Abstract
This study examined the effects of injectable vitamin C (VC) before transport and duration of transit on feedlot performance, inflammation, and muscle fatigue in cattle. One hundred thirty-two Angus-cross steers (393 ± 4 kg) were stratified by body weight (BW) to a 2 × 2 factorial of intramuscular injection (INJ; 20 mL/steer): VC (250 mg sodium ascorbate/mL) or saline (SAL) and road transit duration (DUR): 18 h (18-h; 1,770 km) or 8 h (8-h; 727 km). On day 0, steers were weighed and given INJ of VC or SAL immediately before transport. Upon return (day 1), BW and blood were collected before steers returned to pens equipped with GrowSafe bunks. Steers were weighed on days 0, 1, 7, 15, 30, 31, 54, and 55. Data were analyzed via ProcMixed of SAS (experimental unit = steer; 32 to 34 steers/treatment) with fixed effects of INJ, DUR, and the interaction. Blood was collected on days −5, 1, 2, 3, and 7 (n = 9 steers/treatment); blood parameters were analyzed as repeated measures with the repeated effect of day. Area under the curve (AUC) for plasma ferric reducing antioxidant power (FRAP) was calculated using R. Final BW was greater for 8 h compared to 18 h (P = 0.05) with no effect of INJ or interaction (P ≥ 0.51). Dry matter intake (DMI) from days 1 to 7 was greater for VC-8, intermediate for VC-18 and SAL-18, and least for SAL-8 (P = 0.02). Overall, DMI tended to be greatest for SAL-18, intermediate for VC-18 and VC-8, and least for SAL-8 (P = 0.08). Days 7 to 31 gain:feed (G:F) was greatest for VC-18 compared to other treatments (INJ × DUR, P = 0.05), and there was no effect of treatment on overall G:F (P ≥ 0. 19). There was no INJ or INJ × DAY (P ≥ 0.17) effect on serum lactate, haptoglobin, or non-esterified fatty acid. However, these blood parameters were greater on day 1 for 18 h compared to 8 h, and both treatments returned to near baseline by day 3 (DUR × DAY, P < 0.01). Plasma ascorbate concentrations on day 1 were greater for VC compared to SAL and returned to baseline by day 2 (INJ × DAY, P < 0.01). Plasma FRAP AUC from days −5 to 3 was greatest for VC-18, intermediate for VC-8 and SAL-8, and least for SAL-18 (INJ × DAY, P = 0.02). This suggests an antioxidant prior to long-haul transit positively influenced antioxidant capacity; however, VC did not improve overall post-transit performance. Although longer transit duration increased indicators of muscle fatigue and inflammation, post-transit performance was not appreciably different between transit durations.
Keywords: antioxidant system, beef cattle, stress, transportation, vitamin C
Introduction
Transportation of cattle is unavoidable due to the segmentation of the beef industry. In a calf’s lifetime, they may be transported four to six times between different farms and auction markets (González et al., 2012). Each transit event can induce physiological and psychological stress on the calf. Increased heart rate, inflammation (Arthington et al., 2003), and oxidative stress (Chirase et al., 2004; Deters and Hansen, 2019) are signs of physiological stress following transit. These stressors can be destructive to animal health and performance, resulting in decreased growth efficiency leading to increased cost of gain and break-even prices, decreasing the economical net-return for producers.
Antioxidant properties of vitamin C (VC) have been shown to improve performance following a transportation event. Deters and Hansen (2020) found an injection of VC prior to transit resulted in a ~7 kg advantage in body weight 56-d post-transit compared to steers not receiving a VC injection. This reveals injectable VC as a cost-effective strategy to add value to cattle with a simple addition to the management protocol, yet the mechanisms by which injectable VC affects growth need to be further investigated.
Long-duration transit can result in oxidative stress from an imbalanced redox status when the production of oxidants exceeds that of antioxidants (Finkel and Holbrook, 2000; Chirase et al., 2004). Transportation has been shown to decrease the status of certain antioxidants, such as decreased plasma VC and vitamin E (Chirase et al., 2004; Deters and Hansen, 2020). Consequently, the reduction of antioxidants can impair immune system activation and the body’s ability to mount a defense against infections (Hughes, 1999; Marques et al., 2012). Oxidative stress causes the animal to repartition energy away from growth to repair oxidatively damaged molecules, further hindering the efficiency of growth. Furthermore, markers of oxidative stress along with serum lactate are common indicators of muscle fatigue.
Additionally, the duration of transit experienced by cattle is extremely variable and may stimulate different physiological responses. It is critical to understand how cattle respond to varying transit durations to develop strategies that lessen the negative implications of transit on animal health and production. It was hypothesized that cattle transported for a longer duration would exhibit greater markers of muscle fatigue, and injectable VC prior to transit would mitigate the transit-induced oxidative stress and positively influence post-transit growth performance. Thus, the objectives of the study were to determine the effects of injectable VC prior to transit and transit duration (8 vs. 18 h) on feedlot performance, VC status, and inflammation, as well as muscle fatigue in beef cattle.
Material and Methods
Experimental procedures were approved by the Iowa State University Animal Care and Use Committee (#IACUC-19-180).
Experimental design
One hundred sixty newly weaned, Angus-cross steers were transported from a single ranch in North Dakota to the Iowa State University Beef Nutrition Farm (Ames, IA) in October 2019. Upon arrival steers were offered long-stem grass hay top-dressed with the corn silage-based receiving diet. No additional hay was offered after the second full day. Prior to initiation of the trial, steers were weighed, received visual and electronic identification tags, and given booster vaccinations for clostridial (Ultrabac 7, Zoetis, Parsippany-Troy Hills, NJ) diseases and injectable dewormer (Dectomax, Zoetis) for internal and external parasite control. Steers were fed a common corn silage-based diet and housed in dirt lot pens.
Ninety-eight days after arrival (day −12 relative to trial initiation) steers were moved to partially covered concrete pens (24 pens, n = 6 steers/pen) equipped with GrowSafe bunks (GrowSafe Systems Ltd., Airdrie, Alberta, Canada) for a pre-trial acclimation period and started on a higher-energy total mixed ration (TMR; Table 1). On day −5, 132 steers most uniform in weight (393 ± 4 kg), disposition, and health were stratified by BW and randomly assigned to a 2 × 2 factorial. Steers were given intramuscular injection (INJ; 20 mL/steer; 10 mL/injection site on opposite sides of the neck) of VC (Vet One, Boise, ID; 250 mg sodium ascorbate/mL; 5 g/steers) or saline (SAL) approximately 2 h prior to transit, and prior to injections steers had ad libitum access to feed and water; 18 h consumed 34% of the previous day’s DMI while 8 h steers consumed 99% of the previous day’s DMI. Following pre-transit injections, steers were loaded onto a commercial livestock trailer (Silverstar PSDLC-402; Wilson Trailer Company, Sioux City, IA) and transported for two different durations (DUR) of 18 h (18-h; 1,770 km) or 8 h (8-h; 727 km). During transport, steers did not have access to feed or water. All 18-h steers were loaded onto a single trailer at 1300 h and 8-h steers were loaded onto a separate single trailer at 2300 h, on staggered days, to ensure the same return time for both DUR and a consistent sampling time of day for all treatments. At the time of loading, several steers were removed from the study to align with the legal weight limit of the trailer (2 VC-18, 3 SAL-18, 3 VC-8, and 4 SAL-8); this was due to calves having heavier BW than expected.
