Abstract
Purpose
Macrophages are believed to promote choroidal neovascularization (CNV) in neovascular age-related macular degeneration (nvAMD); however, the underlying proangiogenic mechanism is poorly understood. Therefore, we examined this mechanism in proinflammatory macrophages derived from patients with nvAMD.
Methods
Monocytes were isolated from patients with nvAMD and polarized to form an M1 proangiogenic phenotype. We then screened for the role of proangiogenic cytokines expressed by these macrophages, including TNF-α, VEGF, IL-6, IL-8, and IL-1β, using an ex vivo choroid sprouting assay and an in vivo rodent model of laser-induced CNV (LI-CNV). We also examined the value of inhibiting TNF-α inhibition with respect to reducing the proangiogenic effects of M1 macrophages. Finally, we analyzed the macrophage cytokine expression database to evaluate the feasibility of modulating the expression of TNF-α.
Results
The cytokines above are expressed at high levels in patient-derived M1 macrophages. However, among the cytokines tested only TNF-α significantly increased choroid sprouting. Moreover, adoptive intravitreal transfer of M1 macrophages significantly increased LI-CNV, and blocking TNF-α abolished the proangiogenic effects of M1 macrophages in both models. An analysis of cytokine expression revealed that >50% of TNF-α expression is determined by modifiable factors.
Conclusions
Blocking TNF-α can reduce the proangiogenic effects of M1 macrophages in nvAMD. Thus, activated macrophages may represent a potential therapeutic target for altering TNF-α expression in nvAMD.
Introduction
Monocytes and monocyte-derived inflammatory macrophages have been implicated in the pathogenesis of neovascular age-related macular degeneration (nvAMD) [1-11]. Although studies suggest that inflammatory macrophages may be associated with increased choroidal neovascularization (CNV) [6,7], the mechanisms that underlie this process are poorly understood. We recently reported that activated inflammatory macrophages derived from patients with nvAMD express several proangiogenic and proinflammatory cytokines, accelerating the pathogenesis of ex vivo and in vivo experimental models of angiogenesis and CNV [11]. We also found that monocytes obtained from patients with nvAMD and activated by culturing with IFN-γ and LPS to form proinflammatory M1 macrophages have a more robust proangiogenic effect compared to other macrophage phenotypes and macrophages derived from age-matched controls [11].
Vascular endothelial growth factor (VEGF) plays a major role in the development of CNV, and anti-VEGF compounds are the primary therapy for nvAMD. Although anti-VEGF therapy can improve visual outcomes in some patients with nvAMD, other patients develop a substantial loss of visual acuity despite receiving this therapy [12].
Inflammatory macrophages express VEGF and a variety of other cytokines that are potentially proangiogenic in the context of CNV; however, whether myeloid-derived VEGF is solely responsible for the contribution of macrophages to the development of CNV remains unclear [13-17]. Additional cytokines may mediate the proangiogenic effects of macrophages in the context of nvAMD. Thus, identifying these cytokines may provide important insights into the pathogenesis of nvAMD and may reveal new therapeutic targets for treating this disease.
Previous studies identified markers for M1 (i.e., classically activated proinflammatory) and M2 (alternatively activated) macrophages in the eyes of patients with AMD, and recent studies illustrated how inhibiting M1 macrophages can suppress laser-induced CNV (LI-CNV) [18-21]. In addition, we recently reported high levels of the M1 macrophage marker inducible nitric oxide synthase (iNOS) in a rodent model of LI-CNV [22].
We examined the role of various cytokines in mediating the proangiogenic properties of activated macrophages in nvAMD by screening cytokines that are expressed by activated macrophages derived from patients with nvAMD [11]. Specifically, we examined the effect of these cytokines in ex vivo and in vivo models of neovascularization that recapitulate many of the features of nvAMD. We then tested the ability of anti-TNF-α therapy to reduce the proangiogenic effects of activated macrophages in nvAMD. Finally, we analyzed cytokine expression data to determine whether the production of TNF-α by macrophages can be modified, providing a potential therapeutic route for inhibiting TNF-α in nvAMD.
Methods
Patients
Two elderly (92 and 86 years of age) male patients with nvAMD were recruited from the Retina Clinic in the Department of Ophthalmology at Hadassah-Hebrew University Medical Center. The patients’ diagnosis of AMD was based on criteria established by the Age-Related Eye Disease Study (AREDS) [23], and CNV was confirmed using fluorescein angiography and optical coherence tomography. Overall, the lesions comprised at least 50% active CNV and <25% subretinal hemorrhage. The patients did not present with any other retinal disease or any other possible causes of CNV, such as myopia, trauma, or uveitis. In addition, the patients did not present with any major systemic illness, such as cancer, autoimmune disease, congestive heart failure, or unmanaged diabetes.
Ethics
All experimental protocols and studies involving human subjects were approved by the local Committee on Research Involving Human Subjects of the Hebrew University-Hadassah Medical School, the Helsinki Committee of Hadassah Medical Organization, and the Israel Ministry of Health’s Helsinki Committee for Genetic Experiments on Human Subjects (File #22–03.08.07). Patients and control subjects provided written informed consent in accordance with the tenets of the Declaration of Helsinki prior to participating in the study, in accordance to the guidelines of the Association for Research in Vision and Ophthalmology (ARVO). All methods used in the study were performed in accordance with approved study guidelines while ensuring the participants’ privacy.
