Abstract
Objectives
To examine the association between β-blocker prescription and first primary-care consultation for knee OA, hip OA, knee pain and hip pain.
Methods
Data source: Clinical Practice Research Datalink. Participants aged ≥40 years in receipt of new oral β-blocker prescriptions were propensity score (PS) matched to an unexposed control. Cox proportional hazard ratios (HRs) and 95% CIs were calculated, and adjusted for non-osteoporotic fractures, number of primary-care consultations for knee or hip injury, and, the number of primary-care consultations, out-patient referrals and hospitalizations in the 12 months preceding cohort entry. Analysis was stratified according to β-blocker class and for commonly prescribed drugs. P < 0.05 was considered statistically significant.
Results
A total of 111 718 β-blocker–exposed participants were 1:1 PS matched to unexposed controls. β-blocker prescription was associated with reduced cumulative risk of knee OA, knee pain, and hip pain consultations [with a HR (95% CI) of 0.90 (0.83, 0.98), 0.88 (0.83, 0.92) and 0.85 (0.79, 0.90), respectively]. Propranolol and atenolol were associated with a lower incidence of knee OA and knee pain consultations with a HR of between 0.78 and 0.91. β-blockers were associated with reduced incidence of consultation for large-joint lower-limb OA/pain as a composite outcome, defined as the earliest of knee OA, knee pain, hip OA or a hip pain consultation [with a HR (95% CI) of 0.87 (0.84, 0.90)].
Conclusion
Commonly used β-blockers have analgesic properties for musculoskeletal pain. Atenolol might be a therapeutic option for OA and cardiovascular co-morbidities in which β-blockers are indicated, while propranolol may be suitable for people with co-morbid anxiety. A confirmatory randomized controlled trial is needed before clinical practice is changed.
Keywords: osteoarthritis, pain, β-blockers, anti-nociceptive, comorbidity
Rheumatology key messages
In this large study, β-blockers reduced consultations for knee OA, and knee or hip pain.
Atenolol could be considered for people with osteoarthritis and co-morbidities for which β-blockers are indicated.
Propranolol may be a suitable analgesic for people with co-morbid anxiety.
Introduction
OA is the commonest form of arthritis and affects approximately half of all adults aged >50 years [1, 2]. The pharmacologic management of OA is centred around optimizing analgesia, but first-line drugs only have modest efficacy [3]. Additionally, NSAIDs may cause gastrointestinal, cardiovascular, and renal side effects, particularly in the age groups affected by OA [4]. People with OA are already at high risk of these adverse events due to multi-morbidity [5, 6]. Consequently, the use of opioids for OA pain has increased recently [7]. However, opioids are poorly tolerated and may cause serious side effects and dependency, and evidence for their efficacy in OA pain is limited [8, 9]. Thus, there is an unmet need for developing a safe analgesic for OA.
Small uncontrolled studies suggest that β-adrenoreceptor blocking drugs (β-blockers) have anti-nociceptive effects in FM, temporo-mandibular dysfunction, and migraine [10–12]. Additionally, polymorphisms in the β2-adrenoreceptor gene is associated with chronic painful conditions [13–15]. Recently, we reported a negative association between β-blocker prescription and severe knee pain and opioid prescription in adults with knee or hip OA awaiting total joint replacement [16]. However, these results were not confirmed in another study [17], and, whether the analgesic effect is specific to a subclass of β-blockers is not known.
Thus, the purpose of this study was to investigate the analgesic potential of β-blockers in a primary-care cohort. The specific objectives were to examine the association between β-blocker prescription and first primary-care consultation for knee OA (primary outcome), hip OA, knee pain and hip pain. Additionally, we explored the data to identify the class of β-blockers, and specific drugs, that are most likely to have an analgesic effect.
Methods
Study design: cohort study
Data source
The Clinical Practice Research Datalink (CPRD) is a longitudinal anonymized electronic database containing health records of >10 million people in the UK [18]. CPRD participants are representative of the UK population in terms of age, sex and ethnicity [18]. The CPRD contains details of diagnoses, symptoms and signs; referral details stored as Read code, and records of primary-care prescriptions are stored as drug names.
Ethics approval
Approval was obtained from the Independent Scientific Advisory Committee for the Medicines and Healthcare products Regulatory Agency (Reference: 18_227R).
Study population
The study population consisted of CPRD-registered participants aged ≥40 years who had contributed data from general practice surgeries that met the data quality standards of the CPRD between 1 January 1990 and 31 December 2017. This age cut-off was chosen because both the probability of exposure and outcome is low in the under 40s.
First-ever continuous β-blocker prescription was defined as ≥2 prescriptions for any oral β-blocker within a 60-day period. In the UK, primary-care prescriptions are usually issued every 4 weeks. We selected participants with ≥2 prescriptions within 60 days to exclude those who experienced side effects and discontinued treatment shortly after it was commenced.
Participants without prescription of β-blockers were considered to be unexposed.
It is common to choose active comparators in pharmacoepidemiology studies. We did not use active comparator controls, because there is a hierarchy in the use of drugs for the treatment of cardiovascular diseases driven by NICE guidelines in the UK. For instance, NICE recommend β-blockers for resistant hypertension that has failed to respond to other anti-hypertensive agents. In contrast, they recommend β-blockers as first-line treatment for atrial fibrillation, angina and heart failure. Thus, an active comparator study would introduce greater bias by comparing people with different severity of cardiovascular illnesses.