Table 1.
Ingredient composition of common diet fed from days −12 to 55
| DM% | 57 |
| Ingredient, % DM basis | |
| Sweet Bran1 | 35 |
| Corn silage | 30 |
| Dried distillers grains with solubles | 18.04 |
| Dry-rolled corn | 15 |
| Limestone | 1.5 |
| Salt | 0.31 |
| Rumensin2 | 0.015 |
| Vitamin A and E premix3 | 0.11 |
| Trace mineral premix4 | 0.024 |
| Analyzed composition5 | |
| Crude protein | 17.5 |
| Neutral detergent fiber | 28.4 |
| Ether extract | 4.6 |
| Calculated composition6 | |
| Net energy for gain, Mcal/kg | 1.32 |
1Branded wet corn gluten feed (Cargill Corn Milling, Blair, NE).
2Provided monensin (Elanco Animal Health, Greenfield, IN) at 27 g/ton.
3Provided 2,200 IU vitamin A and 25 IU vitamin E/kg diet DM.
4Provided per kg of diet DM: 10 mg of Cu, 30 mg of Zn, 20 mg of Mn, 0.5 mg of I, 0.1 mg of Se, and 0.1 mg of Co all from inorganic sources.
5Based on total mixed ration analysis from Dairyland, Inc., Arcadia, WI.
6Based on National Academics of Sciences, Engineering, and Medicine (2016) reported net energy for gain values of feedstuffs.
Steers returned to the Iowa State University Beef Nutrition Farm on day 1 (~ 0700 h) and were sorted back into pens equipped with one GrowSafe bunk/pen (6 pens/treatment; 5 to 6 steers/pen) to measure daily individual feed disappearance. From days −12 to 55, all steers were fed a common total mixed ration (Table 1) that was delivered once daily at approximately 0800 h and allowed ad libitum access to feed and water. Steers were weighed on days −5, 0 (pre-transit), 1 (post-transit), 7, 15, 30, 31, 54, and 55. All BW were collected prior to feeding, except day 0 was collected prior to loading and day 1 was collected immediately off the truck to calculate shrink. Steer dry matter intake (DMI), shrink adjusted average daily gain (ADG), and feed efficiency (gain:feed; G:F) were calculated for days 1 to 7, 7 to 31, 31 to 55, and 1 to 55. On day 7, steers were implanted with 80 mg trenbolone acetate, 16 mg estradiol, and 29 mg tylosin tartrate (Component TE-IS, Elanco Animal Health). One steer was removed from the study due to bloat (1 VC-18). Performance data from this steer were included in analysis until the time of removal (day 25) from study.
Sample collection and analytical procedures
Samples of TMR and feed ingredients were collected weekly for dry matter (DM) determination and subsamples were dried in a forced air oven for 48 h at 70 °C. These DM values determined from weekly TMR analysis were used to calculate daily steer DMI based on individual as-fed feed disappearance recorded by the GrowSafe system. After determination of DM values, the dried TMR samples were ground to pass through a 2-mm screen in a Retsch ZM 100 grinding mill (Retsch GmbH, Haan, Germany) and subsamples were composited for analysis by a commercial laboratory (Dairyland Laboratories, Inc., Arcadia, WI) for nitrogen (crude protein; AOAC 1999b; method 990.03), neutral detergent fiber (AOAC, 2005; method 2002.04), and ether extract (AOAC, 1999a; method 920.39); analyzed compositions are presented in Table 1.
Nine steers representative of the average BW across all treatments were selected from each treatment for blood sample collection at all sampling time points, and steers were distributed across pens (n = 6 pens/treatment; n =1–2 samplers/pen). Sample collection started at 0700 h and prior to feeding; blood was collected via jugular venipuncture into vacuum tubes (sodium heparin, #455051, Greiner Bio-One, Monroe, NC; serum, #368045, Becton Dickinson, Franklin Lakes, NJ) on days −5, 1 (post-transit), 2 (24 h post-return), 3 (48 h post-return), and 7 and transported to the laboratory on ice. Sodium heparin tubes were centrifuged at 1,000 × g × 20 min at 4 °C; plasma was then removed and aliquoted into microcentrifuge tubes and stored at −80 °C for future analysis of ascorbate, cortisol, and ferric reducing antioxidant power (FRAP). For prevention of ascorbate degradation, plasma for ascorbate analysis was stabilized with diethylenetriaminepentaacetic acid prior to freezing and all samples were analyzed for ascorbate within 40 d of sample collection. Plasma for ascorbate was carefully handled to avoid direct exposure to light. Commercially available kits were used for analysis of plasma ascorbate (#700420, Cayman Chemical, Ann Arbor, MI; intra-assay CV = 5.16%, inter-assay CV = 8.0%) and FRAP (#K043-H1, Arbor Assays; intra-assay CV = 2.52%, inter-assay CV = 3.68%) concentrations, and cortisol was analyzed using an enzyme immunoassay kit (K003-H1/H5, Arbor Assays, Ann Arbor, MI; intra-assay CV = 6.43%, inter-assay CV = 8.91%). Serum samples were left to clot at room temperature for 90 min prior to centrifugation at 1,000 × g × 20 min at 4 °C; serum was aliquoted into microcentrifuge tubes and stored at −80 °C for future analysis of non-esterified fatty acids (NEFA), haptoglobin (HP), and lactate. Serum NEFA and L-lactate concentrations were analyzed using commercially available colorimetric kits (Wako Diagnostics, Mountain View, CA; intra-assay CV = 1.27%, inter-assay CV = 6.72%; Biomedical Research Service Center, Buffalo, NY; intra-assay CV = 4.31%, inter-assay CV = 4.93%, respectively), and HP concentrations were analyzed using a bovine specific ELISA kit (Hapt-11, Life Diagnostics, Inc., West Chester, PA; intra-assay CV = 5.30%, inter-assay CV = 5.22%).
