Neill G. Polymerase chain reaction for the detection of toxigenic Corynebacterium diphtheriae. Pathology. 1995;27:71–73. doi: 10.1080/00313029500169512. [DOI] [PubMed] [Google Scholar]
- 2.Bates D L. Enzyme amplification: the quest for the zeptomole. Int Labmate. 1995;20:11–13. [Google Scholar]
- 3.Colman G, Weaver E, Efstratiou A. Screening tests for pathogenic corynebacteria. J Clin Pathol. 1992;45:46–48. doi: 10.1136/jcp.45.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley T, Milne D, Arumainayagam J T, Paul I D, Caul E O. The laboratory diagnosis of male Chlamydia trachomatis infections—a time for change? J Infect. 1992;25:69–75. doi: 10.1016/0163-4453(92)92099-5. [DOI] [PubMed] [Google Scholar]
- 5.Efstratiou A, George R C. Microbiology and epidemiology of diphtheria. Rev Med Microbiol. 1996;7:31–42. [Google Scholar]
- 6.Efstratiou A, George R C. Laboratory guidelines for the diagnosis of infections caused by Corynebacterium diphtheriae and C. ulcerans. Commun Dis Public Health. 1999;2:250–257. [PubMed] [Google Scholar]
- 7.Efstratiou A, Engler K H, Dawes C S, Sesardic D. Comparison of phenotypic and genotypic methods for the detection of diphtheria toxin among isolates of pathogenic corynebacteria. J Clin Microbiol. 1998;36:3173–3177. doi: 10.1128/jcm.36.11.3173-3177.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efstratiou A, Engler K H, Mazurova I K, Glushkevich T, Vuopio-Varkila J, Popovic T. Current approaches to the laboratory diagnosis of diphtheria. J Infect Dis. 2000;181(Suppl. 1):S138–S145. doi: 10.1086/315552. [DOI] [PubMed] [Google Scholar]
- 9.Efstratiou A, George R G, Begg N T. Non-toxigenic C. diphtheriae in England. Lancet. 1993;341:1592–1593. doi: 10.1016/0140-6736(93)90727-x. [DOI] [PubMed] [Google Scholar]
- 10.Engler K H, Glushkevich T, Mazurova I K, George R C, Efstratiou A. A modified Elek test for the detection of toxigenic corynebacteria. J Clin Microbiol. 1997;35:495–498. doi: 10.1128/jcm.35.2.495-498.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funke G, Altwegg M, Frommelt L, von Gravenitz A. Emergence of related nontoxigenic C. diphtheriae biotype mitis strains in Western Europe. Emerg Infect Dis. 1999;5:477–480. doi: 10.3201/eid0503.990326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galazka A M, Robertson S E, Oblapenko G P. Resurgence of diphtheria. J Epidemiol. 1995;11:95–105. doi: 10.1007/BF01719954. [DOI] [PubMed] [Google Scholar]
- 13.Hallas G, Harrison T G, Samuel D, Colman G. Detection of diphtheria toxin in culture supernates of Corynebacterium diphtheriae and C. ulcerans by immunoassay with monoclonal antibody. J Med Microbiol. 1990;32:247–253. doi: 10.1099/00222615-32-4-247. [DOI] [PubMed] [Google Scholar]
- 14.Hauser D, Popoff M R, Kiredjian M, Boquet P, Bimet F. Polymerase chain reaction assay for diagnosis of potentially toxinogenic Corynebacterium diphtheriae strains: correlation with ADP-ribosylation activity assay. J Clin Microbiol. 1993;31:2720–2723. doi: 10.1128/jcm.31.10.2720-2723.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hay P E, Thomas B J, Gilchrist E, Palmer H M, Gilroy C B, Taylor-Robinson D. The value of urine samples from men with non-gonococcal urethritis for the detection of Chlamydia trachomatis. Genitourin Med. 1991;67:124–128. doi: 10.1136/sti.67.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa E, Imagawa M, Hashida S, Yoshitake S, Hamaguchi Y, Ueno T. Enzyme-labeling of antibodies and their fragments for enzyme immunoassay and immunohistochemical staining. J Immunoassay. 1983;4:209–327. doi: 10.1080/15321818308057011. [DOI] [PubMed] [Google Scholar]
