Abstract
Background: Sphingosine kinase has been identified as playing a central role in the immune cascade, being a common mediator in the cellular response to a variety of signals. The different effects of sphingosine kinase 1 and 2 (SphK1 and SphK2, respectively) activity have not been completely characterized. Aim: To determine the different roles played by SphK1 and SphK2 in the regulation of immune-mediated disorders. Methods: Nine groups of mice were studied. Concanavalin A (ConA) injection was used to induce immune-mediated hepatitis. Mice were treated with SphK1 inhibitor (termed SphK-I) and SphK2 inhibitor (termed ABC294640), prior to ConA injection, and effects of treatment on liver enzymes, subsets of T lymphocytes, and serum levels of cytokines were observed. Results: While liver enzyme elevation was ameliorated by administration of SphK1 inhibitor, SphK2 inhibitor-treated mice did not show this tendency. A marked decrease in expression of CD25+ T-cells and Foxp+ T-cells was observed in mice treated with a high dose of SphK1 inhibitor. Alleviation of liver damage was associated with a statistically significant reduction of serum IFNγ levels in mice treated with SphK1 inhibitor and not in those treated with SphK2 inhibitor. Conclusions: Early administration of SphK1 inhibitor in a murine model of immune-mediated hepatitis alleviated liver damage and inflammation with a statistically significant reduction in IFN-γ levels. The data support a dichotomy in the anti-inflammatory effects of SphK1 and SphK2, and suggests that isoenzyme-directed therapies can improve the effect of targeting these pathways.
Keywords: sphingosine kinase 1, sphingosine kinase 2, immune-mediated hepatitis, sphingolipids
Introduction
Sphingolipids are molecules with lipid chains forming part of the cell lipid membrane and have been identified in recent years as inflammatory regulators in multiple cellular pathways.1-3 Ceramide, a major sphingolipid, is released during cellular metabolism and degrades to Sphingosine. 4 Sphingosine is in turn phosphorylated by the isoenzymes Sphingosine Kinase 1 and 2 (SphK1 and SphK2, respectively) to Sphingosine-1-Phosphate (S-1P).5,6 S-1P has been shown to play a major role in inflammatory diseases and processes.7,8 S-1P levels have been shown to be elevated in systemic lupus erythematosus9,10 and in coronary artery disease. 11 S-1P signaling also plays a role in the pathogenesis of fibrotic diseases of lung, liver, and heart.12,13 In cancer, S-1P is a highly potent molecule, acting mainly via the a cellular receptor of S-1P (S-1PR) to act both directly on cancer cells and in the context of tumor-associated chronic inflammation.8,14,15
In the liver, SphK and S-1P have been associated with both acute and chronic liver inflammation.16,17 Alleviation of lipopolysaccharide/D-galactosamine-induced acute liver failure was achieved by inhibiting mitogen-activated protein kinases pathway. 18 SphK1 inhibition was associated with upregulation of SphK1 expression and with decreased liver enzymes, serum TNF-alpha, and IL-6.17,19
Injection of concanavalin A (ConA) in mice induces a cytokine and T-cell-mediated hepatitis. 20 The S1P receptor agonist KRP-203 alleviated ConA-induced liver damage via lymphocyte sequestering from the liver to secondary lymph nodes while reducing the number of liver CD4 (+) lymphocytes, alleviating liver damage. 21
The different effects of SphK1 and SphK2 activity remain incompletely understood, despite intense research. While the exact mechanism and causality is unclear, there is much data suggesting a unique role for each of these enzymes, which may not be cross-reactive.22,23
In this study, our overall aim was to determine the role of the SphK/S-1P axis in immune-mediated disorders and study the different roles played by SphK1 and SphK2 in immune regulation. We sought to study the potential role of SphK1 and ABC294640, oral inhibitors of SphK1 and SphK2, respectively, in reduction of inflammation when administered to mice in a ConA model of immune-mediated hepatitis. Our data showed inhibition of SphK1, but not of SphK2, and indicated a trend towards reduced hepatic enzyme elevations, lower levels of inflammatory cytokines in peripheral blood, and altered regulatory lymphocyte trafficking.
Methods and materials
Animals
Male C57BL/6 mice (11–12 weeks old), were purchased from Harlan Laboratories and housed in the Hadassah Ein-Kerem Sharett institute SPF animal unit. Animals were housed in the Animal Core of the Hadassah-Hebrew University Medical School on a 12 h light–dark cycles with free access to water and standard chow diet. Animal experiments were performed according to the guidelines and with the approval of the Hebrew University-Hadassah Institutional Committee for Care and Use of Laboratory Animals (MD-17-15333-3).
Experimental groups
Nine groups of mice were studied as shown in Table 1 (n = 5/group; n = 3 in untreated control group). ConA, diluted from commercially available powdered solution in an appropriate stock solution (Merck Inc.) to a concentration of 5 mg/mL was instilled via injection to tail veins in a dose of 100 μl per injection. Table 1 summarizes the experimental and control groups.