Statistical analysis
Data were analyzed as a complete 2 × 2 factorial using the Mixed procedures of SAS 9.4 (SAS Institute Inc., Cary, NC) with fixed effects of INJ, DUR, and the interaction of INJ and DUR. Steer served as the experimental unit (n = 32 to 34 steers/treatment for all performance variables and 9 steers/treatment for blood variables). Day 0 (pre-transit) DMI was utilized as a covariate in shrink analysis, and each respective treatment adjusted shrink was applied to days 7, 15, 30/31, and 54/55 BW; shrink adjusted BW was used to calculate ADG. Steer BW from day −5 was used as a covariate in analysis of subsequent BW samples dates, ADG, and G:F, and pre-trial (days −7 through −1) DMI utilized as the covariate in analysis of subsequent DMI data. Blood parameters (n = 9 steers/treatment) were analyzed as repeated measures with fixed effects of INJ, DUR, and the interaction, with the repeated effect of day. The heterogenous autoregressive covariance structure was utilized for analysis of serum NEFA and HP and plasma cortisol, whereas autoregressive covariance structure was utilized for analysis of serum lactate and compound symmetry covariance structure was used in analysis of plasma ascorbate based on lowest Akaike’s information criterion (Littell et al., 1998). Area under the curve (AUC) was calculated using R for FRAP from days −5 to 3. Data were tested for normality using the Shapiro−Wilk test; serum HP and NEFA concentrations were natural log transformed to meet the assumption of normality and back transformed means and SEM are presented. Data were tested for outliers using Cook’s D statistic and removed if Cook’s D ≥ 0.30; one VC-8 steer was removed from analyses of DMI and G:F. Data are presented as least square means ± SEM and the difference between means was determined using the PDIFF statement in SAS. Significance was declared at P ≤ 0.05 and tendencies from 0.05 < P ≤ 0.10.
Results
Feedlot performance
Initial BW on day −5 was not different between treatments (Table 2; P ≥ 0.56); however, pre-transit (day 0) and post-transit (day 1) BW were greater for 8-h steers than 18-h steers (P ≥ 0.01). There were no effect of INJ × DUR or INJ on shrink (P ≥ 0.67), but steers transported for steers transported for 18-h had greater shrink compared to 8-h steers (DUR P < 0.01). Body weights were greatest on day 7 for VC-8, intermediate for SAL-8, and least for steers transported 18 h regardless of INJ treatment (INJ × DUR P = 0.03). Average daily gain from days 1 to 7 was greatest for SAL-18 and VC-8, intermediate for 8-SAL, and least for VC-18 (INJ × DUR P = 0.02). Day 15 BW were greatest for VC-8, intermediate for SAL-steers regardless of transit duration, and least for VC-18 (INJ × DUR P < 0.01). Midpoint (days 30/31) and final BW (days 54/55) were greater for 8-h than 18-h steers (P ≤ 0.05) with no effect of INJ or INJ × DUR (P ≥ 0.35). For ADG, 18-h steers exhibited greater ADG (P ≤ 0.04) from days 7 to 31 and days 1 to 55 compared to 8-h steers with no effect of INJ or INJ × DUR (P ≥ 0.18). Steer DMI from days 1 to 7 was greatest for VC-8, intermediate for steers transported for 18-h regardless of injection, and lowest for SAL-8 (INJ × DUR P = 0.02). From days 7 to 31, SAL-18 continued to have greater DMI with VC-8 being intermediate, and VC-18 and SAL-8 having the lowest intakes (INJ × DUR P = 0.03). There were no treatment effects for DMI on d 31 to 55 (P ≥ 0.35); however overall DMI tended to be greatest for SAL-18, intermediate for VC-18 and VC-8, and lowest for SAL-8 steers (INJ × DUR P = 0.08). Days 1 to 7 G:F was greater for VC-8 and SAL-18 and lesser for SAL-8 and VC-18 (INJ × DUR P = 0.03). However, from days 7 to 31, G:F was greatest for VC-18 compared to all other treatments (INJ × DUR P = 0.05). There were no effects of INJ, DUR, or the interaction (P ≥ 0.19) on G:F from days 31 to 55 or overall.
Table 2.
Injectable vitamin C and transit duration effects on feedlot performance by beef steers
| DUR1 | 18-h | 8-h | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| INJ2 | VC | SAL | VC | SAL | SEM3 | INJ | DUR | INJ × DUR |
| Steer (n) | 34 | 33 | 33 | 32 | ||||
| Body weight, kg | ||||||||
| d −5 | 391 | 392 | 392 | 395 | 4.2 | 0.56 | 0.59 | 0.79 |
| d 04 | 406 | 405 | 414 | 412 | 1.0 | 0.18 | 0.01 | 0.65 |
| d 14 | 378 | 377 | 387 | 385 | 0.8 | 0.07 | 0.01 | 0.89 |
| Shrink5,6,% | 7.33 | 7.44 | 5.95 | 5.91 | 0.26 | 0.83 | 0.01 | 0.67 |
| d 74 | 379c | 380c | 390a | 386b | 1.0 | 0.20 | 0.01 | 0.03 |
| d 154 | 397c | 400b | 406a | 402b | 1.1 | 0.85 | 0.01 | 0.01 |
| d 30/314 | 426 | 427 | 433 | 431 | 1.5 | 0.86 | 0.01 | 0.35 |
| d 54/554 | 471 | 474 | 476 | 477 | 2.2 | 0.51 | 0.05 | 0.62 |
| ADG4, kg/d | ||||||||
| d 1 to 7 | 0.10 | 0.46 | 0.46 | 0.15 | 0.151 | 0.87 | 0.87 | 0.02 |
| d 7 to 31 | 1.96 | 1.96 | 1.79 | 1.86 | 0.059 | 0.52 | 0.02 | 0.53 |
| d 31 to 55 | 1.89 | 1.95 | 1.82 | 1.90 | 0.055 | 0.19 | 0.27 | 0.80 |
| d 1 to 55 | 1.72 | 1.79 | 1.65 | 1.69 | 0.039 | 0.18 | 0.04 | 0.66 |
| DMI7, kg/d | ||||||||
| d 1 to 7 | 8.5ab | 8.6ab | 8.8a | 8.4b | 0.12 | 0.25 | 0.84 | 0.02 |
| d 7 to 31 | 9.4b | 9.9a | 9.6ab | 9.5b | 0.13 | 0.19 | 0.31 | 0.03 |
| d 31 to 55 | 10.4 | 10.7 | 10.4 | 10.4 | 0.15 | 0.37 | 0.36 | 0.35 |
| d 1 to 55 | 9.8xy | 10.1x | 9.9xy | 9.8y | 0.13 | 0.32 | 0.29 | 0.08 |
| G:F4, kg/kg | ||||||||
| d 1 to 7 | 0.010 | 0.048 | 0.058 | 0.017 | 0.0172 | 0.91 | 0.63 | 0.03 |
| d 7 to 31 | 0.212a | 0.194b | 0.189b | 0.195b | 0.0062 | 0.34 | 0.07 | 0.05 |
| d 31 to 55 | 0.186 | 0.180 | 0.178 | 0.182 | 0.0050 | 0.86 | 0.61 | 0.35 |
| d 1 to 55 | 0.180 | 0.174 | 0.172 | 0.172 | 0.0039 | 0.48 | 0.19 | 0.42 |
1DUR: 18-h, steers loaded at 1300 h and transported for 18 h (1,770 km); 8-h, steers loaded at 2300 h and transported for 8 h (727 km). d 0 BW = BW collected pre-transit; d 1 BW = BW collected post-transit.
