Abstract
Objective
The objective was to compare the efficacy of azithromycin and clarithromycin in combination with beta-lactams to treat community-acquired pneumonia among hospitalized adults.
Methods
Five databases (PubMed, Google Scholar, Trip, Medline, and Clinical Key) were searched to identify randomized clinical trials with patients exposed to azithromycin or clarithromycin in combination with a beta-lactam. All articles were critically reviewed for inclusion in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
Seven clinical trials were included. The treatment success rate for azithromycin–beta-lactam after 10 to 14 days was 87.55% and that for clarithromycin–beta-lactam after 5 to 7 days of therapy was 75.42%. Streptococcus pneumoniae was commonly found in macrolide groups, with 130 and 80 isolates in the clarithromycin-based and azithromycin-based groups, respectively. The length of hospital stay was an average of 8.45 days for patients receiving a beta-lactam–azithromycin combination and 7.25 days with a beta-lactam–clarithromycin combination.
Conclusion
Macrolide inter-class differences were noted, with a higher clinical success rate for azithromycin-based combinations. However, a shorter length of hospital stay was achieved with a clarithromycin–beta-lactam regimen. Thus, a macrolide combined with a beta-lactam should be chosen using susceptibility data from the treating facility.
Keywords: Community-acquired pneumonia, azithromycin, respiratory tract infection, meta-analysis, macrolide, clarithromycin, beta-lactam, Streptococcus pneumoniae, susceptibility data
Introduction
Community-acquired pneumonia (CAP) is a common lower respiratory tract infection that is associated with high morbidity and mortality. 1 The World Health Organization has indicated that CAP is responsible for almost 3 million deaths annually. 2 Higher mortality rates occur in hospitalized patients (almost 6%–20%) depending on the disease severity and the treatment settings. 3 The high rate of antimicrobial resistance by Streptococcus pneumoniae has increased the rate of hospitalization due to CAP and made treating this infection increasingly complex.4,5 In 2019, the Infectious Disease Society of America (IDSA) recommended the use of combination therapy for hospitalized patients with CAP. 6 The standard treatment regimens that were recommended for nonsevere inpatient CAP are a beta-lactam with a macrolide or fluoroquinolone monotherapy, and treatment regimens recommended for the severe inpatient CAP are a combination of beta-lactam/macrolide or beta-lactam/fluoroquinolone. Owing to the emerging resistance pattern of CAP pathogens, combination regimens are superior to monotherapy particularly in patients with severe CAP, and this was supported by several studies. 7 Because it is not clear which combination therapy is the most effective, some studies have examined the efficacy of beta-lactam and a macrolide 8 and many have studied the outcomes of beta-lactam and a macrolide versus fluoroquinolone. 9 However, only a few studies have investigated the clinical outcomes of a specific macrolide (azithromycin or clarithromycin) in combination with a beta-lactam. 10 Macrolides have been shown to be effective against bacteria that cause lower respiratory tract infections. 11 Bacteriostatic antimicrobials work by reversibly binding to the P site on the 50S subunit of bacterial ribosomes, but at a higher concentration, they have bactericidal properties. 12 A clinical trial was performed in patients with severe CAP class IV using the Pneumonia Severity Index (PSI), and this trial showed that monotherapy with a beta-lactam was inferior to combination therapy using a beta-lactam with macrolides and that patients who received monotherapy had delayed clinical stability compared with the combination therapy. 13 Another study in a cohort setting was performed retrospectively using CAP patients with PSI class V, and this study showed better outcomes with a beta-lactam and macrolide combination compared with fluoroquinolone monotherapy. 14 Several studies with an observational design suggested that initial therapy with a macrolide combined with a beta-lactam may reduce mortality rates and reduce the number of hospitalization days.15–17
Despite these previous studies, there are limited data on the macrolides inter-class differences when treating patients with CAP that might influence the choice of macrolide when availability is not a concern. The objective of this article is to systematically review the literature on the comparative efficacy of azithromycin and clarithromycin in combination with beta-lactams to treat patients with CAP among hospitalized adults and to evaluate the outcomes that are used to determine drug efficacy to provide clinical recommendations.
