Abstract
Objectives
To evaluate the 1-year cost-effectiveness between three different initial treatment strategies in autoantibody-negative RA patients, according to 2010 criteria.
Methods
For this analysis we selected all RA patients within the intermediate probability stratum of the treatment in the Rotterdam Early Arthritis Cohort (tREACH) trial. The tREACH had a treat-to-target approach, aiming for low DAS <2.4, and treatment adjustments could occur every 3 months. Initial treatment strategies consisted of MTX 25 mg/week (initial MTX, iMTX), iHCQ 400 mg/day or an oral glucocorticoids tapering scheme without DMARDs (iGCs). Data on quality-adjusted life-years, measured with the European Quality of Life 5-Dimensions 3 Levels (EQ-5D-3L), healthcare and productivity costs were used.
Results
Average quality-adjusted life-years (s.d.), for iMTX, iHCQ and iGCs were respectively 0.71 (0.14), 0.73 (0.14) and 0.71 (0.15). The average total costs (s.d.) for iMTX, iHCQ and iGCs were, respectively, €10 832 (14.763), €11 208 (12.801) and €10 502 (11.973). Healthcare costs were mainly determined by biological costs, which were significantly lower in the iHCQ group compared with iGCs (P < 0.05). However, costs due to presenteeism were the highest in the iHCQ group (55%) followed by iMTX (27%) and iGCs (18%). The incremental cost-effectiveness ratios did not differ between treatment strategies. At a willingness-to-pay level of €50 000, the Dutch threshold for reimbursement of medical care, iHCQ had the highest probability (38.7%) of being cost-effective, followed by iGCs (31.1%) and iMTX (30.2%).
Conclusion
iHCQ had the lowest healthcare and highest productivity costs, resulting in a non-significant incremental cost-effectiveness ratio. However, iHCQ had the highest chance of being cost-effective at the Dutch willingness-to-pay threshold for healthcare reimbursement. Therefore, we believe that iHCQ is a good alternative to iMTX in autoantibody-negative RA patients, but validation is needed.
Clinical trial registration number
ISRCTN26791028
Keywords: RA, economic evaluation, cost-utility analysis, autoantigens and autoantibodies, QALY, healthcare and productivity costs, worker productivity
Rheumatology key messages
Initial HCQ and MTX show similar (early) treatment responses in autoantibody-negative RA.
Clinical and economic evaluation show that initial HCQ is a valid alternative to MTX in autoantibody-negative RA.
Treatment of RA might be stratified on autoantibody status, but validation is needed.
Introduction
The diversity of the clinical phenotype of RA is increasing due to the emphasis on early diagnosis and treatment [1]. This has resulted in an increased number of RA patients without autoantibodies, also known as autoantibody-negative RA [2]. Literature has already shown that autoantibody-negative RA patients have a better response in terms of disease activity with similar therapy, and even suggests that these patients can be treated with less aggressive therapy [1, 3, 4].
We recently showed that initial HCQ (iHCQ) and MTX (iMTX) had similar early treatment responses in autoantibody-negative RA and that these responses were better than an initial oral glucocorticoid tapering scheme without DMARDs (iGCs) [5].
Although from a clinical perspective treatment responses are similar for iHCQ and iMTX, it is also important to evaluate treatment efficacy from a societal point of view, especially since healthcare costs for RA have increased substantially in the last decades due to the introduction of biologicals [6, 7]. Despite that, the total economic burden has not increased due to increased worker productivity [8]. Various studies have already shown that a treat-to-target approach with biologicals is cost-effective, but cost-effectiveness analyses in autoantibody-negative RA are lacking [9–12].
Therefore, the aim of this study is to evaluate the cost-effectiveness between three different initial treatment strategies in autoantibody-negative RA patients, according to 2010 criteria.
Methods
Patients
We used the 1-year data from the treatment in the Rotterdam Early Arthritis Cohort (tREACH) trial [13]. The tREACH was a multicentre, stratified single-blinded trial [14]. At each participating centre the medical ethics committee approved the tREACH study protocol, and before inclusion all patients gave written informed consent (MEC-2006-252).
Patients included in the original tREACH trial had to have at least one arthritis and a symptom duration <1 year [13]. Eligible patients were stratified into three groups according to their likelihood of progressing to persistent arthritis based upon the prediction model of Visser et al. [15]. The three strata (low, intermediate, high) correspond to probability tertiles of developing persistent arthritis. Further details can be found in the publications of Claessen et al. [13] and Visser et al. [15]. Within the original low, intermediate and high probability strata there were, respectively, 13 (8%), 116 (73%) and 29 (18%) patients who fulfilled the 2010 ACR/EULAR criteria for RA at baseline and who were also autoantibody-negative (simultaneously RF and ACPA negative). Autoantibody-negative RA was only presented in small numbers in the low and high probability strata, and therefore these patients were excluded from our analysis [5]. Thus, for this study we only included the RA patients from the intermediate probability stratum of the tREACH trial [1, 5, 13].