Table 1:
Experimental and control groups.
| Group name | ConA injection | Treatment | N |
|---|---|---|---|
| Control | — | — | 3 |
| Negative control | + | — | 5 |
| Positive control | + | Dexamethasone 1.25 mg | 5 |
| Low dose SphK1-I | + | SphK1-I 20 mg/kg | 5 |
| High dose SphK1-I | + | SphK1-I 50 mg/kg | 5 |
| SphK2 | + | ABC 294640 50 mg/kg | 5 |
| SphK1 low dose | — | SphK1-I 20 mg/kg | 5 |
| SphK1 high dose | — | SphK1-I 50 mg/kg | 5 |
| SphK2 group | — | ABC 294640 50 mg/kg | 5 |
SphK1: Sphingosine Kinase 1; SphK2: Sphingosine Kinase 2
Assessment the role of sphingosine axis immune-mediated hepatitis
In order to determine the potential role of SphK1 and SphK2 in immune-mediated hepatitis, the SphK1 inhibitor (SphK1-I, Enzo Biochem, NYC, NY) and SphK2 inhibitor (BPS Biosciences, San Diego, CA, ABC 294640), were tested. SphK inhibitors were orally administered to treated mice by gavage, with doses differing between groups (Table 1). Positive control mice received an intra-peritoneal (IP) injection of dexamethasone dosed at 1.25 mg per mouse. Mice were treated 2 h prior to ConA injection.
Assessment the effect of cohousing on liver damage
14 h after ConA injection, mice were sacrificed according to standard practices in accordance with IACUC standards. Blood samples were drawn prior to or just after sacrifice. Whole spleens were harvested ex vivo.
Liver enzymes
Biochemical analysis of blood serum for measurement of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was determined using a Reflotvet Plus clinical chemistry analyzer (Roche).
Flow cytometry on isolated splenocytes and hepatic lymphocytes
Spleen-derived lymphocytes were isolated as previously described in Refs. 24 and 25. In short, harvested spleens were supplemented with FCS buffer, pooled, and centrifuged. Following lysis of red blood cells, flow cytometry was performed on lymphocytes resuspended in 1 mL of FACS buffer (PBS + 1% BSA + 0.1% sodium azide) using an LSR-II machine and analyzed using FSC Express software. Splenocytes were stained for CD4, CD8, CD25, NK1.1, and Foxp3 epitopes. Cell harvested from all mice in each group were pooled and analyzed together.
Cytokine measurement
Cytokine assessment was performed on serum samples using commercially available ELISA kits (mouse IFN-gamma qunatikine ELISA kit, MIF00; R&D systems) for quantification of Interferon-γ levels.
Statistical analysis
Statistical analysis of quantitative variables was carried out using Kruskal–Wallis and Mann–Whitney tests. The threshold p-value for statistical significance was predetermined at p < 0.05. Statistical analysis was performed using SPSS software.
Results
The role of the sphingosine axis in immune-mediated hepatitis was examined by determining the effects of SphK-1 and SphK-2 inhibition on liver damage.
Effect of SphK inhibition on liver damage:
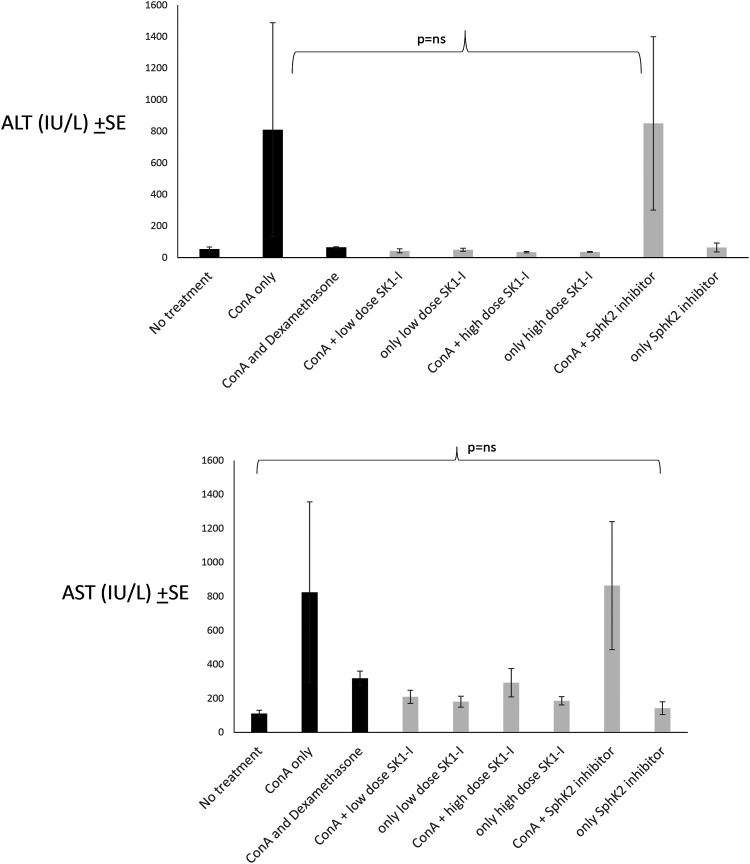
Figure 1(a) A shows the effect of sphingosine inhibition on liver enzyme levels. The mean ALT level ± SE (IU/l) in the ConA only control group was 810 ± 677, and, following administration of low-dose and high-dose SphK1-I, was reduced to 42 ± 12 and 34 ± 3, respectively. In contrast, following administration of SphK2-I, the ALT level was elevated to 850 ± 549 (p=0.09).
