Abstract
Objectives
To examine incidence of treatment changes due to abnormal blood-test results and, to explore rates of treatment changes due to liver, kidney and haematological blood-test abnormalities in autoimmune rheumatic diseases (AIRD) treated with low-dose MTX or LEF.
Methods
Data for people with AIRDs prescribed MTX or LEF were extracted from the Clinical Practice Research Datalink. Participants were followed-up from first prescription of MTX or LEF in primary care. Primary outcome of interest was drug discontinuation, defined as a prescription gap of ≥90 days following an abnormal (or severely abnormal) blood-test result. Dose reduction was examined between consecutive prescriptions. Incidence rates per 1000 person-years were calculated.
Results
15, 670 and 2,689 participants contributing 46, 571 and 4,558 person-years follow-up were included in MTX and LEF cohorts, respectively. The incidence of MTX and LEF discontinuation with abnormal (severely abnormal) blood-test was 42.24 (6.16) and 106.53 (9.42)/1000 person-years in year 1, and 22.44 (2.84) and 31.69 (4.40)/1000 person years, respectively, thereafter. The cumulative incidence of MTX and LEF discontinuation with abnormal (severely abnormal) blood tests was 1 in 24 (1 in 169), 1 in 9 (1 in 106) at 1 year; and 1 in 45 (1 in 352), 1 in 32 (1 in 227) per-year, respectively, thereafter. Raised liver enzymes were the commonest abnormality associated with drug discontinuation. MTX and LEF dose reduction incidence were comparable in year 1, however, thereafter MTX dose was reduced more often than LEF [16.60 (95% CI 13.05, 21.13) vs 8.10 (95% CI 4.97, 13.20)/1000 person-years].
Conclusion
MTX and LEF were discontinued for blood-test abnormalities after year 1 of treatment, however, discontinuations for severely abnormal results were uncommon.
Keywords: autoimmune rheumatic diseases, methotrexate, leflunomide, blood-test monitoring
Rheumatology key messages
Treatment discontinuation and dose reductions were more common in the first year of treatment and occurred at a stable but lower rate thereafter.
Severely abnormal blood-test results were uncommonly associated with methotrexate and leflunomide discontinuations.
Elevated liver enzymes were the commonest reason for discontinuing methotrexate and leflunomide.
Introduction
Autoimmune rheumatic diseases (AIRDs) affect >1% adults and are treated with DMARDs [1–5]. These drugs can cause cytopenia, raised liver enzymes and AKI and, fortnightly to monthly monitoring blood-tests are recommended when initiating treatment with less frequent testing thereafter [6]. In the UK, DMARDs are initiated in a rheumatology clinic with prescriptions dispensed from the hospital and fortnightly blood monitoring overseen by the rheumatology team. Once an effective, tolerated and stable dose is reached, the responsibility for prescribing and arranging 2–3 monthly blood-tests is handed to the GP under shared-care policy supported by the British Society for Rheumatology (BSR) and Royal College of General Practitioners [6]. The Rheumatology team is contacted if there are side-effects, including blood-test abnormalities and oversee treatment changes. Monitoring blood-tests are discontinued after 2 years for SSZ while long-term testing is continued for low-dose MTX and LEF [6, 7]. Whether such long-term testing influences the decision to discontinue treatment is not known because most clinical trials are shorter than 1 year, and many observational studies report cumulative toxicity including outcomes from the treatment initiation phase during which reversible drug-induced target organ injury is common [8–10]. However, evidence from a large 2-year clinical trial suggests that DMARD discontinuation due to target organ damage becomes less common with increasing duration of treatment [10]. With growing use of DMARDs and the corresponding increased burden and cost of testing, it is important to evaluate the benefit from regular monitoring blood-tests for long-term low-dose MTX or LEF treatment [11]. Thus, the objectives of this study were to examine the incidence of drug discontinuation and dose reduction with abnormal blood-test results in AIRDs treated with long-term low-dose MTX or LEF. We also explored the data to examine whether the incidence of MTX discontinuation due to any abnormal blood-test result, elevated liver enzymes, AKI or cytopenia differed in RA and PsA as there is evidence that psoriasis increases the risk of hepatotoxicity from MTX [12, 13].
Methods
Data source
Data from Clinical Practice Research Datalink (CPRD) Gold was used. Incepted in 1987, CPRD-Gold is a longitudinal anonymized electronic database of health records from over 19 million participants in 927 general practice surgeries across the UK and covers 4.52% of UK residents currently. CPRD participants are representative of the UK population in terms of age, sex and ethnicity [14]. CPRD includes information on demographic details, lifestyle factors (e.g. smoking, alcohol intake), diagnoses, results of investigations including blood tests and physical examination and details of all primary-care prescriptions [14]. Diagnostic and prescription data are recorded as Read codes and product codes, respectively. Blood-test results are stored as numeric values. Additionally, general practitioners (GPs) may record abnormal blood-test results using Read codes.
Approvals
Independent Scientific Advisory Committee of the MHRA (Reference: 19_275R).
Study design
Cohort study: Two separate cohorts were constructed comprising of participants prescribed MTX and LEF, respectively.
Study duration: 1 January 2007 to 31 December 2019
The study began on 1 January 2007 as the BSR guidelines recommending aggressive treatment of RA and shared-care monitoring of DMARDs were published in 2006 [4, 15].
Inclusion criteria
Participants were required to meet the following criteria:
diagnosed with either RA, SLE, PsA, ReA, AS, IBD associated arthritis, GCA, PMR or CTDs at age ≥18 years, within the study period;
≥1 GP prescription of MTX (oral or subcutaneous) or LEF after the first record of AIRD diagnosis in CPRD and
continuous registration for ≥1 year before the first AIRD diagnosis date in a GP practice contributing research quality data to CPRD.
