Abstract
Introduction
Studies using different assays and technologies showed highly promising diagnostic value of plasma phosphorylated (P-)tau levels for Alzheimer’s disease (AD). We aimed to compare six P-tau Simoa assays, including three P-tau181 (Eli Lilly, ADx, Quanterix), one P-tau217 (Eli Lilly), and two P-tau231 (ADx, Gothenburg).
Methods
We studied the analytical (sensitivity, precision, parallelism, dilution linearity, and recovery) and clinical (40 AD dementia patients, age 66±8years, 50%F; 40 age- and sex-matched controls) performance of the assays.
Results
All assays showed robust analytical performance, and particularly P-tau217 Eli Lilly; P-tau231 Gothenburg and all P-tau181 assays showed robust clinical performance to differentiate AD from controls, with AUCs 0.936–0.995 (P-tau231 ADx: AUC = 0.719). Results obtained with all P-tau181 assays, P-tau217 Eli Lilly assay, and P-tau231 Gothenburg assay strongly correlated (Spearman’s rho > 0.86), while correlations with P-tau231 ADx results were moderate (rho < 0.65).
Discussion
P-tau isoforms can be measured robustly by several novel high-sensitive Simoa assays.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-021-00939-9.
Keywords: Alzheimer’s disease, Blood biomarkers, P-tau181, P-tau217, P-tau231, Phosphorylated tau proteins, Simoa, Ultra-sensitive immunoassays, Analytical validation, Clinical validation
Background
Alzheimer’s disease (AD) neuropathology is defined by amyloid-β (Aβ) accumulation in extracellular plaques and hyper-phosphorylated tau (P-tau) accumulation in neurofibrillary tangles [1–3]. Aβ and tau pathology can be visualized on positron emission tomography (PET) or in the cerebrospinal fluid (CSF) to assist AD diagnosis [1]. However, these methods are invasive, expensive, and not widely available in care settings, which hinder implementation and use. Therefore, there is a strong interest to develop blood-based biomarkers for AD.
Different studies using different assays and technologies showed that plasma P-tau isoforms, such as P-tau181, P-tau217, and P-tau231 are highly accurate and specific for detection of PET-confirmed Aβ and tau pathology across the clinical AD continuum [4–12]. Furthermore, plasma P-tau181, P-tau217, and P-tau231 have been reported to be already increased in Aβ-PET-positive but still tau-PET-negative individuals [5, 7, 11], suggesting sensitivity for early Alzheimer’s pathology. Moreover, plasma P-tau has strong value in differential diagnosis, with high accuracy for discriminating patients with AD from frontotemporal lobar degeneration [4, 5, 8, 11–14]. In addition, among patients with dementia with Lewy bodies, plasma P-tau identifies amyloid co-pathology [14, 15].
There is a debate whether specific P-tau isoforms have favorable accuracies for diagnosis of AD dementia. For example, some studies in CSF showed higher accuracy of P-tau217 than P-tau181 in CSF to detect AD pathological changes [16, 17]. Likewise, in a comparative plasma study, it was suggested that plasma P-tau217 might be a better biomarker as compared to P-tau181 [8], though this was not observed by an independent study with an updated form of this P-tau181 assay [18]. Plasma P-tau231 on the other hand was suggested to have greatest potential to detect AD pathology in the earliest disease stages [11]. The different plasma P-tau assays that are currently available employ different platforms and different antibody pairs for detection of the different isoforms. It is not yet known whether there are technical reasons, such as differences in binding affinities and specificity of antibodies or sensitivity of the platforms or biological reasons that explain potential differences in clinical performance between the P-tau isoforms.
Here, we present a head-to-head analytical and clinical comparison of six novel P-tau assays that were developed on the high-sensitivity Simoa platform, including three different P-tau181 assays (Eli Lilly, ADx NeuroSciences, and Quanterix), one P-tau217 assay (Eli Lilly), and two P-tau231 assays (ADx NeuroSciences, Gothenburg).
Methods
Plasma samples
For the analytical validation, left-over K2EDTA plasma of routine diagnostic measurements at the Clinical Chemistry department was used. No clinical data were collected. The plasma tubes were centrifuged at 1800xg for 10 min. To reach a high volume, the plasma of different tubes derived from different individuals was pooled. The different plasma pools were aliquoted into polypropylene tubes (Sarstedt, Germany) and stored at −80°C until use.
For the clinical validation, we selected 40 participants with AD dementia from the Amsterdam Dementia Cohort [19, 20] (average±standard deviation (SD) age 66±8 years, n = 20 (50%) female), who were diagnosed according the NIA-AA diagnostic AD criteria [21] and had a CSF biomarker-confirmed AD diagnosis. We selected 40 age- and sex-matched (age 66±8 years, n = 20 (50%) female) cognitively healthy control participants from the Dutch Brain Research Registry (Hersenonderzoek.nl; CSF AD biomarker status unknown) [22]. The minimal sample size required was calculated based on three different studies, one study for each of the three P-tau forms that were measured in our study (P-tau181, P-tau217, P-tau231) [4, 11, 23]. The power calculation showed that a study with a minimum sample size of 4–8 participants per group would be sufficient to achieve a power (β) of 80% and a level of significance (α, two sided) of 5% for detecting true differences in values between the patients with AD and the controls. K2EDTA plasma samples were obtained from all participants through venipuncture. After a 10-min centrifugation at 1800xg, plasma was aliquoted into 0.5-mL portions in polypropylene storage tubes (Sarstedt, Germany) and stored at −80°C until use.
Prior to the P-tau analyses, plasma samples were shortly thawed, vortexed, and centrifuged at 10,000xg for 10 min. All analyses were performed on the Simoa HD-X platform (Quanterix, Billerica, MA, USA) according to manufacturer’s instructions. All measurements for analytical and clinical validation of the P-tau assays were performed at the Neurochemistry Laboratory, Amsterdam UMC, VUmc, Amsterdam, the Netherlands, except for the P-tau231 Gothenburg assay, for which the measurements were performed at the Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden. All P-tau analyses were performed in duplicates.
P-tau assays
The study included three P-tau181 assays (Eli Lilly, ADx, and Quanterix), one P-tau217 assay (Eli Lilly), and two P-tau231 assays (ADx, Gothenburg). The P-tau181 assay of Quanterix is commercially available (#103714, Simoa® P-tau-181 V2 Advantage Kit), which is a modification of an earlier published set-up using the same antibodies and calibrator [5]. Both Eli Lilly assays are prototype Simoa assays based on the earlier published Meso Scale Discovery (MSD) assay set-up [18]. Both ADx assays are new prototype assays including in-house developed antibodies. The P-tau231 Gothenburg assay is a prototype assay developed by the Neurochemistry Laboratory of the University of Gothenburg [11]. Assay characteristics are summarized in Table 1.
Table 1.