2INJ: VC, injection of 20 mL of sodium ascorbate (250 mg/mL) and SAL, injection of 20 mL of saline.
3Highest SEM of any treatment reported.
4Body weight from day −5 applied as a covariate for BW, ADG, and G:F analyses.
5Steer DMI from day 0 prior to loading used as a covariate in analysis of shrink.
6Percent BW covariate adjusted shrink after transit event was applied to subsequent BW to be comparable to the post-transit BW conditions.
7Average steer DMI from days −7 through −1 utilized as a covariate in analysis.
a–cLeast square means in a row without common superscripts differ (P ≤ 0.05).
x,yValues with unlike superscripts tend to differ (P ≤ 0.10).
Blood metabolites
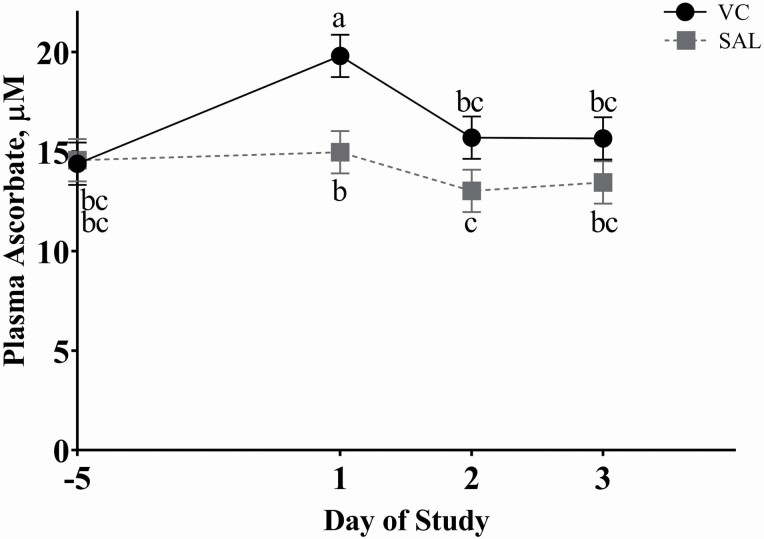
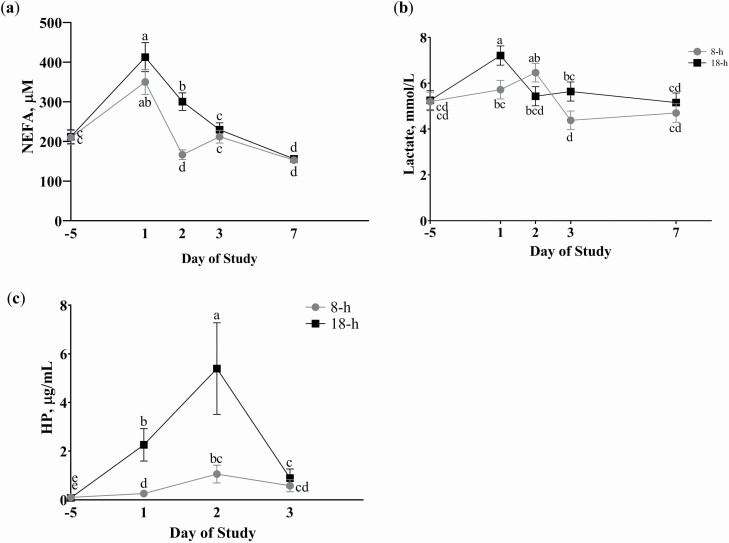
Treatment means from repeated measure analysis for plasma ascorbate concentrations and simple means for plasma FRAP concentrations are presented in Table 3. Initial plasma ascorbate concentrations were similar among treatments, and VC-steers exhibited greater plasma ascorbate concentration immediately post-transit (day 1) compared to SAL-steers (INJ × DAY, P < 0.01; Figure 1). By day 2, plasma ascorbate concentration had returned to near baseline concentrations. There was no effect of DUR× DAY or DUR (P ≥ 0.13) on plasma ascorbate concentrations. Area under the curve from days −5 to 3 for plasma FRAP was greatest for VC-18, intermediate for steers transported for 8 h regardless of INJ treatment, and lowest for SAL-18 (INJ × DUR P = 0.02, Figure 2). There was no effect of INJ or INJ × DAY (P ≥ 0.17) on serum NEFA, lactate, or HP. Duration impacted serum NEFA concentrations where both DUR treatments exhibited increased NEFA concentration on day 1, and 18-h steers NEFA concentrations remained increased through day 2 while 8-h steers returned to below baseline concentrations (DUR × DAY, P < 0.01; Figure 3a). Serum lactate exhibited a DUR × DAY interaction (P < 0.01) with 18-h steers having greater serum lactate concentration on day 1 while 8-h steers peaked on day 2 and both returned to initial concentrations by day 3 (Figure 3b). In addition, DUR impacted serum HP concentrations (DUR × DAY, P < 0.01), where steers transported for 18 h had greater serum HP concentration on days 1 and 2 compared to 8-h steers and returned to near initial concentrations for both DUR treatments by day 3 (Figure 3c).
Table 3.
Repeated measure analysis means for plasma ascorbate concentrations and simple means for FRAP concentrations
| DUR1 | 18-h | 8-h | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INJ2 | VC | SAL | VC | SAL | SEM3 | INJ | DUR | INJ × DUR | Day | INJ × Day | DUR × Day | INJ × DUR ×Day |
| Steer (n) | 9 | 9 | 9 | 9 | ||||||||
| Plasma ascorbate4, µM | ||||||||||||
| Overall | 14.8 | 13.6 | 18.0 | 14.4 | 1.28 | 0.07 | 0.13 | 0.38 | 0.01 | 0.01 | 0.41 | 0.50 |
| d -5 | 12.3 | 14.6 | 16.5 | 14.5 | ||||||||
| d 1 | 18.1 | 13.5 | 21.5 | 16.4 | ||||||||
| d 2 | 14.8 | 13.0 | 16.6 | 13.1 | ||||||||
| d 3 | 14.1 | 13.3 | 17.2 | 13.6 | ||||||||
| Plasma FRAP5, µM | SD | |||||||||||
| d −5 | 371 | 363 | 339 | 365 | 47.3 | |||||||
| d 1 | 344 | 309 | 327 | 324 | 70.6 | |||||||
| d 2 | 370 | 328 | 390 | 356 | 68.6 | |||||||
| d 3 | 368 | 307 | 337 | 376 | 60.7 |
1DUR: 18-h, steers loaded at 1300 h and transported for 18 h (1,770 km); 8-h, steers loaded at 2300 h and transported for 8 h (727 km)
2INJ: VC, injection of 20 mL of sodium ascorbate (250 mg/mL) and SAL, injection of 20 mL of saline.