Preparation of macrophages
Blood samples (30 ml each) from the patients with nvAMD were collected into EDTA-containing tubes (BD Biosciences). Monocytes were isolated from the whole blood samples, differentiated into M0 macrophages, and then activated to form M1 macrophages by stimulation with IFN-γ and LPS as previously described [11,24-28]. We chose this specific macrophage subtype because we previously found that M1 macrophages cause increased neovascularization in vitro and in vivo [11]. The medium was harvested from the cultured macrophages and stored at −20 °C for use in the choroid sprouting assay (CSA). Macrophages were collected using 0.25% trypsin (Sigma-Aldrich, Munich, Germany), washed with RPMI 1640 medium (Biological Industries Israel Beit Haemek Ltd) containing fetal calf serum (FCS), washed 3 times with phosphate-buffered saline (1X PBS; 135 mM NaCl, 2.7 mM KCl, 10 mM Na2PO4, 1.5 mM KH2PO4, pH 7.4), and used immediately in the in vivo experiments.
Animal experiments
All protocols involving animals were approved by the Authority for Biologic and Pre-Clinical Models (ABBM) and the University Ethics Committee for the Care and Use of Laboratory Animals of Hebrew University, which is certified by the Association for Accreditation of Laboratory Animal Care (AAALAC; approval number: MD-16–14796–3, NIH approval number: OPRR-A01–5011). All researchers working with laboratory animals were approved by the ethics committee of the ABBM. All guidelines with respect to the humane and ethical treatment of laboratory animals based on the Association for Research in Vision and Ophthalmology (ARVO) were strictly followed, and all methods used in this study were performed in accordance with approved study guidelines.
Choroid sprouting assay
This ex vivo angiogenesis assay was performed as previously described [29] to assess the putative angiogenic effects of candidate cytokines collected from the supernatant of cultured M1 macrophages prepared from patients with nvAMD as described above [11]. Cytokines were added to the medium at the maximum concentration based on previous enzyme-linked immunosorbent assay (ELISA) results measured using the culture medium of M1 macrophages [11]. Specifically, the following five cytokines (all from PeproTech, Rocky Hill, NJ) were tested at the indicated concentrations (n = 8 replicates for each cytokine): VEGF, 0.25 ng/µl; IL-8, 0.50 ng/µl; IL-6, 2.50 ng/µl; IL-1β, 0.05 ng/µl; and TNF-α, 0.85 ng/µl. Where indicated, anti-TNF-α (infliximab, 100 µg/µl; Remsima, Celltrion Inc., Incheon, South Korea) and anti-VEGF (aflibercept, 40 µg/µl; Bayer Pharma AG, Berlin, Germany) were included in the presence or absence of M1 macrophage‒derived medium and were used at concentrations based on previous studies [30].
For the CSA assay, C57BL/6J mice (4-6 weeks old) were euthanized by cervical dislocation following anesthesia with inhalation liquid Isoflurane USP 100% (provided by Piramal pharma solutions, Mumbai, India), and the eyes were removed immediately and placed in ice-cold endothelial cell growth medium (ECGM, Cat. C-22010; PromoCell, Heidelberg, Germany) containing 100 units/ml penicillin-streptomycin (Biological industries, Beit Haemek, Israel) and 1% glutamine (Biological industries). The choroid-sclera complex was then gently dissected along with the retinal pigment epithelium (RPE) layer. The tissue was then cut into 5-6 1-mm long pieces, which were embedded in 30 µl of growth factor‒reduced Matrigel (Cat. 354230, BD Biosciences, Cowley, Oxford, UK354230) in 24-well plates. The thickness of the Matrigel layer was approximately 0.4 mm. The plates were then incubated without medium for 10 min at 37 °C in 5% CO2 to solidify the Matrigel, after which 500 µl ECGM containing 2.5% supplement mix (C-9215, PromoCell), 5% FCS, 100 units/ml penicillin-streptomycin, and 1% glutamine was added to each well. For the experimental groups containing the supernatant from cultured macrophages, the medium was replaced with 250 µl of this supernatant. The medium in each well was changed every 3 days, and the cultures were fixed with 4% paraformaldehyde (PFA) on day 8. The cultures were viewed using an inverted Olympus CKX41 microscope, and images were captured using a DP70 digital camera (Olympus, Tokyo, Japan).
ImageJ (NIH, Bethesda, MD) was used to quantify the sprouting area [31]. The sprouting area was selected using the software and measured after the choroid tissue was excluded. The background (a control well in each plate containing medium only) was subtracted from each sample. For each well, the ratio between the experimental group and its respective control sample from the same eye was calculated, and replicates were averaged. Although we did not label the sprouting cells in the CSA, previous works have suggested that these sprouts are mostly composed of endothelial cells [2-4].
Laser-induced CNV
For the LI-CNV model, Long-Evans rats (8–12 weeks old) were anesthetized with an intraperitoneal injection of a mixture containing 85% ketamine (Bedford Laboratories, Bedford, OH) and 15% xylazine (InoVet, Arendonk, Belgium). Topical anesthesia (Oxybuprocaine HCL 0.4%; Fisher Pharmaceuticals, Tel-Aviv, Israel) was applied to each eye 10 min before intravitreal injection and laser photocoagulation.
Laser photocoagulation burns (five to seven spots per eye) were applied as previously described [32]. Two days later, the respective compounds were administered by intravitreal injection using a PLI-100 Pico-Injector (Medical System Corp., Greenvale, NY) to induce CNV [33]; these injections were repeated every 2 days for a total of 10 days. Where applicable, macrophages were injected only once (2 days after laser photocoagulation). The entire procedure, including evidence showing lack of a xenograft-induced immune response, has been published previously [11].