Treatment protocol
In the intermediate stratum patients received one of the following initial treatment strategies: iMTX 25 mg per week (n = 50), iHCQ 400 mg daily (n = 40) or a 10-week oral iGCs tapering scheme, starting with 15 mg daily, without any csDMARDs (n = 41). All patients using MTX were given folic acid (10 mg/week). During the first 3 months osteoporosis prophylaxis (risedronate 35 mg/week and calcium/vitamin D combination 500/400 mg/IU/day) was given to patients in the iGCs group. None of the patients within the iMTX or iHCQ group received GC bridging therapy.
A treat-to-target approach was used, aiming for low disease activity (DAS <2.4). Treatment alterations could occur every 3 months and in case of very active disease, based on the rheumatologist’s insight, an earlier visit could be planned. Treatment was intensified in the following order if the RA was still active (DAS ≥2.4): (i) triple DMARD therapy, consisting of MTX, SSZ (2 g/day) and HCQ; (ii) MTX + etanercept (50 mg/week, s.c.); (iii) MTX + adalimumab (40 mg/2 weeks, s.c.); and (iv) MTX + abatacept (500–1000 mg/4 weeks, i.v., weight dependent). Medication was tapered if DAS was <1.6 at two consecutive visits. Hierarchically ordered tapering steps were: (i) biological agent; (ii) SSZ; (iii) MTX; and (iv) HCQ. Biological agent(s), MTX and SSZ were gradually discontinued, whereas HCQ was stopped immediately. A flare during tapering, defined as DAS ≥2.4, resulted in restarting full treatment, according to the stage in the protocol.
Outcomes and cost assessment
For the cost-effectiveness analyses, the primary outcome was the incremental cost-effectiveness ratio (ICER), which is the ratio between the difference in costs divided by the difference in quality-adjusted life years (QALYs) between two of the three initial treatment strategies. Reimbursement of medical care by Dutch health insurance companies depends on costs per QALY. In the Netherlands, the required threshold per additional QALY gained to be funded for a (new) intervention is €50 000 [16–18]. QALYs represent the impact of the disease on patients’ health over time. A QALY of 1 corresponds with living in perfect health for 1 year, whereas a QALY of 0 reflects death [19]. QALYs were determined by calculating the area under the curve of the European Quality of Life 5-Dimensions 3 Levels (EQ-5D-3L) over a period of 1 year [20].
Total costs were analysed from a societal perspective, using both healthcare costs, comprising of costs of treatment and medical consumption, and productivity costs, which includes costs due to sick leave or unemployment (absenteeism) and costs due to working while sick (presenteeism) [8, 21].
Medication costs were calculated from dosages reported in the patients’ case records, valued according to the Dutch college of health insurances (supplementaryTableS1, available at Rheumatology online) [22]. Hospitalization duration and admission diagnoses were recorded 3 monthly. The Dutch average length of stay by diagnosis was used if hospitalization duration was unknown. Medical consumption, including hospitalization, was valued at Dutch standard prices, except for costs of complementary and alternative medicine, which were based upon US data, due to the lack of Dutch data (supplementaryTableS2, available at Rheumatology online) [23, 24].
Productivity costs were calculated from 3-monthly questionnaires about loss of productivity. In addition, every 6 months questionnaires were filled out about work time and unemployment. The working population was defined as being between 15 and 75 years of age and having paid work. Productivity costs were calculated with the friction cost method, which assumes that every employee is replaceable in time and stands for the time between the occurrence of a job vacancy due to long-term sick leave and filling it. Sick leave costs are solely counted during this period, which comprises 85 days in the Netherlands [18]. Productivity losses were valued at age- and sex-dependent standard costs per hour (supplementaryTableS3, available at Rheumatology online) [25, 26]. Healthcare and productivity costs were valued at 2019 prices.
Statistical analysis
Multiple imputations with chained equations, with 40 imputations, were used to handle missing data in baseline and follow-up variables. Imputation regression models were constructed for EQ-5D-3L, medical consumption, unemployment, number of working hour and productivity loss due to absenteeism and presenteeism, in which DAS, HAQ, age, gender and baseline values were the independent variables. The choice of the appropriate imputation model (i.e. linear, logistic and Poisson regression) was based on the distribution of the individual variables.
The total number of dropouts was highest in the iGCs group (11/37, 29.7%), followed by iMTX (6/44, 13.6%) and iHCQ (1/35, 2.9%). We recently showed that iGCs were less effective after 3 months of therapy compared with the other initial treatment strategies, which might have caused the higher dropout ratio [5]. Therefore, we hypothesized an underestimation of costs due to dropouts, especially in patients treated with iGCs. A sensitivity analysis was performed to compare costs and QALYs of complete cases with the original analysis, to ensure that our findings are valid (supplementaryTableS4 and Fig. S1, available at Rheumatology online).
