Abstract
Objective
To describe the baseline characteristics, biologic DMARD (bDMARD) response and drug survival of axial SpA (axSpA) patients in the British Society for Rheumatology Biologics Register in Ankylosing Spondylitis (BSRBR-AS) according to radiographic status.
Methods
The BSRBR-AS is a national prospective cohort including axSpA participants classified according to the Assessment of SpondyloArthritis international Society criteria. In this analysis, baseline data of patients starting bDMARDs were compared. Ankylosing Spondylitis Disease Activity Scores (ASDASs) for low disease status, clinically important improvement (CII) and major improvement (MI) at 1 year were used to assess treatment response. Cox proportional hazards analysis was performed after adjusting for clinically relevant confounders.
Results
A total of 1145 axSpA patients were included. Higher male prevalence, older age and longer disease duration were seen in the radiographic axSpA (r-axSpA) subgroup. Based on a complete case analysis (290 patients), two-thirds of patients achieved an ASDAS low disease state at 1 year regardless of radiographic status [non-radiographic axSpA (nr-axSpA) 64.2% vs r-axSpA 66.1]. No statistically significant differences were seen between the subgroups in attaining ASDAS CII (nr-axSpA 50.7% vs r-axSpA 44.7%) or MI (nr-axSpA 20% vs r-axSpA 18.7%). Drug survival probability curves were similar for both subgroups and the hazard ratio for nr-axSpA/axSpA was 0.94 (95% CI 0.69, 1.28) when adjusted for sex, age, baseline ASDAS with CRP, smoking status, disease duration, HLA-B27 and prescribed biologic.
Conclusions
Although there appeared to be some differences in the baseline characteristics when exploring this cohort according to radiographic status, which are likely related to the natural history of the disease, the level of biologic response and drug survival was comparable between nr-axSpA and r-axSpA.
Keywords: ankylosing spondylitis, axial spondyloarthritis, drug survival, biological therapy, epidemiology
Rheumatology key messages
This is the largest prospective study comparing nr-axSpA and r-axSpA showing similar baseline characteristics.
Drug response evaluated by ASDAS and drug survival was comparable between nr-axSpA and r-axSpA.
These results add evidence that similar treatment strategies should be followed in nr-axSpA and r-axSpA.
Introduction
AS is the established phenotype of axial SpA (axSpA), an inflammatory condition affecting primarily the enthesis and axial skeleton, with a usually earlier, more heterogeneous phenotype classified as non-radiographic axSpA (nr-axSpA) [1]. Nr-axSpA has caused much controversy in recent years, with some arguing that it represents an earlier disease stage that might progress to AS, called radiographic axSpA (r-axSpA), while others believe that it represents a separate entity that should be treated distinctively. Following the introduction of the Assessment of Spondyloarthritis international Society (ASAS) classification criteria [2], the rheumatology community has an increased awareness of the diagnostic issues in axSpA if such criteria are misused, particularly in the non-radiographic patient subgroup. Yet, despite growing evidence that nr-axSpA and r-axSpA show a comparable burden of disease [3], different treatment strategies are still suggested [4].
Biologic DMARDs (bDMARDs) have completely changed the outlook for patients with axSpA, with significant numbers achieving long-term remission or low disease activity over time. There are ample data on the efficacy of TNF inhibitors (TNFis) in nr-axSpA coming from phase III trials [5–8]. However, only a handful of trials (RAPID-axSpA and ESTHER trial) [5, 6] and a post-hoc analysis of the INFAST study looked at the whole axSpA spectrum [9], including patients with both non-radiographic and radiographic disease and showing comparable results across both subgroups. Real-life data are even more scarce, with only a couple of small studies [10, 11] and two larger cohorts, the DANBIO register and the Swiss Clinical Quality Management (SCQM) Cohort [12, 13] published to date.
The British Society for Rheumatology Biologics Registry for Ankylosing Spondylitis (BSRBR-AS) [14] holds a large volume of data comprising both patient subgroups (AS and nr-axSpA), with significant numbers exposed to biologic agents. Based on the hypothesis that both subgroups are part of the same disease continuum and hence have a comparable response to treatment, the aims of this study were to explore the baseline characteristics of the two populations in the BSRBR-AS cohort and to evaluate the level of disease control according to the Ankylosing Spondylitis Disease Activity Score (ASDAS) and the drug survival of the first bDMARD at 1 year.