Methods
Eligibility criteria
To determine the eligibility criteria of this review, we developed a strategy using Population, Intervention, Comparison, Context, Outcome, Study Design (PIC2OS) and determined the inclusion and exclusion criteria, which are listed in Table 1. Additionally, we included studies that were published in English only and that were published from 2000 to 2020. This was a systematic review, so ethics approval and patient consent were not required.
Table 1.
Inclusion and exclusion criteria.
| Eligibility criteria (PIC2OS) | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Patients diagnosed with community-acquired pneumonia | Patients diagnosed with ventilator-associated or hospital-acquired pneumonia |
| Intervention | Azithromycin or clarithromycin in combination with a beta-lactam | |
| Comparison | Compared with control group or other comparator | |
| Context | Hospitalized patients | Patients treated on an outpatient basis |
| Outcomes | Primary: Clinical success rate at the end of therapy Secondary: Length of hospital stay, mortality, morbidity, and days of clinical stability | |
| Study Design | RCT only | Systematic reviews, meta-analyses, single case studies, cross-sectional studies, qualitative studies, case reports, audits of guidelines, conference abstracts and letters to editors. |
RCT, randomized controlled trial; PIC2OS, Population, Intervention, Comparison, Context, Outcome, Study Design.
Information source and search strategy
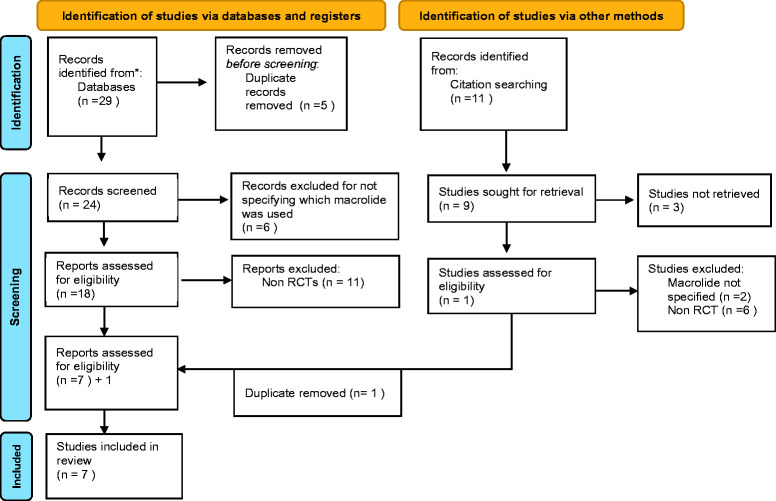
For the primary search, the reviewers (JAS and RKM) independently performed a comprehensive search of five databases (PubMed, Google Scholar, Trip, Medline, and Clinical Key) to identify the relevant studies. Only the full text studies were included. A manual search of the references cited by the relevant articles was performed as a secondary search strategy, as shown in Figure 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart for the identified articles.
Search
A highly sensitive search strategy was used, which included the following words and medical subject heading (MeSH) terms: “azithromycin”, “clarithromycin”, community-acquired pneumonia”, “macrolides”, and “beta-lactam” while searching the selected data bases. Specified beta-lactams were also used to retrieve more studies, these include: “cephalosporin”, “ceftriaxone”, “cefepime”, “cefuroxime”, “penicillin”, “amoxicillin”, and “Amoxiclav”.
Study selection
Articles that were identified using the titles and abstracts (n = 40) were then screened for the eligibility against the inclusion criteria checklist by both reviewers. At the end of the screening process, only full text articles were included (n = 7), and any disagreement in the selection by any of the authors was resolved by discussion. The detailed PRISMA diagram is provided in Figure 1.
Data collection process
All the data from the selected articles were collected using a data extraction tool that was designed in accordance with the objective of this review. These data are illustrated in Table 2.
Table 2.