Figure 1.
Effect of sphingosine inhibition on liver enzymes: (a) ALT and (b) AST. Serum levels at the end of the study were measured by photometry in all treatment and control groups and are presented in IU/L±SE. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase
Figure 1(b) shows the effect of treatment on AST serum levels. The mean AST level ± SE (IU/l) was 824 ± 532 in the ConA group, and was reduced to 209 ± 38 in the low-dose SphK1-I group, and to 292 ± 83 in the high-dose SphK1-I group. Administration of SphK2 inhibitor did not reduce AST levels, and the tested level was 864 ±376 (p=0.13). While liver enzyme elevation was ameliorated by administration of SphK1 inhibitors in all treatment groups, SphK2 inhibitor-treated mice did not show this tendency. Kruskal–Wallis analysis of ALT and AST levels showed that their reduction following both SphK1 and SphK2 inhibition did not reach statistical significance.
To determine the potential hepatotoxic effect of SphK inhibition, the inhibitors were administered to mice without induction of ConA hepatitis. There was no elevation of ALT in groups treated with SphK inhibitors without induction of ConA hepatitis, as compared to control mice that received no SphK inhibitors.
Effect of SphK inhibition on T lymphocyte subsets
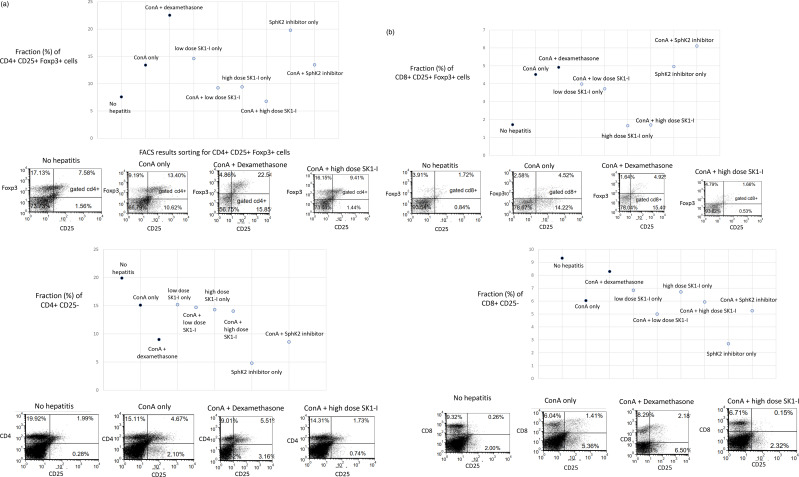
T-cell-mediated immune responses play a critical role in a variety of liver injuries, including autoimmune hepatitis. ConA-induced liver damage is T-cell-mediated. We determined the effect of SphK inhibition on T-cell populations. Figure 2 shows FACS assessment of splenocyte population by interventional group, indicating a marked decrease in expression of T-cells positive for CD25 cell-surface marker and for the Foxp intracellular protein in mice treated with the high-dose SphK1 inhibitor. These differences were maintained across both CD4 and CD8 cell gating.
Figure 2.
Effect of sphingosine inhibition on lymphocyte subsets: FACS analysis was performed on splenocytes harvested from mice in all treatment and control groups at the end of the study. Isolated cells were stained for CD4, CD25 and Foxp3 epitopes and counted using cell sorter. Data are presented in percentages of fractions of cells for the four subsets of lymphocytes. Representative FACS plots are displayed. (a): Fraction (%) of CD4-gated Foxp+ CD25+ Tregs across treatment groups, (b): Fraction (%) of CD8-gated Foxp3+ CD25+ Tregs across treatment groups, (c): Fraction (%) of CD4+ CD25-cells, and (d): Fraction (%) of CD8+ CD25-cells.
Figure 2(a) shows differential fractions of CD4+ CD25+ Foxp3+ regulatory T-cells (Tregs) in the spleen in mice treated with various experimental materials, compared to control mice. Induction of ConA was associated with an increase in CD4+ CD25+ Foxp3+ Tregs from 7.5% in naïve mice to 13.4%. In dexamethasone-treated mice, the level rose further, to 22.5%. Treatment with high-dose SphK1 inhibitor reduced this proportion to 6.7%. The levels in those treated with low-dose SphK1-I and SphK2-I were 14.5% and 19.7%, respectively.
Figure 2(b) shows the effects of treatments on CD8+ CD25+ Foxp3+ Tregs levels in the spleen. The proportion of the CD8 populations showing positive for CD25 and Foxp3 was 1.7% in naïve mice and rose to 4.5% in mice with treated with ConA. Dexamethasone treatment did not alter that fraction, remaining at 4.9%. Treatment with high-dose SphK1-I reduced the level to 1.6%. Treatment with low-dose SphK1-I and/or SphK2 increased the CD8+ CD25+ Foxp3+ Tregs levels to 3.9% and 4.9%, respectively.
