Abstract
Background
Contradicting assumptions have been made about the effectiveness of SARS-CoV-2 vaccines in patients with multiple sclerosis (MS) receiving immunomodulatory disease-modifying therapies (DMTs) based on the quantification of humoral and cellular immune responses. This study aimed to understand changes in the risk of SARS-CoV-2 infection among the total population of patients receiving MS DMTs in England following mass vaccination.
Methods
This is a retrospective analysis of national data collected prospectively and longitudinally. National Health Service (NHS) England and NHS Improvement (NHSE/I) hold prescribing data on all commissioned MS DMTs in England. United Kingdom Health Security Agency (UKHSA) has been collecting data on all registered SARS-CoV-2 test results, including polymerase chain reaction and rapid antigen tests. All patients receiving MS DMTs were identified using NHSE/I datasets. All patients receiving MS DMTs with SARS-CoV-2 infection (i.e., positive test) from March 2020 to August 2021 were identified by merging NHSE/I and UKHSA datasets. Similar data for the general population were captured using publicly available datasets of the United Kingdom government. The incidence rate ratios (IRR) of SARS-CoV-2 infection among patients receiving MS DMTs compared to the general population during the pre-vaccination (November 2020 to January 2021) and post-vaccination (June to August 2021) periods were calculated.
Results
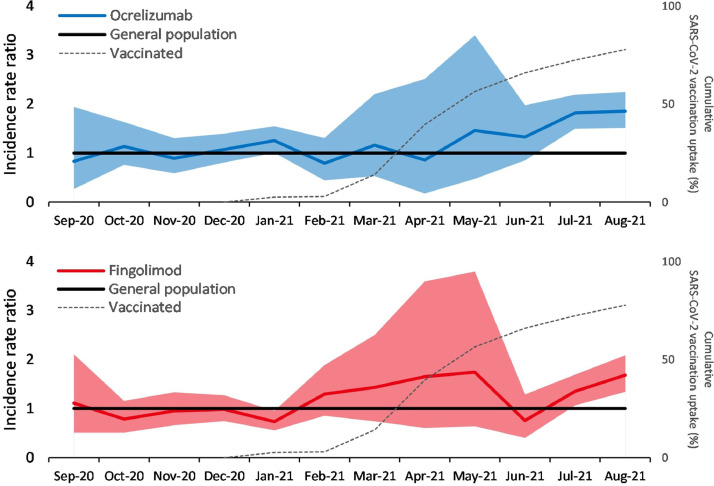
A mean (standard deviation) of 41,208 (4,301) patients received an MS DMT in England during each month from March 2020 to August 2021. The IRR (95% confidence interval) of infection in patients taking ocrelizumab versus the general population increased from 1.13 (0.97–1.31) during the pre-vaccination period to 1.79 (1.57–2.03) during the post-vaccination period. For patients on fingolimod, it increased from 0.87 (0.73–1.02) to 1.40 (1.20–1.63) during the same periods. There were no significant changes for patients on other MS DMTs.
Conclusion
SARS-CoV-2 vaccines offer less protection against infection to patients taking ocrelizumab or fingolimod, who have an impaired immune response to vaccines, than the general population. These findings will have implications for vaccination policies.
Keywords: Multiple sclerosis, Covid-19, SARS-CoV-2, Vaccination, Disease-modifying therapies
1. Introduction
While real-world data in the general population continue to show that SARS-CoV-2 vaccination is effective in preventing infections (Pritchard et al., 2021), it is still unclear whether it offers the same level of protection to multiple sclerosis (MS) patients receiving immunomodulatory disease-modifying therapies (DMTs). Immunological studies are reporting on humoral and cellular immune responses to SARS-CoV-2 vaccines among patients on MS DMTs (Achiron et al., 2021; Apostolidis et al., 2021; Brill et al., 2021; Gadani et al., 2021; Sormani et al., 2021), but they lack findings on the effectiveness of vaccines in preventing infections in this population. It is important that monitoring the population effect of SARS-CoV-2 vaccination is inclusive of patients on immunomodulatory treatments (Pritchard et al., 2021), especially as COVID-19 restrictions are being relaxed.
The present study aimed to understand the impact of mass SARS-CoV-2 vaccination in preventing SARS-CoV-2 (symptomatic and asymptomatic) infections on the entire population of patients taking MS DMTs in England.
2. Materials and methods
This is a retrospective analysis of prospectively and longitudinally collected national data by the National Health Service (NHS) England and NHS Improvement (NHSE/I) and the United Kingdom Health Security Agency (UKHSA).