Characteristics of the included studies.
| Study | Study design | PSI Score | Arm 1 with Dose (n) | Arm 2 with Dose (n) | Culture type | Main outcome |
|---|---|---|---|---|---|---|
| Lin et al., 2007 19 | Randomized open-label (single center) | Not mentioned | Amoxiclav + clarithromycin 500 mg/100 mg IV Q8h (n = 24) | Levofloxacin 500 mg IV Q24h (n = 26) | Sputum | Clarithromycin + BL regimen was as clinically effective as levofloxacin. |
| Garin et al., 2014 13 | Open-label, noninferiority, randomized trial (multicenter) | III, IV | Cefuroxime 1.5 g IV Q8h or Amoxiclav 1.2 g IV Q6h + Clarithromycin 500 mg IV/PO Q12h (n = 289) | Cefuroxime 1.5 g IV Q8h or Amoxiclav 1.2 g IV Q6h (n = 291) | Blood, sputum, and pleural fluid | Beta-lactam monotherapy was not inferior to the combined therapy. Patients infected with atypical pathogens or with PSI category IV pneumonia had delayed clinical stability with monotherapy. |
| Dean et al., 2006 20 | Randomized, prospective, open-label (multicenter) | I, II, III | Clarithromycin 400 mg IV/PO Q12h + Ceftriaxone 1 g IV Q24h (n = not specified) | Gatifloxacin 400 mg IV/PO Q24h (n = not specified) | Blood and sputum | Clarithromycin-based regimen was similar to gatifloxacin in clinical outcomes. |
| Tamm et al., 2007 23 | Prospective, randomized, open-label (multicenter) | III, IV, V | Ceftriaxone 1–2 g/day IV + azithromycin 500 mg Q24h (n = 135) | Ceftriaxone 1–2 g/day IV + clarithromycin 500 mg Q12h IV (n = 143) | Blood | Ceftriaxone plus azithromycin may be a better treatment option in terms of reducing the duration of therapy and LOS because it is as effective as clarithromycin based regimen. |
| Zervos et al., 2004 21 | Randomized, open-label (multicenter) | III, IV, V | Ceftriaxone 1 g Q24h IV + azithromycin 500 mg Q24h IV (n = 110) | Levofloxacin 500 mg IV Q24h (n = 102) | Blood and sputum | The combination of a third-generation cephalosporin and a macrolide is at least as efficacious as monotherapy with a fluoroquinolone with enhanced anti-pneumococcal activity for hospitalized patients with moderate to severe CAP. |
| Frank et al., 2002 22 | Open-label, randomized trial (multicenter) | Not mentioned | Ceftriaxone 1 g IV Q24h + azithromycin 500 mg IV Q24h (n = 121) | Levofloxacin 500 mg IV Q24h (n = 115) | Blood and sputum | Levofloxacin monotherapy was at least as effective as a combination regimen of azithromycin and ceftriaxone in providing coverage against the current causative pathogens in CAP. |
| Rubio et al., 2008 24 | Open-label, non-comparative (multicenter) | III and IV | Ceftriaxone 1 g/day IV + azithromycin 500 mg Q24h (n = 86) | Same patients switched to oral azithromycin only 500 mg (n = 86) | Blood and sputum | Combination treatment was effective and well tolerated in hospitalized patients with CAP. |
PSI, Pneumonia Severity Index; IV, intravenous; PO, orally; CAP, community-acquired pneumonia; Q6h, every 6 hours; Q8h, every 8 hours; Q12h, every 12 hours; Q24h, every 24 hours; BL, beta-lactam; LOS, length of stay.
Data items
The following data were extracted: article characteristics (author, year); study design; study sites (single or multiple); intervention arm; comparator arm; sample size in each arm; dosage regimens used; identified organisms; primary outcome; secondary outcome; and PSI scores.
Quality assessment and risk of bias
The Scottish Intercollegiate Guidelines Network (SIGN) tool was used to evaluate the quality of the included studies. The tool consists of a checklist with two sections to assess in the internal validity and provide an overall assessment of the included studies as high, medium, or low quality.