Figure 2(c) shows the effects of treatments on the fraction (%) of CD4+ CD25-cells in the spleen. Untreated mice showed a fraction of 20%, lowering to 15% in ConA mice and 5.5% in mice treated with dexamethasone. Mice treated with SphK1 inhibitor after hepatitis showed 15% in the low dose group and 14% in the high dose group. SphK2 inhibitor-treated mice showed a lower fraction of 4.79%.
Figure 2(d) shows the effect of treatment on the fraction (%) of CD8+ CD25-cells in the spleen. The proportion of splenic CD8+ cells was highest in untreated control mice—9.2%. Administration of ConA lowered this fraction to 6%. In the dexamethasone-treated group, the fraction again rose to 8%. Administration of SphK inhibitors to ConA mice showed similar results in the low and high dose SphK1 inhibitor groups (6.8% and 6.7%, respectively). In the SphK2 inhibitor group, 2.7% of cells expressed these surface markers.
The CD3+ NK1.1+ subset of lymphocytes was unchanged across treatment groups. This group accounted for 0.3% of naïve mice, 0.3% of ConA mice, 0.2% of the low-dose SphK1-I group, 0.3% of the high-dose SphK1-I group and 0.9% of the SphK2-I group.
While pooling of splenocytes did not allow for statistical analysis of differences due to minimization of group sizes, a descriptive assessment of splenocyte populations showed a return of these populations to pre-treatment levels, an association not seen under dexamethasone and SphK2 inhibitor treatments, and seen modestly under low-dose SphK1 inhibitor treatment (20 mg/kg).
Effect of SphK inhibition on interferon levels:
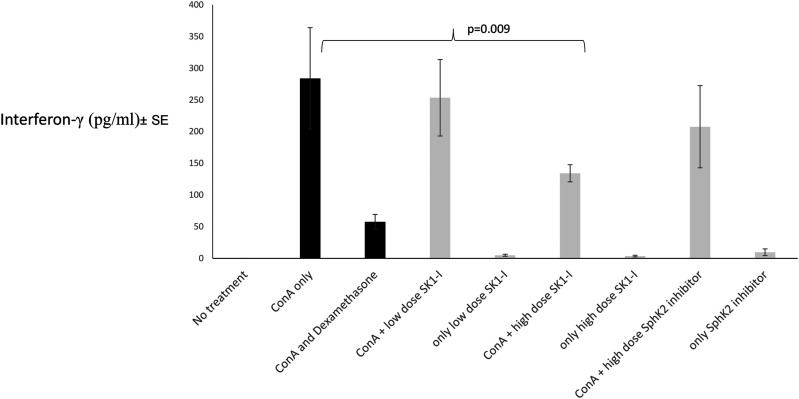
ConA hepatitis liver damage is cytokine-mediated. Figure 3 shows the effect of SphK inhibition on IFN-γ serum levels. IFN-γ levels were unmeasurable in naïve mice in which hepatitis was not induced. Following induction of ConA hepatitis, IFN-γ serum levels increased to a mean of 283.9 ± 178 pg/mL. In treated groups serum levels were reduced to 57 ± 25 in dexamethasone-treated mice, 253 ± 134 in mice treated with low-dose SphK1-I, 134 ± 30 in mice treated with high-dose SphK1-I, and 207 ± 144 in mice treated with SphK2-I. Mann–Whitney analysis of mice treated with high-dose SPphk1-I versus untreated ConA hepatitis mice revealed a statistically significant reduction of IFN-γ (p=0.008 by one-sided Mann–Whitney test).
Figure 3.
Effect on sphingosine inhibition on IFNγ serum levels: Serum IFNγ levels were measured by ELISA in all treatment and control groups at the end of the study. Data are presented in pg/ml ±SE IFN-γ: Interferon
The data of all studies is included in the supplementary file. Data of flow cytometry experiments is included in Supplementary files A, B, and C.
Discussion
The results of the present study show that early administration of SphK1 inhibitors in a murine model of immune-mediated hepatitis ameliorated liver damage, reduced IFNγ levels, and altered the distribution of subsets of lymphocytes in the spleen. A dichotomy in the anti-inflammatory effects between the SphK1 and SphK2 inhibition pathways was documented. Inhibiting the SphK1 and not the SphK2 pathways decreased hepatocyte death, as measured by the levels of the enzymes AST and ALT in the serum. Similarly, SphK1 inhibition was associated with a statistically significant reduction in IFN-γ levels, while SphK2 inhibition was not. FACS analysis of splenocytes showed alteration of the distribution of lymphocyte subsets following inhibition of SphK1. CD25+ Foxp3+ Tregs were depleted in the spleen in mice treated with high-dose SphK1 inhibitor. A much smaller effect was observed in mice treated with SphK2 inhibitor.
A recent study has shown the role of SphK1 in hepatocyte injury showing that deletion of SphK1, but not SphK2, attenuated GalN/LPS-induced liver damage, hepatic apoptosis, serum alanine aminotransferase levels, and mortality rate. 26 Knockout of SphK1 in mouse model significantly alleviated damage after hepatic I/R injury via inhibiting inflammation and oxidative stress. 27 The present study confirms a role for Sphk1 inhibition and not Sphk2 inhibition in preventing liver injury and that inhibition of Sphk1 has a role on preventing liver injury.