Analysis of uncertainty in the estimation of the ICER was illustrated with cost-effectiveness planes via bootstrapping with 1000 replications using a Monte Carlo simulation. Cost-effectiveness acceptability curves were made to decide which initial treatment strategy had the highest chance of being cost-effective for different willingness-to-pay (WTP) levels. WTP thresholds for coverage of drugs differ per country and as mentioned previously the Dutch threshold is €50 000 [16–18, 27].
Statistical analyses were done in STATA v.15.1. A P ≤ 0.05 was considered statistically significant.
Ethical approval and consent for publication
The study was approved by the Medical Ethics committee of the Erasmus MC (MEC-2006-252). All patients gave written informed consent before inclusion (MEC-2006-252). Patients and/or the public were involved in the design and conduct plans of this research.
Results
Patients
The baseline characteristics of the 116 autoantibody-negative RA patients within the intermediate stratum of the tREACH trial are given in Table 1. Patients were predominantly female (70%) with an average age of 54.8 years and a median (interquartile range) symptom duration of 134 days (95–205) (Table 1). At baseline, 58/116 (50%) patients had paid work (Table 1). Respectively, 44, 35 and 37 patients were initially treated with iMTX, iHCQ or iGCs.
Table 1.
Baseline characteristics of autoantibody-negative patients, stratified for induction therapy
| iMTX (n = 44) | iHCQ (n = 35) | iGCs (n = 37) | |
|---|---|---|---|
| Demographic | |||
| Age, mean (s.d.), years | 56 (14) | 55 (14) | 53 (14) |
| Sex, female, n (%) | 33 (75) | 22 (63) | 26 (70) |
| Disease characteristics | |||
| Symptom duration, median (IQR), days | 137 (85–209) | 140 (101–213) | 124 (94–192) |
| DAS, mean (s.d.) | 3.51 (0.92) | 3.00 (0.85) | 3.57 (0.94) |
| SJC44, median (IQR) | 9 (6–13) | 6 (2–10) | 8 (4–15) |
| Erosive disease, n (%)a | 1 (2) | 0 (0) | 0 (0) |
| Work-related outcomes | |||
| Paid work, n (%) | 17 (44) | 21 (66) | 20 (61) |
| Working hours per week, mean (s.d.)b | 32 (10) | 29 (15) | 29 (13) |
| Retired, n (%) | 5 (11) | 3 (9) | 4 (11) |
| EQ-5D-3L, mean (s.d.) | 0.61 (0.24) | 0.68 (0.22) | 0.64 (0.24) |
Erosive disease is defined as having an erosion score >1 in three separate joints [28].
Working hours per week among the working population.
EQ-5D-3L: European Quality of Life 5-Dimensions 3 Levels; iGCs: initial glucocorticoids; iHCQ: initial HCQ; iMTX: initial MTX; IQR: interquartile range; SJC44: swollen joint count (44 joints).
Healthcare costs
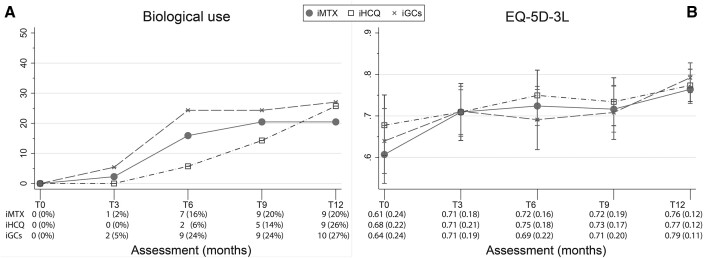
Average healthcare costs (s.d.) for treatment with iMTX, iHCQ and iGCs were, respectively, €2584 (2196), €2123 (2172) and €3.050 (3461). There was no significant difference in total healthcare costs between the three initial treatment strategies (Tables 2 and 4). However, medication costs, which comprise 54.8% of the total healthcare costs, were significantly higher in the iGCs group (mean €1960, 95% CI 982, 2938) compared with the iHCQ group (mean €821, 95% CI €302, €1339, P < 0.05; Table 2), which was caused by the difference in biological use over time (Fig. 1A).
Table 2.