Propensity score matching
As participants prescribed β-blockers are likely to have co-morbidities and be older, a PS for β-blocker prescription was calculated and 1:1 matching undertaken to ensure unexposed and exposed participants were otherwise comparable. The PS included:
demographic factors: age, sex, current smoker (yes, no), general practice surgery level index of multiple deprivation score;
co-morbidities: overweight or obese [body mass index (BMI) ≥25 kg/m2], hypertension, angina, myocardial infarction, heart failure, atrial fibrillation, stroke, chronic kidney disease, diabetes, anxiety, migraine, and duration in years of each cardiovascular comorbidity prior to cohort entry; and
prescriptions: calcium channel blockers, ACE inhibitors, angiotensin II receptor antagonists, bendroflumethiazide, aldosterone antagonists, loop diuretics, alfa-adrenoreceptor blocking drugs, aspirin, clopidogrel, statins, fibrates.
Outcomes
Outcomes included primary-care consultation for knee OA, hip OA, knee pain, and hip pain (Supplementary Table S2, available at Rheumatology online: Codelist). A primary-care diagnosis of OA at either the knee, hip or hands has a positive predictive value of 79.8–82% in validation studies in the CPRD and similar primary-care databases [19, 20].
Index date
The index date was defined as the date of the first of two consecutive prescriptions in the exposed participants (new user design). Unexposed participants were assigned the index date of their matched exposed participant.
Exclusion criteria
Exclusion criteria included consultation for any of the following prior to the index date:
OA at any joint
knee, hip, neck or back pain
autoimmune inflammatory rheumatic diseases, or gout
radiculopathy, or neuropathy
FM
contra-indications to β-blockers: asthma, chronic obstructive pulmonary disease, peripheral vascular disease, heart block, aortic stenosis, hypertrophic obstructive cardiomyopathy
two prescriptions for opioids, NSAIDs, gabapentin, pregabalin, duloxetine or amitriptyline in any 60-day period prior to the index date.
Additionally, participants with <2 years of registration data before the index date were excluded to reduce the chance of prevalent conditions (e.g. long-standing OA or pain) being considered as incident outcomes.
It is typical to require one-year disease-free registration as entry criteria in studies using consultation-based databases. However, people with OA may not consult their GP in a given 12-month period. Thus, a disease-free registration of 2 years prior to cohort entry was chosen in consultation with the GP-expert in the team to minimize the chance of prevalent OA cases being classified as incident outcome(s).
Follow-up
Exposed and unexposed participants were followed-up from the index date until the earliest occurrence of: outcome of interest, death date, transfer out date, date of last data collection, study end date (31 December 2017) or date of last prescription of β-blockers plus 28 days (typical duration of primary-care prescriptions in UK) in the exposed, and an assigned pseudo-end date for each unexposed participant using the end date of their matched exposed person. The follow-up period of participants not experiencing an outcome was censored. Given the well-known effects of propranolol on pain sensitivity [21], we anticipated β-blockers to have an analgesic effect in the short term, therefore follow-up period >28 days after the date of the last β-blocker prescription was disregarded from primary analysis a priori. In a secondary analysis, we extended the follow-up period to the earliest occurrence out of: outcome of interest, death date, transfer out date, date of last data collection, and study end date (31 December 2017).
Statistical analysis
The PS was calculated using a cumulative logit regression model. Greedy nearest neighbour 1:1 matching without replacement, specifying a maximum calliper width of 0.001 was undertaken. Participants with missing data on smoking and BMI were classified as non-smokers and as having normal BMI, respectively. This approach was chosen due to >50% of data being missing for these variables, and because they are not missing at random in consultation-based databases such as CPRD [22–25]. Mean (s.d.), n (%) and standardized difference (d) were used to examine the covariate balance between exposed and unexposed participants. If d was more than +0.10 or less than −0.10, the variable was included in the model as a covariate, as per Nguyen et al. [26].
Cox proportional hazard ratios (HRs) and 95% CIs were calculated for each outcome after checking that proportional hazard assumptions were met using log-log plots and a formal test to assess departure from proportional hazards (Supplementary Fig. S1, available at Rheumatology online). Nelson–Aalen graphs were plotted. Covariates likely to influence outcomes but not related to exposure (i.e. number of GP consultations for knee or hip injury, and non-osteoporotic fractures prior to the index date) or which reflect general health-seeking behaviour and may influence consultation for musculoskeletal pain (i.e. number of GP consultations, out-patient hospital referrals, and hospital admissions in the 12-month period preceding cohort entry) were included in the model. Non-osteoporotic fractures were included as a surrogate for knee or hip injury. They were defined in this study as fractures between the ages of 19 and 49 years in women, and between the ages of 19 and 59 years in men. Vertebral, femoral and distal radius fractures were excluded, because these are target sites for osteoporotic fractures.
The analyses were stratified according to the class of β-blocker used, namely β1 selective or non-selective, intrinsic sympathomimetic activity (ISA) present or absent, membrane-stabilizing effect (MSE) present or absent, and high- or low-lipophilic properties; and commonly prescribed β-blocker drugs. The robustness of results was assessed using the first of OA or consultation for knee or hip pain as an outcome.
Sensitivity analysis
Given the extent of the missing data for smoking status and BMI, a complete case analysis was performed. In this, exposed and unexposed participants with missing data were excluded, 1:1 PS matching was performed, and the analysis was adjusted for the a priori selected covariates listed above. Data management and analysis were performed using Stata (v15). Statistical significance was considered to occur for P < 0.05.
