Abstract
Staphylococcus saprophyticus is one of the coagulase‐negative staphylococcus species. It is the second most frequent causative microorganism in acute uncomplicated urinary tract infections in young women. However, it is potentially capable of causing more invasive infections including bacteremia, particularly secondary to pyelonephritis. We present a young, previously healthy lady who presented with urinary symptoms and hemodynamic instability and was found to have multiple renal and ureteric calculi with pyelonephritis. Later, blood and urine cultures isolated methicillin‐resistant S. saprophyticus. The patient was successfully treated with a course of antibiotics targeting the organism with a favorable outcome. The clinical presentations and management of this rare entity of S. saprophyticus bacteremia‐related pyelonephritis are outlined. In addition, the literature on similar cases was reviewed to raise awareness and avoid devastating consequences.
Keywords: bacteremia, kidney calculi, pyelonephritis, Staphylococcus saprophyticus
Bacteremia due to Staphylococcus saprophyticus is a rare occurrence and appears to represent the end of the spectrum of urinary tract infection with this organism. The timely identification of methicillin‐resistant S. saprophyticus and the use of targeted antimicrobials therapy is crucial to avoid consequences and treatment failure.
1. INTRODUCTION
Staphylococcus saprophyticus is a gram‐positive, novobiocin‐resistant, coagulase‐negative staphylococcus species. 1 It is the second most frequent causative microorganism in acute uncomplicated urinary tract infections in young, sexually active women. 2 However, it can present with a variety of complicated genitourinary tract infections which include prostatitis, pyelonephritis, and epididymitis. 3 Staphylococcus saprophyticus bacteremia rarely complicates the involvement of the urinary tract, particularly in immunocompetent hosts. 4 The clinical presentation and diagnosis are usually undistinguished S. saprophyticus bacteremia secondary to pyelonephritis from typical uropathogens. 5
Typically, S. saprophyticus is sensitive to most antimicrobials used to treat UTIs. However, there is rising resistance of S. saprophyticus to empirically and commonly used antibiotics to treat cystitis, hence, rendering the management more challenging. 6 Herein, we report an unusual highly resistant case of S. saprophyticus pyelonephritis leading to bacteremia in an otherwise healthy young female patient who was successfully treated with a course of vancomycin and daptomycin. In addition, we reviewed the literature for similar cases.
2. CASE PRESENTATION
A 28‐year‐old lady, previously well presented to the hospital with a 2 days history of fever, dysuria, and left flank pain. She had no chronic medical condition or previous similar episodes. On examination, vital signs showed a fever of 38.2 with normal BP 115/71 and HR of 87. Abdominal examination revealed suprapubic and costovertebral tenderness. Basic investigation revealed high inflammatory markers with pyuria and acute kidney injury as depicted in the below (Table 1).
TABLE 1.
Basic laboratories
| Detail | Value w/Units | Normal range |
|---|---|---|
| WBC | 30 × 103/μl | 4.0–10.0 |
| Hgb | 15.4 gm/dl | 13.0–17.0 |
| Platelet | 400 × 103/μl | 150–400 |
| Absolute Neutrophil count Auto# (ANC) | 28 × 103/μl | 2.0–7.0 |
| Neutrophil Auto % | 93.4% | |
| INR | 1.2 | |
| Urea | 10.1 mmol/L | 2.8–8.1 |
| Creatinine | 130 μmol/L | 62–106 |
| Bicarbonate | 26 mmol/L | 22–29 |
| CRP | 65 | 0–5 |
Computed tomography scan revealed bilateral renal calyceal and ureteric stones causing hydronephrosis and obstructive uropathy (Figure 1). The patient was started on meropenem as per local antibiogram for presumptive urosepsis awaiting the results of the cultures. Both blood and urine cultures grew highly resistant staphylococcus saprophyticus that was sensitive only to vancomycin, daptomycin, gentamycin, and linezolid. Hence, the patient's antibiotic was changed to vancomycin. An echocardiogram was done, and it ruled out cardiac vegetations, and repeated blood cultures after 3 days were negative. A bilateral percutaneous nephrostomy was inserted which relieved her urinary obstruction. Subsequently, the patient improved clinically with normalization of her kidney parameters. Following removal of the percutaneous nephrostomy after passing the stones, she was discharged on daptomycin for ease of outpatient administration to complete a total of 14 days of antibiotics. On follow‐up visits, she had no recurrence of her urinary symptoms, and normal kidney function was restored.