Methods
Longitudinal data from the prospective BSRBR-AS cohort study were used for this analysis. The BSRBR-AS cohort has been previously described [14]. Briefly, it includes axSpA patients meeting the ASAS criteria or the modified New York criteria for AS from 83 rheumatology centres across the UK recruited between December 2012 and December 2017. To enter the registry, patients with axSpA were required to be biologic naïve and were subsequently included in the ‘biologic cohort’ if starting a bDMARD (comprised only of TNFis at the time, mainly originator adalimumab, etanercept, infliximab or certolizumab pegol) or remained in the ‘non-biologic cohort’ otherwise. Clinical data and patient-reported questionnaires were retrieved at 3, 6 and 12 months and annually thereafter in the biologic cohort.
For this analysis, we included all axSpA patients starting a bDMARD who were categorized in the r-axSpA (participants with documented X-ray evidence of sacroiliitis as per the modified New York criteria in their medical notes) or the nr-axSpA subgroup (no such evidence). The primary outcome of our study was response to bDMARDs at 1 year follow-up defined as 12 months (s.d. 4) from the baseline visit and drug survival of the first initiated bDMARD. Treatment response was assessed with the ASDAS-CRP (calculated using collected CRP values and relevant patient-reported outcomes items). Where CRP was normal or <0.2 mg/dl, the value of 0.2 was used in the formula as recommended by Machado et al. [15]. Different scenarios were explored: patients achieving a low disease state (ASDAS <2.1), an ASDAS reduction of ≥2.0 [major improvement (MI)] or an ASDAS reduction of ≥1.1 [clinically important improvement (CII)]. Analysis was restricted to patients with an ASDAS available at baseline. We performed an additional analysis classifying patients as responders if they achieved an ASDAS low disease state or showed an ASDAS MI or CII. Where the 12 month assessment was missing but individuals remained on a drug, they were considered as responders if they demonstrated a response at 6 months.
Statistical analysis
Baseline characteristics were compared between both subgroups (nr- vs r-axSpA). Student’s t- or Mann–Whitney U test for continuous variables and chi-squared test for categorical variables were used. The proportion of patients attaining an ASDAS low disease state, MI or CII were compared when the ASDAS was available for both the baseline and 1 year time point (complete case analysis).
Drug survival was defined as the time from initiation to the end of the first bDMARD (switches to biosimilars were not considered a treatment discontinuation) or to the last available follow-up date (censoring) and were explored using Kaplan–Meier plots and the log-rank test. Cox proportional hazards analysis was performed after adjusting for clinically relevant confounders (sex, age, baseline ASDAS, smoking status, disease duration, HLA-B27 and prescribed biologic) to assess the possible impact of radiographic status on response to bDMARD therapy. The proportional hazards assumption was not violated after analytical and graphical testing. All analysis was conducted using Stata version 16.1 (StataCorp, College Station, TX, USA).
Results
Baseline characteristics were available in 1145 patients (Table 1), of whom 727 (63.5%) had radiographic sacroiliitis and were classified as r-axSpA. Regarding the nr-axSpA population, 90% (n = 378) had a positive SIJ MRI, as per the standardized ASAS definition, while only 40 patients were classified according to the clinical arm [2]. Compared with nr-axSpA, those with r-axSpA were more likely to be male, older and had longer disease duration. Uveitis was more frequently reported in the r-axSpA population, who also were more likely to be ever-smokers. Baseline BASFI and CRP levels were higher in the r-axSpA subgroup. When exploring comorbidities, these were statistically more frequent in the r-axSpA subgroup, with the main difference seen in the prevalence of hypertension (see supplementary material 1, available at Rheumatology online).
Table 1.