The following control group and five experimental groups were included in this study: PBS (4 µl, control); infliximab (1 µl of a 100 µg/µl solution); aflibercept (1 µl of a 100 µg/µl solution); infliximab plus 105 M1 macrophages (1 µl and 4 µl, respectively); aflibercept plus 105 M1 macrophages (1 µl and 4 µl, respectively); and 105 M1 macrophages (4 µl). The concentration of each compound injected was based on previous studies [34]. For each group, both eyes in four rats were injected (for a total of eight eyes per group). After each injection, antibiotic ointment (5% chloramphenicol) was applied to each eye. After the final injections (i.e., 10 days after laser photocoagulation), choroid-RPE and retinal flat mounts were prepared as previously described [11,35] and analyzed as described below.
Quantification of CNV
Choroid-RPE flat mounts were fixed for 1 h in 4% PFA and suspended overnight in Isolectin GS-IB4 Alexa Fluor 594 staining solution (Molecular Probes, Eugene, OR) containing 200 mM NaN3 and 1 mM CaCl2. The flat mounts were then washed six times for 20 min each in PBS and mounted on a slide using mounting medium. The area of CNV surrounding each laser injury was measured using ImageJ as previously described [11].
Basic statistical analyses
Data were analyzed using the biostatistics software package InStat (GraphPad, San Diego, CA). Differences with a p-value <0.05 were considered statistically significant. Outliers (±2 SD) were excluded from the statistical analysis (less than 5%) [39]. Appropriate statistical tests were used based on the results of a test for normality, the sample distribution, and parameters.
Analysis of macrophage modulation
Using data from the present study and a previous study [11], we examined the role of age, gender, disease status (nvAMD versus control), and macrophage subtype (M0 versus M1 versus M2) on mRNA levels, protein levels, and proangiogenic properties in the CSA and LI-CNV models. We included in the analysis the protein levels of PDGF, TNF-α, VEGF, MCP1 (i.e., CCL2), and ICAM (measured using ELISA), as well as the mRNA levels of PDGF (Gene ID 5154, OMIM 173430), TNF-α (Gene ID 7124, OMIM 191160), and VEGF (Gene ID 3383, OMIM 147840) measured using qPCR) of all patient-derived polarized human macrophages. These data were obtained from our previous study [11] and included 34 patients with nvAMD (21 women and 13 men) with a mean (± standard error of the mean [SEM]) age of 75.9±1.50 years (range: 59–93 years) and 25 age-matched unaffected controls (ten women and 15 men) with a mean age of 72.3±1.70 years (range: 59–89 years).
We then evaluated the contribution of the patient (“Cell Origin”) and the laboratory manipulation of cultured macrophages (“Environment”) to the protein and mRNA levels measured in the macrophages. These contributions were compared using an analysis of variance (ANOVA) with the software program R (and R studio) together with the “reshape2” package. We then calculated the percentage of variance that was due to age, gender, disease status, and environment (and the interaction between cell origin and environment), as well as any unexplained variance, using a mixed-design ANOVA with repeated measures in R.
Results
TNF-α and anti-TNF-α therapy increases and decreases, respectively, the proangiogenic effects of human M1 macrophages on choroid sprouting in an ex vivo model
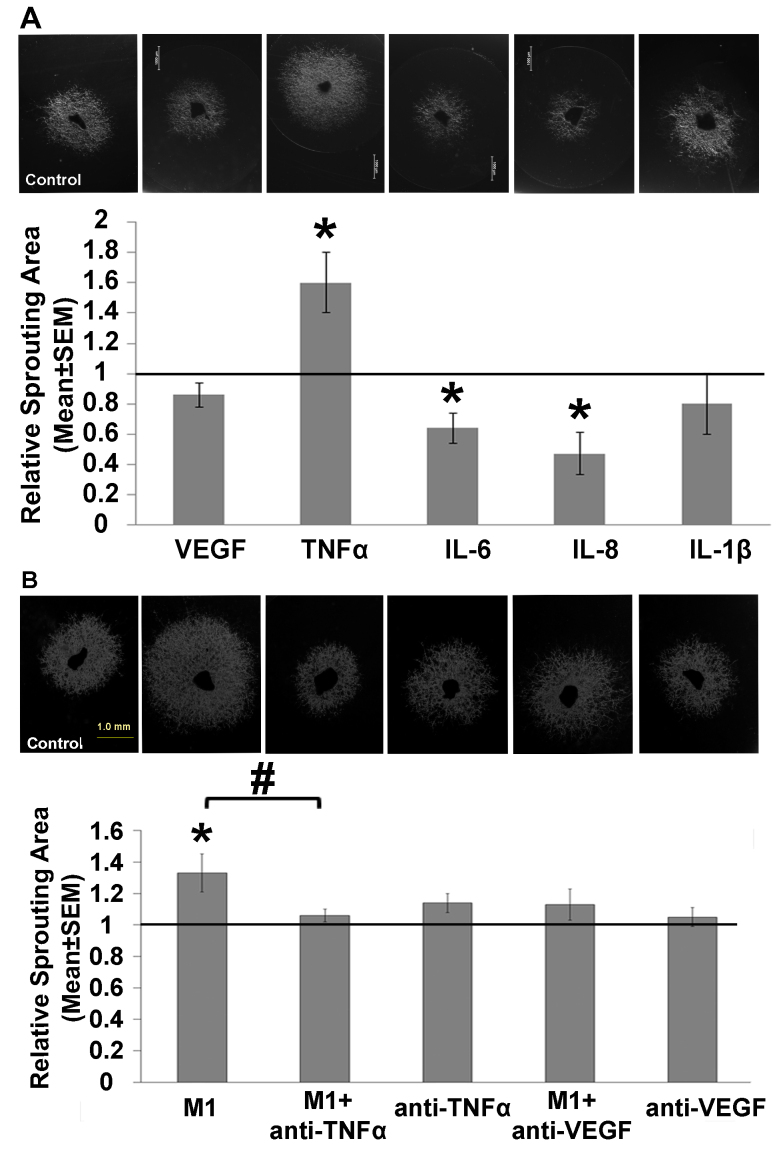
First, using an ex vivo choroid sprouting assay, we examined whether the addition of exogenous cytokines affects neovascularization. We found that addition of TNF-α to the culture medium statistically significantly increased sprouting compared to the untreated wells (Figure 1A). Specifically, the mean (± SEM) ratio of the relative sprouting area between the TNF-α-treated and untreated wells was 1.6±0.2 (n = 8; p=0.01, Student t test). In contrast, addition of either IL-6 or IL-8 statistically significantly decreased the sprouting area (Figure 1A), with a relative ratio between treated and untreated wells of 0.64±0.1 (n = 9; p=0.01, Student t test) and 0.47±0.14 (n = 8; p=0.007, Student t test), respectively. Addition of either IL-1β or VEGF had no effect on choroid sprouting (Figure 1A). The lack of an effect of VEGF in this model may have been due to the high levels of VEGF present in the growth medium [29], which may have obscured any possible effect of VEGF added to the medium.