FIGURE 1.
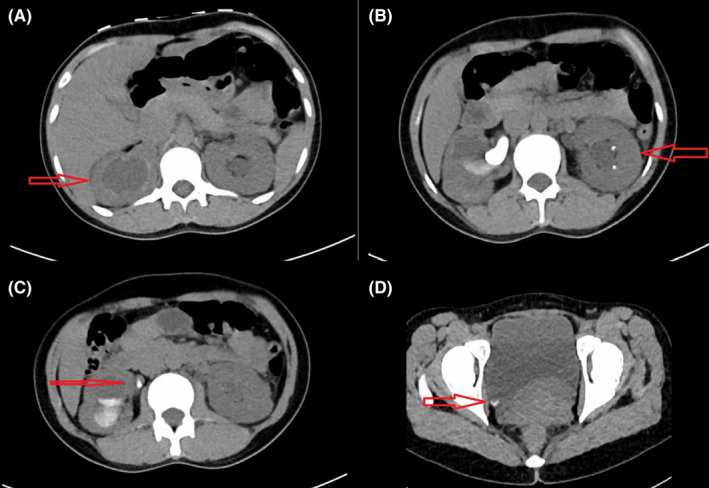
CT KUB findings of (A) Hydronephrosis, (B) Renal calculi with evidence of hydronephrosis, (C) Ureteric calculi, and (D) Urinary bladder stone
3. DISCUSSION
Staphylococcus saprophyticus is a gram‐positive organism that possesses multiple virulence factors, most importantly hemagglutinin and catalase. It usually attaches to the epithelium of the urogenital tract utilizing hemagglutinin and adhesins that help to anchor the bacteria to the cell wall allowing it to escape the immune system. Furthermore, catalase protects S. saprophyticus from being killed by reactive oxygen species. 7 The existence of a renal tract obstruction would favorably enable S. saprophyticus to ascend more proximally toward the renal pelvis causing pyelonephritis as in our case. 8 Staphylococcus saprophyticus is a well‐recognized cause of uncomplicated cystitis after E. coli and has been isolated in 42% of young females with uncomplicated cystitis. 2 , 9 The usual course of uncomplicated Staphylococcus saprophyticus infection is mild and responds well to antibiotics either orally or intravenously in more severe cases. 3 Nevertheless, complicated genitourinary infections including prostatitis, pyelonephritis, and epididymitis have rarely been reported in certain high‐risk patients. 8 Many risk factors have been reported for complicated Staphylococcus saprophyticus UTIs such as immunocompromised hosts, obstructive nephrolithiasis, indwelling urethral catheters, and the presence of renal tract anomalies. 10 However, it rarely causes infection in immunocompetent adults.
Although Staphylococcus saprophyticus is a well‐established cause of uncomplicated UTIs, it is pathogenic significance, role in complicated UTIs, and clinical significance when isolated from blood culture has not been well defined. 4 , 10 It has been postulated that S. sapro is of low virulence due to multiple reasons, including the absence of coagulase, unlike other staphylococci. Coagulase degrades fibrin and results in clotting activation, and coats the organism helping to escape phagocytosis. Furthermore, Staphylococcus saprophyticus lacks ATPase; hence, it is difficult to grow in low potassium environments such as the blood, whereas urine is a suitable medium because it is potassium contents. 8 For these reasons, the pathogenicity of S. saprophyticus might be lower in the blood than in urine because of its physiological function and activity. Even though, when bacteremia occurs, the significance is still not well established, and further studies are required to delineate the course. 4
Staphylococcus saprophyticus is generally sensitive to most antibiotics including beta‐lactams. However, some strains isolated from complicated UTIs were generally more resistant to broad‐spectrum antibiotics than those isolated from uncomplicated infections. 11 This explains the high resistance profile isolated from our patient.