Healthcare utilization and costs average per patient during 1 year of follow-up
| iMTX (n = 44) |
iHCQ (n = 35) |
iGCs (n = 37) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Utilization | Quantity | Costs (€) | Utilization | Quantity | Costs (€) | Utilization | Quantity | Costs (€) | |
| Medication | |||||||||
| DMARDs | 43 (98) | – | 265 (377) | 35 (100) | – | 209 (309) | 27 (73) | – | 232 (337) |
| Glucocorticoids | 17 (39) | – | 3 (5) | 9 (26) | – | 2 (4) | 35 (95) | – | 8 (6) |
| Biological | 10 (23) | – | 1098 (2139) | 5 (14) | – | 492 (1336) | 10 (27) | – | 1647 (2875)* |
| Analgesia | 35 (80) | – | 100 (103) | 32 (91) | – | 116 (107) | 25 (68) | – | 57 (70) |
| Co-medication | 38 (86) | – | 3 (3) | 5 (14) | – | 0 (1) | 35 (95) | – | 15 (9) |
| Hospitalization | 3 (7) | 0 (8) | 141 (634) | 3 (9) | 0 (6) | 169 (602) | – | – | – |
| Medical consumption | |||||||||
| Standard healthcare | |||||||||
| Primary care physician | 4 (9) | 0 (10) | 11 (40) | 4 (11) | 0 (20) | 39 (134) | 3 (8) | 0 (10) | 19 (66) |
| Specialist | 44 (100) | 7 (13) | 375 (623) | 35 (100) | 7 (14) | 657 (975) | 37 (100) | 8 (16) | 786 (1081) |
| Nurse practitioner/physician assistant | 13 (30) | 1 (7) | 36 (64) | 8 (23) | 1 (5) | 25 (49) | 12 (32) | 1 (8) | 39 (62) |
| Paramedical care | |||||||||
| Physical therapy | 2 (5) | 0 (44) | 46 (242) | 1 (3) | 0 (108) | 108 (639) | 1 (3) | 0 (48) | 45 (276) |
| Complementary medicine | |||||||||
| Alternative medical systems | – | – | – | 1 (3) | 0 (3) | 3 (16) | – | – | – |
| Total healthcare costs | 2584 (2196) | 2123 (2172) | 3050 (3461) | ||||||
Results shown are, respectively, n (%) for utilization, median (maximum) for quantity and mean (s.d.) for costs.
Co-medication consists of folic acid, risedronate and calcium carbonate/colecalciferol.
P < 0.05 iHCQ vs iGCs.
iGCs: initial glucocorticoids; iHCQ: initial HCQ; iMTX: initial MTX.
Table 4.
QALYs and (specified) costs per patient during the first year of follow-up
| QALYs and costs | iMTX (n = 44) | iHCQ (n = 35) | iGCs (n = 37) |
|---|---|---|---|
| QALYs (EQ-5D-3L, AUC) | 0.71 (0.14) | 0.73 (0.14) | 0.71 (0.15) |
| Total costs, € | 10 832 (14 763) | 11 208 (12 801) | 10 502 (11 973) |
| Total healthcare costs, € | 2584 (2196) | 2123 (2172) | 3050 (3461) |
| Medication, € | 1471 (2136) | 821 (1510) | 1960 (2933) |
| Medical consumption, € | 972 (717) | 1133 (1392) | 1090 (1080) |
| Hospitalization, € | 141 (634) | 169 (602) | − |
| Total productivity costs, € | 8249 (14 171) | 9085 (11 571) | 7453 (10 446) |
| Absenteeism, € | 6750 (12 804) | 6070 (7984) | 6469 (9506) |
| Presenteeism, € | 1499 (2921) | 3015 (5157) | 984 (1693) |
Results shown are mean (s.d.).
AUC: area under the curve; EQ-5D-3L: European Quality of Life 5-Dimensions 3 Levels; iGCs: initial glucocorticoids; iHCQ: initial HCQ; iMTX: initial MTX; QALYs: quality-adjusted life years.
Fig. 1.
Biological use and EQ-5D-3L over time
Panels (A) and (B) show the biological use and EQ-5D-3L over time, respectively. Error bars indicate 95% CIs. EQ-5D-3L: European Quality of Life 5-Dimensions 3 Levels; iGCs: initial glucocorticoids; iHCQ: initial HCQ; iMTX: initial MTX.
Productivity costs
Average productivity costs (s.d.) for treatment with iMTX, iHCQ and iGCs were, respectively, €8249 (14 171), €9085 (11 571) and €7453 (10 446) (Tables 3 and 4). The difference in productivity costs was mainly caused by dissimilarity in presenteeism, which were lowest in patients with iGCs. Although less long-term sick leave was seen in patients treated with iHCQ (14%) compared with the other groups (43%), we do see a higher occurrence of sick leave in patients with iHCQ (18 times) compared with the other groups (13–15 times). After 1 year, the average workweek of the working population was 36, 36 and 29 h with an average decrease of 9, 5 and 7 contract hours per week for, respectively, iMTX, iHCQ and iGCs. During the first year of follow-up patients using iMTX, iHCQ and iGCs were on average 15.7, 23.6 and 9.7 days, respectively, less productive than usual. On these days productivity (presenteeism) decreased by 18, 52 and 20%, respectively (Table 3).
Table 3.
Productivity loss and costs after 1 year of follow-up
| Productivity loss | iMTX (n = 44) | iHCQ (n = 35) | iGCs (n = 37) |
|---|---|---|---|
| Employment | |||
| Paid work | 17 (39) | 21 (60) | 19 (51) |
| Became unemployed | 0 (0) | 0 (0) | 1 (3) |
|
Absenteeism during 1 year of follow-upa |
|||
| Occurrence | 13 (76) | 18 (86) | 15 (75) |
| Long-term sicknessb | 3 (18) | 1 (5) | 3 (15) |
| Days absent, median (IQR) | 11 (5–63) | 11 (7–64) | 6 (4–121) |
|
Contract hour,a mean (s.d.) |
|||
| Working hours per week | 36 (27–40) | 36 (16–37) | 29 (20–38) |
| Change in working hours per week | −9 (16) | −5 (14) | −7 (15) |
| Amount of reduction, days | 22.9 (51.1) | 6.2 (28.4) | 19.5 (47.6) |
|
Presenteeism during 1 year of follow-up,c mean (s.d.) |
|||
| Number of days per year | 15.7 (15.8) | 23.6 (23.4) | 9.7 (11.7) |
| Average productivity loss, proportion (s.d.), % | 18 (36) | 52 (86) | 20 (45) |
| Total productivity costs, mean (s.d.), € | 8249 (14 171) | 9085 (11 571) | 7453 (10 446) |
Results shown are n (%) unless stated otherwise.