Figure 1.
Select cytokines and M1 macrophages affect choroid sprouting. Sprouting was measured using an inverted microscope and is expressed relative to control. A: Example images and summary of choroid sprouting measured in the presence of the indicated recombinant cytokines previously identified in the culture medium of macrophages activated with IFN-γ and LPS. B: Example images and summary of choroid sprouting measured under the indicated conditions; where indicated, the culture medium supernatant from M1 macrophages was applied. n = 7–8/group. *p<0.05 versus the corresponding control group; #p<0.05 versus M1 supernatant alone.
Next, we examined whether supernatant collected from cultured M1 macrophages affects choroid sprouting and found that addition of this supernatant statistically significantly increased the sprouting area to 1.33±0.12 (n = 7, p=0.04, Student t test) relative to the untreated wells (Figure 1B). Moreover, this increase was prevented by adding either anti-TNF-α or anti-VEGF to the M1 supernatant, although treatment with either anti-TNF-α or anti-VEGF alone had no statistically significant effect on choroid sprouting (Figure 1B).
Taken together, these findings suggest that TNF-α—and possibly VEGF as well—mediates the proangiogenic effect of M1 macrophages in choroid sprouting. Moreover, the increase in sprouting induced by M1 supernatant, but not VEGF alone, suggests that macrophages can induce choroid sprouting via a VEGF-independent pathway.
Anti-TNF-α therapy prevents the proangiogenic effect of human MI/L macrophages in an in vivo model
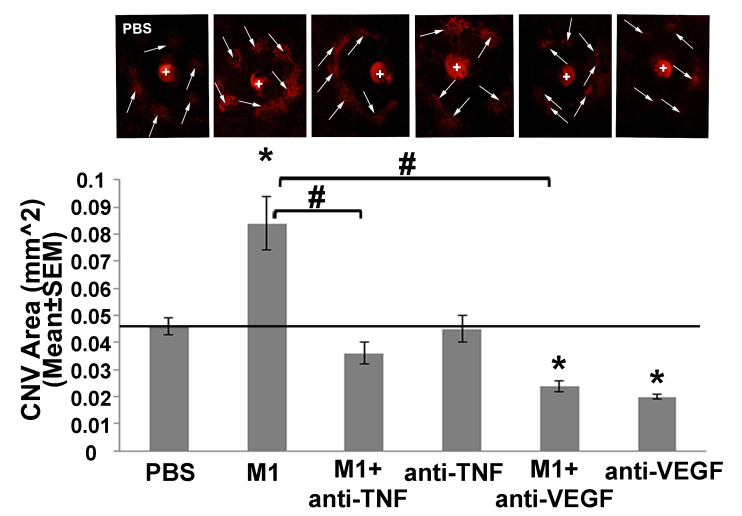
Next, we used an in vivo model of LI-CNV to examine whether intravitreal injections of anti-TNF-α can reduce the proangiogenic effects of M1 macrophages. We found that intravitreal adoptive transfer of M1 macrophages statistically significantly increased the CNV area compared to controls (Figure 2). Moreover, this increase was eliminated by coinjecting either anti-TNF-α or anti-VEGF together with M1 macrophages. Consistent with the ex vivo results, we also found that injecting anti-TNF-α alone had no effect on CNV (Figure 2). Interestingly, and in contrast with the ex vivo results, we also found that intravitreal injection of anti-VEGF alone statistically significantly reduced the CNV area (Figure 2).
Figure 2.
Anti-TNF-α and anti-VEGF therapy reduced the proangiogenic effects of M1 macrophages in an in vivo rat model of laser-induced CNV. Fluorescence images and summary of the choroidal neovascularization (CNV) area (in square millimeters) measured following the indicated treatments in the laser-induced CNV (LI-CNV) model (see Materials and Methods for details). The laser injury sites are indicated with arrows, and the optic disc is indicated with a “+.” n = 8 eyes in four rats/group. *p<0.05 versus PBS; #p<0.05 versus eyes injected with M1 macrophages alone.
Cell origin and environment can explain part of the variance in TNF-α expression at the mRNA and protein levels
Next, we examined our previous data regarding the expression profile of activated macrophages obtained from patients and controls [11] to determine whether cell origin, or the environment, or both can explain the variance in the expression of TNF-α and other cytokines in macrophages, thus indicating that expression is modifiable. At the mRNA level (Figure 3A), we found that 29% of the variance in the expression of TNF-α, VEGF, and PDGF measured in three macrophage subtypes cultured from patients with nvAMD (n = 7) and age-matched controls (n = 9) was explained by cell origin (i.e., patient-derived factors). However, 16%, 20%, and 9% of the variance in the TNF-α, VEGF, and PDGF mRNA levels, respectively, was explained by environmental factors (i.e., the cell culture conditions used to generate the macrophage phenotypes). Finally, 55%, 51%, and 62% of the variance in the TNF-α, VEGF, and PDGF mRNA levels, respectively, was either unexplained or was explained by the interaction between the cell origin and the environment.