Results
Data for 223 436 1:1 PS-matched, β-blocker–exposed (n = 111 718) and –unexposed (n = 111 718) participants were included (Supplementary Fig. S2, available at Rheumatology online). The mean (s.d.) follow-up period while receiving β-blocker prescription and total follow-up period, including when not prescribed β-blockers, was 2.75 (4.03) and 11.29 (6.59) years, respectively, in the exposed participants. The corresponding follow-up period for the unexposed participants was 2.35 (3.17) and 10.02 (6.38) years, respectively. There was covariate balance after PS matching on all variables, except for age, for which there was imbalance (d = −0.147, Supplementary Table S1, available at Rheumatology online). Age was included in the model to account for the imbalance. After PS matching, exposed and unexposed participants had similar numbers of primary-care consultations in the preceding 12 months, with a mean (s.d.) of 5.27 (7.05) and 5.81 (6.92) visits, respectively (d = 0.08).
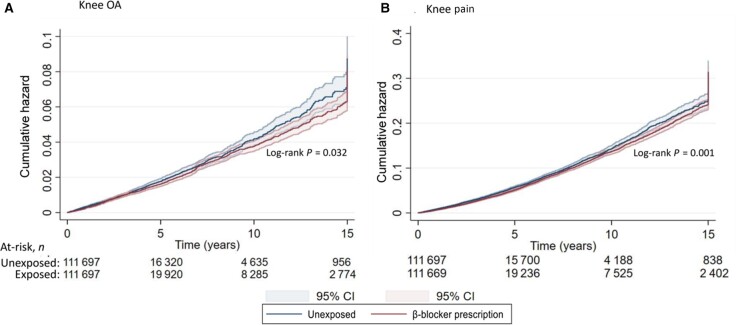
β-blocker prescription was associated with a reduced cumulative risk of incident primary-care consultation for knee OA [aHR (95% CI) of 0.90 (0.83, 0.98)], knee pain [aHR (95% CI) 0.88 (0.83, 0.92)] and hip pain [aHR (95% CI) of 0.85 (0.79, 0.90)] (Table 1, Fig. 1). On secondary analysis, there was no association between β-blocker prescription and primary-care consultation for knee OA or hip OA when the follow-up period extended beyond the end of β-blocker prescription, while there was an increased incidence of primary-care consultation for knee pain or hip pain (Table 2).
Table 1.
The association between β-blocker prescription and primary-care consultation for incident OA and joint pain: follow-up period restricted to end of β-blocker prescription (n = 223 436)
| Outcomes | Exposed | Events (n) | Person-time (years) | Event rate (95% CI) / 1000 person-years | PS-matched HR (95% CI) | PS-matched and adjusted HR (95% CI)a |
|---|---|---|---|---|---|---|
| Knee OA | No | 986 | 262 003 | 3.76 (3.54, 4.01) | 1.00 | 1.00 |
| Yes | 1101 | 307 231 | 3.58 (3.38, 3.80) | 0.90 (0.83, 0.99) | 0.90 (0.83, 0.98) | |
| Hip OA | No | 451 | 263 753 | 1.71 (1.56, 1.87) | 1.00 | 1.00 |
| Yes | 530 | 310 045 | 1.71 (1.57, 1.86) | 0.94 (0.83, 1.06) | 0.94 (0.83, 1.07) | |
| Knee pain | No | 3074 | 255 003 | 12.06 (11.64, 12.49) | 1.00 | 1.00 |
| Yes | 3560 | 297 027 | 11.99 (11.60, 12.37) | 0.91 (0.87, 0.96) | 0.88 (0.83, 0.92) | |
| Hip pain | No | 1767 | 259 515 | 6.81 (6.50, 7.13) | 1.00 | 1.00 |
| Yes | 1981 | 304 454 | 6.51 (6.23, 6.80) | 0.87 (0.82, 0.93) | 0.85 (0.79, 0.90) |
Adjusted for age, number of GP consultations, hospital out-patient referrals, hospital admissions in the 12-month period preceding cohort entry, total number of GP consultations for knee or hip injury prior to cohort entry, and non-osteoporotic fractures.
Fig. 1.
Cumulative hazard of (A) knee OA and (B) knee pain consultation in β-blocker–exposed and –unexposed participants
Data restricted to the last prescription of β-blocker.
Table 2.
The association between β-blocker prescription and primary-care consultation for incident OA and joint pain: follow-up period not restricted to end of β-blocker prescription (n = 223 436)
| Outcomes | Exposed | Events | Person-time (years) | Event rate (95% CI) / 1000 person-years |
PS-matched
HR (95% CI) |
PS-matched and adjusted
HR (95% CI) a |
|---|---|---|---|---|---|---|
| Knee OA | No | 4809 | 1 118 936 | 4.30 (4.12, 4.42) | 1.00 | 1.00 |
| Yes | 5330 | 1 261 516 | 4.23 (4.11, 4.34) | 0.96 (0.92, 1.00) | 0.97 (0.93, 1.01) | |
| Hip OA | No | 2253 | 1 137 529 | 1.98 (1.90, 2.06) | 1.00 | 1.00 |
| Yes | 2512 | 1 282 641 | 1.96 (1.88, 2.04) | 0.96 (0.91, 1.02) | 0.98 (0.93, 1.04) | |
| Knee pain | No | 15 921 | 1 049 982 | 15.16 (14.93, 15.40) | 1.00 | 1.00 |
| Yes | 19 473 | 1 168 291 | 16.67 (16.44, 16.90) | 1.07 (1.05, 1.09) | 1.03 (1.01, 1.05) | |
| Hip pain | No | 9392 | 1 095 747 | 8.57 (8.40, 8.75) | 1.00 | 1.00 |
| Yes | 11 532 | 1 225 992 | 9.41 (9.24, 9.58) | 1.06 (1.03, 1.09) | 1.04 (1.02, 1.07) |
Adjusted for age, number of GP consultations, hospital out-patient referrals, hospital admissions in the 12-month period preceding cohort entry, total number of GP consultations for knee or hip injury prior to cohort entry, and non-osteoporotic fractures.