Baseline characteristics of patients of the BSRBR-AS cohort according to radiographic status
| Variables | Level | nr-axSpA (n = 418) | r-axSpA (n = 727) | P-value |
|---|---|---|---|---|
| Age, mean (s.d.), years | 39.7 (12.3) | 46.1 (13.4) | <0.001 | |
| Sex, n (%) | Male | 239 (57) | 529 (73) | <0.001 |
| Symptom duration, mean (s.d.), years | 11.3 (10.9) | 16.7 (12.9) | <0.001 | |
| Diagnostic delay, median (IQR), years | 3.0 (1.0–10.0) | 3.0 (0.0–11.0) | 0.83 | |
| HLA-B27 (missing = 325) | 227 (73) | 387 (76) | 0.40 | |
| Inflammatory back pain, n (%) | 405 (97) | 697 (97) | 0.40 | |
| Uveitis, n (%) | 92 (22) | 205 (30) | 0.003 | |
| Crohn’s/colitis, n (%) | 55 (13) | 113 (17) | 0.11 | |
| Psoriasis, n (%) | 79 (19) | 115 (17) | 0.43 | |
| BMI, mean (s.d.) | 27.5 (5.6) | 28.2 (5.8) | 0.10 | |
| Comorbidity count, mean (s.d.) | 0.6 (0.9) | 0.7 (1.0) | 0.016 | |
| Smoking status, n (%) | Never smoked | 148 (43) | 218 (38) | 0.040 |
| Ex-smoker | 96 (28) | 207 (36) | ||
| Current smoker | 100 (29) | 154 (27) | ||
| CRP, median (IQR), mg/dL | 0.5 (0.1–1.3) | 0.9 (0.3–2.5) | <0.001 | |
| BASDAI, median (IQR) | 6.7 (5.3–7.8) | 6.5 (5.0–7.7) | 0.12 | |
| BASFI, median (IQR) | 5.9 (4.2–7.8) | 6.5 (4.4–8.3) | 0.043 | |
| BAS-G, mean (s.d.) | 7.0 (2.0) | 6.8 (2.0) | 0.056 | |
| ASDAS-CRP, mean (s.d.) | 2.8 (0.8) | 2.8 (0.9) | 0.32 | |
| ASQOL, median (IQR) | 13.0 (9.0–16.0) | 13.0 (9.0–15.5) | 0.29 | |
| Concomitant NSAID use, n (%) | 311 (75) | 560 (77) | 0.43 | |
| Biologic (to start), n (%) | Adalimumab | 238 (57) | 436 (60) | 0.20 |
| Etanercept | 131 (31) | 220 (30) | ||
| Certolizumab | 35 (8) | 47 (6) | ||
| Golimumab | 5 (1) | 12 (2) | ||
| Secukinumab | 7 (2) | 10 (1) | ||
| Infliximab | 2 (<1) | 2 (<1) |
ASQOL: Ankylosing Spondylitis Quality of Life questionnaire.
Disease activity and treatment response
Disease activity measures and functional index at baseline and the 1 year time point are presented in supplementary material 2, available at Rheumatology online. Follow-up ASDAS was available in only 290 patients, so we explored the baseline characteristics of patients with missing values and found no significant differences in baseline ASDAS-CRP, concomitant NSAID or TNF drug used (supplementary material 3, available at Rheumatology online). Of note, patients with missing values were significantly younger and had a shorter disease duration. Overall, two-thirds of the patients with available follow-up ASDAS data achieved a low disease state (ASDAS <2.1) at 1 year regardless of radiographic status [nr-axSpA 64.2% vs r-axSpA 66.1%; difference −1.9% (95% CI −13.7, 9.8)]. Further, no significant differences were seen between the subgroups in attaining ASDAS CII [nr-axSpA 50.7% vs r-axSpA 44.7%; difference 6.0% (95% CI −7.8, 19.8)] or MI [nr-axSpA 20% vs r-axSpA 18.7%; difference 1.3% (95% CI −9.7, 12.3)]. Additionally, no differences were seen between the r- and nr-axSpA subgroups when patients were classified as responders (ASDAS low disease state, CII or MI) or non-responders [nr-axSpA 76.2% vs r-axSpA 72.6 responders; difference 3.6% (95% CI −5.2, 12.3)].
Drug survival
The median follow-up was 24 months (IQR 12–39). The first bDMARD stop time was available for 1122 patients. A total of 387 patients (33.8%) stopped their first bDMARD due to adverse events (nr-axSpA 34%, r-axSpA 37%) and lack of efficacy (nr-axSpA 35%, r-axSpA 30%) as the most frequent reasons for discontinuation, with no statistically significant differences found between both subgroups. Kaplan–Meier curves were similar for both subgroups (log-rank test P = 0.12), with a median survival time of 39.5 months (95% CI 33.7, 48.1) in the nr-axSpA subgroup vs 41.4 months (95% CI 38.5, 49.4) in the r-axSpA subgroup (Fig. 1). In the multivariable analysis, the hazard ratio for nr-axSpA/axSpA was 0.94 (95% CI 0.69, 1.28) when adjusted for sex, age, baseline ASDAS-CRP, smoking status, disease duration, HLA-B27 status and prescribed biologic. Interaction terms with gender and HLA-B27 were added into the model and did not show significant differences. When subdividing the nr-axSpA population into those fulfilling the ASAS imaging or clinical criteria, survival curves were similar for the three subgroups (supplementary material 4, available at Rheumatology online).