Analytical characteristics of the six P-tau assays
| P-tau181 | P-tau181 | P-tau181 | P-tau217 | P-tau231 | P-tau231 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay characteristics | |||||||||||||
| Assay | Provider | Eli Lilly | ADx NeuroSciences | Quanterix | Eli Lilly | ADx NeuroSciences | Gothenburg (NA) | ||||||
| Status | Prototype | Prototype | Commercial | Prototype | Prototype | Prototype | |||||||
| Catalogue number | N/A | N/A | 103714 | N/A | N/A | N/A | |||||||
| Biofluid | EDTA plasma | EDTA plasma | EDTA plasma | EDTA plasma | EDTA plasma | EDTA Plasma /Serum | |||||||
| Platform | Simoa | Simoa HD-X | Simoa HD-X | Simoa HD-X | Simoa HD-X | Simoa HD-X | Simoa HD-x/HD-1 | ||||||
| Antibodies | Name capture | AT270 | ADx252 | AT270 | FAb2 of IBA493 | ADx253 | ADx253 | ||||||
| Epitope capture (AA) according to tau 441 numbering | Sequence 176-PPAPKT(p)P-182 phosphorylated specifically at threonine-181 | Phospho-Thr 181 and no cross-reactivity with phospho-Thr175 | Sequence 176-PPAPKT(p)P-182 phosphorylated specifically at threonine-181 | Peptide phosphorylated at Thr217 | Phosphorylated tau at T231 | Phosphorylated tau at T231 | |||||||
| Name detector | LRL | ADx204 | Tau12 | 4G10E2 | ADx204 | Tau12 | |||||||
| Epitope detector (AA) | 111–130 according to the Tau441 sequence | N-terminal, that recognizes all forms of tau except those phosphorylated at Tyr 18 | N-terminal epitope 6-QEFEVMEDHAGT-18 | 111–130 according to the Tau441 sequence | N-terminal, that recognizes all forms of tau except those phosphorylated at Tyr 18 | N-terminal epitope 6-QEFEVMEDHAGT-18 | |||||||
| Assay protocol | Steps | 2-step assay | 2-step assay | 2-step assay | 3-step assay | 2-step assay | 3-step assay | ||||||
| Incubation times, min | 60-5 | 60-10.5 | 35-5 | 30-5.15-5.15 | 60-5.15 | 40-7-7 | |||||||
| Sample/calibrator volume, μL | 100 | 135 | 100 | 100 | 100 | 100 | |||||||
| Beads volume, μL | 25 | 25 | 25 | 25 | 25 | 25 | |||||||
| Detector volume, μL | 20 | 20 | 20 | 100 | 20 | 100 | |||||||
| SBG volume, μL | 100 | 100 | 100 | 100 | 100 | 100 | |||||||
| Assay reagents | Helper beads, % of beads | 66% | 50% | 60% | 50% | 50% | 0% | ||||||
| SBG, pM | 150 pM | 50pM | 150 pM | 150 pM | 50 pM | 300 | |||||||
| Detector, μg/mL | 1 | 0.6 | Unknown | 0.1 | 0.6 | 2 | |||||||
| Calibrator | Type | Synthetic peptide | Synthetic peptide | Recombinant protein | Synthetic peptide | Synthetic peptide | Recombinant protein | ||||||
| No. of calibrator points | 9 | 8 | 7 | 8 | 8 | 8 | |||||||
| Range, pg/mL | 0.226-52 | 0.625-50 | 0.177-86 | 0.04-180 | 0.3125-40 | 0-64 | |||||||
| Curve fit | 1/y2-weighted 5PL | 1/y2-weighted 5PL | 1/y2-weighted 4PL | 1/y2-weighted 4PL | 1/y2-weighted 5PL | 1/y2-weighted 4PL | |||||||
| Sample dilution | Fold-dilution | 4 | 5 | 4 | 2 | 5 | 2 | ||||||
| Recommended method | Automated | Manual | Automated | Automated | Manual | Automated | |||||||
| Assay sensitivity and precision results | |||||||||||||
| Sensitivity | Analytical LLOQ, pg/mL | 1.55 | 2.36 | 0.24 | 0.15 | 0.56 | 3.95 | ||||||
| Functional LLOQ, pg/mL | 6.2 | 11.8 | 0.96 | 0.3 | 2.8 | 7.9 | |||||||
| Concentrations of QC and KC panels | QC1: high, pg/mL | 15 | 26.8 | 3.8 | 2 | 7.4 | 26.7 | ||||||
| QC2: intermediate, pg/mL | 6.9 | 13.3 | 1.3 | 0.6 | 4.7 | 17.1 | |||||||
| QC3: low, pg/mL | 5.9 | 9.6 | 1.1 | 0.2 | 2.6 | 8.3 | |||||||
| KC1, pg/mL | 4.05 | 9.75 | 3.22 | 0.64 | 1.41 | NA | |||||||
| KC2, pg/mL | 16.69 | 17.87 | 70.35 | 1.99 | 5.72 | NA | |||||||
| KC3, pg/mL | 142.01 | N/A | N/A | 61.6 | N/A | NA | |||||||
| Precision QCs | Average Intra-assay %CV | 6.6 | 14.5 | 7.7 | 13.5 | 16.8 | 3.7 | ||||||
| Average Inter-assay %CV | 10 | 15.2 | 19.5 | 14.1 | 27.7 | 5.1 | |||||||
| Precision KCs | Average Intra-assay %CV | 5.6 | 16 | 6 | 9 | 19.9 | NA | ||||||
| Average Inter-assay %CV | 9 | 23 | 29.5 | 10.5 | 30.5 | NA | |||||||
| Clinical samples measurements | Number | 80 | 80 | 80 | 80 | 80 | 80 | ||||||
| Range concentration, pg/mL | 2.69–21.65 | 1.91–77.29 | 0.89–8.65 | 0.04–1.93 | 1–16.07 | 5.68–25.8 | |||||||
| Within calibrator range, % | 100% | 100% | 100% | 100% | 100% | 100% | |||||||
| Range, CV% | 0.00–23.23 | 0.33–69.08 | 0.1–15.91 | 0.07–64 | 0.05–51.34 | 0.01–14.49 | |||||||
| Average CV% | 5.74 | 12.20 | 5.83 | 14.20 | 8.25 | 3.35 | |||||||
| n measured <LLOQ | 21 | 14 | 1 | 39 | 1 | 7 | |||||||
| n measured > 20%CV | 1 | 13 | 0 | 16 | 3 | 0 | |||||||
| Other validation results | |||||||||||||
| Parallelism | Average slope of samples | 0.67 | 0.43 | 0.49 | 0.59 | 0.61 | 0.90 | ||||||
| Range of slopes of samples | 0.55–0.75 | 0.33–0.61 | 0.39–0.61 | 0.53–0.68 | 0.48–0.72 | 0.85–0.97 | |||||||
| Average slope of calibrator | 0.67 | 0.39 | 0.52 | 0.60 | 0.72 | 0.78 | |||||||
| Parallelism, % | 99.6 | 110.3 | 94.6 | 98.7 | 84 | 116 | |||||||
| Dilution linearity | Spiked concentration, pg/ml | 150 | 150 | 150 | 11 | 150 | |||||||
| Df (x) | Mean %L | Df (x) | Mean %L | Df (x) | Mean %L | Df (x) | Mean %L | Df (x) | Mean %L | Df (x) | Df (x) | ||
| Linear dilution factor with mean %Linearity | 1 | - | 1 | - | 1 | - | 1.00 | - | 1 | - | 1 | 1 | |
| 5 | 173 | 5 | 54 | 5 | 200 | 2.80 | 118 | 5 | 147 | 5 | 5 | ||
| 25 | 132 | 25 | 139 | 25 | 117 | 7.84 | 125 | 25 | 81 | 25 | 25 | ||
| 125 | 120 | 125 | 117 | 125 | 118 | 21.95 | 115 | 125 | 112 | 125 | 125 | ||
| 625 | 294 | 625 | 376 | 625 | 153 | 61.47 | 109 | 625 | 150 | 625 | 625 | ||
| 3125 | 426 | 3125 | 541 | 3125 | 265 | 172.10 | 134 | 3125 | 196 | 3125 | 3125 | ||
| Recovery | Spike | Mean %R | Spike | Mean %R | Spike | Mean %R | Spike | Mean %R | Spike | Mean %R | Spike | Mean %R | |
|
Spiked concentration (pg/mL) With mean %Recovery |
0.8 | 102 | 0.85 | 54 | 0.8 | 72 | 0.4 | 108 | 0.85 | 131 | 1 | 150 | |
| 4.0 | 95 | 4.27 | 67 | 4.0 | 82 | 1 | 107 | 4.27 | 139 | 6 | 124 | ||
| 20.0 | 149 | 21.33 | 67 | 20.0 | 83 | 4 | 113 | 21.33 | 147 | 24 | 113 | ||