The optimal treatment for bacteremia due to S. saprophyticus is not yet well defined given it is a rarity. In our case, we treated the patient with antibiotics for 2 weeks given the highly resistant nature of the organism and the presence of urinary obstruction. 4
Our search of the literature yielded a total of nine cases of S. saprophyticus bacteremia originating from the urinary tract (Table 2). Cases ranged between 14 and 53 years of age and were predominantly female. Half of them had underline urolithiasis while no immunosuppressed status was reported. Pyelonephritis is mostly involved in bacteremia rather than uncomplicated UTIs. Of the cases identified, only two cases reported some form of resistant staphylococcus saprophyticus as in our case. The duration of therapy ranged from 7 days to 21 days. All cases achieved complete recovery and clearance of the bacteremia with no complications (Table 2).
TABLE 2.
Summary of previously reported cases of S. saprophyticus bacteremia originating from the urinary tract
| Case | Sex/age | Comorbidities | Predisposing factor | Source | Empirical treatment | Definite treatment | Duration of Abx | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Golledge 12 | F/14 | None | Sexual activity | Pyelonephritis | Amoxicillin, cloxacillin, | penicillin | 10 days | Recovered |
| 2 | Golledge 12 | F/49 | None | Sexual activity/Uretric obstruction recurrent UTI | Pyelonephritis | Cephalothin, gentamicin | penicillin | 7 days | Recovered |
| 3 | Glimaker 13 | F/19 | None | Sexual activity | Pyelonephritis | Co‐trimoxazole | cloxacillin, flucloxacillin | 3 weeks | Recovered |
| 4 | Glimaker 13 | F/33 | None | Ureteric Stones Sexual activity | Pyelonephritis | Co‐trimoxazole | 14 days | Recovered | |
| 5 | Olafsen 14 | F/27 | None | Pregnancy, ureteric calculus | Pyelonephritis | Ampicillin | amoxicillin | 12 days | Recovered |
| 6 | Chen 15 | F/38 | None | Unknown | Pyelonephritis | Gentamicin | 14 days | Recovered | |
| 7 | Lee 16 | F/38 | None | Pregnancy | Urinary tract infection | cefazolin | cephalexin | 7 days | Recovered |
| 8 | M Hofmans 17 | F/53 | None | Ureterolithiasis | Urinary tract infection | Temocillin | ciprofloxacin | 10 days | Recovered |
| 9 | Our case | None | Ureterolithiasis | Pyelonephritis | Meropenem | Vancomycin then Daptomycin | 14 days | Recovered |
4. CONCLUSION
Pyelonephritis‐associated staphylococcus saprophyticus bacteremia in an immunocompetent host is a rare clinical entity that demonstrates the ability of the organism to manifest as invasive infections. S. saprophyticus is generally sensitive to most antibiotics commonly used to treat uncomplicated community‐acquired UTIs; however, resistance strain is raising which necessitating caution when dealing with the invasive infection of this organism. The treatment should be guided by the pattern of antimicrobials sensitivity, and the optimal treatment duration remains unknown.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Ahmad Matarneh, Gawahir A. Ali, and Wael Goravey: Clinical care, literature review, and manuscript write up.
ETHICAL APPROVAL
The case report was approved by Hamad medical corporation, MRC number MRC‐04‐21‐242.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGEMENTS
We thank the internal medicine department at Hamad Medical Corporation for giving us the opportunity and support to conduct this work.