The latter two criteria prevents prevalent AIRD cases on long-term DMARDs that have recently changed GP surgeries from entering the cohort as incident cases.
Exclusion criteria
Chronic liver disease (autoimmune hepatitis, primary sclerosing cholangitis, hepatitis B or C, cirrhosis); haematological malignancies (lymphoma, leukaemia); myelodysplasia; haemolytic anaemia, neutropenia, idiopathic thrombocytopenic purpura or chronic kidney disease (CKD) stage ≥4 prior to cohort entry.
Cohort entry
First shared-care GP prescription of MTX or LEF, respectively.
Cohort exit
The earliest of date of outcome, death, transfer out of the GP practice, last data collection from the GP practice, or 31 December 2019. For the dose reduction analysis, follow-up was censored on the first prescription date at which dose data were missing.
Outcomes
Drug discontinuation with abnormal blood-test result: Prescription gap of ≥90 days, with an abnormal blood-test result or Read code indicating abnormal blood-test result within ±60 days of the date of last prescription. The thresholds for abnormal blood-test results were: white blood cells (WBCs) <3.5 × 109/l; neutrophils <1.6 × 109/l; platelets <140 × 109/l; ALT/AST >100 IU/l (6); and kidney function decline defined as either CKD progression based on Read codes entered by the GP using Kidney Disease Improving Global Outcomes (KDIGO) CKD guidelines [16], or a creatinine increase of >26 μmol/l, the threshold for consideration of AKI [17].
Drug discontinuation with severe abnormal blood-test result: Prescription gap of ≥90 days, with severely abnormal monitoring blood-test result within ±60 days of the date of last prescription. Severe blood-test abnormalities were defined as: WBCs <2.5 × 109/l; neutrophils <1.0 × 109/l; platelets <50 × 109/l; ALT or AST >200 IU/l or serum creatinine >2 times the previous value. These thresholds were selected as they reflect grade-3 cytopenia according to Common Terminology Criteria for Adverse Events, stage 2 acute kidney injury according to the KDIGO guidelines and meet the criteria for drug induced liver injury with ALT or AST >5 times upper limit of normal [17–19].
Dose reduction with abnormal, and severely abnormal blood-test result: Dose reduction between two consecutive prescriptions.
Drug discontinuation (any reason): Gap of ≥90 days between the last prescription date and the earliest of date of death, transfer out of the GP practice, last data collection from GP practice or 31 December 2019.
Data management
Read code and product code lists were developed to ascertain AIRDs, inclusion and exclusion criteria, prescriptions and outcomes (available on request).
Outcome validation
A random sample (40%) of MTX discontinuations with a blood-test abnormality was drawn. Data for all consultations within ±60 days of the abnormal blood-test result was extracted. A.A. (Consultant Rheumatologist trained in General Medicine and Rheumatology) screened all Read codes to exclude administrative codes e.g. reminder letter sent, telephone appointment, etc. All clinical experts in the study team [two rheumatologists, one nephrologist, one hepatologist, one gastroenterologist, one haematologist (C.F.) and one academic GP] reviewed the remaining Read codes. Each expert could vote in any condition if they felt that the condition, its treatment or its complications could cause blood, liver or kidney injury. The final list was reviewed by all clinicians and four Read codes were excluded as they were non-specific or could imply DMARD side effects if used alone (Supplementary Table S1, available at Rheumatology online).
Statistical analyses
Mean (s.d.) and n (%) were used for descriptive purposes. The proportion of MTX discontinuations with blood test abnormality that could potentially be explained by an underlying illness was determined. Survival analysis was undertaken to calculate the incidence of outcomes [95% confidence intervals (CIs)] per 1000 person-years for entire follow-up period, first 12 months of follow-up and the subsequent period. Incidence of drug discontinuation or dose reduction with individual blood-test abnormalities were calculated. Missing data on doses were not imputed as they were missing not at random and imputation could create spurious outcomes. Life tables were constructed to estimate the cumulative incidence at 1- and 5-year follow-up. Cumulative hazards were plotted using Nelson-Aalen graphs.
Sensitivity analysis
The incidence of MTX discontinuation for blood-test abnormalities was examined in a sensitivity analysis after excluding cases with SLE and other CTDs as these conditions can cause cytopenia. The incidence of MTX discontinuation with abnormal blood-test results was calculated separately for RA and PsA. Data management and analysis were performed in Stata v16.
Results
Data for 24 871 and 3897 participants with AIRDs prescribed MTX and LEF were ascertained. Of these, 15 670 and 2689 participants contributing 46 571 and 4558 person-years follow-up were included in the MTX and LEF cohorts, respectively (Supplementary Figs S1 and S2). The median (IQR) MTX and LEF dose at cohort entry was 10 (7.5–15) mg/week and 10 (10–20) mg/day, respectively. 2.1% participants were prescribed both drugs at cohort entry or within the first 6 months. The majority of participants in the MTX cohort had RA (65.8%), were female (64.6%) and their mean (s.d.) age was 57 (15) years. In the LEF cohort, 63.9% had RA, 67.3% were female and the mean (s.d.) age was 57 (13) years (Table 1). The median (IQR) follow-up in the MTX and LEF cohorts was 2.31 (0.82–4.92) and 1.03 (0.33–2.94) years, and there were 1262 and 259 drug discontinuations due to abnormal monitoring blood test results, respectively. Of these 95.6 and 95% were ascertained using values of blood-test results while the remainder were ascertained using Read codes. The 40% random sample of MTX discontinuations with blood-test abnormalities consisted of 505 cases and yielded 27 (5.35%) discontinuations that could potentially be explained by another underlying illness or its treatment or complications.