3Highest SEM of any treatment reported
4Plasma ascorbate analyzed as repeated measures with INJ × Day effect (P < 0.01) shown in Figure 1
5Simple means for plasma FRAP shown; FRAP analyzed as area under the curve for days −5 to 3 displayed in Figure 2
Figure 1.
Effect of injectable VC and day relative to transit event on day 0 (INJ × Day P < 0.01) on plasma ascorbate concentrations based upon repeated measures analysis of samples collected on days −5 and 1 (post-transit), days 2 and 3 (24 and 48 h post-transit); values with unlike superscripts differ (P ≤ 0.05). VC = 20 mL of sodium ascorbate (250 mg/mL) administered intramuscularly (IM) immediately prior to transit. SAL = 20 mL of saline administered IM immediately prior to transit. There was no effect of DUR or INJ × DUR on plasma ascorbate concentration (P ≥ 0.13). N = 18 steers per INJ treatment.
Figure 2.
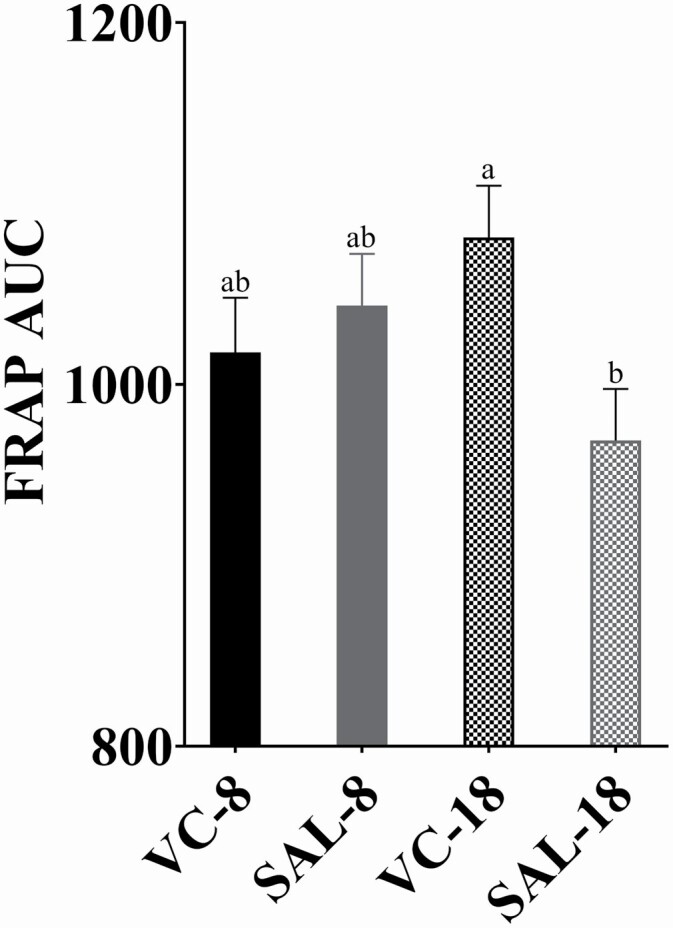
Effect of INJ × DUR (P = 0.02) on plasma ferric reducing antioxidant power (FRAP) area under the curve (AUC) from days −5 and 1 (post-transit), days 2 and 3 (24 and 48 h post-transit); values with unlike superscripts differ (P ≤ 0.05). VC-18 = 20 mL of sodium ascorbate (250 mg/mL) administered intramuscularly (IM) immediately prior to 18 h transit. VC-8 = 20 mL of sodium ascorbate (250 mg/mL) administered IM immediately prior to 8 h transit. SAL-18 = 20 mL of saline administered IM immediately prior to 18 h transit. SAL-8 = 20 mL of saline administered IM immediately prior to 8 h transit. N = 9 steers per treatment.
Figure 3.
Effect of DUR by day (P < 0.01) relative to transit event (day 0) on (a) serum non-esterified fatty acid (NEFA), (b) lactate, and (c) haptoglobin (HP) concentrations based upon repeated measures analysis of samples collected on days −5 and 1 (post-transit), days 2 and 3 (24 and 48 h post-transit, respectively), and day 7. Within a panel, values with unlike superscripts differ (P ≤ 0.05). 8-h = steers transported for 8 h (727 km) and 18-h = steers transported for 18 h (1,770 km). There was no effect of INJ, INJ × Day, or INJ × DUR × Day (P ≥ 0.17). N = 18 steers per DUR treatment.
Discussion
Transportation is unavoidable within the beef industry and can cause psychological and physiological stress on the animal. This stress may culminate in immunosuppression, leading to increased susceptibility to respiratory disease, increased rates of cortisol release, and decreased cattle performance (Lykkesfeldt and Svendsen, 2007; Marques et al., 2012). During transport, calves are deprived of feed and water and experience physical exertion due to long periods of standing. This induces decreased antioxidant capacity, and increased circulating concentration of NEFA, as well as the acute phase protein HP, an indicator of inflammation (Arthington et al., 2003; Knowles et al., 2014; Van Engen and Coetzee, 2018). Collectively, transit stress can result in poor cattle growth, reduced feed efficiency, and increased health risk. This study was designed to explore a proactive approach to mediate transit-induced oxidative stress and potentially improve cattle resiliency to, and recovery from, transit stress.
One approach to increase the animal’s antioxidant capacity is to administer injectable VC (ascorbate), a water-soluble antioxidant. Vitamin C protects against cellular damage induced by free radicals, and VC aids in regeneration of the fat-soluble antioxidant, vitamin E (Packer et al., 1979). Deters and Hansen (2020) found steers given injectable VC prior to an 18-h transit event had greater ADG and a 7 kg BW advantage 56-d post-transit compared to steers given saline. This study was among the first to examine the effects of injectable VC and transit duration on inflammation and muscle fatigue in beef cattle, along with growth performance in the feedlot. It was hypothesized that a pre-transit injection of VC would mediate transit-induced inflammation and maintain antioxidant status, while cattle trucked for 8 h would experience lesser muscle fatigue and oxidative stress than those trucked for 18 h.