2.1. Population data
NHSE/I acquire prescribing data on all commissioned MS DMTs in England (National Health Service England, 2018 2021). The total number of patients on MS DMTs (including alemtuzumab, beta-interferons, cladribine, dimethyl fumarate, fingolimod, glatiramer acetate, natalizumab, ocrelizumab, and teriflunomide) during each month from March 2020 to August 2021 was estimated based on their last DMT prescription any time before and including the last day of each month since January 2019.
The total population of England was captured from publicly available data (Office for National Statistics, 2021). The population of adults aged 20 years or above was used to match the MS population who over 98% of them are adults (The Multiple Sclerosis International Federation, 2020).
2.2. SARS-CoV-2 infection data
UKHSA has collected data on all registered SARS-CoV-2 test results, including polymerase chain reaction (PCR) and rapid antigen tests (RAT), from the start of the pandemic which is publicly available for the general population (United Kingdom Government, 2021). The datasets of NHSE/I and UKHSA were merged to identify all patients taking MS DMTs who tested positive for SARS-CoV-2 during each month from March 2020 to August 2021. The last prescribed MS DMT any time before the date of a positive test was used to determine the DMT an MS patient was taking when they tested positive.
The available data on SARS-CoV-2 infections for both MS patients and the general population included people with 1) positive PCR, 2) positive RAT confirmed by positive PCR taken within 72 h, or 3) positive RAT when PCR was not done within 72 h (89.8%, 7.4%, and 2.8% of cases in the general population by the end of August 2021, respectively) (United Kingdom Government, 2021). People with positive RAT but negative PCR within 72 h were not included as a case of SARS-CoV-2 infection. People with more than one positive test were counted once and the date of their first positive test was used.
2.3. Statistical analysis
The incidence rate of SARS-CoV-2 infection was calculated for the general population and patients on MS DMTs. The incidence rate ratio (IRR) was calculated as the incidence rate of SARS-CoV-2 infection among patients taking MS DMTs divided by the incidence rate among the general population. The 95% confidence interval (CI) was estimated using Stata 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.).
In England, mass SARS-CoV-2 vaccination started in December 2020 and COVID-19 restrictions were gradually lifted from March to July 2021. The IRR and 95% CI were calculated for each month during the study period as well as during two waves of the COVID-19 pandemic: (1) three months around the start time of mass SARS-CoV-2 vaccination (November 2020 to January 2021) referred to as pre-vaccination, and (2) three months after the start of mass vaccination (June to August 2021) referred to as post-vaccination.
3. RESULTS
A mean (standard deviation) of 41,208 (4301) MS patients received DMTs in England during each month from March 2020 to August 2021. A total of 3524 patients taking MS DMTs had SARS-CoV-2 infection during this period.
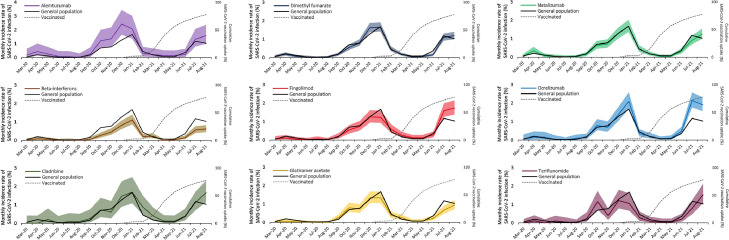
The monthly incidence rate of SARS-CoV-2 infection among patients on MS DMTs and the general population is presented in Fig. 1 and Supplementary Material 1. The IRR (95% CI) of infection for patients on ocrelizumab versus the general population significantly increased from 1.13 (0.97–1.31), pre-vaccination, to 1.79 (1.57–2.03), post-vaccination (Fig. 2 ). For patients on fingolimod, this also significantly increased from 0.87 (0.73–1.02) to 1.40 (1.20–1.63) (Fig. 2). There were no significant changes for patients on other MS DMTs (Supplementary Material 2 and 3).
Fig. 1.
Monthly incidence rate of SARS-CoV-2 infection among multiple sclerosis (MS) patients receiving disease-modifying therapies (DMTs) versus the general population of 20-years-old or above in England. Data for individual DMTs are presented in separate graphs. The coloured line in each graph is the incidence rate of infection during each month per 100 MS patients taking each DMT, and the shaded areas are the 95% confidence intervals. The black line is the incidence rate of infection during each month per 100 people aged 20 years or above in the general population. The dashed gray line is the cumulative SARS-CoV-2 vaccination (both doses) uptake during each month among the adult population in England.