Absenteeism and contract hours is over working population.
Long-term sickness is defined as absence from work for longer than 85 days (Dutch friction period).
Presenteeism is over entire population.
iGCs: initial glucocorticoids; iHCQ: initial HCQ; iMTX: initial MTX; IQR: interquartile range.
Cost-utility analyses
Quality of life (EQ-5D-3L) over time is shown in Fig. 1B. No significant differences in QALYs were seen between treatment arms. Average QALYs (EQ-5D-3L, s.d.), for iMTX, iHCQ and iGCs were, respectively, 0.71 (0.14), 0.73 (0.14) and 0.71 (0.15) (Table 4).
Average total costs for iMTX, iHCQ and iGCs were, respectively, €10 832(€14 763), €11 208 (€12 801) and €10 502 (€11 973). Total costs, which are comprised of 24% healthcare costs and 76% productivity costs, did not differ between the three initial treatment strategies (Table 4).
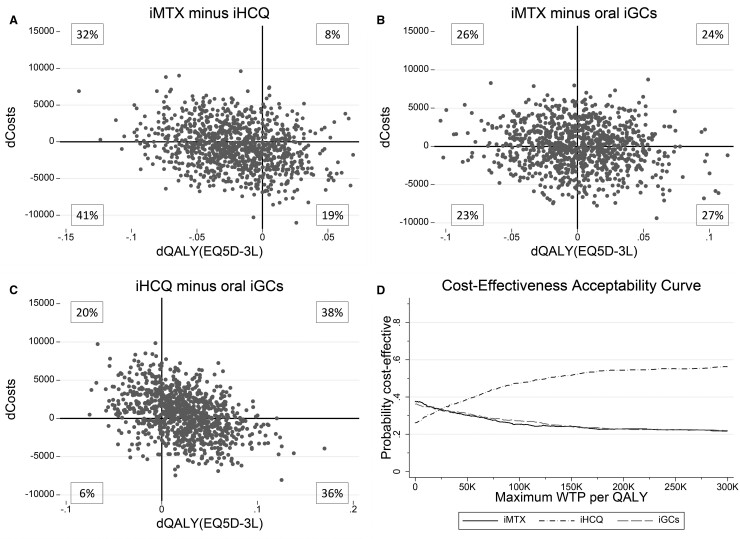
The ICER (95% CI) between iHCQ and iMTX was −€50 390(−€393 581, €292 802), between iHCQ and iGCs was −€830 022 (−€1 700 498, €40 455) and between iMTX and iGCs was −€14 336 (−€109 402, €80 731), and did not differ between groups. Fig. 2 shows the cost-effectiveness planes and cost-effectiveness acceptability curves for the different initial treatment strategies compared with each other. Uncertainty of the ICER was visualized in the cost-effectiveness planes in Fig. 2A–C. The probability of being cost-effective at a WTP level of €50 000 was 30.2, 38.7 and 31.1% for, respectively, iMTX, iHCQ and iGCs. This indicates that for the Dutch WTP level of €50 000, iHCQ has the highest chance of being cost-effective [16–18, 27]. For WTP levels >€25 600 iHCQ has the highest probability of being cost-effective, while iMTX and iGCs had the highest probability of being cost-effective ≤€25 600 (Fig. 2D).
Fig. 2.
Summary of economic evaluation
(A–C) Results of 1000 bootstrapped replications, presented in cost-effectiveness planes indicating the uncertainty of the ICER for the comparison of the three initial treatment strategies. Differences in costs and differences in QALYs are indicated for the comparison of (A) iMTX minus iHCQ, (B) iMTX minus oral iGCs and (C) iHCQ minus oral iGCs. (D) The cost-effectiveness acceptability curve indicates the probability of being cost-effective at several levels of willingness to pay per QALY for the three separate initial treatment strategies. EQ5D-3L: European Quality of Life 5-Dimensions 3 Levels; ICER: incremental cost-effectiveness ratio; iGCs: initial glucocorticoids; iHCQ: initial HCQ; iMTX: initial MTX; QALY: quality-adjusted life year; WTP: willingness-to-pay.