The incidence of MTX and LEF discontinuation for any reason, with any blood-test abnormality, and with any severe blood-test abnormality was highest in the first 12-months of shared-care prescribing (Table 2 and Fig. 1). The cumulative annual incidence of discontinuing MTX with abnormal, and severely abnormal blood-test results was 1 in 24 and 1 in 169 at 1 year, and this reduced to 1 in 45 and 1 in 352 per-year, respectively, thereafter. Similarly, for LEF, the cumulative annual incidence of discontinuing treatment with abnormal and severely abnormal blood-test results was 1 in 9 and 1 in 106 at 1 year, reducing to 1 in 32 and 1 in 227 per-year, respectively, thereafter. The proportion discontinuing MTX with abnormal blood test results was lower than that of LEF at 1-year and 5-year follow-up, being 4.2% (95% CI 3.7, 4.4%) for MTX vs 9.3% (8.1, 10.7%) for LEF at 1 year, and 12.2% (11.5, 12.9%) for MTX vs 20.5% (17.8, 23.5%) for LEF at 5-year (Supplementary Fig. S3, available at Rheumatology online). However, the cumulative incidence of MTX and LEF discontinuation with severe blood-test abnormalities were comparable at both time points.
Table 2.
The incidence of MTX and LEF discontinuation
| MTX |
LEF |
|||||
|---|---|---|---|---|---|---|
| Outcome | Events (n) | Person-time (years) | Event rate (95% CI) /1000 person-years | Events (n) | Person-time (years) | Event rate (95%CI) /1000 person-time |
| Any reason | ||||||
| Ever | 3584 | 46 571 | 76.96 (74.48, 79.52) | 946 | 4,558 | 207.54 (194.73, 221.20) |
| First 12 months | 2185 | 12 327 | 177.25 (169.97, 184.84) | 765 | 1,593 | 480.10 (447.26, 515.36) |
| After 12 months | 1399 | 34 244 | 40.85 (38.81, 43.00) | 181 | 2,965 | 61.05 (53.01, 70.30) |
| With any blood-test abnormality | ||||||
| Ever | 1262 | 45 435 | 27.78 (26.29-29.35) | 259 | 4,449 | 58.22 (51.55, 65.76) |
| First 12 months | 517 | 12 239 | 42.24 (38.75, 46.05) | 168 | 1,577 | 106.53 (91.58, 123.92) |
| After 12 months | 745 | 33 196 | 22.44 (20.90, 24.09) | 91 | 2,872 | 31.69 (25.89, 38.79) |
| With severe blood-test abnormality | ||||||
| Ever | 170 | 46 466 | 3.66 (3.15, 4.25) | 28 | 4,548 | 6.16 (4.25, 8.92) |
| First 12 months | 73 | 12 317 | 5.93 (4.71, 7.45) | 15 | 1,592 | 9.42 (5.68, 15.63) |
| After 12 months | 97 | 34 149 | 2.84 (2.32, 3.47) | 13 | 2,956 | 4.40 (2.56, 7.56) |
Fig. 1.
Nelson–Aalen cumulative hazard estimates for MTX and LEF discontinuation due to: any reason (A), any abnormal blood-test results (B), any severely abnormal blood-test results (C).
Table 1.
Baseline characteristics of participants in the MTX (n = 15 670) and LEF (n = 2,689) cohorts
| Characteristics | MTX | LEF |
|---|---|---|
| Age at cohort entry, mean (s.d.) | 57.2 (14.8) | 57 (13.4) |
| Female, n (%) | 10 115 (64.6) | 1807 (67.3) |
| Smoking status, n (%) | ||
| Non-smoker | 7339 (46.8) | 1221 (45.5) |
| Current smoker | 3300 (21.1) | 555 (20.7) |
| Ex-smoker | 4972 (31.7) | 902 (33.6) |
| Missing | 59 (0.4) | 8 (0.3) |
| Alcohol use, n (%) | ||
| Non-user | 3132 (20) | 606 (22.6) |
| Low | 8714 (55.6) | 1452 (54.1) |
| Medium | 573 (3.7) | 80 (3.0) |
| Hazardous | 875 (5.6) | 140 (5.2) |
| EX-user | 563 (3.6) | 149 (5.6) |
| Missing | 1813 (11.6) | 259 (9.6) |
| AIRD type, n (%) | ||
| RA | 10 306 (65.8) | 1715 (63.9) |
| Lupus/other CTD | 468 (3.0) | 26 (1.0) |
| PMR/GCA | 1597 (10.2) | 203 (7.6) |
| Spondyloarthropathy | 3299 (21.1) | 742 (27.6) |
| Other DMARDs, n (%) | ||
| LEF | 331 (2.1) | −/− |
| MTX | −/− | 57 (2.1) |
| SSZ | 2660 (17.0) | 395 (14.7) |
−/−: value <5. Alcohol and smoking status were derived from categorical data in CPRD Additional Clinical file. Alcohol consumption was classified as low (1-14 units/week), medium (15-21 units/week) and hazardous (>21 units/week).