Fig. 2.
Incidence rate ratio of SARS-CoV-2 infection among multiple sclerosis (MS) patients taking ocrelizumab and fingolimod compared to the general population aged 20 years or above in England. Data for ocrelizumab (top) and fingolimod (bottom) are presented in separate graphs. The coloured line in each graph is the incidence rate ratio and the shaded area is the 95% confidence interval. The black line demarcates the incidence rate ratio in the general population which is always one and serves as a reference line. The dashed gray line is the cumulative SARS-CoV-2 vaccination (both doses) uptake during each month among the adult population in England.
4. Discussion
This study presents the incidence of SARS-CoV-2 infection for the entire population of MS patients receiving DMTs in England and compares their risk of infection to the general population before implementation of mass SARS-CoV-2 vaccination and when at least 74% and 56% of the adult population had received their first and second doses of vaccine, respectively (United Kingdom Government, 2021). To our knowledge, this is the first study to report changes in the risk of SARS-CoV-2 infection in relation to mass vaccination in a population under immunomodulatory therapies. Although individual-level data on SARS-CoV-2 vaccination was not available at the time of the study, the MS population were expected to have a similar pattern of vaccination to the general population as they had a high willingness to be vaccinated (Huang et al., 2021), or may have been vaccinated earlier (patients with severe neurological disabilities or those taking alemtuzumab or ocrelizumab) (UK Health Security Agency, 2020). The study used positive SARS-CoV-2 test results which includes both symptomatic and asymptomatic infections.
The findings of this study show a substantial increase in the risk of SARS-CoV-2 infection among patients on ocrelizumab or fingolimod compared to the general population following the liberalization of COVID-19 restrictions and despite mass vaccination. There were no obvious changes in the risk of infection among patients taking other MS DMTs.
Patients on ocrelizumab and rituximab show reduced antibody and memory B-cell responses to SARS-CoV-2 vaccines (Achiron et al., 2021; Apostolidis et al., 2021; Brill et al., 2021; Gadani et al., 2021; Sormani et al., 2021). Nevertheless, they can mount a T-cell response to these vaccines (Apostolidis et al., 2021; Brill et al., 2021; Gadani et al., 2021). It is unknown how this interplay between humoral and cellular immune responses translate into protecting patients on these anti-CD20 B-cell depleting therapies from infection. Fingolimod also seems to prevent the production of antibodies in response to SARS-CoV-2 vaccination (Achiron et al., 2021; Sormani et al., 2021). So far, assumptions about the impact of MS DMTs on the effectiveness of SARS-CoV-2 vaccines are based on experiences with previous vaccinations and these immunological studies rather than population-based studies (Achiron et al., 2021; Apostolidis et al., 2021; Brill et al., 2021; Cabreira et al., 2021; Sormani et al., 2021). Cohort studies to assess the effectiveness of SARS-CoV-2 vaccines in patients taking MS DMTs have been set up, but it will be a while before they are concluded (Sormani et al., 2021). The findings of our study suggest that the humoral immune response to vaccines, which is suppressed by ocrelizumab and fingolimod and preserved by other MS DMTs (Achiron et al., 2021; Apostolidis et al., 2021; Brill et al., 2021; Sormani et al., 2021), may be mainly responsible for the protection provided against SARS-CoV-2 infection.
We also noted that the risk of SARS-CoV-2 infection associated with beta-interferons was lower than the general population, both pre- and post-vaccination, which is not unexpected given their antiviral effects (Dumitrescu et al., 2021).
The effectiveness of SARS-CoV-2 vaccination in preventing symptomatic infections and severe disease among patients taking MS DMT is yet to be determined. The timing of vaccination in relation to administration of some MS DMTs, such as alemtuzumab, cladribine, and ocrelizumab, can affect the development of an immune response to vaccines (Cabreira et al., 2021) which was not applied in the present study because of individual-level data not being available at the time of this study. Also, other potential confounders, such as age, sex, or place of residence, could not be considered in the analysis because of the same reason.
5. Conclusions
These preliminary findings suggest that SARS-CoV-2 vaccines offer minimal protection against infection to patients taking ocrelizumab or fingolimod. Population studies using individual-level data on vaccination (including interval between vaccination and SARS-CoV-2 infection), antibody levels, infections, and disease severity are required to establish the benefits of current vaccination programmes and offering third dose vaccines to patients with drug-induced immunosuppression.