Sensitivity analysis
Our sensitivity analysis comparing the original analysis with complete cases showed lower costs for patients receiving iMTX and iHCQ, but higher costs for patients receiving iGCs. The highest average total costs were seen in complete cases receiving iGCs €12 294 (15 926), followed by iHCQ €8887 (12 673) and iMTX €8109 (12 634). The costs of the complete cases receiving iGCs are higher, which validates our hypothesis that missing values were not at random (supplementary Table S4, available at Rheumatology online). The probability of being cost-effective for each WTP threshold was in favour of iHCQ (∼85%) (supplementary Fig. S1, available at Rheumatology online).
Discussion
In this study we evaluated the 1-year cost-effectiveness between three different initial treatment strategies; iMTX, iHCQ or a 10-week oral glucocorticoid tapering scheme without DMARDs (iGCs) in newly diagnosed autoantibody-negative (simultaneously RF and ACPA negative) RA patients. Healthcare costs were mainly determined by biological use, which was significantly lower in the iHCQ group compared with iGCs (P < 0.05). On the other hand, costs due to presenteeism were the highest in the iHCQ group (55%) followed by iMTX (27%) and iGCs (18%). Therefore, the ICERs did not differ between the initial treatment strategies. At a WTP level of €50 000, the Dutch threshold for reimbursement of medical care, iHCQ had the highest probability (38.7%) of being cost-effective, followed by iGCs (31.1%) and iMTX (30.2%).
Therefore, the current economic evaluation as well as our previous clinical evaluation show that iHCQ is a valid alternative for iMTX in autoantibody-negative RA patients [5]. However, WTP thresholds may differ per country. For example, the WTP thresholds can range from €4000 per QALY in Thailand to €158 000 per QALY in Norway [29]. Therefore, one could argue that the most cost-effective therapy depends on the nation’s prosperity.
Another problem is that differences in legislation, healthcare systems and treatment prices between countries make it hard to generalize our results, but also to compare them with other cost-effectiveness analyses. For example, legislation in the Netherlands makes it relatively difficult to fire employees. On the other hand, Dutch people often work more part-time, which reduces workload and therefore enables employees to preserve their work productivity longer. Besides the aforementioned reasonsing, differences in the time periods studied also makes it hard to compare cost-effectiveness analyses with each other. Luckily, there are several Dutch trials with cost-effectiveness analyses from a similar time period with which we can carefully compare our results [9, 30, 31].
The burden of disease, measured as the area under the curve of the EQ-5D-3L, within our trial is comparable to previous Dutch strategy trials [9, 30, 31]. The tREACH trial, for example, reported a first year QALY of 0.73 for RA patients, 84% autoantibody-positive, who received iMTX plus a 10-week oral GC tapering scheme, which is comparable to our QALY for iMTX. However, the average total cost was higher for the aformentioned RA patients (€15 442) compared with our autoantibody-negative RA patients treated with iMTX (€10 832) [9]. The Behandelstrategieën in RA (BeSt) study on the other hand showed a QALY of ∼0.65 [31]. Our QALYs are higher compared with the BeSt study, which might be due to earlier detection of disease and less severe disease characteristics. To illustrate, our baseline DAS is lower compared with the BeST study (3.4 vs 4.5). Contrary to the BeSt study, the Combinatietherapie bij RA (COBRA)-light trial reported a similar QALY (0.68) to our trial [30]. However, healthcare costs were higher, while productivity costs were lower in the COBRA-light trial compared with our analyses. The difference in healthcare costs is explained by the difference in biological use. The difference in productivity costs is probably caused by the fact that besides cost due to absenteeism, we also included costs due to presenteeism [30].
Although clinical outcomes have improved enormously in the last decades, productivity loss still occurs often in RA patients, which is a huge issue for patients. A recent systematic review, for example, showed that in the first year 66% of the employed RA patients experienced productivity loss due to the burden of disease [32]. Moreover, previous studies demonstrated that productivity loss expenditures exceeded healthcare costs in determining overall RA costs, which is in agreement with our results (76 vs 24%) [11, 33]. Another recent systematic review reported that presenteeism reduces work productivity to a greater extent than absenteeism [8]. It is therefore important to include presenteeism as well as absenteeism as productivity loss when assessing productivity costs in cost-effectiviness analyses [34]. None of the aforementioned Dutch cost-effectiveness trials took presenteeism into account in the calculation of the productivity costs [9, 30, 31].
A limitation of this study is that differences in legislation and regulations between countries make it difficult to generalize our results. Despite these differences between countries, the fact that iHCQ was more cost-effective than iMTX for all WTP thresholds >€25 600 is in our opinion generalizable. Moreover, our results could serve as an inspiration for others who wish to carry out similar cost-effectiveness analyses and adapt them to their country of interest.
Secondly, we only selected autoantibody-negative RA patients within the intermediate stratum of the tREACH trial, because most of the autoantibody-negative RA patients belonged to this stratum (73%) and their numbers were low in the other strata (respectively 13 and 29 RA patients in the low and high strata). This might have introduced a selection bias, and therefore we compared the baseline characteristics of autoantibody-negative RA patients in the low, intermediate and high probability strata in supplementary Table S5, available at Rheumatology online. Although disease activity and quality of life differ between the RA patients from the three strata, we have already shown that after 12 months of treatment DAS, functional ability and radiological progression did not seem to differ between RA patients from the intermediate and high strata [5]. This suggests that even in autoantibody-negative RA patients within the high group, less intensive treatment is sufficient. However, validation is needed.