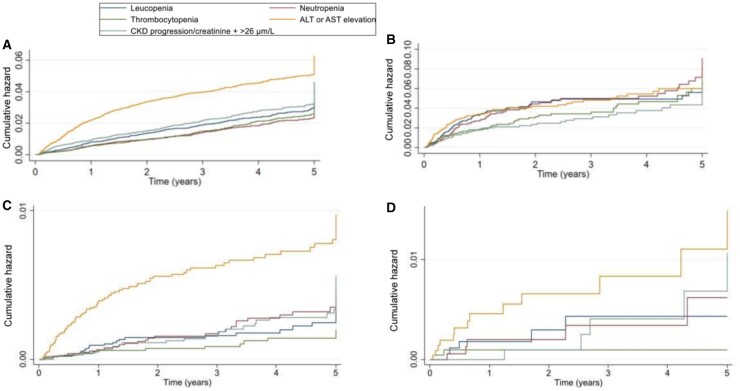
The incidence of MTX discontinuation with raised liver enzymes, and decline in kidney function was higher in the first 12 months than subsequently, whereas the incidence of MTX discontinuation with cytopenia remained stable throughout (Table 3 and Fig. 2; Supplementary Fig. S4, available at Rheumatology online). On the contrary, the incidence of LEF discontinuation with cytopenia, elevated liver enzymes, and kidney function decline was higher in the first 12 months (Table 3 and Fig. 2). LEF discontinuation with severe individual blood-test abnormalities was numerically more common in the first 12 months than subsequently (Table 3).
Table 3.
The incidence of MTX and LEF discontinuation due to individual blood-test abnormalities
| MTX |
LEF |
|||||
|---|---|---|---|---|---|---|
| Outcome | Events (n) | Person-time (years) | Event rate (95% CI) /1000 person-years | Events (n) | Person-time (years) | Event rate (95%CI) /1000 person-time |
| Leucopenia (WBC <3.5 × 109/l) | ||||||
| Ever | 286 | 46 425 | 6.16 (5.49, 6.92) | 76 | 4535 | 16.76 (13.39, 20.98) |
| First 12 months | 97 | 12 322 | 7.87 (6.45, 9.61) | 55 | 1590 | 34.58 (26.55, 45.04) |
| After 12 months | 189 | 34 103 | 5.54 (4.81, 6.39) | 21 | 2945 | 7.13 (4.66, 10.91) |
| Severe leucopenia (WBC <2.5×109/l) | ||||||
| Ever | 28 | 46 555 | 0.60 (0.42, 0.87) | 5 | 4558 | 1.10 (0.45, 2.64) |
| First 12 months | 11 | 12 327 | 0.89 (0.49, 1.61) | –/– | –/– | 1.88 (0.61, 5.84) |
| After 12 months | 17 | 34 228 | 0.50 (0.31, 0.80) | –/– | –/– | 0.68 (0.17, 2.70) |
| Neutropenia (Neutrophil <1.6×109/l) | ||||||
| Ever | 216 | 46 476 | 4.65 (4.07, 5.31) | 77 | 4528 | 17.01 (13.60, 21.26)) |
| First 12 months | 70 | 12 324 | 5.68 (4.49, 7.18) | 45 | 1589 | 28.31 (21.14, 37.92) |
| After 12 months | 146 | 34 152 | 4.28 (3.63, 5.03) | 32 | 2939 | 10.89 (7.71, 15.37) |
| Severe neutropenia (Neutrophil <1.0×109/l) | ||||||
| Ever | 31 | 46 552 | 0.67 (0.47, 0.95) | 5 | 4557 | 1.10 (0.46, 2.64) |
| First 12 months | 8 | 12 326 | 0.65 (0.32, 1.30) | –/– | –/– | 1.88 (0.61, 5.84) |
| After 12 months | 23 | 34 226 | 0.67 (0.45, 1.01) | –/– | –/– | 0.67 (0.17, 2.70) |
| Thrombocytopenia (Platelet <140 × 109/l) | ||||||
| Ever | 264 | 46 428 | 5.69 (5.04, 6.42) | 59 | 4537 | 13.00 (10.08, 16.78) |
| First 12 months | 66 | 12 323 | 5.36 (4.21, 6.82) | 32 | 1591 | 20.11 (14.22, 28.44) |
| After 12 months | 198 | 34 105 | 5.81 (5.05, 6.67) | 27 | 2946 | 9.17 (6.30, 13.34) |
| Severe thrombocytopenia (Platelet <100×109/l) | ||||||
| Ever | 14 | 46 571 | 0.30 (0.18 , 0.51) | –/– | –/– | 0.44 (0.11, 1.75) |
| First 12 months | 6 | 12 327 | 0.49 (0.22, 1.08) | –/– | –/– | 1.26 (0.31, 5.02) |
| After 12 months | 8 | 34 244 | 0.23 (0.12, 0.47) | 0 | 2965 | 0 |
| ALT or AST >100 IU/l | ||||||
| Ever | 517 | 46 209 | 11.19 (10.26, 12.20) | 80 | 4524 | 17.68 (14.20, 22.02) |
| First 12 months | 272 | 12 292 | 22.13 (19.65, 24.92) | 60 | 1586 | 37.84 (29.38, 48.73) |
| After 12 months | 245 | 33 917 | 7.22 (6.38, 8.18) | 20 | 2938 | 6.81 (4.40, 10.54) |
| ALT or AST >200 IU/l | ||||||
| Ever | 83 | 46 526 | 1.78 (1.44, 2.21) | 13 | 4549 | 2.86 (1.66 , 4.92) |
| First 12 months | 48 | 12 321 | 3.90 (2.94, 5.17) | 8 | 1592 | 5.03 (2.51, 10.05) |
| After 12 months | 35 | 34 205 | 1.02 (0.73, 1.42) | 5 | 2957 | 1.69 (0.70, 4.06) |
| CKD progression/creatinine + >26 μmol/l | ||||||
| Ever | 312 | 46 081 | 6.77 (6.06, 7.57) | 51 | 4532 | 11.25 (8.55, 14.81) |
| First 12 months | 118 | 12 284 | 9.61 (8.02, 11.51) | 30 | 1590 | 18.87 (13.19, 26.98) |
| After 12 months | 194 | 33 797 | 5.74 (4.99, 6.60) | 21 | 2942 | 7.14 (4.66, 10.93) |
| Creatinine >2 times previous value | ||||||
| Ever | 32 | 46 534 | 0.69 (0.49, 0.97) | 5 | 4558 | 1.10 (0.46, 2.64) |
| First 12 months | 6 | 12 325 | 0.49 (0.22, 1.08) | 0 | 0 | 0 |
| After 12 months | 26 | 34 209 | 0.76 (0.52, 1.12) | 5 | 4558 | 1.10 (0.46, 2.64) |
Fig. 2.