Phospho-specific antibody FAb2 of IBA493 and anti-tau antibodies LRL and 4G10E2 are property of Eli Lilly and Company. Phospho-specific antibody ADx252, ADx253, and anti-tau antibody ADx204 are property of ADx NeuroSciences. Phospho-specific AT270 is of ThermoFischer Scientific, and Tau-specific Tau12 is of Sigma Aldrich. Analytical LLOQ was calculated as the mean signal of 16 blanks plus 10 times the SD, with the P-tau concentration extrapolated from the calibration curve. This was multiplied by the sample dilution factor to obtain the functional LLOQ. QC samples are EDTA plasma pools and specific to each assay. KC samples were from the providers and specific to each assay, either synthetic peptide or recombinant protein spiked in buffer (both Eli Lilly assays and P-tau181 Quanterix assay, respectively) or remnant EDTA plasma sample (both ADx assays). KCs were not available for the P-tau231 Gothenburg assay. Average intra-and inter-assay variation was derived from measuring the QC and KC panels over four independent runs (except only two runs for the high QC sample with the P-tau181 ADx). With each assay, 80 clinical samples were measured, but due to technical reasons duplicate results were obtained, for 66 samples with P-tau181 Eli Lilly, for 74 with P-tau181 ADx, for 79 with P-tau217 Eli Lilly, for 79 with P-tau231 ADx and for 74 with P-tau231 Gothenburg. No results were obtained for 3 samples with P-tau181 Eli Lilly, for 1 sample with P-tau181 ADx and for 3 samples with P-tau231 Gothenburg. For parallelism, with each assay, four samples were measured after being four-times 2-fold serially diluted (P-tau181 Eli Lilly, P-tau181 ADx, and P-tau231 ADx: starting dilution 5-fold, reaching 40-fold; P-tau181 Quanterix: starting dilution 4-fold, reaching 32-fold; P-tau217 Eli Lilly and P-tau231 Gothenburg: starting dilution 2-fold, reaching 16-fold). For dilution linearity, three samples were spiked with high recombinant protein concentration, subsequently measured undiluted, and serially diluted until low P-tau concentrations below the LLOQs of the assays. With the P-tau181 ADx assay, two out of three of the undiluted samples were not measurable, likely due to matrix effect. With the P-tau231 Gothenburg, signals were not detected for the lowest two dilutions with the three samples
P-tau Phosphorylated tau, SBG Streptavidin β-galactosidase, PL Polynomial, LLOQ Lower limit of quantification, QC Quality control, KC Kit control, CV Coefficient of variation, %L % linearity, %R % recovery, NA Not applicable
All assays use different capture and detector antibodies or different combinations of them. All assays were calibrated with seven to nine calibrator points using a synthetic peptide, except the P-tau181 Quanterix assay that used a recombinant protein (full-length recombinant tau1-441 phosphorylated in vitro by glycogen synthase kinase 3β; TO8–50FN; SignalChem, Vancouver, BC, Canada) [5]. For the Eli Lilly assays (P-tau181 and P-tau217), two different synthetic peptides were used. For both ADx assays (P-tau181 and P-tau231), one single synthetic peptide with a phosphorylation on both threonine 181 and 231 was used. For P-tau231 Gothenburg, full-length recombinant tau 441 phosphorylated in vitro by glycogen synthase kinase 3β was used as the calibrator.
Analytical validation of the P-tau assays
We validated the sensitivity (lower limit of quantification; LLOQ), precision, parallelism, dilution linearity, and recovery of all P-tau assays according to the method developed by the BIOMARKAPD consortium [23] (details in Table 1).
For each assay, LLOQ was calculated from the mean signal of 16 blanks plus 10x SD, with the P-tau concentration interpolated from the calibration curve (i.e., the analytical LLOQ) and subsequently multiplied by the sample dilution factor (i.e., the functional LLOQ). Intra-assay precision was derived from the duplicate measurements of the 80 clinical samples. Inter-assay precision was calculated by measuring assay-specific quality control (QC) panels of three pooled EDTA plasma samples (high, medium, and low) and two or three kit controls (provided by the manufacturers; except for the P-tau231 Gothenburg assay that did not include KCs) over four runs (except for QC high with P-tau181 ADx, which was measured twice). Parallelism was calculated as the average %-agreement of the slope of the assay calibrator with the slopes of four and 4-times serially diluted plasma samples. For P-tau181 Eli Lilly, P-tau181 ADx, and P-tau231 ADx, the starting dilution was 5-fold, with a subsequent 2-fold serial dilution until 40-fold. For P-tau181 Quanterix, the starting dilution was 4-fold with a subsequent 2-fold dilution until 32-fold. For P-tau217 Eli Lilly and P-tau231 Gothenburg assays, the starting dilution was 2-fold, with a subsequent 2-fold serial dilution until 16-fold. Parallelism results measured below the LLOQ were not excluded from the calculations. Dilution linearity was assessed using three samples with an assay-specific spike that were measured undiluted and serially diluted until ultimately low levels below LLOQ were reached. The average %-agreement of P-tau concentration in a serially diluted sample was calculated in comparison to the P-tau concentration measured in the previous dilution. Recovery of each assay was determined by measuring P-tau in four plasma samples that were diluted according to their standard dilution factor and spiked with a low, medium, and high spike of the assay-specific calibrator, in comparison to non-spiked sample.
Data analysis
Acceptance criteria were < 20% coefficient of variation (CV) for precision, or between 80 and 120% for %-agreement calculations. For the clinical results, we used Spearman’s correlations to assess the agreement of P-tau measurements between the assays. We used non-parametric Mann-Whitney U tests to compare P-tau levels between the AD dementia and control groups. Since we included 6 assays in this study for P-tau measurement, the p value that we considered significant for these group comparisons after Bonferroni correction was p < 0.05/6 = p < 0.0083. We applied receiver operating characteristic curve (ROC) analysis to calculate the accuracy of the P-tau assays to discriminate between AD dementia and controls and calculated cutoffs at the Youden’s indices [24]. We applied repeated measures ANOVA using natural log-transformed and Z-transformed P-tau levels as within-subject variables and group (AD dementia versus control) as between-subject variables, to investigate if the assays have a different discriminatory potential. As a post hoc analysis, we explored the difference in discriminatory accuracy of the assays by comparing their area under the curves (AUC) obtained with the ROC analyses using the DeLong test [25], in which we regarded p < 0.05 as significant and p < 0.10 as a trend. For all P-tau measurements below LLOQ, we used interpolated concentration as assigned by the Simoa. As a sensitivity analysis, we re-ran our analyses in the set with complete P-tau data for all assays (n = 37 controls, n = 36 patients with AD dementia), which did not change the findings (results not shown). We used R version 4.0.3 and SPSS version 26 for statistical analysis and construction of graphs.