Figure 3.
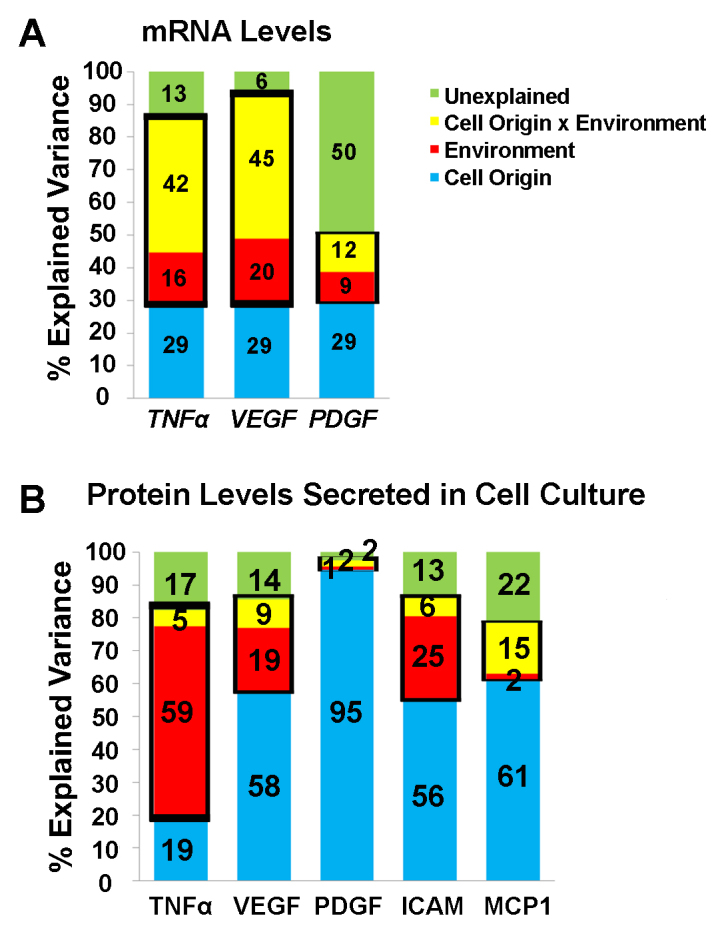
Summary of the factors that explain the variance in cytokine expression at the mRNA and protein levels. A: A mixed-design ANOVA with repeated measures was used to evaluate the effect of cell origin (blue), environment (red), and the interaction between cell origin and environment (yellow) on the mRNA levels of the indicated cytokines. B: A mixed-designed ANOVA with repeated measures on the protein levels of the indicated cytokines. Unexplained variance is shown in green. The thick black boxes are used to highlight the total variance explained by Environment and the interaction between cell origin and environment.
Using a mixed-design ANOVA with repeated measures, we combined non-modifiable factors, such as the patient’s age, gender, and disease status, and found that the interaction between the environment and the cell origin and the environment alone explained 58%, 65%, and 21% of the variance in the TNF-α, VEGF, and PDGF mRNA levels, respectively. Finally, 13%, 6%, and 50% of the variance in the TNF-α, VEGF, and PDGF mRNA levels, respectively, was unexplained (Figure 3A). These results suggest that more than 50% of the variance in the expression of TNF-α and VEGF at the mRNA level can be modified by factors such as cell origin and environment.
With respect to the variance in protein levels (based on 13 patients with nvAMD and 13 age-matched controls), we found that only 19% of the variance in the TNF-α protein was explained by cell origin, whereas 59% of the variance was explained by the environment (Figure 3B). In contrast, 58%, 95%, 56%, and 61% of the variance in VEGF, PDGF, ICAM, and MCP1, respectively, was explained by cell origin (Figure 3B). These results suggest that among these five cytokines, the expression of TNF-α is the most modifiable in activated macrophages.
Macrophage function in two models of CNV is modifiable
Using a mixed-design ANOVA with repeated measures, we found that macrophage function was modifiable in the in vivo and ex vivo models of CNV. Specifically, we found that cell origin explained only 17% and 35% of the variance in the effect of the three macrophage subtypes in the LI-CNV and CSA models, respectively (eight patients with nvAMD and nine age-matched controls), whereas 55% and 50%, respectively, was explained by environment and the interaction between the cell origin and the environment (Figure 4).
Figure 4.
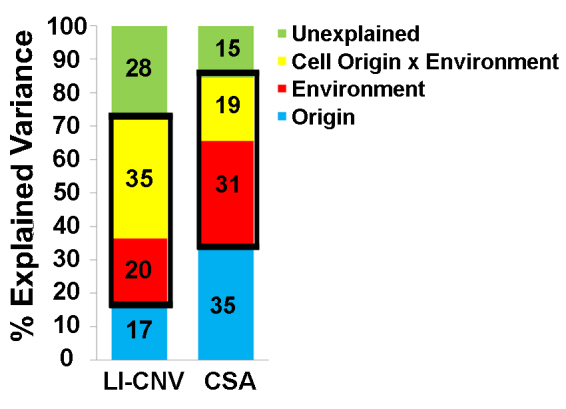
Summary of the factors that explain the variance of the effect of inflammatory macrophages obtained using the LI-CNV and CSA models. A mixed-design ANOVA with repeated measures was used to evaluate the effect of cell origin (blue), environment (red), and the interaction between cell origin and environment (yellow). Unexplained variance is shown in green. The thick black boxes are used to highlight the total variance explained by the environment and the interaction between the cell origin and the environment.