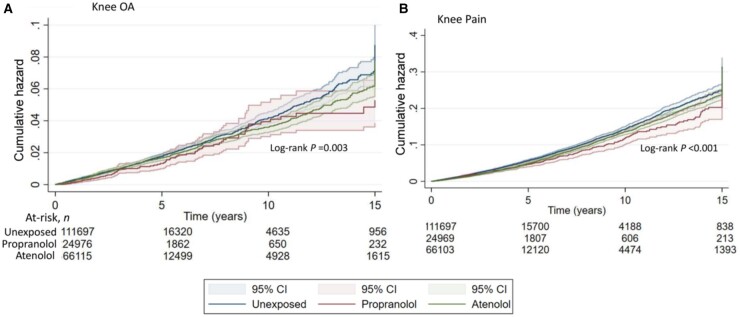
Of the β-blocker classes that could be assessed, high-lipophilic non-selective β-blockers were associated with a lower cumulative incidence of primary-care consultation for knee OA and knee pain, with an aHR (95% CI) of 0.78 (0.63, 0.95) and 0.80 (0.72, 0.89), respectively (Table 3). Similarly, low-lipophilic, β1-selective drugs without MSE or ISA reduced the cumulative incidence of primary-care consultation for knee pain [aHR (95% CI) of 0.88 (0.80, 0.93)] and knee OA [aHR (95% CI) of 0.92 (0.84, 1.01)]. Additionally, lipophilic β1-selective and low-lipophilic non-selective β-blockers, without MSE or ISA, were associated with a reduced cumulative incidence of primary-care consultation for knee pain [aHR (95% CI) of 0.81 (0.66, 1.00)] and 0.85 (0.71, 1.02), respectively]. There was a trend for similar effects when hip OA and hip pain consultations were the outcomes of interest (Table 3; Supplementary Fig. S3, available at Rheumatology online). When data were stratified according to individual drugs, there was a significant protective effect for propranolol and atenolol for knee OA and knee pain consultations, and for atenolol for hip pain consultations (Table 4, Fig. 2). There was a trend for propranolol prescription to be associated with a lower cumulative risk of hip pain consultation (Table 4; Supplementary Fig. S4).
Table 3.
The association between β-blocker prescription and incident OA and pain: stratified according to drug class
| β-blocker classb | Events (n) | Person- time (years) | Event rate (95% CI) / 1000 person-years |
PS-matched and adjustedc HR (95% CI) | Events (n) |
Person- time (years) | Event rate (95% CI) / 1000 person-years |
PS-matched and adjusted HRc (95% CI) |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Knee OA | Knee pain | |||||||||
| Unexposeda | 986 | 262 003 |
3.76 (3.54, 4.01) |
1 | 3074 | 255 003 |
12.06 (11.64, 12.49) |
1 | ||
| Non-selective, low-lipophilic | 39 | 10 462 |
3.73 (2.72, 5.10) |
0.84 (0.60, 1.17) |
124 | 10 127 |
12.24 (10.27, 14.60) |
0.85 (0.71, 1.02) |
||
| Non-selective, high lipophilic, MSE | 101 | 38 419 |
2.63 (2.16-3.20) |
0.78 (0.63, 0.95) |
392 | 37 508 |
10.45 (9.47, 11.54) |
0.80 (0.72, 0.89) |
||
| β1selective, low-lipophilic | 900 | 240 757 |
3.74 (3.50, 4.00) |
0.92 (0.84, 1.01) |
2860 | 232 271 |
12.31 (11.87, 12.77) |
0.88 (0.83, 0.93) |
||
| β1selective, high lipophilic | 33 | 8635 |
3.82 (2.72, 5.38) |
0.95 (0.67, 1.35) |
88 | 8370 |
10.51 (8.53, 12.96) |
0.81 (0.66, 1.00) |
||
| Hip OA | Hip pain | |||||||||
| Unexposeda | 451 | 263 753 |
1.71 (1.56, 1.88) |
1 | 1767 | 259 515 |
6.81 (6.50, 7.13) |
1 | ||
| Non-selective, low-lipophilic | 15 | 10 567 |
1.42 (0.86, 2.35) |
0.74 (0.44, 1.23) |
73 | 10 345 |
7.06 (5.61, 8.88) |
0.84 (0.67, 1.07) |
||
| Non-selective, high lipophilic, MSE | 46 | 38 600 |
1.19 (0.89, 1.59) |
0.79 (0.58, 1.07) |
216 | 38 035 |
5.68 (4.97, 6.49) |
0.88 (0.76, 1.01) |
||
| β1selective, low-lipophilic | 433 | 243 134 |
1.78 (1.62, 1.96) |
0.96 (0.84, 1.10) |
1557 | 238 680 |
6.53 (6.22, 6.86) |
0.83 (0.77, 0.89) |
||
| β1selective, high lipophilic | 20 | 8678 |
2.30 (1.49, 3.57) |
1.26 (0.80, 1.97) |
68 | 8498 |
8.00 (6.31, 10.15) |
1.07 (0.84, 1.36) |
||
Comparison group is unexposed to β-blockers;
β-blocker properties
Propensity score–matched and adjusted for age, number of GP consultations, hospital out-patient referrals, hospital admissions in the 12-month period preceding cohort entry, total number of GP consultations for knee or hip injury prior to cohort entry, and non-osteoporotic fractures; ISE: intrinsic sympathomimetic effect; MSE: membrane-stabilizing effect. Drugs from the rest of the β-blocker class combinations are not used in clinical practice. Lipophilic non-selective β-blockers, lipophilic non-selective β-blockers with ISE and MSE, low-lipophilic non-selective β-blockers with ISE and MSE, low-lipophilic β1-selecive blockers with ISE and MSE were excluded, because the numbers of outcome events were fewer than 50 for both knee pain and knee OA.