Nelson–Aalen cumulative hazard estimates for drug discontinuation due to the individual abnormal blood-test results: MTX discontinuation due to mild abnormal blood-test results (A), severely abnormal blood-test results (C); LEF discontinuation due to mild abnormal blood-test results (B), severely abnormal blood-test results (D).
Sensitivity analysis
On excluding people with SLE or CTDs, the incidence (95% CI) of MTX discontinuation was 27.40 (25.90, 28.98)/1000 person-years for any blood-test abnormality and, 5.88 (5.21, 6.63)/1000 person-years for leucopenia, 4.40 (3.83, 5.05)/1000 person-years for neutropenia and 5.75 (5.09, 6.49)/1000 person-years for thrombocytopenia. This was comparable to that observed in the entire dataset (Table 2). There were no differences when the analyses were stratified for duration of follow-up (data not shown).
The incidence of MTX discontinuation due to elevated liver enzymes was higher in psoriatic arthritis than RA. This difference was present both early and late in the treatment course and for any or severely elevated liver enzymes. However, the incidence of MTX discontinuation due to cytopenia or renal function decline was comparable in the two populations (Table S2).
Dose reduction
85.3% and 30% participants in the MTX and LEF cohorts had at least one dose data missing, and their follow-up was censored at this time point. MTX dose was reduced more often with abnormal blood-test results than LEF after the first 12 months (Table 4). However, the cumulative incidence of dose-reduction with any blood test abnormality and with severe blood-test abnormalities were comparable for MTX and LEF at 1 and 5 years. (Supplementary Fig. S3, available at Rheumatology online). On evaluating individual blood-test abnormalities, LEF dose was more likely to be reduced with neutropenia than MTX, and less likely to be reduced with abnormal LFTs (Table 4).
Table 4.
Incidence of MTX and LEF dose reduction
| MTX |
LEF |
|||||
|---|---|---|---|---|---|---|
| Outcome | Events (n) | Person-time (years) | Event rate (95% CI) /1000 person-years | Events (n) | Person-time (years) | Event rate (95%CI) /1000 person-time |
| Any abnormal blood-test result | ||||||
| Ever | 142 | 6679 | 21.26 (18.04, 25.06) | 47 | 3151 | 14.92 (11.21, 19.85) |
| First 12 months | 77 | 2764 | 27.86 (22.29, 34.84) | 31 | 1176 | 26.36 (18.54, 37.48) |
| After first 12 months | 65 | 3915 | 16.60 (13.05, 21.13) | 16 | 1975 | 8.10 (4.97, 13.20) |
| Leucopenia (WBC <3.5×109/l) | ||||||
| Ever | 36 | 6785 | 5.31 (3.83, 7.36) | 19 | 3185 | 5.97 (3.80, 9.35) |
| First 12 months | 19 | 2779 | 6.84 (4.36, 10.72) | 14 | 1181 | 11.85 (7.02, 20.02) |
| After first 12 months | 17 | 4006 | 4.24 (2.64, 6.82) | 5 | 2004 | 2.50 (1.04, 5.99) |
| Neutropenia (Neutrophil <1.6×109/l) | ||||||
| Ever | 21 | 6798 | 3.09 (2.01, 4.74) | 23 | 3177 | 7.24 (4.81, 10.89) |
| First 12 months | 14 | 2779 | 5.04 (2.98, 8.51) | 13 | 1182 | 11.00 (6.39, 18.94) |
| After first 12 months | 7 | 4019 | 4.24 (2.64, 6.82) | 10 | 1995 | 5.04 (2.71, 9.35) |
| Thrombocytopenia (Platelet <140×109/l) | ||||||
| Ever | 26 | 6784 | 3.83 (2.61, 5.63) | 9 | 3200 | 2.81 (1.46, 5.41) |
| First 12 months | 11 | 2782 | 3.95 (2.19, 7.14) | 8 | 1183 | 6.77 (3.38, 13.53) |
| After first 12 months | 15 | 4002 | 3.75 (2.26, 6.21) | –/– | –/– | 0.50 (0.07, 3.52) |
| ALT or AST >100 IU/l | ||||||
| Ever | 56 | 6758 | 8.29 (6.38, 10.77) | 9 | 3197 | 2.82 (1.46, 5.41) |
| First 12 months | 31 | 2776 | 11.17 (7.85, 15.88) | 7 | 1184 | 5.91 (2.82, 12.40) |
| After first 12 months | 25 | 3982 | 6.28 (4.25, 9.28) | –/– | 2013 | 1.00 (0.25, 3.97) |
| CKD progression/creatinine + >26 μmol/l | ||||||
| Ever | 33 | 6787 | 4.86 (3.46, 6.84) | 6 | 3199 | 1.88 (0.84, 4.17) |
| First 12 months | 19 | 2777 | 6.84 (4.36, 10.73) | –/– | –/– | 1.69 (0.42, 6.76) |
| After first 12 months | 14 | 4010 | 3.49 (2.07, 5.89) | –/– | –/– | 1.99 (0.75, 5.28) |
WBC: white blood cells
Seven participants prescribed MTX had dose reduction for severely abnormal blood-test results with incidence of 1.03 (0.49, 2.16) per 1000 person-years. Fewer than five participants prescribed LEF had dose reduction for severely abnormal blood-test results during shared care prescribing. Due to office for national statistics and CPRD policy to avoid accidental identification, we are unable to present incidence for outcomes with <5 events.