Discussion
We previously reported that activated macrophages—particularly M1 macrophages derived from patients with nvAMD—are proangiogenic in ex vivo and in vivo models of CNV. In addition, we reported that TNF-α is upregulated in these macrophages [11]. In this report, we show that TNF-α is associated with increased choroid sprouting in an ex vivo model. Furthermore, we found that anti-TNF-α therapy abolished the proangiogenic effects of M1 macrophages in the ex vivo and in vivo models, whereas anti-VEGF therapy was effective in vivo and only partially effective in the ex vivo model. Finally, we found that the expression of TNF-α and the proangiogenic effects of M1 macrophages are largely modifiable in both models.
In the clinical setting, anti-VEGF therapy alone is often not sufficient to suppress CNV in nvAMD over either the short- or long-term [36]. Liyanage et al. reported that VEGF expression in inflammatory macrophages is not involved in the development of laser-induced CNV [13]. Moreover, Wang et al. reported that TNF-α can increase the release of VEGF from RPE cells [37]. Taken together, these findings suggest that additional macrophage-derived cytokines and growth factors may underlie the proangiogenic properties of these cells in the context of nvAMD.
Previous studies yielded inconsistent results regarding the effect of anti-TNF-α therapy in nvAMD, including improved anatomic and visual outcomes, a lack of effect, and even the development of intraocular inflammation [38-40]. In addition, the release of TNF-α has been suggested to characterize activated monocytes in nvAMD, and differences in this release may underlie differences in the development of CNV [41]. The present results suggest that TNF-α may underlie the pathophysiological role of inflammatory macrophages in nvAMD and suggest that anti-TNF-α therapy may reduce macrophage-CNV. Thus, a therapeutic window of opportunity may exist for using anti-TNF-α in nvAMD, particularly given that macrophages play a major role in the development of CNV.
Targeting inflammatory macrophages using anti-TNF-α compounds may complement anti-VEGF-based treatment strategies for nvAMD. The putative benefits of targeting macrophages have been examined in other diseases, including cancer [42-44], lupus nephritis [45], and other inflammatory diseases [46]. Moreover, Zandi et al. suggested that inhibiting Rho-associated kinases, which have been implicated in activating macrophages, might also serve as a viable strategy in nvAMD [47].
We previously examined the effects of cross-species cytokine transplantation [11] based on the high degree of homology between human and rodent cytokines (ranging from 64% to 97% similarity) [48]. In addition, we found that human macrophages transplanted into the rat eye not only survive but also migrate across the retina to the lesion area and even can cross the retina to the RPE [11]. Importantly, ophthalmoscopy, immunostaining, and FACS analysis showed that rat eyes do not appear to develop an immune response to transplanted human cells, possibly because the eye is an immune-privileged organ.
This study has several caveats that warrant discussion. First, the rat model of LI-CNV involves a wound-healing reaction induced by damage at the level of Bruch’s membrane; thus, the response relies heavily on inflammation [6,7] and therefore, may not directly reflect the precise pathogenesis of nvAMD. In addition, because the rodent eye lacks a defined macula, this model may not fully mimic the complex pathology present in patients [49]. Nevertheless, this model has been found to be suitable for testing the efficacy of new drugs delivered either systemically or locally (i.e., by intraocular injection) and has been used to predict drug effects in patients with AMD (e.g., aflibercept) [50,51]. Furthermore, although we found that anti-VEGF abolished the development of CNV in the LI-CNV model, anti-VEGF has been reported to prevent angiogenesis in human CNV [12]. This may explain why we found similar results with respect to the CNV area between the group that received anti-VEGF alone and the group that received macrophages and anti-VEGF.
In conclusion, the present results indicate that targeting TNF-α may serve as a viable therapeutic strategy for nvAMD. Such approaches may involve the use of anti-TNF-α therapy or modulation of the production of TNF-α by macrophages or both. Therefore, future studies should focus on identifying the therapeutic window during which anti-TNF-α therapy would provide the highest benefit with respect to preventing the pathogenesis of nvAMD.
Acknowledgments
This study was supported in part by grants from the Israel Science Foundation (#1006/13) and the Israeli Ministry of Health (#9184). These funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The raw data used in this study will be made available upon reasonable request to the corresponding author at (chowers@hadassah.org.il).