Table 4.
The association between commonly prescribed β-adrenoreceptor blocking drugsand incident OA and pain
| β-blockersc | Event |
Person-time
(years) |
Event rate
(95% CI) / 1000 person-years |
PS-matched and adjusted b HR (95% CI) | Event | Person- time (years) |
Event rate
(95% CI) / 1000 person-years |
PS-matched and adjusted HR b (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Knee OA |
Knee pain
|
|||||||
| Unexposeda | 986 | 262 003 |
3.76 (3.54, 4.01) |
1 | 3074 | 255 003 |
12.06 (11.64, 12.49) |
1 |
| Atenolol | 686 | 191 455 |
3.58 (3.32, 3.86) |
0.91 (0.82, 1.00) |
2138 | 185 636 |
11.52 (11.04, 12.02) |
0.86 (0.81, 0.91) |
| Propranolol | 93 | 35 663 |
2.61 (2.13, 3.20) |
0.78 (0.63, 0.97) |
342 | 34 948 |
9.79 (8.80, 10.88) |
0.78 (0.69, 0.87) |
| Bisoprolol | 204 | 47 037 |
4.34 (3.78, 4.98) |
0.99 (0.85, 1.16) |
695 | 44 469 |
15.63 (14.51, 16.84) |
0.98 (0.91, 1.08) |
| Sotalol | 38 | 10 328 |
3.68 (2.68, 5.06) |
0.81 (0.58, 1.14) |
124 | 9990 |
12.41 (10.41, 14.80) |
0.88 (0.73, 1.05) |
| Metoprolol | 33 | 8635 |
3.82 (2.72, 5.38) |
0.96 (0.67, 1.35) |
88 | 8370 |
10.51 (8.53, 12.96) |
0.82 (0.66, 1.01) |
| Hip OA | Hip pain | |||||||
| Unexposeda | 451 | 263 753 |
1.71 (1.56, 1.88) |
1 | 1767 | 259 515 |
6.81 (6.50, 7.13) |
1 |
| Atenolol | 327 | 193 111 |
1.69 (1.52, 1.89) |
0.94 (0.81, 1.08) |
1153 | 190 067 |
6.07 (5.73, 6.43) |
0.80 (0.74, 0.86) |
| Propranolol | 43 | 35 833 |
1.20 (0.89, 1.62) |
0.81 (0.59, 1.11) |
195 | 35 348 |
5.52 (4.79, 6.35) |
0.89 (0.76, 1.03) |
| Bisoprolol | 99 | 47 733 |
2.07 (1.70, 2.53) |
1.02 (0.82, 1.28) |
386 | 46 380 |
8.32 (7.53, 9.20) |
0.92 (0.82, 1.03) |
| Sotalol | 14 | 10 432 |
1.34 (0.79, 2.27) |
0.71 (0.42, 1.21) |
72 | 10 210 |
7.05 (5.60, 8.88) |
0.85 (0.67, 1.08) |
| Metoprolol | 20 | 8678 |
2.30 (1.49, 3.57) |
1.26 (0.81, 1.98) |
68 | 8498 |
8.00 (6.31, 10.15) |
1.07 (0.84, 1.36) |
Comparison group is unexposed to β-blockers.
Propensity score–matched and adjusted for age, number of GP consultations, hospital out-patient referrals, hospital admissions in the 12-month period preceding cohort entry, total number of GP consultations for knee or hip injury prior to cohort entry, and non-osteoporotic fractures.
cRestricted to drugs with 10 or more outcome events.
Fig. 2.
Cumulative hazard of (A) knee OA and (B) knee pain consultation in atenolol and propranolol–exposed and –unexposed participants
Data restricted to the last prescription of β-blocker.
β-blockers were associated with a reduced cumulative risk of primary-care consultation for large-joint lower-limb OA and/or pain, defined as the earliest of knee OA, knee pain, hip OA or hip pain [aHR (95% CI) 0.87 (0.84, 0.90)]. The aHR (95% CI) was 0.80 (0.73, 0.87) for propranolol, and 0.85 (0.82, 0.89) for atenolol. On complete case PS-matched analysis, all covariates were balanced. Exposure to β-blockers was associated with a lower cumulative incidence of primary-care consultation for knee OA [aHR (95% CI) 0.85 (0.76, 0.96)], knee pain [0.77 (0.72, 0.82)], hip pain [0.70 (0.64, 0.76)] and hip OA [0.85 (0.72, 1.02)], adjusted for the a priori selected covariates.