The immune system is influenced by bioactive sphingolipids.1,28 Sphingolipids have been shown to influence both innate and adaptive immune cells.13,29,30 They are involved in the inflammatory capacity of cells and immune cell trafficking.31,32 Sphingolipid manipulation, involving inhibitors of sphingosine kinase, has been shown to have potential to reduce inflammation in many studies.33,34 Administration of glycosphingolipids (i.e., sphingolipids attached to a sugar group) has been shown to play a positive anti-inflammatory role in immune-mediated disorders both in animal models and humans, possibly mediated via their effect on lipids rafts and natural killer T-cells.35-37
Sphingosine kinase has been suggested to play a central role in the immune cascade, being a common mediator in the cellular response to variety of signals. 31 With activity of SphK tied strongly to generation of ceramide and S-1P, it has been observed to act in several mechanisms. Cellular G-protein-coupled receptors for sphingosine phosphate, of which several exist, termed S-1PRs, mediate some of the activity of S-1P as an inflammatory mediator.38-40 The most well-known of the drugs targeting these receptors is Fingolimod, approved for use in multiple sclerosis, which exerts antagonistic effects on S-1PR1, beneficially influencing lymphocyte trafficking via this crucial receptor. 41 Its effects have also been observed to be mediated directly through modulation of NLRP3 inflammasome and of basic cellular processes including oxidative phosphorylation in a manner not requiring an activity of S-1PR.42,43
The dichotomy in the effect of sphingolipids on immune pathways has been proposed to be associated with environmental factors, or with subsets of target immune cells which they affect.28,36,44,45 The present data support a notion that targeting isoenzymes can serve as a method for potentiating the effect of immunomodulatory agents. While much studied, differences between physiological and pathological actions of SphK1 and SphK2 have not been completely characterized. Some studies suggest that SphK1 inhibition more robustly influences inflammation, and that SphK2 may play the role of an auxiliary enzyme.46,47 Other studies suggest SphK2 may play a counter-regulatory function to SPHK1, driving apoptosis where SphK1 drives proliferation and survival. 48 The data from the present study do not support a role for inhibition of SphK2 in attenuating inflammation or in changing immune cell signatures in a model of immune-mediated liver injury.
Our study suggests a role for altered Tregs trafficking in attenuation of inflammation in ConA-induced hepatitis. Tregs have been known to promote tolerance in the context of experimental ConA hepatitis.49,50 Localization to inflamed tissue has been observed in Tregs and is thought to promote their regulatory function in inflammation.51-56 While the specific importance of SphK1 in Tregs has not been extensively studied, our results showing attenuated inflammation concomitant with reduced splenic Tregs may suggest that SphK1 inhibition during acute inflammation promotes Tregs recruitment from blood and splenic pools to the liver, reducing tissue inflammation. Future experiments may test this hypothesis by performing immunohistochemical stains on harvested liver samples. If these stains reveal an increased Tregs population in the liver, this will point towards enhanced Tregs localization as a driving force behind reduced inflammation. Of note, administration of SphK2 inhibitor, but not of SphK1 inhibitor, lowered fractions of CD4+ CD25-and CD8+ CD25-cells in spleen compared to their levels in ConA only mice.
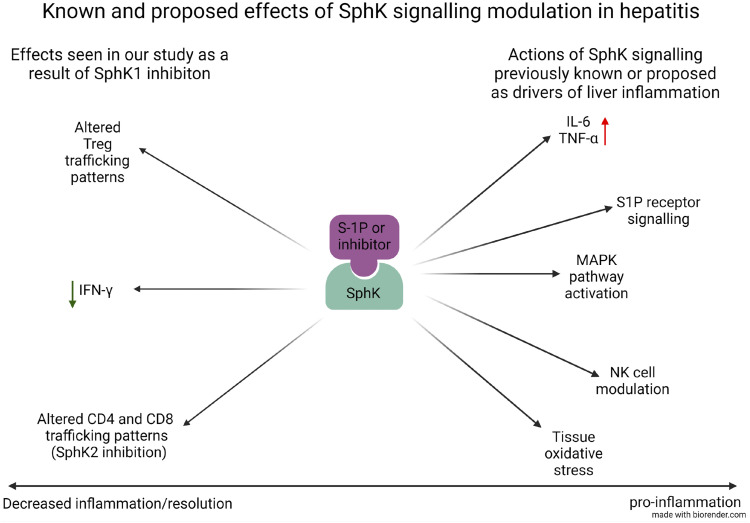
Figure 4 shows a potential mechanism for the dichotomy of the two inhibitors.
Figure 4.
The potential mechanism for the effect of SK inhibitors in hepatitis.
Pre-clinical and clinical studies suggested that targeting sphingolipids via the oral route can exert an effect on the systemic immune system.28,37,44,57-60 Whether the effect is exerted by absorption or via an effect on the gut immune system cannot be determined based on the current data. Future studies will determine whether adequate levels are reached in blood and which cells in the intestine are affected by oral administration.