Discussion
This is the largest study to examine the incidence of treatment changes with abnormal blood-test results during long-term MTX or LEF therapy. In comparison, the largest systematic review (SR) of low-dose MTX included data on liver and bone-marrow toxicity from 3806 and 3463 participants from 29 studies, and the previous largest study of LEF included data for 3325 participants [9, 20].
This study focused on patients successfully initiated on long-term DMARDs as there is lack of data on benefit from monitoring during this period [6]. It reports that treatment changes with abnormal blood-test results are common in the first 12 months after hospital-supervised treatment initiation and stabilization, and becomes less frequent thereafter. Treatment changes with severe blood-test abnormalities were uncommon and became less frequent over time.
Our observation that 3.3% participants discontinued low-dose MTX with elevated liver enzymes are comparable to the 3.7% incidence reported in the SR [9], and, are higher than those in the CORRONA registry [12]. In our study, 2.8% participants discontinued MTX with cytopenia. This is lower than the 6.7% cumulative incidence of cytopenia during MTX therapy in the SR [9]. The incidence of MTX discontinuation with leucopenia (0.6% vs 1.2%) and neutropenia (0.5% vs 1.8%) at 1 year was lower than the cumulative incidence reported in a recent SR of clinical trials that included events from the treatment initiation phase [21]. This may be due to the fact that our outcome definition required drug discontinuation with cytopenia, whereas the SRs reported on the incidence of any cytopenia, including those not requiring treatment discontinuation [9, 21].
Solomon et al. [22] reported a lower cumulative incidence of elevated liver enzymes (0.56%) and haematological abnormalities (0.95%) using data from a 3-year trial of low-dose MTX for preventing cardiovascular events in a population without AIRD. The lower incidence may be due to non-prescription of other DMARDs and less NSAID use [22].
As reported previously, raised liver enzymes were the commonest abnormality associated with MTX discontinuation, and the risk reduced after 12 months [9, 10, 22–24]. On the contrary, the incidence of MTX discontinuation with cytopenia was similar throughout the treatment period. Previous 2-year trials of MTX have reported cytopenia only occasionally, and unrelated to treatment duration [10, 23].
The cumulative incidence of LEF discontinuation with elevated liver enzymes (3.0% vs 3.1%), and with either cytopenia or elevated liver enzymes (7.7% vs 7.0%) were comparable to previous reports [12, 20]. As reported previously, there was a higher incidence of LEF discontinuation with blood-test abnormalities in the first 12 months [10].
LEF was more likely to be discontinued with abnormal blood-tests than MTX. These findings are contrary to the results of a trial in which folate supplementation was not mandatory for participants randomized to MTX [10]. However, in another trial where folate supplementation was mandatory for participants randomized to MTX there were more LEF than MTX discontinuations for elevated liver enzymes (7.7% vs 4.4%) [25]. Folic acid supplementation was recommended in the BSR guidelines and became common practice in the early 2000s, and our findings of greater liver toxicity with LEF are expected.
MTX and LEF discontinuation with kidney function decline was uncommon, though more frequent in the first 12 months, raising the possibility that these drugs may be nephrotoxic. However, published data suggests that nephrotoxicity is uncommon with these drugs. Only one case of reversible kidney failure due to MTX was reported in a clinical trial, and there is one case report of LEF induced interstitial nephritis but this was associated with chronic over-dosing and with no cases of LEF nephrotoxicity reported in clinical trials [10, 25–28]. The largest clinical trial to examine the side-effects from low-dose MTX albeit in a non-AIRD population reported an average 1.9 ml/min/1.73 m2 improvement in estimated glomerular filtration rate, and 15% lower risk of renal adverse events compared with placebo [22]. A previous 2-year clinical trial reported no change in creatinine with LEF, and only a marginal increase in creatinine with MTX [10]. These findings suggest that there is low risk of nephrotoxicity with LEF and low-dose MTX.
MTX was twice as likely to be discontinued with elevated liver enzymes in PsA than in RA, as reported previously [12, 13]. However, the rates of MTX discontinuation due to cytopenia and renal function decline were comparable suggesting this risk is target-organ specific. Further research is required to understand the underlying mechanism. However, these findings suggest that PsA patients treated with MTX should be monitored carefully for hepatotoxicity and advised to minimize risk factors for the latter.
Most treatment discontinuations in this study were not due to abnormal blood-test results. The cumulative incidence of all-cause MTX and LEF discontinuation at 1- and 5 years in this study were comparable to previous reports [29–31]. Given a wide MTX dosing range, dose reduction was more common for MTX than for LEF.
Strengths of this study include large sample size allowing us to provide precise estimates for anticipated low event rates. Additionally, this study used real-world data, thus increasing generalizability. Outcomes were stratified according to their severity and time-course to add granularity to the results and increase clinical utility. Data from the period when MTX or LEF was commenced were excluded by design and the results are applicable to long-term maintenance treatment where the greatest burden of testing lies. Although this may be viewed as a limitation, it does not reduce the validity of our findings. Missing outcome data is a concern with studies using consultation-based databases. However, the cumulative estimates of drug discontinuation reported in this study are consistent with those from previous trials and observational studies. Additionally, our validation exercise revealed that only 5% outcomes were potentially related to another condition, its complication or its treatment. We used a parsimonious list of conditions in this exercise including those for which there was only a remote possibility of abnormal blood-test results.