References
- 1.Grunin M, Hagbi-Levi S, Chowers I. The role of monocytes and macrophages in age-related macular degeneration. Adv Exp Med Biol. 2014;801:199–205. doi: 10.1007/978-1-4614-3209-8_26. [DOI] [PubMed] [Google Scholar]
- 2.Qian B-Z, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarks SH, Van Driel D, Maxwell L, Killingsworth M. Softening of drusen and subretinal neovascularization. Trans Ophthalmol Soc U K. 1980;100:414–22. [PubMed] [Google Scholar]
- 4.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 5.Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 2006;3:e310. doi: 10.1371/journal.pmed.0030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–92. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 7.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–85. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 8.Kataoka K, Nishiguchi KM, Kaneko H, van Rooijen N, Kachi S, Terasaki H. The roles of vitreal macrophages and circulating leukocytes in retinal neovascularization. Invest Ophthalmol Vis Sci. 2011;52:1431–8. doi: 10.1167/iovs.10-5798. [DOI] [PubMed] [Google Scholar]
- 9.Robertson MJ, Erwig LP, Liversidge J, Forrester JV, Rees AJ, Dick AD. Retinal microenvironment controls resident and infiltrating macrophage function during uveoretinitis. Invest Ophthalmol Vis Sci. 2002;43:2250–7. [PubMed] [Google Scholar]
- 10.Scholl HP, Charbel Issa P, Walier M, Janzer S, Pollok-Kopp B, Borncke F, Fritsche LG, Chong NV, Fimmers R, Wienker T, Holz FG, Weber BH, Oppermann M. Systemic complement activation in age-related macular degeneration. PLoS One. 2008;3:e2593. doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagbi-Levi S, Grunin M, Jaouni T, Tiosano L, Rinsky B, Elbaz-Hayoun S, Peled A, Chowers I. Pro-Angiogenic Characteristics of Activated Macrophages from Patients with Age-related Macular Degeneration. Neurobiol Aging. 2017;51:71–82. doi: 10.1016/j.neurobiolaging.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 13.Liyanage SE, Fantin A, Villacampa P. Lange C a., Denti L, Cristante E, Smith AJ, Ali RR, Luhmann UF, Bainbridge JW, Ruhrberg C. Myeloid-Derived Vascular Endothelial Growth Factor and Hypoxia-Inducible Factor Are Dispensable for Ocular Neovascularization-Brief Report. Arterioscler Thromb Vasc Biol. 2016;36:19–24. doi: 10.1161/ATVBAHA.115.306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsutsumi C, Sonoda KH, Egashira K, Qiao H, Hisatomi T, Nakao S, Ishibashi M, Charo IF, Sakamoto T, Murata T, Ishibashi T. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003;74:25–32. doi: 10.1189/jlb.0902436. [DOI] [PubMed] [Google Scholar]
- 15.Krause TA, Alex AF, Engel DR, Kurts C, Eter N. VEGF-production by CCR2-dependent macrophages contributes to laser-induced choroidal neovascularization. PLoS One. 2014;9:94313. doi: 10.1371/journal.pone.0094313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawanaka N, Yamamura M, Aita T, Morita Y, Okamoto A, Kawashima M, Iwahashi M, Ueno A, Ohmoto Y, Makino H. CD14+,CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2002;46:2578–86. doi: 10.1002/art.10545. [DOI] [PubMed] [Google Scholar]
- 17.Song J, Lee K, Park SW, Chung H, Jung D, Na YR, Quan H, Cho CS, Che JH, Kim JH, Park JH, Seok SH. Lactic acid upregulates VEGF expression in macrophages and facilitates choroidal neovascularization. Invest Ophthalmol Vis Sci. 2018;59:3747–54. doi: 10.1167/iovs.18-23892. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Tu Y, Wang Y, Xu X, Sun X, Xie L, Zhao Q, Guo Y, Gu Y, Du J, Du S, Zhu M, Song E. Prodrug of epigallocatechin-3-gallate alleviates choroidal neovascularization via down-regulating HIF-1α/VEGF/VEGFR2 pathway and M1 type macrophage/microglia polarization. Biomed Pharmacother. 2020;121:1–9. doi: 10.1016/j.biopha.2019.109606. [DOI] [PubMed] [Google Scholar]
- 19.Cao X, Shen D, Patel MM, Tuo J, Johnson TM, Olsen TW, Chan C-CC. Macrophage polarization in the maculae of age-related macular degeneration: A pilot study. Pathol Int. 2011;61:528–35. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly J, Khan AA, Yin J, Ferguson TA, Apte RS, Ali Khan A, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest. 2007;117:3421–6. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Liu F, Tang M, Yuan M, Hu A, Zhan Z, Li Z, Li J, Ding X, Lu L. Macrophage polarization in experimental and clinical choroidal neovascularization. Sci Rep. 2016;6:30933–45. doi: 10.1038/srep30933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagbi-Levi S, Abraham M, Tiosano L, Rinsky B, Grunin M, Eizenberg O, Peled A, Chowers I. Promiscuous Chemokine Antagonist (BKT130) Suppresses Laser-Induced Choroidal Neovascularization by Inhibition of Monocyte Recruitment. J Immunol Res. 2019;•••:8535273. doi: 10.1155/2019/8535273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Age-Related Eye Disease Study Research G. The Age-Related Eye Disease Study (AREDS) design implications. AREDS report no. 1. Control Clin Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelinas L, Falkenham A, Oxner A, Sopel M, Legare JF. Highly purified human peripheral blood monocytes produce IL-6 but not TNF{alpha} in response to angiotensin II. J Renin Angiotensin Aldosterone Syst. 2011;12:295–303. doi: 10.1177/1470320310391332. [DOI] [PubMed] [Google Scholar]