Study limitations
While several metrics in the present study did not reach statistical significance, likely due to small sample sizes and the preliminary nature of the study, the noted trends support further studies in additional models for better understanding of the SphK1/SphK2 dichotomy. Future studies will focus on measuring of SphK activities in target cells and on the cellular effect in mRNA and protein levels in immune cells. The effect of SphK pathway on recruitment and activation of NKT cells and of conventional T-cells, and on additional pro-inflammatory cytokines, which contribute to development and progression of Con A-induced damage is yet to be determined along with histology and Tunnel assays on liver tissues.
Conclusions
Sphingosine Kinase-dependent pathways play a major role in the pathogenesis of inflammatory pathways. The data from the present study suggest different roles for SphK1 and SphK2 in these pathways. The data support a potent role for SphK1 inhibition in immune-mediated hepatitis model, suggesting that targeting these pathways requires differentiation between SphK1 and SphK2 inhibitory pathways.
ORCID iD
Yaron Ilan https://orcid.org/0000-0003-0802-1220
References
- 1.Ilan Y. (2016) Compounds of the sphingomyelin-ceramide-glycosphingolipid pathways as secondary messenger molecules: new targets for novel therapies for fatty liver disease and insulin resistance. American Journal of Physiology-Gastrointestinal and Liver Physiology 310: G1102–G1117. [DOI] [PubMed] [Google Scholar]
- 2.Hannun YA, Obeid LM. (2018) Sphingolipids and their metabolism in physiology and disease. Nature Reviews Molecular Cell Biology 19: 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinville BM, Deschenes NM, Ryckman AE, et al. (2021) A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis. International journal of molecular sciences 22: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ventura AE, Mestre B, Silva LC. (2019) Ceramide Domains in Health and Disease: A Biophysical Perspective. Bioactive Ceramides in Health and Disease 1159: 79–108. [DOI] [PubMed] [Google Scholar]
- 5.Sukocheva OA, Furuya H, Ng ML, et al. (2020) Sphingosine kinase and sphingosine-1-phosphate receptor signaling pathway in inflammatory gastrointestinal disease and cancers: A novel therapeutic target. Pharmacology and Therapeutics 207: 107464. [DOI] [PubMed] [Google Scholar]
- 6.Zheng X, Li W, Ren L, et al. (2019) The sphingosine kinase-1/sphingosine-1-phosphate axis in cancer: Potential target for anticancer therapy. Pharmacology and Therapeutics 195: 85–99. [DOI] [PubMed] [Google Scholar]
- 7.Snider AJ. (2013) Sphingosine kinase and sphingosine-1-phosphate: regulators in autoimmune and inflammatory disease. International journal of clinical rheumatology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagahashi M, Abe M, Sakimura K, et al. (2018) The role of sphingosine‐1‐phosphate in inflammation and cancer progression. Cancer Science 109: 3671–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson L, Tullus K, Marks SD, et al. (2012) Increased serum concentration of sphingosine-1-phosphate in juvenile-onset systemic lupus erythematosus. Journal of Clinical Immunology 32: 1019–1025. [DOI] [PubMed] [Google Scholar]
- 10.Mike EV, Makinde HM, Der E, et al. (2018) Neuropsychiatric Systemic Lupus Erythematosus Is Dependent on Sphingosine-1-Phosphate Signaling. Frontiers in Immunology 9: 2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mihanfar A, Nejabati HR, Fattahi A, et al. (2019) The role of sphingosine 1 phosphate in coronary artery disease and ischemia reperfusion injury. Journal of Cellular Physiology 234: 2083–2094. [DOI] [PubMed] [Google Scholar]
- 12.Wang E, He X, Zeng M. (2019) The Role of S1P and the Related Signaling Pathway in the Development of Tissue Fibrosis. Frontiers in pharmacology 9: 1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishay Y, Nachman D, Khoury T, et al. (2020) The role of the sphingolipid pathway in liver fibrosis: an emerging new potential target for novel therapies. American Journal of Physiology-Cell Physiology 318: C1055–C1064. [DOI] [PubMed] [Google Scholar]
- 14.Nagahashi M, Yamada A, Katsuta E, et al. (2018) Targeting the SphK1/S1P/S1PR1 Axis That Links Obesity, Chronic Inflammation, and Breast Cancer Metastasis. Cancer Research 78: 1713–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang P, Yuan Y, Lin W, et al. (2019) Roles of sphingosine-1-phosphate signaling in cancer. Cancer Cell International 19: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleuser B. (2018) Divergent Role of Sphingosine 1-Phosphate in Liver Health and Disease. International journal of molecular sciences 19: 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohrbach T, Maceyka M, Spiegel S. (2017) Sphingosine kinase and sphingosine-1-phosphate in liver pathobiology. Critical reviews in biochemistry and molecular biology 52: 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian T, Tian W, Yang F, et al. (2016) Sphingosine kinase 1 inhibition improves lipopolysaccharide/D‐galactosamine‐induced acute liver failure by