Vitamin C is an antioxidant that many species, including cattle, are capable of producing in the liver via glucose due to the presence of l-gulonolactone oxidase. Some species lack the enzyme, thus requiring exogenous VC supplementation (Bánhegyi et al., 1997). Cattle do not have an established VC requirement (NASEM, 2016), likely because of their ability to endogenously produce VC. However, an animal may not be able to synthesize sufficient VC when oxidants exceed the antioxidants available or when the animal is under stress and glucose is redirected towards growth and immune function.
Initial plasma ascorbate concentrations of steers in this study were 14.5 ± 1.1 µM/L, below the reference range of 17.1 to 28.2 µM/L for beef cattle proposed by Matsui (2012). Steers receiving injectable VC had a 38% increase in plasma ascorbate concentration immediately post-transit (day 1) compared to steers not given VC (VC = 16.2; SAL = 14.2 ± 0.9 µM/L). By day 2, approximately 24-h post-transit, plasma ascorbate concentration had returned to baseline concentration. The quick return to baseline plasma concentration could be a result of VC being taken up by tissues to combat reactive oxygen species (ROS) produced during transit to prevent oxidative cellular damage. Contrary to Deters and Hansen (2020), who observed a 10% decline in plasma ascorbate concentration of saline-injected steers following an 18-h transit event, steers in the present study given saline did not change plasma ascorbate status following trucking, regardless of duration. This absence of change in ascorbate status could be due to the steers having below normal plasma ascorbate concentration pre-transit and the body tightly regulating plasma concentration by mobilizing VC from tissue pools preventing the decrease in plasma concentrations. Toutain et al. (1997) found VC can be rapidly mobilized from tissues if arterial concentrations suddenly drop, for instance VC can be mobilized from lung tissues within ~42 min while liver and muscle stores take ~17.5 h to mobilize VC.
Additionally, stress from weaning, housing conditions, and heat has decreased plasma ascorbate concentration (Nakano and Suzuki, 1984). Cummins and Brunner (1991) observed male Holstein calves housed in metal pens versus calves housed in commercial calf hutches had 17% lesser plasma ascorbate concentrations (24.4 vs. 29.5 µM/L); where metal pens are considered to add environmental stress. A study by Padilla et al. (2006) investigated heat stress in lactating Holstein cows and found that when compared with controls, heat-stressed cows had a 51% decrease in plasma ascorbate concentration.
Plasma FRAP was measured as an indicator of antioxidant capacity to evaluate if VC, a known cellular antioxidant, impacted total antioxidant potential. In the current study, plasma FRAP concentrations were decreased 9.4% immediately post-transit. Similarly, Chirase et al. (2004) and Deters and Hansen (2020) observed decreases in plasma FRAP between 9.5% and 9.7% in beef steers following a transit event of 19 h 40 min or 18 h, respectively. Interestingly, steers in the current study had greater pre-trucking antioxidant capacity (FRAP = 360 µM) compared to steers in the Deters and Hansen (2020) study (FRAP = 330 µM). The greater initial antioxidant capacity of steers in the current study may explain the general lack of performance differences between steers given injectable VC and saline, as these steers may have had sufficient antioxidants available and did not benefit from exogenous VC. There is not an established reference range for FRAP in cattle, but if these results were repeatable, it could indicate FRAP concentrations of 330 µM are below the normal antioxidant capacity of the animal. The AUC for FRAP from days −5 to 3 was greatest for steers given injectable VC and transported for 18 h, intermediate for 8-h steers regardless of INJ, and least for steers transported for 18 h and given saline prior to transit. The lack of difference in FRAP AUC between injection treatments for steers transported for 8 h suggests that a shorter transit duration may induce less physiological stress upon the animal compared to steers transported for 18 h and given saline. However, the administration of injectable VC, a potent antioxidant, prior to a long-duration transit event mitigated the decrease in antioxidant capacity to concentrations comparable to steers transported for 8 h. This suggests administration of an antioxidant prior to a long-duration transit equipped cattle with antioxidants to combat the ROS produced from the physiological stress during transit.
A longer duration transit event of 18 h induced a greater inflammatory response compared to steers transported for only 8 h, as shown by increased concentrations of the acute phase protein HP on days 1 and 2. Longer duration transit events have been shown to increase HP concentrations in beef steers (Marques et al., 2012; Cooke et al., 2013; Van Engen et al., 2014). The current study observed no effect of VC on HP concentrations, similar to the study by Deters and Hansen (2020) where steers given injectable VC pre- or post-transit had no difference in HP concentration following a transit event, but the transit event did increase HP concentration.
Furthermore, this duration effect was observed in serum lactate and NEFA concentrations. Steers transported for 18 h had a 37% increase in serum lactate concentration and 8-h steers had only a 10% increase immediately following transit, indicating greater muscle fatigue for those animals transported for a longer duration. The extended period of standing during transit can lead to physical exertion, resulting in the up-regulation of anaerobic glycolysis from aerobic metabolism to meet the increased energy demand of the muscle (Sahlin, 1986). This increase in anaerobic glycolysis is less efficient and produces less adenosine triphosphate (ATP) per molecule of glucose. In anaerobic glycolysis, glucose is converted to pyruvate and ultimately lactic acid, which dissociates into lactate and H+ in the cell cytosol (Wan et al., 2017). Excess lactate that is not utilized by other cells and converted back into pyruvate to produce ATP is exported to the serum, resulting in increased serum lactate concentrations.
Similarly, Chacon et al. (2005) found lactate concentrations following transit to be positively correlated with transport time for steers of 30 min, 3 h, or 6 h. Steers in the current study returned to baseline lactate concentrations by day 2 for 18-h and day 3 for 8-h. Duration affected serum NEFA concentrations where both transit duration treatments peaked on day 1, indicating an increase in fatty acid mobilization due to energy deficiency from the feed restriction and increased energy need during transit. Steers transported for 18 h took an additional day to return to baseline NEFA concentrations compared to 8-h steers, suggesting that a longer duration transit requires more time for an animal to recover. Similarly, Cooke et al. (2013) found steers transported for 1280 km had greater NEFA concentrations following transit compared to steers that were not transported and had full access to feed and water, with concentrations remaining increased over controls through day 7 post-transit.