Matarneh A, Ali GA, Goravey W. Pyelonephritis‐associated Staphylococcus saprophyticus bacteremia in an immunocompetent host: Case report and review of the literature. Clin Case Rep. 2021;9:e05183. doi: 10.1002/ccr3.5183
Funding information
Qatar national library—Qatar foundation
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Raz R, Colodner R, Kunin CM. Who are you ‐ Staphylococcus saprophyticus? Clin Infect Dis. 2005;40(6):896‐898. [DOI] [PubMed] [Google Scholar]
- 2. Hovelius B, Mårdh PA. Staphylococcus saprophyticus as a common cause of urinary tract infections. Rev Infect Dis. 1984;6(3):328‐337. [DOI] [PubMed] [Google Scholar]
- 3. Goldenring JM. Urinary tract infection with Staphylococcus saprophyticus . J Adolesc Heal Care. 1986;7(6):417‐418. [DOI] [PubMed] [Google Scholar]
- 4. Choi SH, Woo JH, Jeong JY, et al. Clinical significance of Staphylococcus saprophyticus identified on blood culture in a tertiary care hospital. Diagn Microbiol Infect Dis. 2006;56(3):337‐339. [DOI] [PubMed] [Google Scholar]
- 5. Wallmark G, Arremark I, Telander B. Staphylococcus saprophyticus: a frequent cause of acute urinary tract infection among female outpatients. J Infect Dis. 1978;138(6):791‐797. [DOI] [PubMed] [Google Scholar]
- 6. Pailhoriès H, Cassisa V, Chenouard R, Kempf M, Eveillard M, Lemarié C. Staphylococcus saprophyticus: which beta‐lactam? Int J Infect Dis. 2017;65:63‐66. [DOI] [PubMed] [Google Scholar]
- 7. Silva KCS, Silva LOS, Silva GAA, et al. Staphylococcus saprophyticus proteomic analyses elucidate differences in the protein repertories among clinical strains related to virulence and persistence. Pathogens. 2020;9(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hur J, Lee A, Hong J, et al. Staphylococcus saprophyticus bacteremia originating from urinary tract infections: a case report and literature review. Infect Chemother. 2016;48(2):136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chabrière E, Raoult D, Fenollar F. Direct identification of pathogens in urine by use of a specific matrix‐assisted laser desorption ionization‐time of flight spectrum database. J Clin Microbiol. 2019;57(4):e01678–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ishihara S, Yokoi S, Ito M, Kobayashi S, Deguchi T. Pathologic significance of Staphylococcus saprophyticus in complicated urinary tract infections. Urology. 2001;57(1):17‐20. [DOI] [PubMed] [Google Scholar]
- 11. Widerström M, Wiström J, Ferry S, Karlsson C, Monsen T. Molecular epidemiology of Staphylococcus saprophyticus isolated from women with uncomplicated community‐acquired urinary tract infection. J Clin Microbiol. 2007;45(5):1561–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Golledge CL. Staphylococcus saprophyticus bacteremia. J Infect Dis. 1988;157(1):215. [DOI] [PubMed] [Google Scholar]
- 13. Glimåker M, Granert C, Krook A. Septicemia caused by staphylococcus saprophyticus . Scand J Infect Dis. 1988;20(3):347‐348. [DOI] [PubMed] [Google Scholar]
- 14. Olafsen LD, Melby K. Urinary tract infection with septicaemia due to Staphylococcus saprophyticus in a patient with a ureteric calculus. J Infect. 1986;13(1):92‐93. [DOI] [PubMed] [Google Scholar]
- 15. Chen CH. Staphylococcus saprophyticus bacteremia with pyelonephritis cured by gentamicin. J Formos Med Assoc. 2014;113(7):483‐484. [DOI] [PubMed] [Google Scholar]
- 16. Lee W, Carpenter RJ, Phillips LE, Faro S. Pyelonephritis and sepsis due to staphylococcus saprophyticus . J Infect Dis. 1987;155(5):1079‐1080. [DOI] [PubMed] [Google Scholar]
- 17. Hofmans M, Boel A, Van Vaerenbergh K, De Beenhouwer H. Staphylococcus saprophyticus bacteremia after ESWL in an immunocompetent woman. Acta Clin Belgica. 2015;70:215–217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