Data extraction was performed using Microsoft Excel (Microsoft Corp., Redmond, WA, USA) where the primary outcome from all the studies was the clinical success rate, which is defined as the time (in days) to reach the following criteria: 1) systolic blood pressure above 90 mmHg; 2) heart rate <100 beats per minute; 3) respiratory rate <22 breaths per minute; 4) temperature <38.3°C; and/or 5) oxygen saturation >90%. 18 Data on the causative organism and length of hospital stay were also collected.
Results
Study selection
Forty studies were identified from the primary and secondary search. Among them, six studies were duplicates and 17 studies had a design other than a randomized controlled trial (RCT). Finally, seven full text RCT studies were included.13,19–24 Figure 1 summarizes the study selection process.
Study characteristics
All of the included studies were conducted between 2002 and 2014. All of the studies were also multicenter trials except for Lin et al. 19 Three of the studies were from the USA,20–22 and one each was from China, 19 Switzerland, 13 the Netherlands, 23 and Brazil. 24 The intervention arm in each study has a combination of a macrolide and a beta-lactam, while the comparator group was either a fluoroquinolone (n = 4) or another macrolide (n = 2), except if the study comparator was a control group. The length of hospital stay was reported in five studies,19–24 and the clinical success rate was reported in all of the included studies.13,19–24
Beta-lactam selection and use in combination therapy
In this systematic review, all of the seven included studies were published between 2002 and 2014, as shown in Table 2. Patients who were taking azithromycin received a dose of 500 mg per day. Those who received clarithromycin were administered a dose between 300 mg and 1000 mg per day. Similar doses of clarithromycin were administered using the oral route compared with the intravenous (IV) route, where the oral route was equivalent to the IV route for patients who were being treated for CAP. 25 Azithromycin was administered IV while clarithromycin was administered either orally or IV. The macrolide combination included either with a penicillin–lactamase inhibitor or a cephalosporin-based beta-lactam, and ceftriaxone was commonly used (Figure 2).
Figure 2.
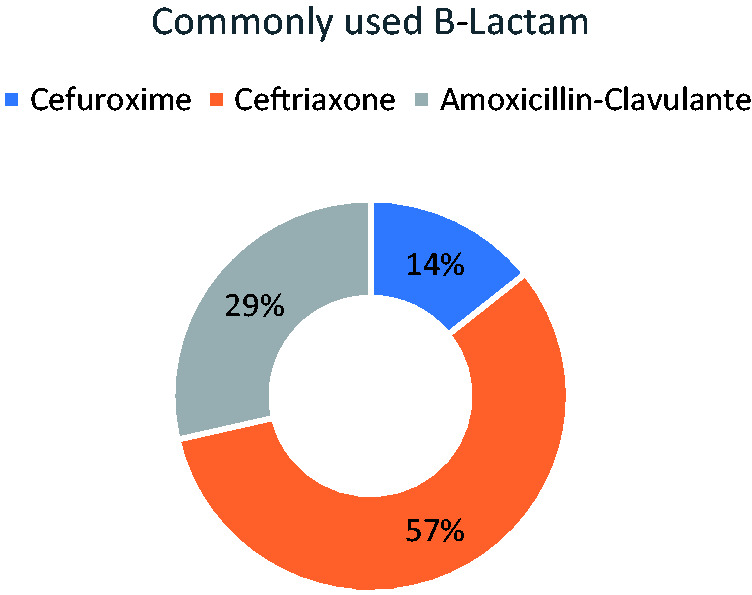
Commonly used beta-lactams in combination with macrolides.