Results
Analytical performance of the P-tau assays
LLOQ and precision
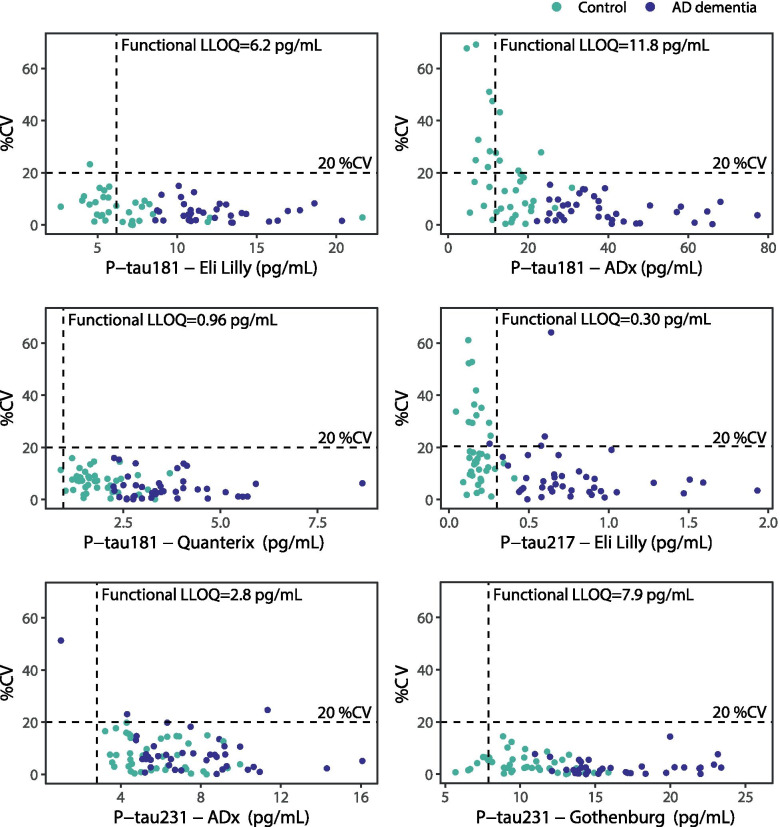
Functional LLOQ and intra-assay precision results are presented in Table 1 and Fig. 1. With P-tau181 Eli Lilly, 21 samples (27%; controls) were measured below the functional LLOQ and one sample (1%) was measured with a %CV > 20%. For P-tau181 ADx, levels of 14 samples (18%) were below LLOQ and 13 samples (18%) had a %CV > 20%. For P-tau181 Quanterix, level of only one sample (1%) was below LLOQ, and none of the samples had a %CV > 20%. For P-tau217 Eli Lilly, levels of 39 samples (49%; 37 of which were controls) were below LLOQ and 16 samples (20%) had a %CV > 20%. For P-tau231 ADx, level of 1 sample (1%) was below LLOQ and 3 samples (4%) had a %CV > 20%. For P-tau231 Gothenburg, 7 samples (9%) were measured below LLOQ and none of the samples had a %CV > 20%.
Fig. 1.
Precision plots of the six P-tau assays. For each assay, concentrations were plotted against the variation in their duplicate measurements (%CV), color-coded for clinical group. Due to technical reasons, duplicate results were obtained for 66/80 samples with P-tau181 Eli Lilly, for 74/80 with P-tau181 ADx, for 79/80 with P-tau217 Eli Lilly, for 79/80 with P-tau231 ADx, and for 77/80 with P-tau231 Gothenburg. Horizontal dashed lines were set at CV 20%, vertical dashed lines were set at the functional LLOQ for each assay (i.e., analytical LLOQ multiplied by sample dilution factor). CV coefficient of variation; P-tau phosphorylated tau, LLOQ lower limit of quantification
For both the in-house plasma quality controls (QC) and the manufacturer-provided kit controls (KC), average inter-assay precision was acceptable for all assays (Table 1), ranging from 5.1 (P-tau231 Gothenburg) to 27.7% (P-tau231 ADx) for the QCs (individual values in supplementary table 1) and from 9 (P-tau181 Eli Lilly) to 30.5% (P-tau231 ADx) for the KCs (individual values in supplementary table 2).
Parallelism, dilution linearity, and recovery
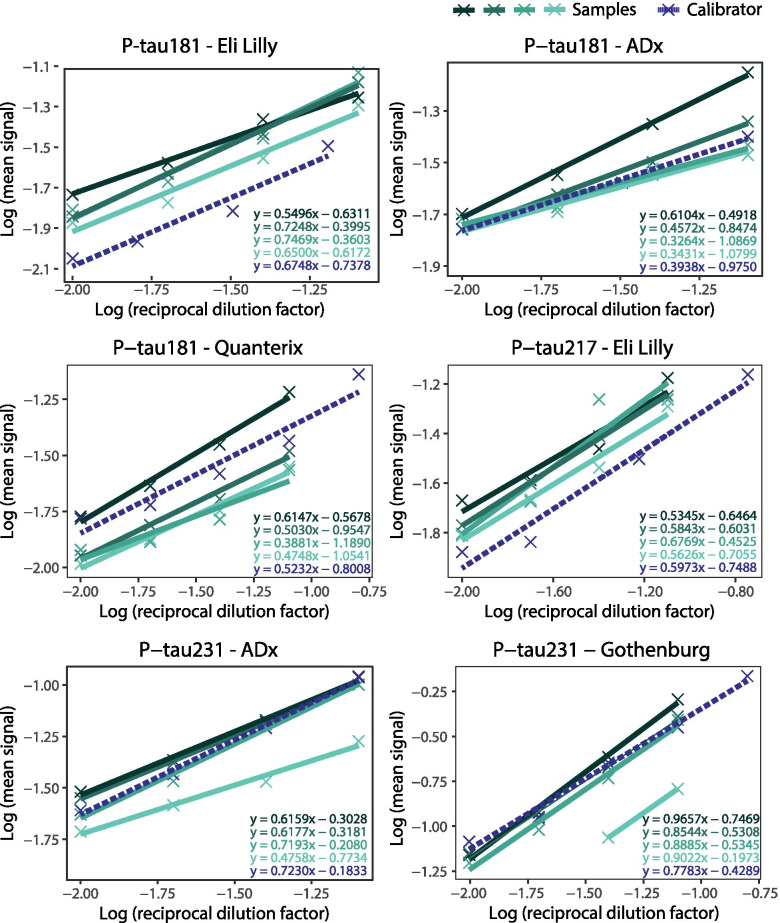
All P-tau assays showed good parallelism (Table 1, Fig. 2), with average parallelism ranging from 84% (P-tau231 ADx) to 116% (P-tau231 Gothenburg).
Fig. 2.
Parallelism of the six P-tau assays. Serial dilution of four plasma samples (in green, solid lines) and one calibrator (in purple, dashed line) was performed for each of the P-tau assays. Plasma samples with relatively high endogenous P-tau concentrations were selected for the parallelism experiment. For P-tau231 Gothenburg, one sample showed no signals upon dilution for the 8- and 16-fold dilutions. Crosses represent the individual measurements. A linear slope was fitted for each sample and for the calibrator, the equation of which is presented in the figures
Dilution linearity responses of the spiked samples upon serial dilution varied between the P-tau assays (Table 1). The P-tau217 Eli Lilly assay showed the broadest dilution range (i.e., linear responses from undiluted until 61.5-fold diluted), followed by P-tau181 Quanterix and P-tau231 ADx (5-fold until 125-fold diluted). The dilution range with acceptable linearity was narrowest for P-tau181 Eli Lilly (25-fold until 125-fold), P-tau181 ADx (25-fold until 125-fold), and P-tau231 Gothenburg (5-fold until 25-fold). No hook effect at spiked concentrations above the highest calibrator point of the assays was observed for any of the assays.
Average %-recovery varied between the assays (Table 1). For P-tau217 Eli Lilly, all spikes (low, intermediate, and high) showed acceptable recovery. For P-tau181 Eli Lilly, the low and intermediate spikes showed acceptable recovery. For P-tau181 Quanterix, the intermediate and high spikes showed acceptable recovery. For P-tau181 ADx, P-tau231 ADx, and P-tau231 Gothenburg, none of the low, intermediate, or high spikes showed acceptable recovery.