Fig. 1.
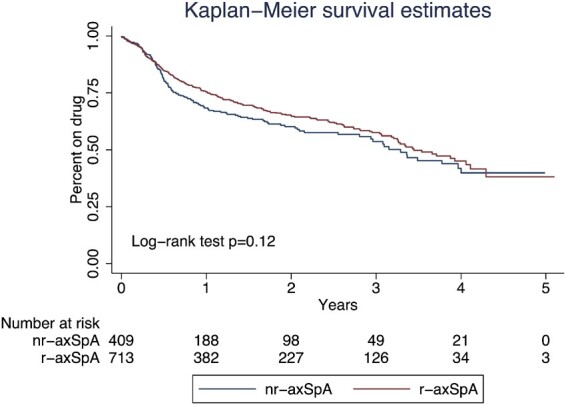
Kaplan–Meier survival curves of nr-axSpA vs r-axSpA
Discussion
Publication of the ASAS classification criteria led to considerable debate over the last decade as to whether both nr-axSpA and r-axSpA should be considered the same entity. Incidentally, the ASAS criteria were never created to separate, but to encompass the whole axSpA continuum, facilitating the identification of homogeneous cohorts in clinical trials. In our analysis of real-world data from a prospective multicentre cohort, baseline demographic and clinical characteristics were broadly similar between nr-axSpA and r-axSpA (AS). Further, the level of bDMARD response according to the ASDAS was comparable at 1 year between subgroups, as was the survival time of the first bDMARD, even in the adjusted multivariable analysis. Baseline characteristics of our cohort were similar to previously published reports, although some particularities are worth mentioning. In our study, HLA-B27 prevalence was similar between nr- and r-axSpA, as shown in the SCQM cohort, while r-axSpA patients from the DANBIO study had a higher prevalence of positive HLA-B27 [12, 13]. There is a rationale to assuming that nr- and r-axSpA have the same genetic background as part of the whole axSpA continuum. The differences with the Danish registry might be explained by the heterogeneity of the included patients, as recruitment started in 2000, predating the publication of the ASAS criteria, which led to the cohort being classified retrospectively for the analysis. In the BSRBR-AS cohort, r-axSpA patients are more frequently male than nr-axSpA patients and this is in line with published literature [3]. In addition, CRP levels and smoking history were different between subgroups and might explain a higher likelihood of progressing to r-axSpA, as these have been postulated as radiographic progression factors [16]. The higher radiographic damage of r-axSpA might relate to higher BASMI and BASFI scores found in this subgroup as part of the natural history of axSpA. Moreover, comorbidity count was statistically higher in patients with r-axSpA, mainly because of the prevalence of hypertension. Older age and longer disease duration in the r-axSpA subgroup might explain these findings [17].
We centred our analysis on the ASDAS response, as this has been shown to have good discriminatory power in both AS (r-axSpA) and nr-axSpA [18]. Similar to our real-world data, a recent clinical trial including patients with nr-axSpA and r-axSpA treated with certolizumab achieved the same treatment response at week 48 measured by ASDAS [19]. When exploring the available evidence in observational cohorts, the 1 year treatment response as per the ASDAS was higher in the r-axSpA subgroup in the SCQM cohort, although this was not statistically significant [13]. In the DANBIO study, the ASDAS response was similar between both subgroups, although this was evaluated at the 3 and 6 month time points. Differences in drug survival have been explored in a few cohorts [10, 12, 20]. Overall, all reports show similar treatment adherence in nr-axSpA and r-axSpA as outlined in our study. Interestingly, a small retrospective study from Italy did show lower drug survival in nr-axSpA [20] and poorer adherence in patients with nr-axSpA was seen in the DANBIO cohort [12], although this was not confirmed in the multivariate analysis. Most patients were classified as nr-axSpA due to a positive SIJ MRI (ASAS imaging arm), so conclusions on drug survival similarities between the clinical and imaging arms should be interpreted with caution.