Additionally, in our trial the dropout rates were higher in the iGCs group (11/37, 29.7%) compared with the iMTX (6/44, 13.6%) and iHCQ (1/35, 2.9%) groups, which might imply that missing values are not at random but possibly related to initial treatment. We recently showed that iGCs were less effective after 3 months of therapy compared with the other initial treatment strategies, which might have caused the higher dropout ratio. Therefore, we hypothesized an underestimation of costs due to dropouts, especially in patients treated with iGCs [5]. To ensure that our findings are valid, we performed a sensitivity analysis using only complete cases. This analysis confirmed our hypothesis and showed that costs were higher for patients receiving iGCs and that this therapy is also not indicated for this group of patients. However, the sensitivity analysis still showed that iHCQ is a valid alternative for iMTX in autoantibody-negative RA patients.
Finally, we only used 1 year of follow-up data and we do not know what the results would be if we looked at longer follow-up periods. However, we do know that most treatment alterations and productivity loss occurs in the first year after diagnosis [4, 5, 9, 14, 32, 35].
The strength of this analysis is that it is the first study that provides insight in the cost-effectiveness of different induction treatment strategies, including initial GCs without csDMARDs, in autoantibody-negative RA patients [11]. Moreover, this study provides extensive data on cost-effectiveness, including absenteeism and presenteeism.
In conclusion, iHCQ had the lowest healthcare and highest productivity costs, resulting in a non-significant ICER. However, iHCQ had the highest chance of being cost-effective at the Dutch WTP threshold for healthcare reimbursement. These results underscore the possibility of stratified treatment in RA based upon autoantibody status, in which iHCQ might be a good alternative for iMTX in autoantibody-negative RA, but validation is needed.
Supplementary Material
Acknowledgements
We especially thank the participating patients in the tREACH trial for their willingness to contribute to the study and for their cooperation. We thank all rheumatologists from the following participating centers: Erasmus MC, Rotterdam; Maasstad ziekenhuis, Rotterdam; Sint Fransiscus Gasthuis & Vlietland, Rotterdam & Schiedam; Albert Schweitzer ziekenhuis, Dordrecht; Admiraal de Ruyter ziekenhuis, Goes en Vlissingen; and Zorgsaam ziekenhuis, Terneuzen. We also thank all study nurses, laboratory personnel, co-investigators and others who were involved with the tREACH study. All authors contributed to the conception or design of the trial; or the acquisition, analysis or interpretation of data for the work; drafting or revision of the manuscript; and final approval of the manuscript for publication. All authors contributed to refinement of the article.
Funding: The tREACH trial was supported by an unrestricted grant from Pfizer bv. (WI229707). Pfizer had no involvement in the study design; in collection, analysis and interpretation of data; writing of the report; or decision to submit for publication. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication. Data management was sponsored by the Dutch Arthritis Society (16-3-101).
Disclosure statement: The authors declare no competing interests.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Smolen JS, Landewe RBM, Bijlsma JWJ. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 2. Boeters DM, Mangnus L, Ajeganova S. et al. The prevalence of ACPA is lower in rheumatoid arthritis patients with an older age of onset but the composition of the ACPA response appears identical. Arthritis Res Ther 2017;19:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi ST, Lee KH.. Clinical management of seronegative and seropositive rheumatoid arthritis: a comparative study. PLoS One 2018;13:e0195550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Combe B, Landewe R, Daien CI. et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2017;76:948–59. [DOI] [PubMed] [Google Scholar]
- 5. Luurssen-Masurel N, Weel AEAM, Hazes JMW, de Jong PHP.. Towards stratified treatment of rheumatoid arthritis. Int J Clin Rheumatol 2020;15:73–82. [Google Scholar]
- 6. Manova M, Savova A, Vasileva M. et al. Comparative price analysis of biological products for treatment of rheumatoid arthritis. Front Pharmacol 2018;9:1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Modena V, Bianchi G, Roccatello D.. Cost-effectiveness of biologic treatment for rheumatoid arthritis in clinical practice: an achievable target? Autoimmun Rev 2013;12:835–8. [DOI] [PubMed] [Google Scholar]
- 8. Batko B, Rolska-Wójcik P, Władysiuk M.. Indirect costs of rheumatoid arthritis depending on type of treatment-a systematic literature review. Int J Environ Res Public Health 2019;16:2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Jong PH, Hazes JM, Buisman LR. et al. Best cost-effectiveness and worker productivity with initial triple DMARD therapy compared with methotrexate monotherapy in early rheumatoid arthritis: cost-utility analysis of the tREACH trial. Rheumatology (Oxford) 2016;55:2138–47. [DOI] [PubMed] [Google Scholar]