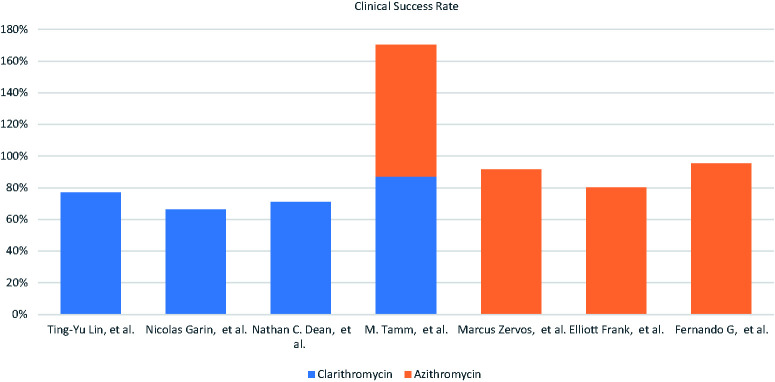
Clinical success rate
The primary outcome was the clinical success rate among all the included trials. The clinical success rate was 87.55% after 10 or 14 days of therapy among the studies with data on azithromycin–beta-lactam and 75.42% after 5 or 7 days of therapy among the studies with data on clarithromycin–beta-lactam (Figure 3). Lin et al. 9 reported a success rate of 77% for clarithromycin, and Rubio et al. 24 reported a success rate of 95.5% for azithromycin.
Figure 3.
Percentage of clinical success rate among both macrolide–beta-lactam-based regimens.
The clinical success rate in the macrolide-based combination trials was defined as a clinical cure at the end of therapy, which is the resolution of signs and symptoms of pneumonia including dyspnea, cough, sputum, and fever. This success rate was reported mostly in patients with a PSI score of III or IV (Figure 4).
Figure 4.
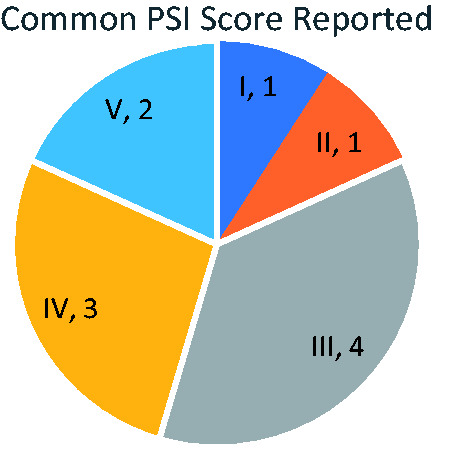
Common Pneumonia Severity Index (PSI) score reported.
Causative microorganism
Five20–24 out of the seven studies investigated the causative organisms in all the treatment arms. Among all the isolated organisms, S. pneumoniae was commonly found in both of the macrolide groups, but more S. pneumoniae was found in the clarithromycin–beta-lactam-based group compared with the azithromycin group. An azithromycin–beta-lactam-based regimen had more isolates of Hemophilus influenza than in the clarithromycin–beta-lactam group. However, both regimens had the same killing effect on Mycoplasma pneumoniae (Figure 5).
Figure 5.
Number of bacteria isolated from each treatment arm.
Length of hospital stay
Data for the length of the hospital stay was retrieved from five clinical trials13,19,20,23,24 (Figure 6). Patient who were treated with an azithromycin-based regimen spent more days in hospital than those treated with a clarithromycin-based regimen. The mean length of the hospital stay was 8.45 and 7.25 days, respectively.
Figure 6.
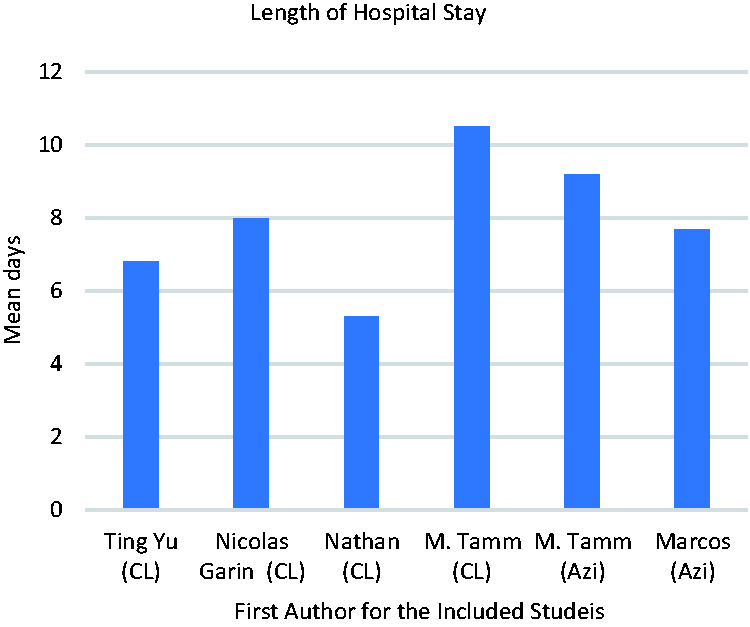
Length of hospital stay (mean number of days).