Discussion
This primary-care–based study reports that β-blocker prescription was associated with reduced primary-care consultation for knee OA, knee pain, and hip pain. Interestingly, the effect disappeared after the end of β-blocker prescription, and participants had more consultations for knee and hip pain in this period. This suggests that the effect of β-blockers may potentially be due to analgesia rather than structure modification. However, we did not assess the latter in this study.
The greatest effect size was observed for propranolol, a non-selective, lipophilic β-blocker with MSE. Analgesic effects of propranolol have been reported. In a randomized, double-blind, placebo-controlled cross-over study (n = 40), propranolol significantly lowered pain scores due to temporomandibular dysfunction [27]. Similar findings were observed in FM and temporomandibular dysfunction in controlled studies shortly after low-dose i.v. propranolol (0.1 mg/kg) [10]. Propranolol also reduces the post-operative analgesic requirement [28]. However, an analgesic effect for propranolol was not demonstrated in people with extensive burns and in other experimental models of pain [29, 30]. Propranolol is used in the treatment of anxiety, and 21% of OA patients have co-morbid anxiety [31], making it particularly attractive in this scenario.
The β1 adrenoreceptor selective drug atenolol was associated with a reduced cumulative risk of primary-care consultation for knee OA, knee pain and hip pain. Identical in properties to atenolol, esmolol also has an analgesic effect [32]. It reduces both intraoperative [standard mean difference (SMD) (95% CI) −1.60 (−2.25, −0.96)] and post-anaesthesia opioid consumption [SMD (95% CI) –1.21 (−1.66, −0.77)] [32]. Atenolol is used for the treatment of cardiovascular conditions such as angina, hypertension and supraventricular tachycardia, and our findings suggest that it might be suitable for the treatment of cardiovascular co-morbidities in symptomatic OA patients. However, confirmation of our findings in a randomized controlled trial (RCT) is needed before practice is changed.
The analgesic effect of β-blockers is mediated by β2 adrenoreceptor blockade. β2 adrenoreceptors are present on peripheral nociceptors, dorsal root ganglia and the superficial dorsal horn, and their stimulation results in hyperalgesia that is blocked by either non-selective or β2 adrenoreceptor-selective drugs [33–35], but not by indomethacin [35]. The analgesic effect of β-blockers does not seem to be mediated by the β1 adrenoreceptor. For example, the hyperalgesic state in low catechol-O-methyl transferase gene activity is blocked by propranolol but not by selective β1-adrenoreceptor blockers [36]. Non-selective β-blockers reduce the negative affective component of pain [37], regulate the firing of periaqueductal grey neurons via a gamma-aminobutyric acid (GABA)-mediated action, and interfere with the chronic sensitization processes in the rostral ventromedial medulla and locus coeruleus [38, 39]. Thus, the analgesic effect of atenolol is likely to be mediated by its β2 adrenoreceptor blocking activity. Although classified as β1 selective, its β1/β2 adrenoreceptor selectivity is relatively modest at 47 [40].
This study suggests that β1-adrenoreceptor selective drugs may also have an analgesic effect. This is consistent with the findings of a previous cross-sectional study [16], and that of another study using data from people undergoing total knee arthroplasty [41]. In the latter study, β-blocker prescription was associated with lower opioid use at day 30 [adjusted OR (95% CI) of 0.89 (0.80, 0.99)] [41]. Ninety percent of participants in this study were prescribed β1-adrenoreceptor selective drugs [41]. However, the findings of these studies are not consistent with those of a study using data from the OA Initiative [17]. That study reported comparable pain score, proportion reporting widespread pain, and opioid consumption in people with knee OA prescribed β-blockers and other anti-hypertensive medications [17]. However, that was a hospital-based study with a different comparator i.e. ‘prescription of another anti-hypertensive drug’, had a relatively modest sample size (n = 1168), and only 15% participants were prescribed β-blockers, resulting in potential type-2 error [17].
Strengths of this study include a large sample size, balanced PS-matched exposed and unexposed groups, and adjustment for covariates that reflect health-seeking behaviour, or are risk factors for OA. GPs are the first physician option for people with chronic conditions in the UK, and it is extremely unlikely that someone with OA will be seen in a hospital service, including in private settings, for the first time, without ever consulting their GP. Only GPs refer patients to NHS hospitals for long-term conditions. Participants with less than 2-year registration in the general practice surgery before the index date were excluded to reduce the chance of prevalent cases being classified as incident outcomes. Finally, we excluded participants with chronic painful conditions and contra-indications to β-blockers to minimize confounding by indication that may not be addressed by PS matching.
However, there are several caveats. First, we could not undertake multiple imputation to account for missing smoking status and BMI data because these were missing in 50.5% and 60.3% of participants, respectively, and multiple imputation is not recommended with such degree of missingness [22, 23]. In addition, smoking and BMI are not missing at random in consultation-based databases; therefore, multiple imputation should not be used [24, 25]. Second, CPRD participants with missing data are likely to be healthier. After PS matching, there was a comparable proportion of people in exposed and unexposed groups with missing data on BMI and smoking, minimizing any potential for confounding. Third, we used GP diagnosis of OA to define our primary outcome. Although this has been validated previously [19, 20], its positive predictive value (PPV) for OA diagnosis is ∼ 80%; thus, some participants may not be diagnosed, limiting the validity of our findings. We used primary-care consultations to define the outcomes. This is later than the onset of symptoms, because most patients defer seeing their GP for chronic musculoskeletal pain. However, there is no reason to suspect that this delay will differ between the groups. Similarly, access to GP surgery and ability to pay for repeat prescription may also affect the results. This is likely to play a small role, because healthcare is free at the point of delivery in the NHS, and socio-economically disadvantaged patients are eligible for free NHS prescriptions. Furthermore, we did not examine the association between β-blocker prescription and total joint replacement in this study, because the mean follow-up was short. Finally, we only dichotomized the exposure as two or more than two prescriptions within 60 days. Further dose–response analysis examining the association between cumulative dose and number of prescriptions is warranted.