To our knowledge, this is the largest prospective cohort study comparing drug response and baseline characteristics between nr-axSpA and r-axSpA. Another strength is the fact that the study inclusion criteria were based on fulfilment of the ASAS classification criteria as opposed to being retrospectively adjudicated, ensuring the homogeneity of the study population. A limitation of our study is mainly the amount of missing data at the 1 year time point. This issue was addressed by analysing excluded patients and finding that there were no differences in baseline disease activity or treatment used. The excluded patients were younger and had a shorter disease duration, suggesting that they were doing well, which might justify why they did not attend follow-up. An additional analysis using a 6 month assessment if they stayed on a bDMARD increased the sample to 407, showing the same proportion of responders. Also, statistical power was adequate (0.89) with this sample size at α = 0.05 to find a 20% difference between subgroups. In addition, we performed the drug survival analysis with most of the population (1122 patients), confirming the hypothesis that there are no differences between subgroups. Another limitation is the absence of regression analysis comparing the ASDAS response between subgroups, as this overlapped with a similar study in this cohort looking at predictors of TNFi response in axSpA at the first follow-up (10 weeks–9 months) [21]. In that analysis, disease criteria were not associated with a lack of response, supporting our results. Moreover, it is well known that SIJ assessment has limited reliability, thus misclassification of nr/r-axSpA [22] may have occurred in some cases, although this cannot be confirmed in the absence of CT or MRI of all patients. However, this study reflects real-life practice whereby clinicians have to routinely consider this possibility.
In conclusion, nr-axSpA and r-axSpA present with similar baseline characteristics in a large multicentre cohort and achieve the same level of response to bDMARDs with analogous drug survival. These results support a unique treatment strategy for axSpA and encourage future clinical trial design to encompass the whole spectrum of axSpA rather than address nr-axSpA and r-axSpA as independent diseases.
Supplementary Material
Acknowledgements
We acknowledge Prof. Gary J. MacFarlane as chief investigator on the BSRBR-AS study. We are grateful to the staff of the BSRBR-AS register and to the recruiting staff at the clinical centres, details of which are available at www.abdn.ac.uk/bsrbr-as. H.M.O. designed the study proposal. X.M. analysed the data and wrote the manuscript, with significant input from all co-authors. G.T.J. is deputy chief investigator on the BSRBR-AS study and together with L.D. discussed results and provided input into drafts of the manuscript. The study was approved by the National Research Ethics Service Committee North East-County Durham and Tees Valley (reference 11/NE/0374) and informed consent was obtained from all participants.
Funding: This work was supported by a research grant from the FOREUM Foundation for Research in Rheumatology. The BSRBR-AS is funded by the British Society for Rheumatology, which received funding for this from Pfizer, AbbVie and UCB. They have no input in determining the topics for analysis or work involved in undertaking it.
Disclosure statement: S.D. and H.M.O. are supported by the National Institute for Health Research (NIHR) Leeds Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR or the UK Department of Health. H.M.O. has received research grants from Janssen and Novartis and consultancy fees/honoraria from AbbVie, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Takeda and UCB. The remaining authors have no conflicts to declare.
Data availability statement
The data underlying this article were provided by the BSRBR-AS register committee. Data will be shared on request to the corresponding author with permission of the above institution.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Sieper J, Poddubnyy D.. Axial spondyloarthritis. Lancet 2017;390:73–84. [DOI] [PubMed] [Google Scholar]
- 2. Rudwaleit M, van der Heijde D, Landewe R. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 3. López-Medina C, Ramiro S, van der Heijde D. et al. Characteristics and burden of disease in patients with radiographic and non-radiographic axial spondyloarthritis: a comparison by systematic literature review and meta-analysis. RMD Open 2019;5:e001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Michelena X, , Marzo-Ortega H.. Axial spondyloarthritis: time to stop the split 10 years on. Nat Rev Rheumatol 2020;16:5–6. [DOI] [PubMed] [Google Scholar]