However, this study has several limitations. First, our findings are not applicable to patients at very high risk of drug toxicity and not transferred to shared-care prescribing e.g. CKD-4, pre-existing chronic liver disease. However, it is extremely uncommon to offer MTX or LEF to such patients, and the results of our study will therefore apply to the vast majority of AIRD patients. Secondly, dose data were missing for the majority of MTX and a large proportion of LEF prescriptions. This limits the validity of dose-reduction analysis. Thirdly, CKD progression and a serum creatinine increase of >26 μm/l, the minimum change required to consider the presence of AKI, was used to ascertain drug discontinuation with kidney function decline [17]. The guideline specifies that the increase in creatinine should occur within 48 h. We were unable to meet this part of the definition due to inherent large gaps between blood-tests, potentially resulting in an overestimate of the incidence of AKI. Our results therefore represent a worst-case scenario with respect to impact on kidney function. Some of the abnormal blood-test results could be due to concurrent prescription of other DMARDs e.g. SSZ. This can potentially elevate the outcome event rate. However, this is unlikely to play a large part as our outcome definition required a prescription gap of at-least 90 days and, it can reasonably be expected that in this period most rheumatologists will be able to ascertain the actual drug responsible for the blood-test abnormality. Moreover, some patients prescribed first-line subcutaneous MTX for RA from the hospital clinic, and stepping down to GP prescribed and monitored oral MTX may appear as incident users of MTX. However, this is likely to be uncommon as most patients with RA in the UK are commenced on oral MTX first-line and, if commenced on subcutaneous MTX first-line may have a contraindication to oral therapy. Research suggests that patients prescribed subcutaneous MTX change to the oral route in <3% instances [32]. Additionally, some treatment discontinuations in people with SLE may be due to increased disease activity e.g. cytopenia resulting in treatment escalation. However, a sensitivity analysis excluding cases with SLE or CTD reported similar event rates as the main analysis. Finally, it is difficult to attribute causality for adverse events and some potential adverse events may be unrelated to the treatment.
MTX and LEF are uncommonly discontinued for blood-test abnormalities after the first year of shared-care prescription and discontinuations for severely abnormal blood-test results are even less frequent. These data will be useful when counselling patients in routine clinical practice. Elevated liver enzymes were the commonest blood-test abnormality to cause treatment discontinuations. This underlines the need to advise patients treated with DMARDs to minimize other risk-factors for hepatotoxicity. Further research is required to identify risk-factors of target-organ damage, and, to develop a prognostic model for risk-stratified blood-test monitoring. This is being evaluated by our team in another ongoing study that will also assess the acceptability and cost-effectiveness of risk-based monitoring.
Funding: This article presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit Programme (Grant Reference Number PB-PG-1217–20030). The views expressed are those of the author(s) and not necessarily those of the NHS, NIHR or the Department of Health and Social Care.
C.D.M. is funded by the NIHR Applied Research Collaboration West Midlands, the NIHR School for Primary Care Research and a NIHR Research Professorship in General Practice (NIHR-RP-2014–04-026) for this research project. The study sponsor did not have any role in the conduct or reporting of this study.
A.A. has received departmental research grants from AstraZeneca and Oxford Immunotec, speaker bureau fees from Menarini, scientific meeting support from Pfizer, consulting fees from Inflazome and author royalties from UpToDate and Springer, unrelated to this work. M.D. has received honoraria for attending Ad hoc advisory boards on gout and osteoarthritis for Grunenthal, Mallinckrodt and Pfizer, and author royalties from UpToDate, and was an investigator in an AstraZeneca-funded, investigator-led, non-drug study (the ‘Sons of Gout’ study), unrelated to this work. His Department have received funding from Bristol Myers Squibb for an unrelated atrial fibrillation study. W.Z. has received honoraria from Regeneron and Eli Lilly for advice on treatment of OA. G.P.A. reports consulting fees from Astrazenca, Amryt Pharma, FRACTYL, Median technologies, Bergen Bio ASA; advisory fees from Kandy therapeutics, GSK, Owlstone, Inventiva Pharma; research grant support from Preglem, Pfiezer inc; and meeting support from Roche Diagnostics.
Disclosure statement: The authors have no conflict of interest to declare.