In summary, both non-selective and selective β-blockers may reduce the cumulative risk of incident OA. Atenolol might be a consideration for people with OA and cardiovascular co-morbidities, while propranolol may be suitable for people with OA and anxiety. However, a RCT is necessary to further evaluate these possibilities before clinical practice is changed.
Supplementary Material
Acknowledgements
A.A. and A.V. conceptualized the study. A.A., A.V., M.J.G., M.A.M., C.M., N.T., W.Z. and M.D. planned the study. A.A., M.J.G., G.N., N.T., W.Z., M.A.M., C.M., A.V. and M.D. developed the analysis plan. G.N. carried out the data management and analysis. A.A. and G.N. wrote the first manuscript draft. M.J.G., M.A.M., G.N., N.T., W.Z., C.M., A.V. and M.D. reviewed the manuscript critically and approved the final version.
Funding: This work was funded by the National Institute for Health Research (grant numbers PB-PG-0816–20025 and NIHR-RP-2014–04-026). C.M. is funded by the National Institute for Health Research (NIHR) Applied Research Collaboration West Midlands, the National Institute for Health Research (NIHR) School for Primary Care Research and a National Institute for Health Research (NIHR) Research Professorship in General Practice (NIHR-RP-2014–04-026) for this research project. The study sponsor did not have any role in the conduct or reporting of this study.
Disclosure statement: This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) programme (grant reference number PB-PG-0816–20025). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. A.A. has received departmental research grants from AstraZeneca, speaker bureau fees from Menarini, scientific meeting support from Pfizer, and author royalties from UpToDate, unrelated to this work. W.Z. has received honoraria from AstraZeneca and Grunenthal, and speaker fees from BioBarica, Regeneron and Hisun, unrelated to this work. The Keele School of Medicine have received funding from Bristol Myers Squibb for advice provided by C.M. on primary-care recruitment to a non-pharmacological Atrial Fibrillation (AF) study. M.D. has received honoraria for attending ad hoc advisory boards on gout for Grunenthal and Mallinckrodt, and author royalties from UpToDate, and is an investigator in an AstraZeneca-funded, investigator-led, non-drug study (the Sons of Gout study), unrelated to this work. The other authors have no conflicts of interest to declare.
Data availability statement
This study used data from the Clinical Research Datalink (CPRD). Due to the CPRD data-sharing policy, data used in this study cannot be shared with the third party. However, access to CPRD data can be requested directly from the CPRD.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Thomas E, Peat G, Croft P.. Defining and mapping the person with osteoarthritis for population studies and public health. Rheumatology (Oxford) 2014;53:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cross M, Smith E, Hoy D. et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323–30. [DOI] [PubMed] [Google Scholar]
- 3. McAlindon TE, Bannuru RR, Sullivan MC. et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363–88. [DOI] [PubMed] [Google Scholar]
- 4. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J.. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis 2018;9:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Puenpatom RA, Victor TW.. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med 2009;121:9–20. [DOI] [PubMed] [Google Scholar]
- 6. Singh G, Miller JD, Lee FH, Pettitt D, Russell MW.. Prevalence of cardiovascular disease risk factors among US adults with self-reported osteoarthritis: data from the Third National Health and Nutrition Examination Survey. Am J Manag Care 2002;8(15 Suppl):S383–91. [PubMed] [Google Scholar]
- 7. Wright EA, Katz JN, Abrams S, Solomon DH, Losina E.. Trends in prescription of opioids from 2003–2009 in persons with knee osteoarthritis. Arthritis Care Res 2014;66:1489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen LH, Hedegaard H, Warner M.. Drug-poisoning deaths involving opioid analgesics: United States, 1999–2011. No. 2014. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. 2014.