- 5. Landewé R, Braun J, Deodhar A. et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 2014;73:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Song I-H, Weiß A, Hermann K-GA. et al. Similar response rates in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis after 1 year of treatment with etanercept: results from the ESTHER trial. Ann Rheum Dis 2013;72:823–5. [DOI] [PubMed] [Google Scholar]
- 7. van der Heijde D, Joshi A, Pangan AL. et al. ASAS40 and ASDAS clinical responses in the ABILITY-1 clinical trial translate to meaningful improvements in physical function, health-related quality of life and work productivity in patients with non-radiographic axial spondyloarthritis. Rheumatology (Oxford) 2016;55:80–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sieper J, van der Heijde D, Dougados M. et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2015;67:2702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sieper J, Rudwaleit M, Lenaerts J. et al. Partial remission in ankylosing spondylitis and non-radiographic axial spondyloarthritis in treatment with infliximab plus naproxen or naproxen alone: associations between partial remission and baseline disease characteristics. Rheumatology (Oxford) 2016;55:1946–53. [DOI] [PubMed] [Google Scholar]
- 10. Corli J, Flipo R-M, Philippe P. et al. Tumor necrosis factor-α inhibition in ankylosing spondylitis and nonradiographic axial spondyloarthritis: treatment response, drug survival, and patient outcome. J Rheumatol 2015;42:2376–82. [DOI] [PubMed] [Google Scholar]
- 11. Wallman JK, Kapetanovic MC, Petersson IF. et al. Comparison of non-radiographic axial spondyloarthritis and ankylosing spondylitis patients—baseline characteristics, treatment adherence, and development of clinical variables during three years of anti-TNF therapy in clinical practice. Arthritis Res Ther 2015;17:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glintborg B, Sørensen IJ, Østergaard M. et al. Ankylosing spondylitis versus nonradiographic axial spondyloarthritis: comparison of tumor necrosis factor inhibitor effectiveness and effect of HLA-B27 status. An observational cohort study from the nationwide DANBIO registry. J Rheumatol 2017;44:59–69. [DOI] [PubMed] [Google Scholar]
- 13. Ciurea A, Scherer A, Exer P. et al. Tumor necrosis factor α inhibition in radiographic and nonradiographic axial spondyloarthritis: results from a large observational cohort. Arthritis Rheum 2013;65:3096–106. [DOI] [PubMed] [Google Scholar]
- 14. Macfarlane GJ, Barnish MS, Jones EA. et al. The British Society for Rheumatology Biologics Registers in Ankylosing Spondylitis (BSRBR-AS) study: protocol for a prospective cohort study of the long-term safety and quality of life outcomes of biologic treatment. BMC Musculoskelet Disord 2015;16:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Machado P, Navarro-Compán V, Landewé R. et al. Calculating the ankylosing spondylitis disease activity score if the conventional c-reactive protein level is below the limit of detection or if high-sensitivity C-reactive protein is used: an analysis in the DESIR cohort. Arthritis Rheumatol 2015;67:408–13. [DOI] [PubMed] [Google Scholar]
- 16. Protopopov M, Poddubnyy D.. Radiographic progression in non-radiographic axial spondyloarthritis. Expert Rev Clin Immunol 2018;14:525–33. [DOI] [PubMed] [Google Scholar]
- 17. Derakhshan MH, Goodson NJ, Packham JC. et al. Increased risk of hypertension associated with spondyloarthritis disease duration: results from the ASAS-COMOSPA study. J Rheumatol 2019;46:701–9. [DOI] [PubMed] [Google Scholar]
- 18. Fernández-Espartero C, de Miguel E, Loza E. et al. Validity of the ankylosing spondylitis disease activity score (ASDAS) in patients with early spondyloarthritis from the Esperanza programme. Ann Rheum Dis 2014;73:1350–5. [DOI] [PubMed] [Google Scholar]
- 19. Landewe R, van der Heijde D, Dougados M. et al. Induction of sustained clinical remission in early axial spondyloarthritis following certolizumab pegol treatment: 48-week outcomes from C-OPTIMISE. Rheumatol Ther 2020;7:581–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopalco G, Venerito V, Cantarini L. et al. Different drug survival of first line tumour necrosis factor inhibitors in radiographic and non-radiographic axial spondyloarthritis: a multicentre retrospective survey. Clin Exp Rheumatol 2019;37:762–7. [PubMed] [Google Scholar]
- 21. Macfarlane GJ, Pathan E, Jones GT. et al. Predicting response to anti-TNFα therapy among patients with axial spondyloarthritis (axSpA): results from BSRBR-AS. Rheumatology (Oxford) 2020;59:2481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christiansen AA, Hendricks O, Kuettel D. et al. Limited reliability of radiographic assessment of sacroiliac joints in patients with suspected early spondyloarthritis. J Rheumatol 2017;44:70–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the BSRBR-AS register committee. Data will be shared on request to the corresponding author with permission of the above institution.