CRediT authorship contribution statement
Afagh Garjani: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing. Sameer Patel: Data curation, Investigation, Methodology, Resources, Validation, Writing – review & editing. Dhiren Bharkhada: Conceptualization, Investigation, Methodology, Project administration, Resources, Validation, Writing – review & editing. Waqar Rashid: Conceptualization, Methodology, Supervision, Writing – review & editing. Alasdair Coles: Conceptualization, Methodology, Supervision, Writing – review & editing. Graham R Law: Formal analysis, Methodology, Visualization, Writing – review & editing. Nikos Evangelou: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interests
Afagh Garjani has received research support from the United Kingdom Multiple Sclerosis Society, speaker honorarium and travel support from the Multiple Sclerosis Academy, and travel support from Novartis and Merck. Sameer Patel declares no competing interests. Dhiren Bharkhada declares no competing interests. Waqar Rashid declares no competing interests. Alasdair Coles declares no competing interests. Graham R Law declares no competing interests. Nikos Evangelou has served as a member of advisory boards for Biogen, Merck, Novartis, and Roche, received grant income from the United Kingdom Multiple Sclerosis Society, Medical Research Council (MRC), Patient-Centered Outcomes Research Institute (PCORI), and National Institute for Health Research (NIHR).
Acknowledgments
Acknowledgments
We would like to thank Dr Trudy Owens and Dr Alain Pitiot for editing advice for the manuscript.
Funding
No funding.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2021.103458.
Appendix. Supplementary materials
References
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., Dolev M., Menascu S., Flechter S., Falb R. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. 17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021:1–12. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill L., Rechtman A., Zveik O., Haham N., Oiknine-Djian E., Wolf D.G., Levin N., Raposo C., Vaknin-Dembinsky A. Humoral and T-Cell Response to SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis Treated With Ocrelizumab. JAMA Neurol. 2021 doi: 10.1001/jamaneurol.2021.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreira V., Abreu P., Soares-dos-Reis R., Guimarães J., Sá M.J. Multiple sclerosis, disease-modifying therapies and covid-19: a systematic review on immune response and vaccination recommendations. Vaccines (Basel) 2021;9(7):773. doi: 10.3390/vaccines9070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu L., Papathanasiou A., Coclitu C., Constantinescu C.S., Popescu B.O., Tanasescu R. Beta interferons as immunotherapy in multiple sclerosis: a new outlook on a classic drug during the COVID-19 pandemic. QJM. 2021 doi: 10.1093/qjmed/hcaa348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani S.P., Reyes-Mantilla M., Jank L., Harris S., Douglas M., Smith M.D., Calabresi P.A., Mowry E.M., Fitzgerald K.C., Bhargava P. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine. 2021;73 doi: 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Rodgers W.J., Middleton R.M., Baheerathan A., Tuite-Dalton K.A., Ford D.V., Nicholas R., Group M.R.R. Willingness to receive a COVID-19 vaccine in people with multiple sclerosis–UK MS Register survey. Mult. Scler. Relat. Disord. 2021;55 doi: 10.1016/j.msard.2021.103175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Health Service England, 2018. National Programmes of Care and Clinical Reference Groups. https://www.england.nhs.uk/commissioning/spec-services/npc-crg/group-d/d04/. (Accessed October 27, 2021.
- Office for National Statistics, 2021. Population estimates for the UK, England and Wales, Scotland and Northern Ireland: mid-2020. https://www.ons.gov.uk/. (Accessed October 27, 2021.
- Pritchard E., Matthews P.C., Stoesser N., Eyre D.W., Gethings O., Vihta K.-.D., Jones J., House T., VanSteenHouse H., Bell I. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat. Med. 2021:1–9. doi: 10.1038/s41591-021-01410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Inglese M., Schiavetti I., Carmisciano L., Laroni A., Lapucci C., Da Rin G., Serrati C., Gandoglia I., Tassinari T. EBioMedicine; 2021. Effect of SARS-CoV-2 mRNA Vaccination in MS Patients Treated With Disease Modifying Therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Multiple Sclerosis International Federation, 2020. Atlas of MS. https://www.atlasofms.org/map/global/epidemiology/number-of-people-with-ms. (Accessed October 27, 2021.
- UK Health Security Agency, 2020. Coronavirus (COVID-19) vaccination information for public health professionals. https://www.gov.uk/government/publications/covid-19-the-green-book-chapter-14a. (Accessed October 27, 2021.
- United Kingdom Government, 2021. Coronavirus (COVID-19) in the UK. https://coronavirus.data.gov.uk/. (Accessed October 27, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.