The longer transit duration increased markers of inflammation and muscle fatigue post-transit, impacting receiving period performance. Body weights collected immediately prior to loading (day 0) were ~7.5 kg lighter for 18-h steers compared to 8-h steers; however, on day −5 when steers were assigned to treatments, there were no differences in BW between treatments. This difference in BW at the time of loading is likely due to gut-fill prior to loading, as the 18-h truck loaded at 1300 h and steers only had ~3 h to eat while the 8-h truck was loaded at 2300 h and steers had full access to the day’s feeding. There was a difference in shrink due to DUR (18-h = 7.4%; 8-h = 5.9%), and it was hypothesized that steers transported for 18 h would have greater shrink than 8-h steers due to a longer period standing and no access to food or water, resulting in greater loss of urine, feces, and body tissue metabolites. Self and Gay (1972) reported that half of the weight lost during transport (~1,020 km) is due to muscle tissue loss, and with 18-h steers having greater shrink compared to 8-h steers, this suggests the 18-h steers experienced more muscle tissue mobilization during transport. Coffey et al. (2001) estimated average shrink rates to be 1%/h during the first 3 to 4 h, decreasing to as low as 0.1%/h after 10 h or more of transit. A study by Warriss et al. (1995) observed shrink of 4.6, 6.5, and 7.0% for steers (341 kg) transported for 5, 10, or 15 h (286, 536, and 738 km), respectively.
Within the first week post-transit, all steers had recovered all BW lost during transit with VC-8 and SAL-18 making a more rapid recovery, and this is reflected in ADG from days 1 to 7. According to a study by Self and Gay (1972), the average time needed for cattle to recover BW lost during transit is 10.6 d with a range between 3 and 30 d. Interestingly, VC-8 had greater BW compared to all other treatments on days 7 and 15, and then by day 31, the 8-h steers in general had greater BW compared to 18-h steers. The 8-h steers had a 4 ± 2.2 kg advantage in final BW over the 18-h steers. Similarly, Meléndez et al. (2020) observed an advantage in BW for steers transported for 12-h compared to 36-h. This slight advantage in BW for steers transported for 8-h was expected, as steers transported for a longer duration were hypothesized to have decreased performance following transit due to experiencing a greater physiological response as indicated through the blood parameters analyzed. In contrast, Cernicchiaro et al. (2012) found steers transported a greater distance to feedlots had greater HCW at harvest, suggesting that animals may be able to adjust their BW gain after long-distance transport.
The spread between pre-transit BW and final BW for 18-h and 8-h steers decreased from days 0 to 55 (BW spread on day 0 = 7.5 kg; day 55 = 4 kg, respectively), and this is reflective of 18-h having greater ADG from days 7 to 31 (18-h = 1.96; 8-h = 1.83 ± 0.06 kg) and overall (18-h = 1.75; 8-h = 1.67 ± 0.04 kg). The improvement in ADG from days 7 to 31 cannot be contributed to greater DMI, as VC-18 steers had lesser DMI compared to all other treatments during this period, and DMI overall is inconsistent between treatments and periods. However, VC-18 steers have greater G:F compared to all other treatments for days 7 to 31, which can be attributed to greater ADG and lesser DMI. It is interesting to note all steers in the current experiment received an anabolic implant on day 7, after which the efficiency improvement in VC-18 was noted. Similarly, Deters and Hansen (2020) saw an advantage in ADG from days 7 to 31 for steers given injectable VC prior to an 18-h transit event, when the same anabolic implant was given to all steers on day 7. To the author’s knowledge, no work has investigated interactions between anabolic implants and VC.
Transit stress greatly impacts the beef industry by impeding animal health and performance. In the current study, injectable VC administered pre-transit increased plasma ascorbate concentration and improved total antioxidant capacity for steers transported for 18 h compared to steers receiving SAL; however, injectable VC did not affect overall performance as greatly as in a previous study. As hypothesized, steers transported for 18 h had greater inflammation and muscle fatigue and even with better interim performance still fell short of final BW of steers transported 8 h. Research is needed to explore the effects of transit duration and the animal’s ability to adjust BW gain following a long-haul transportation event to understand better the physiological changes occurring within the muscle following transit. Additionally, more research is needed to determine reference ranges in beef cattle for antioxidant capacity markers such as FRAP, and how values outside this range may impact the animal physiologically. This research may provide insight for better pre-transit supplementation strategies to improve the physiological response to transit duration.
Acknowledgments
This research was financially supported by the Iowa State Beef Checkoff Program.
Glossary
Abbreviations
- ADG
average daily gain
- ATP
adenosine triphosphate
- AUC
area under the curve
- BW
body weight
- DM
dry matter
- DMI
dry matter intake
- FRAP
ferric reducing antioxidant power
- G:F
gain to feed ratio
- HP
haptoglobin
- NEFA
non-esterified fatty acid
- ROS
reactive oxygen species
- TMR
total mixed ration
- VC
vitamin C
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- AOAC. 1999a. Ether extract in animal feeds. Method 920.39. In: Cunniff P., editor. Official methods of analysis. 16th ed. Vol. 1. Assoc. Off. Anal. Chem., Gaithersburg, MD. Ch. 4. p. 25. [Google Scholar]
- AOAC. 1999b. Protein (crude) in animal feed: combustion method. Method 990.03. In: Cunniff P., editor. Official methods of analysis. 16th ed. Vol. 1. Assoc. Off. Anal. Chem., Gaithersburg, MD. Ch.4. p. 18–19. [Google Scholar]
- AOAC. 2005. Amylase-treated neutral detergent fiber in feeds. Method 2002.04. In: Horwitz W., editor. Official methods of analysis. 18th ed. Assoc. Off. Anal. Chem., Gaithersburg, MD. Ch. 4. p. 49–55. [Google Scholar]
- Arthington, J. D., Eicher S. D., Kunkle W. E., and Martin F. G.. . 2003. Effect of transport and commingling on the acute-phase protein response, growth, and feed intake of newly weaned beef calves. J. Anim. Sci. 81:1120–1125. doi: 10.2527/2003.8151120x [DOI] [PubMed] [Google Scholar]