- 9. Solomon DH, Rassen JA, Glynn RJ. et al. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010;170:1968–76. [DOI] [PubMed] [Google Scholar]
- 10. Light KC, Bragdon EE, Grewen KM. et al. Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain 2009;10:542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wood PB, Kablinger AS, Caldito GS.. Open trial of pindolol in the treatment of fibromyalgia. Ann Pharmacother 2005;39:1812–6. [DOI] [PubMed] [Google Scholar]
- 12. Del Giaccio A, Eblen-Zajjur A.. Cardiovascular drugs in human mechanical nociception: digoxin, amlodipine, propranolol, pindolol and atenolol. Invest Clin 2010;51:77–86. [PubMed] [Google Scholar]
- 13. Kushnir VM, Cassell B, Gyawali CP. et al. Genetic variation in the beta-2 adrenergic receptor (ADRB2) predicts functional gastrointestinal diagnoses and poorer health-related quality of life. Aliment Pharmacol Ther 2013;38:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skouen JS, Smith AJ, Warrington NM. et al. Genetic variation in the beta-2 adrenergic receptor is associated with chronic musculoskeletal complaints in adolescents. Eur J Pain 2012;16:1232–42. [DOI] [PubMed] [Google Scholar]
- 15. Vargas-Alarcon G, Fragoso JM, Cruz-Robles D. et al. Association of adrenergic receptor gene polymorphisms with different fibromyalgia syndrome domains. Arthritis Rheum 2009;60:2169–73. [DOI] [PubMed] [Google Scholar]
- 16. Valdes AM, Abhishek A, Muir K, Zhang W. et al. Association of beta-blocker use with less prevalent joint pain and lower opioid requirement in people with osteoarthritis. Arthritis Care Res 2017;69:1076–81. [DOI] [PubMed] [Google Scholar]
- 17. Zhou L, Kwoh CK, Ran D, Ashbeck EL, Lo-Ciganic WH.. Lack of evidence that beta blocker use reduces knee pain, areas of joint pain, or analgesic use among individuals with symptomatic knee osteoarthritis. Osteoarthritis Cartilage 2020;28:53–61. [DOI] [PubMed] [Google Scholar]
- 18. Herrett E, Gallagher AM, Bhaskaran K. et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferguson RJ, Prieto-Alhambra D, Walker C. et al. Validation of hip osteoarthritis diagnosis recording in the UK Clinical Practice Research Datalink. Pharmacoepidemiol Drug Saf 2019;28:187–93. [DOI] [PubMed] [Google Scholar]
- 20. Rahman MM, Kopec JA, Goldsmith CH, Anis AH, Cibere J.. Validation of administrative osteoarthritis diagnosis using a clinical and radiological population-based cohort. Int J Rheumatol 2016;2016:6475318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Light KC, Bragdon EE, Grewen KM. et al. Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain 2009;10:542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jakobsen JC, Gluud C, Wetterslev J, Winkel P.. When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med Res Methodol 2017;17:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clark TG, Altman DG.. Developing a prognostic model in the presence of missing data: an ovarian cancer case study. J Clin Epidemiol 2003;56:28–37. [DOI] [PubMed] [Google Scholar]
- 24. Marston L, Carpenter JR, Walters KR. et al. Issues in multiple imputation of missing data for large general practice clinical databases. Pharmacoepidemiol Drug Saf 2010;19:618–26. [DOI] [PubMed] [Google Scholar]
- 25. Bhaskaran K, Smeeth L.. What is the difference between missing completely at random and missing at random? Int J Epidemiol 2014;43:1336–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nguyen TL, Collins GS, Spence J. et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol 2017;17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tchivileva IE, Lim PF, Smith SB. et al. Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenet Genomics 2010;20:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harkanen L, Halonen J, Selander T, Kokki H.. Beta-adrenergic antagonists during general anesthesia reduced postoperative pain: a systematic review and a meta-analysis of randomized controlled trials. J Anesth 2015;29:934–43. [DOI] [PubMed] [Google Scholar]
- 29. Orrey DC, Halawa OI, Bortsov AV. et al. Results of a pilot multicenter genotype-based randomized placebo-controlled trial of propranolol to reduce pain after major thermal burn injury. Clin J Pain 2015;31:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petersen KK, Andersen HH, Tsukamoto M. et al. The effects of propranolol on heart rate variability and quantitative, mechanistic, pain profiling: a randomized placebo-controlled crossover study. Scand J Pain 2018;18:479–89. [DOI] [PubMed] [Google Scholar]
- 31. Stubbs B, Aluko Y, Myint PK, Smith TO.. Prevalence of depressive symptoms and anxiety in osteoarthritis: a systematic review and meta-analysis. Age Ageing 2016;45:228–35. [DOI] [PubMed] [Google Scholar]
- 32. Gelineau AM, King MR, Ladha KS. et al. Intraoperative Esmolol as an adjunct for perioperative opioid and postoperative pain reduction: a systematic review, meta-analysis, and meta-regression. Anesth Analg 2018;126:1035–49. [DOI] [PubMed] [Google Scholar]
- 33. Aley KO, Martin A, McMahon T. et al. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci 2001;21:6933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nicholson R, Dixon AK, Spanswick D, Lee K.. Noradrenergic receptor mRNA expression in adult rat superficial dorsal horn and dorsal root ganglion neurons. Neurosci Lett 2005;380:316–21. [DOI] [PubMed] [Google Scholar]
- 35. Khasar SG, McCarter G, Levine JD.. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol 1999;81:1104–12. [DOI] [PubMed] [Google Scholar]
- 36. Nackley AG, Tan KS, Fecho K. et al. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain 2007;128:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deyama S, Katayama T, Ohno A. et al. Activation of the beta-adrenoceptor–protein kinase A signaling pathway within the ventral bed nucleus of the stria terminalis mediates the negative affective component of pain in rats. J Neurosci 2008;28:7728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koella WP. CNS-related (side-)effects of beta-blockers with special reference to mechanisms of action. Eur J Clin Pharmacol 1985;28 (Suppl):55–63. [DOI] [PubMed] [Google Scholar]
- 39. Boyer N, Signoret-Genest J, Artola A, Dallel R, Monconduit L.. Propranolol treatment prevents chronic central sensitization induced by repeated dural stimulation. Pain 2017;158:2025–34. [DOI] [PubMed] [Google Scholar]
- 40. Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol 2005;144:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Starr JB, Backonja M, Rozet I.. Beta-blocker use is associated with a reduction in opioid use 30 days after total knee arthroplasty. Pain Phys 2019;22:e395–e406. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study used data from the Clinical Research Datalink (CPRD). Due to the CPRD data-sharing policy, data used in this study cannot be shared with the third party. However, access to CPRD data can be requested directly from the CPRD.