inhibiting mitogen‐activated protein kinases pathway. United European Gastroenterology Journal 4: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei Y-C, Yang LL, Li W, et al. (2015) Inhibition of sphingosine kinase 1 ameliorates acute liver failure by reducing high-mobility group box 1 cytoplasmic translocation in liver cells. World Journal of Gastroenterology 21: 13055–13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H-X, Liu M, Weng SY, et al. (2012) Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World Journal of Gastroenterology 18: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneko T, Murakami T, Kawana H, et al. (2006) Sphingosine-1-phosphate receptor agonists suppress concanavalin A-induced hepatic injury in mice. Biochemical and Biophysical Research Communications 345: 85–92. [DOI] [PubMed] [Google Scholar]
- 22.Yuza K, Nakajima M, Nagahashi M, et al. (2018) Different Roles of Sphingosine Kinase 1 and 2 in Pancreatic Cancer Progression. Journal of Surgical Research 232: 186–194. [DOI] [PubMed] [Google Scholar]
- 23.Tonelli F, Alossaimi M, Natarajan V, et al. (2013) The roles of sphingosine kinase 1 and 2 in regulating the metabolome and survival of prostate cancer cells. Biomolecules 3: 316–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trop S, Samsonov D, Gotsman I, et al. (1999) Liver-associated lymphocytes expressing NK1.1 are essential for oral immune tolerance induction in a murine model. Hepatology 29: 746–755. [DOI] [PubMed] [Google Scholar]
- 25.Falcone M, Facciotti F, Ghidoli N, et al. (2004) Up-regulation of CD1d expression restores the immunoregulatory function of NKT cells and prevents autoimmune diabetes in nonobese diabetic mice. The Journal of Immunology 172: 5908–5916. [DOI] [PubMed] [Google Scholar]
- 26.Avni D, Harikumar KB, Sanyal AJ, et al. (2021) Deletion or inhibition of SphK1 mitigates fulminant hepatic failure by suppressing TNFα-dependent inflammation and apoptosis. FASEB journal : Official Publication of the Federation of American Societies for Experimental Biology 35: e21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiang G-H, Wang Z-X, Ji A-L, et al. (2019) Sphingosine kinase 1 knockout alleviates hepatic ischemia/reperfusion injury by attenuating inflammation and oxidative stress in mice. Hepatobiliary & Pancreatic Diseases International 18: 255–265. [DOI] [PubMed] [Google Scholar]
- 28.Ilan Y. (2019) β-Glycosphingolipids as Mediators of Both Inflammation and Immune Tolerance: A Manifestation of Randomness in Biological Systems. Frontiers in Immunology 10: 1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Espaillat MP, Kew RR, Obeid LM. (2017) Sphingolipids in neutrophil function and inflammatory responses: mechanisms and implications for intestinal immunity and inflammation in ulcerative colitis. Advances in biological regulation 63: 140–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollmann C, Wiese T, Dennstädt F, et al. (2019) Translational Approaches Targeting Ceramide Generation From Sphingomyelin in T Cells to Modulate Immunity in Humans. Frontiers in Immunology 10: 2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maceyka M, Spiegel S. (2014) Sphingolipid metabolites in inflammatory disease. Nature 510: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiper IV, East JE, Subrahmanyam PB, et al. (2016) Sphingosine 1-phosphate signaling impacts lymphocyte migration, inflammation and infection. Pathogens and disease 74: ftw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzpatrick LR, Green C, Frauenhoffer EE, et al. (2011) Attenuation of arthritis in rodents by a novel orally-available inhibitor of sphingosine kinase. Inflammopharmacology 19: 75–87. [DOI] [PubMed] [Google Scholar]
- 34.Nishiuma T, Nishimura Y, Okada T, et al. (2008) Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. American Journal of Physiology-Lung Cellular and Molecular Physiology 294: L1085–L1093. [DOI] [PubMed] [Google Scholar]
- 35.Varela ARP, Couto AS, Fedorov A, et al. (2016) Glucosylceramide Reorganizes Cholesterol-Containing Domains in a Fluid Phospholipid Membrane. Biophysical journal 110: 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zigmond E, Preston S, Pappo O, et al. (2007) Beta-glucosylceramide: a novel method for enhancement of natural killer T lymphoycte plasticity in murine models of immune-mediated disorders. Gut 56: 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalazar G, Zigmond E, Weksler-Zangen S, et al. (2017) Oral Administration of β-Glucosylceramide for the Treatment of Insulin Resistance and Nonalcoholic Steatohepatitis: Results of a Double-Blind, Placebo-Controlled Trial. Journal of Medicinal Food 20: 458–464. [DOI] [PubMed] [Google Scholar]
- 38.Rivera J, Proia RL, Olivera A. (2008) The alliance of sphingosine-1-phosphate and its receptors in immunity. Nature Reviews Immunology 8: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sassoli C, Pierucci F, Zecchi-Orlandini S, et al. (2019) Sphingosine 1-Phosphate (S1P)/ S1P Receptor Signaling and Mechanotransduction: Implications for Intrinsic Tissue Repair/Regeneration. International journal of molecular sciences 20: 5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gatfield J, Monnier L, Studer R, et al. (2014) Sphingosine-1-phosphate (S1P) displays sustained S1P1 receptor agonism and signaling through S1P lyase-dependent receptor recycling. Cellular Signalling 26: 1576–1588. [DOI] [PubMed] [Google Scholar]