- 17.Jalgaonkar S V, Saoji A M. Coagglutination for rapid testing of toxin producing Corynebacterium diphtheriae. Indian J Med Res. 1993;97:35–36. [PubMed] [Google Scholar]
- 18.Lortholary O, Buu-Hoe A, Gutman L, Acar J. Corynebacterium diphtheriae endocarditis in France. Clin Infect Dis. 1995;31:63–65. doi: 10.1093/clinids/17.6.1072. [DOI] [PubMed] [Google Scholar]
- 19.Lucchini G M, Gruner E, Altwegg M. Rapid detection of diphtheria toxin by the polymerase chain reaction. Med Microbiol Lett. 1992;1:276–283. [Google Scholar]
- 20.Markham R, Young L, Fraser I S. Enzyme amplification of ELISA. Aust J Med Sci. 1996;17:37–40. [Google Scholar]
- 21.Mikhailovich V M, Melnikov V G, Mazurova I K, Wachsmuth I K, Wenger J D, Wharton M, Nakao H, Popovic T. Application of PCR for detection of toxigenic Corynebacterium diphtheriae strains isolated during the Russian diphtheria epidemic, 1990 through 1994. J Clin Microbiol. 1995;33:3061–3063. doi: 10.1128/jcm.33.11.3061-3063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakao H, Popovic T. Development of a direct PCR for detection of the diphtheria toxin gene. J Clin Microbiol. 1997;7:1651–1655. doi: 10.1128/jcm.35.7.1651-1655.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen P B, Koch C, Friss H, Heron I, Prag J, Schmidt J. Double-sandwich enzyme-linked immunosorbent assay for rapid detection of toxin-producing Corynebacterium diphtheriae. J Clin Microbiol. 1987;25:1280–1284. doi: 10.1128/jcm.25.7.1280-1284.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pallen M J. Rapid screening for toxigenic Corynebacterium diphtheriae by the polymerase chain reaction. J Clin Pathol. 1991;44:1025–1026. doi: 10.1136/jcp.44.12.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pallen M J, Hay A J, Puckey L H, Efstratiou A. Polymerase chain reaction for screening clinical isolates of corynebacteria for the production of diphtheria toxin. J Clin Pathol. 1994;47:353–356. doi: 10.1136/jcp.47.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul I D. Chlamydia trachomatis: a review of its laboratory diagnosis and a preliminary evaluation of a new DNA based assay. Public Health Lab Serv Microbiol Dig. 1996;13:223–226. [Google Scholar]
- 27.Pietrzak J, Muehlestein S, Gasser M. Sandwich-dot immunobinding assay (sandwich-DIA), a new immunological method for the detection of diphtheria toxin. Zentbl Bakteriol. 1990;274:61–69. doi: 10.1016/s0934-8840(11)80975-8. [DOI] [PubMed] [Google Scholar]
- 28.Public Health Laboratory Service. Diphtheria acquired during a cruise in the Baltic Sea. Commun Dis Rep CDR Weekly. 1997;7:207. [PubMed] [Google Scholar]
- 29.Tiley S M, Kokiuba K R, Heron L G, Munro R. Infective endocarditis due to non-toxigenic Corynebacterium diphtheriae: report of seven cases and review. Clin Infect Dis. 1993;16:271–275. doi: 10.1093/clind/16.2.271. [DOI] [PubMed] [Google Scholar]
- 30.Toma C, Sisvath L, Iwanaga M. Reversed passive latex agglutination assay for detection of toxigenic Corynebacterium diphtheriae. J Clin Microbiol. 1997;35:3147–3149. doi: 10.1128/jcm.35.12.3147-3149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuber P L F, Gruner E, Altwegg M, von Graevenitz A. Invasive infection with non-toxigenic C. diphtheriae among drug users. Lancet. 1992;339:1359. doi: 10.1016/0140-6736(92)92004-y. [DOI] [PubMed] [Google Scholar]