Clinical performance of the P-tau assays
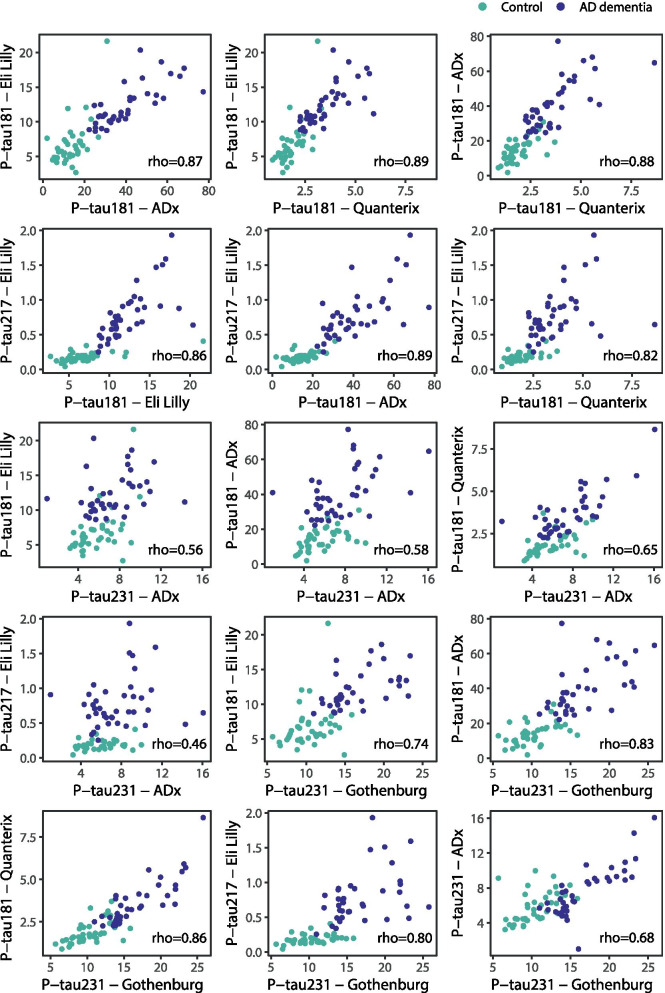
The P-tau levels measured in the plasma samples of 40 controls and 40 patients with AD dementia with the different assays correlated moderately to strongly with each other (Fig. 3; all p < 0.001)). Strong correlations were observed among the results obtained with the P-tau181 assays (range Spearman’s rho 0.87–0.89), for P-tau217 Eli Lilly results with results of all three P-tau181 assays (range 0.82–0.89) and with results of the P-tau231 Gothenburg assay (rho 0.80) and for P-tau231 Gothenburg results with results of all three P-tau181 assays (range 0.74–0.86). Correlations for P-tau231 ADx results with results of all other assays were moderate (range 0.46–0.68).
Fig. 3.
Scatterplots of the six P-tau isoforms, color-coded for diagnostic group. Correlation coefficient rho is calculated using Spearman’s rank correlation. Controls are presented in green and patients with AD dementia in purple. P-tau phosphorylated tau
With all assays, P-tau levels were significantly higher in AD dementia patients compared to controls (all: p < 0.001; Table 2; supplementary fig. 1). For P-tau217 Eli Lilly, median levels were 4.1-fold higher in AD dementia compared to controls, compared with 1.8-fold for P-tau181 Eli Lilly, 2.9-fold for P-tau181 ADx, 1.9-fold for P-tau181 Quanterix, 1.3-fold for P-tau231 ADx, and 1.5-fold for P-tau231 Gothenburg. AUCs for differentiating controls and patients with AD dementia ranged from 0.936 to 0.995 for P-tau217 Eli Lilly, all P-tau181 assays and P-tau231 Gothenburg assay, and was AUC = 0.719 for P-tau231 ADx (Table 2, Fig. 4). Repeated measures ANOVA demonstrated that there were differences between the P-tau assays in their discriminatory potential (interaction P-tau assay*group: Wilks’ Lambda p < 0.001). Post hoc comparison of the AUCs of the ROC analysis showed that P-tau181 ADx and P-tau217 Eli Lilly performed comparably good (DeLong’s p = 0.38), while both assays outperformed P-tau181 Quanterix (P-tau181 ADx: ΔAUC = 0.05, DeLong’s p = 0.03; P-tau217 Eli Lilly: ΔAUC = 0.06, DeLong’s p = 0.02). P-tau217 Eli Lilly outperformed P-tau231 Gothenburg (ΔAUC = 0.05, DeLong’s p = 0.03) and P-tau181 ADx tended to outperform P-tau231 Gothenburg (ΔAUC = 0.04, DeLong’s p = 0.08). Furthermore, both P-tau217 Eli Lilly and P-tau181 ADx tended to perform better than P-tau181 Eli Lilly did (P-tau181 ADx: ΔAUC = 0.05, DeLong’s p = 0.08; P-tau217 Eli Lilly: ΔAUC = 0.06, DeLong’s p = 0.07). The P-tau231 ADx assay was outperformed by all assays (range ΔAUC 0.22–0.28; all p < 0.001).
Table 2.
Clinical performance of the six P-tau assays
| AD dementia | Controls | Differentiation AD dementia versus controls | |||||
|---|---|---|---|---|---|---|---|
| Median [IQR] | Median [IQR] | Fold change | AUC (95% CI) | Cutoff | %Sens | %Spec | |
| P-tau181 Eli Lilly | 11.1 [10.4–13.6] | 6.1 [5.1–7.4] | 1.8 | 0.938 (0.872–1.000) | 8.6 | 100 | 89 |
| P-tau181 ADx | 37.6 [28.8–48.6] | 13.2 [10.3–17.6] | 2.9 | 0.988 (0.969–1.000) | 24 | 100 | 92 |
| P-tau181 Quanterix | 3.4 [2.7–4.1] | 1.6 [1.4–2.2] | 2.0 | 0.936 (0.885–0.987) | 2.2 | 100 | 78 |
| P-tau217 Eli Lilly | 0.7 [0.6–0.9] | 0.17 [0.14–0.2] | 4.1 | 0.995 (0.987–1.000) | 0.42 | 92.5 | 100 |
| P-tau231 ADx | 7.3 [5.6–9.1] | 5.5 [4.5–6.9] | 1.3 | 0.719 (0.607–0.831) | 4.7 | 95 | 43 |
| P-tau231 Gothenburg | 15.3 [13.9–19.8] | 10.3 [8.9–11.9] | 1.5 | 0.943 (0.896–0.991) | 13.4 | 89 | 90 |
Median concentrations are in pg/mL. Fold change was calculated by dividing the median concentration in the AD dementia group over the median concentration in the control group. AUCs were derived from ROC analysis. All group comparisons were significant with p values below the Bonferroni-adjusted p value cutoff of 0.0083. P-tau cutoff was specified at the Youden’s indeces (maximum sum of sensitivity and specificity)
P-tau Phosphorylated tau, AD Alzheimer’s disease, IQR: Interquantile range, AUC Area under the curve, sens Sensitivity, spec Specificity
Fig. 4.