- 25.Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARγ Activation Primes Human Monocytes into Alternative M2 Macrophages with Anti-inflammatory Properties. Cell Metab. 2007;6:137–43. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 27.Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J. 2009;28:2114–27. doi: 10.1038/emboj.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 29.Shao Z, Friedlander M, Hurst CG, Cui Z, Pei DT, Evans LP, Juan AM, Tahiri H, Duhamel F, Chen J, Sapieha P, Chemtob S, Joyal J-SS, Smith LEH. Choroid sprouting assay: an ex vivo model of microvascular angiogenesis. PLoS One. 2013;8:e69552. doi: 10.1371/journal.pone.0069552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grattendick KJ, Nakashima JM, Feng L, Giri SN, Margolin SB. Effects of three anti-TNF-alpha drugs: etanercept, infliximab and pirfenidone on release of TNF-alpha in medium and TNF-alpha associated with the cell in vitro. Int Immunopharmacol. 2008;8:679–87. doi: 10.1016/j.intimp.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43:25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- 32.Tobe T, Okamoto N, Vinores MA, Derevjanik NL, Vinores SA, Zack DJ, Campochiaro PA. Takao Tobe, 1‘2 Naoyuki Okamoto, 1’2 Melissa A. Vinores, 1‘2 Nancy L Derevjanik 1’2, Stanley A. Vinores, 12 Donald J. Zack 1′5, Campochiaro12 PA, Tobe T, Okamoto N, Vinores MA, Derevjanik NL, Vinores SA, Zack DJ, Campochiaro PA. Evolution of Neovascularization in Mice with Overexpression of Vascular Endothelial Growth Factor in Photoreceptors. Invest Ophthalmol Vis Sci. 1998;39:180–8. [PubMed] [Google Scholar]
- 33.Lederman M, Hagbi-Levi S, Grunin M, Obolensky A, Berenshtein E, Banin E, Chevion M, Chowers I. Degeneration modulates retinal response to transient exogenous oxidative injury. PLoS One. 2014;9:e87751. doi: 10.1371/journal.pone.0087751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giansanti F, Ramazzotti M, Vannozzi L, Rapizzi E, Fiore T, Iaccheri B. Degl’Innocenti D, Moncini D, Menchini U. A pilot study on ocular safety of intravitreal infliximab in a rabbit model. Invest Ophthalmol Vis Sci. 2008;49:1151–6. doi: 10.1167/iovs.07-0932. [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Chen M, Manivannan A, Lois N, Forrester JV. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 2008;7:58–68. doi: 10.1111/j.1474-9726.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 36.Beykin G, Grunin M, Averbukh E, Banin E, Hemo Y, Chowers I. Bevacizumab treatment for neovascular age-related macular degeneration in the setting of a clinic: “real life” long-term outcome. BMC Ophthalmol. 2015;15:39. doi: 10.1186/s12886-015-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Han X, Wittchen ES, Hartnett ME. TNF-alpha mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent beta-catenin activation. Mol Vis. 2016;22:116–28. [PMC free article] [PubMed] [Google Scholar]
- 38.Markomichelakis NN, Theodossiadis PG, Sfikakis PP. Regression of neovascular age-related macular degeneration following infliximab therapy. Am J Ophthalmol. 2005;139:537–40. doi: 10.1016/j.ajo.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 39.Theodossiadis PG, Liarakos VS, Sfikakis PP, Vergados IA, Theodossiadis GP. Intravitreal Administration of the Anti-Tumor Necrosis Factor Agent Infliximab for Neovascular Age-related Macular Degeneration. Am J Ophthalmol. 2009;147:825–30. doi: 10.1016/j.ajo.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Nussenblatt RB, Byrnes G, Sen HN, Yeh S, Faia L, Meyerle C, Wroblewski K, Li Z, Liu B, Chew E, Sherry PR, Friedman P, Gill F, Ferris F. A randomized pilot study of systemic immunosuppression in the treatment of age-related macular degeneration with choroidal neovascularization. Retina. 2010;30:1579–87. doi: 10.1097/IAE.0b013e3181e7978e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol. 2004;122:1013–8. doi: 10.1001/archopht.122.7.1013. [DOI] [PubMed] [Google Scholar]
- 42.Panni RZ, Linehan DC, DeNardo DG. Targeting tumor-infiltrating macrophages to combat cancer. Immunotherapy. 2013;5:1075–87. doi: 10.2217/imt.13.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Östling J, Dahan R, Harris RA, Rantalainen M, Klevebring D, Sund M, Brage SE, Fuxe J, Rolny C, Li F, Ravetch JV, Karlsson MCI. Reprogramming Tumor-Associated Macrophages by Antibody Targeting Inhibits Cancer Progression and Metastasis. Cell Reports. 2016;15:2000–11. doi: 10.1016/j.celrep.2016.04.084. [DOI] [PubMed] [Google Scholar]
- 44.Ries CH, Hoves S, Cannarile MA, Ruttinger D. CSF-1/CSF-1R targeting agents in clinical development for cancer therapy. Curr Opin Pharmacol. 2015;23:45–51. doi: 10.1016/j.coph.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Chalmers SA, Chitu V, Ramanujam M, Putterman C. Therapeutic targeting of macrophages in lupus nephritis. Discov Med. 2015;20:43–9. [PubMed] [Google Scholar]
- 46.Patel SK, Janjic JM. Macrophage targeted theranostics as personalized nanomedicine strategies for inflammatory diseases. Theranostics. 2015;5:150–72. doi: 10.7150/thno.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zandi S, Nakao S, Chun KH, Fiorina P, Sun D, Arita R, Zhao M, Kim E, Schueller O, Campbell S, Taher M, Melhorn MI, Schering A, Gatti F, Tezza S, Xie F, Vergani A, Yoshida S, Ishikawa K, Yamaguchi M, Sasaki F, Schmidt-Ullrich R, Hata Y, Enaida H, Yuzawa M, Yokomizo T, Kim YB, Sweetnam P, Ishibashi T, Hafezi-Moghadam A. ROCK-Isoform-Specific Polarization of Macrophages Associated with Age-Related Macular Degeneration. Cell Reports. 2015;10:1173–86. doi: 10.1016/j.celrep.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller JW. Age-related macular degeneration revisited–piecing the puzzle: the LXIX Edward Jackson memorial lecture. Am J Ophthalmol. 2013;155:1–35. doi: 10.1016/j.ajo.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 50.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Saishin Y, Saishin YY, Takahashi K, Silva RLE, Hylton D, Rudge JS, Wiegand SJ, Campochiaro PA. VEGF-TRAPR1R2 suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:241–8. doi: 10.1002/jcp.10246. [DOI] [PubMed] [Google Scholar]