- 41.Huwiler A, Zangemeister-Wittke U. (2018) The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: Recent findings and new perspectives. Pharmacology and Therapeutics 185: 34–49. [DOI] [PubMed] [Google Scholar]
- 42.Zhong M, Wu W, Wang Y, et al. (2020) Inhibition of Sphingosine Kinase 1 Attenuates Sepsis-induced Microvascular Leakage via Inhibiting Macrophage NLRP3 Inflammasome Activation in Mice. Anesthesiology 132: 1503–1515. [DOI] [PubMed] [Google Scholar]
- 43.Pyne S, Edwards J, Ohotski J, et al. (2012) Sphingosine 1-phosphate receptors and sphingosine kinase 1: novel biomarkers for clinical prognosis in breast, prostate, and hematological cancers. Frontiers in oncology 2: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ilan Y. (2019) Immune rebalancing by oral immunotherapy: A novel method for getting the immune system back on track. Journal of Leukocyte Biology 105: 463–472. [DOI] [PubMed] [Google Scholar]
- 45.Ilan Y, Ohana M, Pappo O, et al. (2007) Alleviation of acute and chronic graft-versus-host disease in a murine model is associated with glucocerebroside-enhanced natural killer T lymphocyte plasticity. Transplantation 83: 458–467. [DOI] [PubMed] [Google Scholar]
- 46.Snider AJ, Ruiz P, Obeid LM, et al. (2013) Inhibition of Sphingosine Kinase-2 in a Murine Model of Lupus Nephritis. PLOS ONE 8: e53521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohammed S, Vineetha NS, James S, et al. (2019) Examination of the role of sphingosine kinase 2 in a murine model of systemic lupus erythematosus. The FASEB Journal 33: 7061–7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neubauer HA, Pitson SM. (2013) Roles, regulation and inhibitors of sphingosine kinase 2. FEBS Journal 280: 5317–5336. [DOI] [PubMed] [Google Scholar]
- 49.Erhardt A, Biburger M, Papadopoulos T, et al. (2007) IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology 45: 475–485. [DOI] [PubMed] [Google Scholar]
- 50.Wei H-X, Chuang Y-H, Li B, et al. (2008) CD4+CD25+foxp3+regulatory T cells protect against T cell-mediated fulminant hepatitis in a TGF-β-dependent manner in mice. The Journal of Immunology 181: 7221–7229. [DOI] [PubMed] [Google Scholar]
- 51.Campbell DJ. (2015) Control of regulatory T cell migration, function, and homeostasis. Journal of immunology (Baltimore, Md.: 1950) 195: 2507–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khoury T, Ben Ya’acov A, Shabat Y, et al. (2015) Altered distribution of regulatory lymphocytes by oral administration of soy-extracts exerts a hepatoprotective effect alleviating immune mediated liver injury, non-alcoholic steatohepatitis and insulin resistance. World Journal of Gastroenterology 21: 7443–7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shabat Y, Lichtenstein Y, Zolotarov L, et al. (2013) Hepatoprotective effect of DT56a is associated with changes in natural killer T cells and regulatory T cells. Journal of Digestive Diseases 14: 84–92. [DOI] [PubMed] [Google Scholar]
- 54.Shuvy M, Hershcovici T, Lull-Noguera C, et al. (2008) Intrahepatic CD8+ lymphocyte trapping during tolerance induction using mushroom derived formulations: A possible role for liver in tolerance induction. World Journal of Gastroenterology 14: 3872–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Livovsky DM, Lalazar G, Ya'acov AB, et al. (2008) Administration of β-glycolipids overcomes an unfavorable nutritional dependent host milieu: a role for a soy-free diet and natural ligands in intrahepatic CD8+ lymphocyte trapping and NKT cell redistribution. International Immunopharmacology 8: 1298–1305. [DOI] [PubMed] [Google Scholar]
- 56.Lalazar G, Ben Ya’acov A, Lador A, et al. (2008) Modulation of intracellular machinery by β-glycolipids is associated with alteration of NKT lipid rafts and amelioration of concanavalin-induced hepatitis. Molecular Immunology 45: 3517–3525. [DOI] [PubMed] [Google Scholar]
- 57.Ilan Y. (2016) Oral immune therapy: targeting the systemic immune system via the gut immune system for the treatment of inflammatory bowel disease. Clinical and Translational Immunology 5: e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adar T, Ilan Y. (2008) β-Glycosphingolipids as Immune Modulators. Journal of Immunotoxicology 5: 209–220. [DOI] [PubMed] [Google Scholar]
- 59.Lalazar G, Ben Ya’acov A, Eliakim-Raz N, et al. (2008) β-Glycosphingolipids-mediated lipid raft alteration is associated with redistribution of NKT cells and increased intrahepatic CD8+ T lymphocyte trapping. Journal of Lipid Research 49: 1884–1893. [DOI] [PubMed] [Google Scholar]
- 60.Zigmond E, Zangen SW, Pappo O, et al. (2009) β-Glycosphingolipids improve glucose intolerance and hepatic steatosis of the Cohen diabetic rat. American Journal of Physiology-Endocrinology and Metabolism 296: E72–E78. [DOI] [PubMed] [Google Scholar]