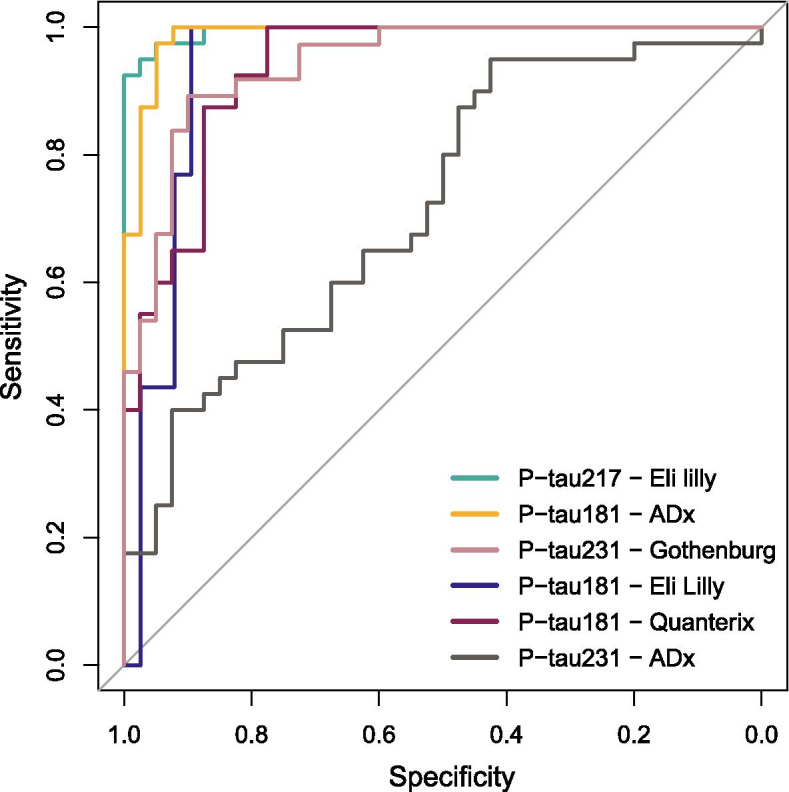
ROC curves discriminating between controls and AD dementia for the six P-tau assays. P-tau: phosphorylated tau
Discussion
We here compared six assays of four providers that detect plasma P-tau isoforms 181, 217, and 231 on the Simoa, of which five are prototype assays and one is currently commercially available. With all assays, P-tau concentrations were measured in all clinical samples above the assay blanks, but for some assays particularly (P-tau181 Eli Lilly, P-tau181 ADx, and P-tau217 Eli Lilly), a large proportion of control samples were measured below the LLOQs of the assays. Furthermore, all assays showed good analytical performance, especially in terms of intra- and inter-assay precision and parallelism. Clinically, we found that P-tau levels with all assays were higher in patients with AD dementia compared to controls and that particularly P-tau217 Eli Lilly assay, all three P-tau181 assays and the P-tau231 Gothenburg assay demonstrated high diagnostic accuracy for AD dementia (AUC > 0.93). The prototype P-tau231 ADx assay showed moderate diagnostic accuracy for AD dementia (AUC = 0.72). Our extensive and systematic assay comparison in terms of analytical characteristics combined with a clinical validation in a small proof-of-concept cohort are relevant for the interpretation of results that are currently being obtained across cohorts and studies using different forms or versions of the available P-tau assays.
Precise quantification of P-tau in all samples, including control samples, is relevant to obtain reliable results, e.g., to detect slight increases in early disease stages. With all six Simoa P-tau assays, we obtained detectable P-tau levels above the assay blanks in all clinical samples, including the samples of the healthy controls. However, especially with the P-tau217 Eli Lilly and P-tau181 ADx assays, many control samples were measured below LLOQ, resulting in higher imprecision (i.e., CV > 20%). This could impose difficulties on the clinical use of the two assays as stand-alone diagnostic tests, especially in early AD stages. By contrast, the P-tau181 Eli Lilly, P-tau181 Quanterix, P-tau231 ADx, and P-tau231 Gothenburg assays showed more robust intra-assay precision profiles. Although with the P-tau181 Eli Lilly assay, almost half of the control samples were measured below LLOQ, and with the P-tau231 Gothenburg 7 samples were measured below LLOQ, none of the measured samples had a CV% > 20%. With both the P-tau181 Quanterix and the P-tau231 ADx assays, only one sample was measured below LLOQ and respectively only one and three samples had a CV > 20%. In agreement with the intra-assay precision plots, particularly QC plasma samples with low concentrations close to or below the LLOQs of the assays showed variability of the results over the independent runs. Similar high variability was also noted for kit controls with concentrations close to or below the LLOQs. For other purposes, we used the P-tau181 Quanterix assay over 61 Simoa runs, using one assay lot, to monitor inter-assay precision over 3 months. We observed reproducible measurements with an average of 9% CV for the plasma QCs over time (data not shown). This extensive inter-assay precision data for P-tau181 Quanterix extends on the findings of our current study confirming that the P-tau181 Quanterix assay is a stable assay.
Analytically, the average parallelism responses for all assays were within the acceptable ranges, indicating good parallelism. Generally, parallelism indicates whether binding abilities of antibodies to endogenous P-tau isoforms is similar to the recombinant P-tau or synthetic P-tau peptide that is used as calibrator in the assays. For the dilution linearity, all P-tau assays showed acceptable dose-response linearity above their LLOQs. Particularly, P-tau217 Eli Lilly showed the broadest dilution linearity range. The range was slightly narrower for P-tau181 Quanterix and P-tau231 ADx and was narrowest for P-tau181 Eli Lilly, P-tau181 ADx, and P-tau231 Gothenburg. Narrow dilution ranges indicate that plasma matrix and/or sample diluent affect the detectability of P-tau isoforms differently; thus, sample dilution might affect accuracy and precision. The spike recoveries of the low, intermediate, and high spikes were all within the acceptable ranges for the P-tau217 Eli Lilly assay, while the recovery of one or more spikes deviated with the other assays. Deviations in recovery responses might suggest that the assay responds differently to endogenous and recombinant protein or synthetic peptide or that there is interference from biological samples leading to less accuracy in detecting true concentration differences between samples. Taking all results of the analytical performance of the assays together, P-tau181 Quanterix might be the favored assay, since precision profiles were robust, along with good parallelism, dilution linearity, and recovery. However, it is to note that all five other assays showed good analytical performance, and the prototype status of the assays should be considered as well. Especially, the P-tau217 had robust analytical performance (parallelism, linearity, recovery); however, the precision profiles on the clinical sample measurements showed less accuracy for measurements in low concentration samples (i.e., the controls).
Clinically, all P-tau181, P-tau217, and P-tau231 Gothenburg results correlated strongly with each other, while P-tau231 ADx correlated only moderately with the other results. The three P-tau181 assays and P-tau217 Eli Lilly and P-tau231 Gothenburg assays performed excellent in discriminating between controls and patients with AD dementia, with AUCs > 0.93, while the prototype P-tau231 ADx assay performed moderately with AUC = 0.72. The high AUCs observed for discriminating patients with AD dementia and controls with plasma P-tau181, P-tau217 Eli Lilly, and P-tau231 Gothenburg assays are in line with several previous reports in large cohorts using the same reagents but employing the MSD platform [4, 8, 16, 18, 26–28]. Likewise, the high AUC obtained with the P-tau181 Quanterix assay is in line with recent publications using a homebrew assay that employs the same antibodies as the Quanterix assay [5, 9]. Interestingly, in our study, the prototype P-tau231 ADx only moderately associated with AD, which contradicts the findings presented in this paper with the P-tau231 Gothenburg assay, or findings published earlier [11]. The assay set-up of the P-tau231 ADx is not exactly the same as the P-tau231 Gothenburg assay set-up, with a different antibody used as the detector and differences in terms of diluents used and assay conditions, such as reagent incubation times. It remains to be investigated if this difference in detector antibody or the differences in the assay set-ups explain the difference in clinical findings between the assays.