- 10. Kowalik K, Węgierska M, Barczyńska T, Jeka S.. Pharmacoeconomic evaluation of costs of rheumatoid arthritis therapy with selected biological treatment. Reumatologia 2018;56:340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schoels M, Wong J, Scott DL. et al. Economic aspects of treatment options in rheumatoid arthritis: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2010;69:995–1003. [DOI] [PubMed] [Google Scholar]
- 12. van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261–3. [PubMed] [Google Scholar]
- 13. Claessen SJ, Hazes JM, Huisman MA. et al. Use of risk stratification to target therapies in patients with recent onset arthritis; design of a prospective randomized multicenter controlled trial. BMC Musculoskelet Disord 2009;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Jong PH, Hazes JM, Han HK. et al. Randomised comparison of initial triple DMARD therapy with methotrexate monotherapy in combination with low-dose glucocorticoid bridging therapy; 1-year data of the tREACH trial. Ann Rheum Dis 2014;73:1331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Visser H, le Cessie S, Vos K, Breedveld FC, Hazes JM.. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum 2002;46:357–65. [DOI] [PubMed] [Google Scholar]
- 16. Cross M, Smith E, Hoy D. et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1316–22. [DOI] [PubMed] [Google Scholar]
- 17. Ubel PA, Hirth RA, Chernew ME, Fendrick AM.. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med 2003;163:1637–41. [DOI] [PubMed] [Google Scholar]
- 18. Zwaap J, Knies S, van der Meijden C, Staal P, van der Heijden L. Kosteneffectiviteit in de praktijk. In: Ministerie van Volksgezondheid WeS, ed. Zorginstituut Nederland, 2015.
- 19. Torrance GW. Measurement of health state utilities for economic appraisal. J Health Econ 1986;5:1–30. [DOI] [PubMed] [Google Scholar]
- 20. Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A.. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ-5D). Br J Rheumatol 1997;36:551–9. [DOI] [PubMed] [Google Scholar]
- 21. Huscher D, Merkesdal S, Thiele K et al.; for the German Collaborative Arthritis Centres. Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann Rheum Dis 2006;65:1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zorginstituut Nederland. Medicijnkosten, 2017. http://www.medicijnkosten.nl (21 March 2019, date last accessed).
- 23. Nahin RL, Barnes PM, Stussman BJ, Bloom B.. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States. Natl Health Stat Report 2007;2009:1–14. [PubMed] [Google Scholar]
- 24. Hakkaart-van Roijen L, Van der Linden N, Bouwmans C, Kanters T, Tan SS. Kostenhandleiding Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. Zorginstitituut Nederland. 2015.
- 25. StatLine CBS . Beloning en arbeidsvolume van werknemers, 2019. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/84165NED/table?dl=217EE&ts=1566303305202 (1 Jun 2019, date last accessed).
- 26. StatLine CBS. Beloningsverschillen tussen mannen en vrouwen, 2019.https://opendata.cbs.nl/statline/#/CBS/nl/dataset/81901NED/table?dl=217EC (1 Jun 2019, date last accessed).
- 27. van Mulligen E, Weel AE, Kuijper TM. et al. Two-year cost effectiveness between two gradual tapering strategies in rheumatoid arthritis: cost-utility analysis of the TARA trial. Ann Rheum Dis 2020;79:1550–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Heijde D, van der Helm-van Mil AH, Aletaha D. et al. EULAR definition of erosive disease in light of the 2010 ACR/EULAR rheumatoid arthritis classification criteria. Ann Rheum Dis 2013;72:479–81. [DOI] [PubMed] [Google Scholar]
- 29. Schwarzer R, Rochau U, Saverno K. et al. Systematic overview of cost-effectiveness thresholds in ten countries across four continents. J Comp Eff Res 2015;4:485–504. [DOI] [PubMed] [Google Scholar]
- 30. Ter Wee MM, Coupe VM, den Uyl D. et al. Cost-utility of COBRA-light versus COBRA therapy in patients with early rheumatoid arthritis: the COBRA-light trial. RMD Open 2017;3:e000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van den Hout WB, Goekoop-Ruiterman YP, Allaart CF. et al. Cost-utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 2009;61:291–9. [DOI] [PubMed] [Google Scholar]
- 32. Burton W, Morrison A, Maclean R, Ruderman E.. Systematic review of studies of productivity loss due to rheumatoid arthritis. Occup Med (London) 2006;56:18–27. [DOI] [PubMed] [Google Scholar]
- 33. Ruof J, Hülsemann JL, Mittendorf T. et al. Costs of rheumatoid arthritis in Germany: a micro-costing approach based on healthcare payer’s data sources. Ann Rheum Dis 2003;62:544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hsieh PH, Wu O, Geue C. et al. Economic burden of rheumatoid arthritis: a systematic review of literature in biologic era. Ann Rheum Dis 2020;79:771–7. [DOI] [PubMed] [Google Scholar]
- 35. Goetzel RZ, Long SR, Ozminkowski RJ. et al. Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting U.S. employers. J Occup Environ Med 2004;46:398–412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.