Data availability statement
This study used data from the Clinical Practice Research Datalink. Due to the CPRD data sharing policy, we unable to share this study’s data. However, access to CPRD data can be directly requested from the CPRD.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
References
- 1. Abhishek A, Doherty M, Kuo CF. et al. Rheumatoid arthritis is getting less frequent-results of a nationwide population-based cohort study. Rheumatology (Oxford, England) 2017;56:736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Springate DA, Parisi R, Kontopantelis E. et al. Incidence, prevalence and mortality of patients with psoriasis: a U.K. Population-based cohort study. Brit J Dermatol 2017;176:650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rees F, Doherty M, Grainge M. et al. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999-2012. Ann Rheum Dis 2016;75:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luqmani R, Hennell S, Estrach C. et al. ; British Health Professionals in Rheumatology Standards, Guidelines and Audit Working Group. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (the first two years). Rheumatology (Oxford, England) 2006;45:1167–9. [DOI] [PubMed] [Google Scholar]
- 5. Judge A, Wallace G, Prieto-Alhambra D, Arden NK, Edwards CJ.. Can the publication of guidelines change the management of early rheumatoid arthritis? An interrupted time series analysis from the United Kingdom. Rheumatology (Oxford, England) 2015;54:2244–8. [DOI] [PubMed] [Google Scholar]
- 6. Ledingham J, Gullick N, Irving K. et al. BSR and BHPR guideline for the prescription and monitoring of non-biologic disease-modifying anti-rheumatic drugs. Rheumatology (Oxford, England) 2017;56:2257. [DOI] [PubMed] [Google Scholar]
- 7. Singh JA, Saag KG, Bridges SL Jr.. et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res 2016;68:1–25. [DOI] [PubMed] [Google Scholar]
- 8. Hazlewood GS, Barnabe C, Tomlinson G. et al. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying anti-rheumatic drugs for rheumatoid arthritis: A network meta-analysis. The Cochrane Database Systematic Rev 2016;2016:Cd010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salliot C, van der Heijde D.. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis 2009;68:1100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emery P, Breedveld FC, Lemmel EM et al.; Group tMLS. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology 2000;39:655–65. [DOI] [PubMed] [Google Scholar]
- 11. Edwards CJ, Campbell J, van Staa T, Arden NK.. Regional and temporal variation in the treatment of rheumatoid arthritis across the UK: a descriptive register-based cohort study. BMJ Open 2012;2:e001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Curtis JR, Beukelman T, Onofrei A. et al. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann Rheum Dis 2010;69:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tilling L, Townsend S, David J.. Methotrexate and hepatic toxicity in rheumatoid arthritis and psoriatic arthritis. Clin Drug Invest 2006;26:55–62. [DOI] [PubMed] [Google Scholar]
- 14. Herrett E, Gallagher AM, Bhaskaran K. et al. Data Resource Profile: linical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chakravarty K, McDonald H, Pullar T. et al. ; British Association of Dermatologists (BAD). BSR/BHPR guideline for disease-modifying anti-rheumatic drug (DMARD) therapy in consultation with the British Association of Dermatologists. Rheumatology (Oxford, England) 2008;47:924–5. [DOI] [PubMed] [Google Scholar]
- 16. Stevens PE, Levin A.; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–30. [DOI] [PubMed] [Google Scholar]
- 17.Section 2: AKI Definition. Kidney Int Suppl 2012;2:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fontana RJ, Seeff LB, Andrade RJ. et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology (Baltimore, Md) 2010;52:730–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Common Terminology Criteria for Adverse Events: (CTCAE). 2010; https://ctep. cancer.gov/protocoldevelopment/electronic_ applications/ctc.htm.
- 20. Siva C, Eisen SA, Shepherd R. et al. Leflunomide use during the first 33 months after food and drug administration approval: experience with a national cohort of 3,325 patients. Arthritis Rheum 2003;49:745–51. [DOI] [PubMed] [Google Scholar]
- 21. Vanni KMM, Lyu H, Solomon DH.. Cytopenias among patients with rheumatic diseases using methotrexate: a meta-analysis of randomized controlled clinical trials. Rheumatology (Oxford, England) 2020;59:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Solomon DH, Glynn RJ, Karlson EW. et al. Adverse effects of low-dose methotrexate: A Randomized Trial. AnnInt Med 2020;172:369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bijlsma JWJ, Welsing PMJ, Woodworth TG. et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): a multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016;388:343–55. [DOI] [PubMed] [Google Scholar]
- 24. Dirven L, Klarenbeek NB, van den Broek M. et al. Risk of alanine transferase (ALT) elevation in patients with rheumatoid arthritis treated with methotrexate in a DAS-steered strategy. ClinRheum 2013;32:585–90. [DOI] [PubMed] [Google Scholar]
- 25. Strand V, Cohen S, Schiff M. et al. ; Leflunomide Rheumatoid Arthritis Investigators Group. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. ArchI Int Med 1999;159:2542–50. [DOI] [PubMed] [Google Scholar]
- 26. Fiehn C. [The other opinion: nephrotoxicity of low-dose methotrexate - a problem which does not exist]. Zeitschrift fur Rheumatol 2011;70:825–6. [DOI] [PubMed] [Google Scholar]
- 27. Smolen JS, Kalden JR, Scott DL. et al. ; European Leflunomide Study Group. Efficacy and safety of leflunomide compared with placebo and sulphasalazine in active rheumatoid arthritis: a double-blind, randomised, multicentre trial. Lancet 1999;353:259–66. [DOI] [PubMed] [Google Scholar]
- 28. Haydar AA, Hujairi N, Kirkham B, Hangartner R, Goldsmith DJ.. Chronic overdose of leflunomide inducing interstitial nephritis. Nephrol Dialy Transplant 2004;19:1334–5. [DOI] [PubMed] [Google Scholar]
- 29. Curtis JR, Bykerk VP, Aassi M, Schiff M.. Adherence and persistence with methotrexate in rheumatoid arthritis: A Systematic Review. J Rheumatol 2016;43:1997–2009. [DOI] [PubMed] [Google Scholar]
- 30. Zink A, Listing J, Kary S. et al. Treatment continuation in patients receiving biological agents or conventional DMARD therapy. Ann Rheum Dis 2005;64:1274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alcorn N, Saunders S, Madhok R.. Benefit-risk assessment of leflunomide: an appraisal of leflunomide in rheumatoid arthritis 10 years after licensing. Drug Safety 2009;32:1123–34. [DOI] [PubMed] [Google Scholar]
- 32. Hazlewood GS, Thorne JC, Pope JE. et al. ; CATCH Investigators. The comparative effectiveness of oral versus subcutaneous methotrexate for the treatment of early rheumatoid arthritis. Ann Rheum Dis 2016;75:1003–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study used data from the Clinical Practice Research Datalink. Due to the CPRD data sharing policy, we unable to share this study’s data. However, access to CPRD data can be directly requested from the CPRD.