Tau proteins are generated from the same gene, and subsequently subjected to post-translational modifications, such as phosphorylation at different sites, producing different forms (e.g., P-tau217, P-tau181, and P-tau231). Some post-translational modifications were reported to occur at earlier stages of the AD disease process in the brain tissue [29]. Therefore, it was suggested that different P-tau forms might be favorable in different contexts of use, for example specifically to investigate ongoing AD pathology in cognitively unimpaired individuals or to discriminate patients with AD dementia from patients with other types of dementia. Recently, using a mass spectrometry approach that simultaneously quantifies different P-tau forms, the different phosphorylation sites that are quantified in this study were not phosphorylated to the same degree, with phosphorylation of P-tau181 present to a high degree in plasma and CSF of controls and with a relative small increase in patients with AD, while P-tau217 seems to be phosphorylated to a very low degree in controls, while the increase in AD is high [6, 17]. This suggests that P-tau217 might be a more sensitive marker than P-tau181 for AD pathological changes. However, our data did not support this, since AUCs for P-tau181 ADx were similar to P-tau217 Eli Lilly. A recent study [11] reported that plasma P-tau231 Gothenburg had similar clinical performance as the P-tau181 Simoa assay [5], similar to the findings or our current work. From our study, it cannot be excluded that technical differences between P-tau assays, such as antibodies and binding specificity may underlie subtle differences observed in large clinical comparison studies. In addition to analytical robustness and clinical validity, there are also other factors to consider, such as widespread availability for research and clinical settings, which usually occurs through commercialization. At this point, only the P-tau181 Quanterix is commercially available as a research assay. The five prototype assays compared in this study showed promising results in terms of analytical and clinical validity; thus, their further development into commercial products will be important, to make these assays available to the wide research community. Improvement of assay sensitivity to increase measurement precision in the low concentration ranges will be important for the assays with many control samples measured below the LLOQ, and buffer adjustments to decrease matrix effects is needed to improve recovery results.
A particular strength of our study is the extensive systematic analytical validation in EDTA plasma samples in one clinical research laboratory, allowing to directly compare the performance of the six novel ultrasensitive Simoa P-tau assays. Extensive comparison of clinical contexts of use of different P-tau assays is subject of several ongoing efforts, but none compared the analytical robustness yet.
Limitations
Regarding the limitations, we did not include all possible assays that arise in this quickly emerging field. For example, we did not include the in-house set-up of the P-tau181 assay as described by Karikari et al. [5], due to suboptimal technical performance of this assay in our lab during pilot testing. However, it is noted that this same pair of antibodies is used in the commercial P-tau181 Quanterix assay. Moreover, we did not compare the Simoa and MSD set-ups for the Eli Lilly assays. Therefore, we cannot conclude if Simoa had added value in terms of increasing assay sensitivity of the MSD assay set-ups. In addition, studies assessing the commutability of the assays, using reference materials for the harmonization of plasma P-tau measurements, will further improve our understanding of potential differences between plasma P-tau assays for clinical use over the Alzheimer’s continuum. Furthermore, the clinical sample set was small and exploratory, limiting the conclusions pertaining to different possible clinical contexts of use. Finally, our controls were not confirmed to be negative for ongoing amyloid pathology as no brain amyloid PET or CSF amyloid-beta42 data was available for these participants. However, since all samples were measured with all assays, this should not affect our comparative analyses.
Conclusions
In conclusion, all investigated assays showed robust analytical performance, and all yielded accurate clinical discrimination, except the current version of P-tau231 ADx assay. Differences in the analytical performance of the six P-tau assays may underlie subtle differences observed in clinical comparison studies, including in our current work.
Supplementary Information
Additional file 1: Supplementary figure 1. P-tau isoforms measured with different assays in AD dementia and control samples. Supplementary table 1. Intra-and Inter-assay %CV for the P-tau assays using QC samples. Supplementary table 2. Intra-and Inter-assay %CV for the P-tau assays using kit controls provided by the manufacturers of the assays.
Acknowledgements
Research of the Neurochemistry lab and Alzheimer center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Recruitment was partly accomplished through the Dutch Brain Research Registry (Hersenonderzoek.nl) that facilitates participant recruitment for neuroscience studies (www.hersenonderzoek.nl). Hersenonderzoek.nl is funded by ZonMw-Memorabel (project no 73305095003), a project in the context of the Dutch Deltaplan Dementie, Gieskes-Strijbis Foundation, the Alzheimer’s Society in the Netherlands and Brain Foundation Netherlands.
Abbreviations
- AD
Alzheimer’s disease
- Aβ
Amyloid-β
- P-tau
Phosphorylated tau
- PET
Positron emission tomography
- CSF
Cerebrospinal fluid
- P-tau181
Phosphorylated tau at threonine 181
- P-tau217
Phosphorylated tau at threonine 217
- P-tau231
Phosphorylated tau at threonine 231
- K2EDTA
Potassium Ethylenediaminetetraacetic acid
- NIA-AA
National Institute on Aging and Alzheimer’s Association
- LLOQ
Lower limit of quantification
- BIOMARKAPD
Biomarkers for Alzheimer’s disease and Parkinson’s disease
- QC
Quality control
- CV
Coefficient of variation
- ROC
Receiver operating characteristic curve
- AUC
Area under the curves
- ANOVA
Analysis of variance
Authors’ contributions
SB, IV, BDD, and CT designed and conceptualized the study, and analyzed and interpreted the data. SB, BDD, and ZH performed the experiments at Amsterdam UMC. IV performed the statistical analysis. SB, IV, and CT wrote the manuscript. JV, ES, EV, and JD developed and provided kits, interpreted the data, and revised the manuscript for intellectual content. NJA, HZ, and KB performed the analytical validation and sample analyses of the P-tau231 Gothenburg assay, interpreted the data, and revised the manuscript for intellectual content. MZ and WF are responsible for the Amsterdam Dementia Cohort and Hersenonderzoek.nl biobanks and databases and revised the manuscript for intellectual content. The authors read and approved the final manuscript.
Funding
IV is appointed on a research grant by Alzheimer Nederland (NL-17004). HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532); the European Research Council (#681712); Swedish State Support for Clinical Research (#ALFGBG-720931); the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862); the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C); the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2019-0228); the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE); and the UK Dementia Research Institute at UCL. KB is supported by the Swedish Research Council (#2017-00915); the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB-201809-2016615); the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243); the Swedish state under the agreement between the Swedish government and the County Councils; the ALF-agreement (#ALFGBG-715986); the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236); and the National Institute of Health (NIH) USA (grant #1R01AG068398-01). Research of CT is supported by the European Commission (Marie Curie International Training Network, grant agreement No 860197 (MIRIADE), and JPND), Health Holland, the Dutch Research Council (ZonMW), Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, Alzheimer Netherlands, and Alzheimer Association. CT and WF are recipients of ABOARD, which is a public-private partnership receiving funding from ZonMW (#73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). More than 30 partners participate in ABOARD. ABOARD also receives funding from Edwin Bouw Fonds and Gieskes-Strijbisfonds. The chair of Wiesje van der Flier is supported by the Pasman stichting. Alzheimer Center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds.
Availability of data and materials
The datasets generated and analyzed during the current study can be made available by the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
All participants gave written informed consent to use medical data and biomaterials for research purposes. Ethical approval of the Amsterdam UMC VUmc medical ethical committee was in place, and the study was in accordance with the Helsinki Declaration of 1975.
Consent for publication
Not applicable.