- Bánhegyi, G., Braun L., Csala M., Puskás F., and Mandl J.. . 1997. Ascorbate metabolism and its regulation in animals. Free Radic. Biol. Med. 23:793–803. doi: 10.1016/s0891-5849(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Cernicchiaro, N., White B. J., Renter D. G., Babcock A. H., Kelly L., and Slattery R.. . 2012. Associations between the distance traveled from sale barns to commercial feedlots in the United States and overall performance, risk of respiratory disease, and cumulative mortality in feeder cattle during 1997 to 20091. J. Anim. Sci. 90:1929–1939. doi: 10.2527/jas.2011-4599. [DOI] [PubMed] [Google Scholar]
- Chacon, G., Garcia-Belenguer S., Villarroel M., and Maria G. A.. . 2005. Effect of transport stress on physiological responses of male bovines. Dtsch. Tierarztl. Wochenschr. 112:465–469. PMID: 16425633. [PubMed] [Google Scholar]
- Chirase, N. K., Greene L. W., Purdy C. W., Loan R. W., Auvermann B. W., Parker D. B., E. F.Walborg, Jr, Stevenson D. E., Xu Y., and Klaunig J. E.. . 2004. Effect of transport stress on respiratory disease, serum antioxidant status, and serum concentrations of lipid peroxidation biomarkers in beef cattle. Am. J. Vet. Res. 65:860–864. doi: 10.2460/ajvr.2004.65.860. [DOI] [PubMed] [Google Scholar]
- Coffey, K. P., Coblentz W. K., Humphry J. B., and Brazle F. K.. . 2001. Review: Basic principles and economics of transportation shrink in beef cattle. Prof. Anim. Sci. 17:247–255. doi: 10.15232/s1080-7446(15)31636-3. [DOI] [Google Scholar]
- Cooke, R. F., Guarnieri Filho T. A., Cappellozza B. I., and Bohnert D. W.. . 2013. Rest stops during road transport: Impacts on performance and acute-phase protein responses of feeder cattle1. J. Anim. Sci. 91:5448–5454. doi: 10.2527/jas.2013-6357. [DOI] [PubMed] [Google Scholar]
- Cummins, K. A., and Brunner C. J.. . 1991. Effect of calf housing on plasma ascorbate and endocrine and immune function. J. Dairy Sci. 74:1582–1588. doi: 10.3168/jds.S0022-0302(91)78320-3. [DOI] [PubMed] [Google Scholar]
- Deters, E. L., and Hansen S. L.. . 2019. Effect of supplementing a Saccharomyces cerevisiae fermentation product during a preconditioning period prior to transit on receiving period performance, nutrient digestibility, and antioxidant defense by beef steers. Transl. Anim. Sci. 3:1227–1237. doi: 10.1093/tas/txz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deters, E. L., and Hansen S. L.. . 2020. Pre-transit vitamin C injection improves post-transit performance of beef steers. Animal 14:2083–2090. doi: 10.1017/S1751731120000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel, T., and Holbrook N. J.. . 2000. Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- González, L. A., Schwartzkopf-Genswein K. S., Bryan M., Silasi R., and Brown F.. . 2012. Factors affecting body weight loss during commercial long haul transport of cattle in North America1. J. Anim. Sci. 90:3630–3639. doi: 10.2527/jas.2011-4786. [DOI] [PubMed] [Google Scholar]
- Hughes, D. A. 1999. Effects of dietary antioxidants on the immune function of middle-aged adults. Proc. Nutr. Soc. 58: 79–84. doi: 10.1079/pns19990012. [DOI] [PubMed] [Google Scholar]
- Knowles, T. G., Warriss P. D., and Vogel K.. . 2014. Stress physiology of animals during transport. In: Grandin T., editor. Livestock Handling and Transport. 4th ed. CABI, Oxfordshire, UK. p. 399–420. [Google Scholar]
- Littell, R. C., Henry P. R., and Ammerman C. B.. . 1998. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt, J., and Svendsen O.. . 2007. Oxidants and antioxidants in disease: oxidative stress infarm animals. Vet. J. 173: 502–511. doi: 10.1016/j.tvjl.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Marques, R. S., Cooke R. F., Francisco C. L., and Bohnert D. W.. . 2012. Effects of twenty-four hour transport or twenty-four hour feed and water deprivation on physiologic and performance responses of feeder cattle. J. Anim. Sci. 90: 5040–5046. doi: 10.2527/jas.2012-5425. [DOI] [PubMed] [Google Scholar]
- Matsui, T. 2012. Vitamin C nutrition in cattle. Asian-Australas. J. Anim. Sci. 25:597–605. doi: 10.5713/ajas.2012.r.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meléndez, D. M., Marti S., Haley D. B., Schwinghamer T. D., and Schwartzkopf-Genswein K. S.. . 2020. Effect of transport and rest stop duration on the welfare of conditioned cattle transported by road. PLoS One 15:e0228492. doi: 10.1371/journal.pone.0228492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano, K., and Suzuki S.. . 1984. Stress-induced change in tissue levels of ascorbic acid and histamine in rats. J. Nutr. 114: 1602–1608. doi: 10.1093/jn/114.9.1602. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM). 2016. Nutrient requirements of beef cattle. 8th Revised ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Packer, J. E., Slater T. F., and Willson R. L.. . 1979. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 278:737–738. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- Padilla, L., Matsui T., Kamiya Y., Kamiya M., Tanaka M., and Yano H.. . 2006. Heat stress decreases plasma vitamin C concentration in lactating cows. Livest. Sci. 101:300–304. doi: 10.1016/j.livprodsci.2005.12.002. [DOI] [Google Scholar]
- Sahlin, K. 1986. Muscle fatigue and lactic acid accumulation. Acta Physiol. Scand. Suppl. 556:83–91. [PubMed] [Google Scholar]
- Self, H. L., and Gay N.. . 1972. Shrink during shipment of feeder cattle. J. Anim. Sci. 35:489–494. doi: 10.2527/jas1972.352489x. [DOI] [Google Scholar]
- Toutain, P. L., Béchu D., and Hidiroglou M.. . 1997. Ascorbic acid disposition kinetics in the plasma and tissues of calves. Am. J. Physiol. 273:R1585–R1597. doi: 10.1152/ajpregu.1997.273.5.R1585. [DOI] [PubMed] [Google Scholar]
- Van Engen, N. K., and Coetzee J. F.. . 2018. Effects of transportation on cattle health and production: a review. Anim. Health Res. Rev. 19:142–154. doi: 10.1017/S1466252318000075. [DOI] [PubMed] [Google Scholar]
- Van Engen, N. K., Stock M. L., Engelken T., Vann R. C., Wulf L. W., Karriker L. A., Busby W. D., Lakritz J., Carpenter A. J., Bradford B. J., . et al. 2014. Impact of oral meloxicam on circulating physiological biomarkers of stress and inflammation in beef steers after long-distance transportation. J. Anim. Sci. 92: 498–510. doi: 10.2527/jas.2013-6857. [DOI] [PubMed] [Google Scholar]
- Wan, J. J., Qin Z., Wang P. Y., Sun Y., and Liu X.. . 2017. Muscle fatigue: general understanding and treatment. Exp. Mol. Med. 49:e384. doi: 10.1038/emm.2017.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warriss, P. D., Brown S. N., Knowles T. G., Kestin S. C., Edwards J. E., Dolan S. K., and Phillips A. J.. . 1995. Effects on cattle of transport by road for up to 15 hours. Vet. Rec. 136:319–323. doi: 10.1136/vr.136.13.319. [DOI] [PubMed] [Google Scholar]