Competing interests
SB, IV, BDD, ZH, MZ, and NA report no conflicts of interest. WF holds the Pasman chair. WF has performed contract research for Biogen MA Inc. and Boehringer Ingelheim. WF has been an invited speaker at Boehringer Ingelheim, Biogen MA Inc., Danone, Eisai, WebMD Neurology (Medscape). WF is a consultant to Oxford Health Policy Forum CIC, Roche, and Biogen MA Inc. WF participated in an advisory board of Biogen MA Inc. WF is associate editor at Alzheimer’s, Research & Therapy. All funding is paid to her institution. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, AZTherapies, CogRx, Denali, Eisai, Nervgen, Pinteon Therapeutics, Red Abbey Labs, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure and Biogen; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. KB has served as a consultant or at advisory boards for Abcam, Axon, Biogen, JOMDD/Shimadzu, Lilly, MagQu, Prothena, Roche Diagnostics and Siemens Healthineers, and as data monitoring committee for Julius Clinical and Novartis. All payment was made to him personally. KB is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. JV and ES are employees of ADx NeuroSciences. EV is co-founder of ADx NeuroSciences. JD a minor stock holder of Eli Lilly and Company and inventor on the patent application used in the Lilly P-tau217 assay. CT has a collaboration contract with ADx Neurosciences and Quanterix and performed contract research or received grants from AC-Immune, Axon Neurosciences, Biogen, Brainstorm Therapeutics, Celgene, EIP Pharma, Eisai, PeopleBio, Roche, Toyama, Vivoryon. CT serves on editorial boards of Medidact Neurologi/Springer, Alzheimer Research and Therapy, Neurology: Neuroimmunology & Neuroinflammation, and is an editor of a Neuromethods book Springer. WF: Research programs of WF have been funded by ZonMW, NWO, EU-FP7, EU-JPND, Alzheimer Nederland, CardioVascular Onderzoek Nederland, Health~Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes-Strijbis fonds, stichting Equilibrio, Pasman stichting, Biogen MA Inc., Boehringer Ingelheim, Life-MI, AVID, Roche NL, Biogen NL, Novartis NL, Philips, Neurocast, Fujifilm, Combinostics.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sherif Bayoumy and Inge M. W. Verberk shared first authorship and contributed equally to this study.
References
- 1.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer’s disease. Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 3.Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chetelat G, Teunissen CE, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387–397. doi: 10.1038/s41591-020-0762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karikari TK, Pascoal TA, Ashton NJ, Janelidze S, Benedet AL, Rodriguez JL, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422–433. doi: 10.1016/S1474-4422(20)30071-5. [DOI] [PubMed] [Google Scholar]
- 6.Barthelemy NR, Horie K, Sato C, Bateman RJ. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J Exp Med. 2020;217(11):e20200861. doi: 10.1084/jem.20200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janelidze S, Berron D, Smith R, Strandberg O, Proctor NK, Dage JL, et al. Associations of plasma phospho-Tau217 levels with tau positron emission tomography in early alzheimer disease. JAMA Neurol. 2021;78(2):149–156. doi: 10.1001/jamaneurol.2020.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):772–781. doi: 10.1001/jama.2020.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karikari TK, Benedet AL, Ashton NJ, Lantero Rodriguez J, Snellman A, Suarez-Calvet M, et al. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s disease neuroimaging initiative. Mol Psychiatry. 2021;26(2):429–442. doi: 10.1038/s41380-020-00923-z. [DOI] [PubMed] [Google Scholar]
- 10.Keshavan A, Pannee J, Karikari TK, Rodriguez JL, Ashton NJ, Nicholas JM, et al. Population-based blood screening for preclinical Alzheimer’s disease in a British birth cohort at age 70. Brain. 2021;144(2):434–449. doi: 10.1093/brain/awaa403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashton NJ, Pascoal TA, Karikari TK, Benedet AL, Lantero-Rodriguez J, Brinkmalm G, et al. Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021;141(5):709–724. doi: 10.1007/s00401-021-02275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, Serrano GE, et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat Med. 2020;26(3):379–386. doi: 10.1038/s41591-020-0755-1. [DOI] [PubMed] [Google Scholar]
- 13.Benussi A, Karikari TK, Ashton N, Gazzina S, Premi E, Benussi L, et al. Diagnostic and prognostic value of serum NfL and p-Tau181 in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2020;91(9):960–967. doi: 10.1136/jnnp-2020-323487. [DOI] [PubMed] [Google Scholar]
- 14.Lantero Rodriguez J, Karikari TK, Suarez-Calvet M, Troakes C, King A, Emersic A, et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020;140(3):267–278. doi: 10.1007/s00401-020-02195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall S, Janelidze S, Londos E, Leuzy A, Stomrud E, Dage JL, et al. Plasma phospho-Tau identifies Alzheimer’s co-pathology in patients with lewy body disease. Mov Disord. 2021;36(3):767–771. doi: 10.1002/mds.28370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janelidze S, Stomrud E, Smith R, Palmqvist S, Mattsson N, Airey DC, et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat Commun. 2020;11(1):1683. doi: 10.1038/s41467-020-15436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barthelemy NR, Bateman RJ, Hirtz C, Marin P, Becher F, Sato C, et al. Cerebrospinal fluid phospho-tau T217 outperforms T181 as a biomarker for the differential diagnosis of Alzheimer’s disease and PET amyloid-positive patient identification. Alzheimers Res Ther. 2020;12(1):26. doi: 10.1186/s13195-020-00596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brickman AM, Manly JJ, Honig LS, Sanchez D, Reyes-Dumeyer D, Lantigua RA, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021;17(8):1353–1364. doi: 10.1002/alz.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Flier WM, Pijnenburg YA, Prins N, Lemstra AW, Bouwman FH, Teunissen CE, et al. Optimizing patient care and research: the Amsterdam Dementia Cohort. J Alzheimers Dis. 2014;41(1):313–327. doi: 10.3233/JAD-132306. [DOI] [PubMed] [Google Scholar]
- 20.van der Flier WM, Scheltens P. Amsterdam dementia cohort: performing research to optimize care. J Alzheimers Dis. 2018;62(3):1091–1111. doi: 10.3233/JAD-170850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwan MD, van der Flier WM, Cleutjens S, Schouten TC, Vermunt L, Jutten RJ, et al. Dutch Brain Research Registry for study participant recruitment: design and first results. Alzheimers Dement (N Y) 2021;7(1):e12132. doi: 10.1002/trc2.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreasson U, Perret-Liaudet A, van Waalwijk van Doorn LJC, Blennow K, Chiasserini D, Engelborghs S, et al. A practical guide to immunoassay method validation. Front Neurol. 2015;6:179. doi: 10.3389/fneur.2015.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 26.Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76(7):791–799. doi: 10.1001/jamaneurol.2019.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moscoso A, Grothe MJ, Ashton NJ, Karikari TK, Rodriguez JL, Snellman A, et al. Time course of phosphorylated-tau181 in blood across the Alzheimer’s disease spectrum. Brain. 2021;144(1):325–339. doi: 10.1093/brain/awaa399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mielke MM, Frank RD, Dage JL, Jeromin A, Ashton NJ, Blennow K, et al. Comparison of plasma phosphorylated tau species with amyloid and tau positron emission tomography, neurodegeneration, vascular pathology, and cognitive outcomes. JAMA Neurol. 2021;78(9):1108–1117. doi: 10.1001/jamaneurol.2021.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wesseling H, Mair W, Kumar M, Schlaffner CN, Tang S, Beerepoot P, et al. Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease. Cell. 2020;183(6):1699–713.e13. doi: 10.1016/j.cell.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary figure 1. P-tau isoforms measured with different assays in AD dementia and control samples. Supplementary table 1. Intra-and Inter-assay %CV for the P-tau assays using QC samples. Supplementary table 2. Intra-and Inter-assay %CV for the P-tau assays using kit controls provided by the manufacturers of the assays.
Data Availability Statement
The datasets generated and analyzed during the current study can be made available by the corresponding author upon reasonable request.