Methodological quality and risk of bias within individual studies
The quality of the studies was assessed using the SIGN tool. 26 Seven full text RCT studies were included in this study13,19–24 on the basis of the SIGN tool’s assessment of quality. Five studies were of high quality13,19,20,22,24 and two studies were of medium quality,21,23 with no study in the low quality category. All the reviewers agreed to include these seven studies for further analysis. All the included studies included were open-label trials, and there was no blinded treatment allocation process. Participants in all of the studies were randomized to the treatment groups, except for one study, Rubio et al., 24 because no comparator was used. Overall, the results of all the studies were directly applicable to the patient group that was targeted in each study.
Discussion
The 2019 IDSA guidelines recommend the combination of a beta-lactam and a macrolide to treat hospitalized patients with moderate to severe CAP. 6
Both azithromycin–beta-lactam and clarithromycin–beta-lactam combinations have shown adequate efficacy in treating patients with CAP, with a higher clinical cure rate at the end of therapy using an azithromycin-based regimen. In our review, a clinical success rate of 87.55% after 10 or 14 days of treatment was reported for studies with an azithromycin–beta-lactam regimen, and a success rate of 75.42% after 5 or 7 days of treatment was reported among studies with a clarithromycin–beta-lactam regimen. In accordance with our study, a prospective study comparing clarithromycin–ceftriaxone (n = 106) and gatifloxacin (n = 99) showed a clinical success rate of 91% and 97%, respectively. 27 A multicenter study was performed to compare moxifloxacin monotherapy (n = 233) to clarithromycin–amoxicillin (n = 134), and that study showed a high clinical success rate of 93.6% and 93.7%, respectively, 7 to 10 days after the end of therapy. Most of the included patients who were in that study (84%) had class a PSI score of I, II, or III. 28 Both studies are consistent with this review, which emphasizes the high clinical rate with a clarithromycin-based regimen. In our study, most patients with a high clinical success rate had a PSI score of III or IV (Figure 4). The high clinical success rate could reflect the need for a potent antimicrobial regimen in patients with a higher PSI score, where the combination of a macrolide and beta-lactam had lower 14 and 30-day mortality compared with fluroquinolone monotherapy. 14 Moreover, macrolide combination therapy showed a significantly lower intensive care unit mortality rate (26.1%) in ventilated patients compared with the combination with fluoroquinolones (46.3%; hazard ratio 0.48, 95% confidence interval 0.23–0.97, p = 0.04). 8 Another multicenter study with a randomized design compared moxifloxacin monotherapy with ceftriaxone with or without azithromycin, and that study showed a similar clinical success rate between both groups (83.3% in the moxifloxacin group [n = 108]; 79.6% in the comparator group [n = 113]), and in the latter group, 70% of the patients received ceftriaxone combined with azithromycin. 29
Combination therapy using a beta-lactam with a macrolide has been retrospectively assessed in many studies that investigated the clinical outcomes and the impact on hospitalization. These studies reported positive clinical outcomes that favored the addition of a macrolide such as erythromycin, clarithromycin, or azithromycin in hospitalized adults, and it also reduced the number of hospitalization days.30–32 A recent open-label randomized trial compared ceftriaxone to ampicillin–sulbactam when clarithromycin or erythromycin was added to either of the beta-lactams in patients with CAP. The results showed a significantly higher effectiveness rate at day 7 in the ampicillin–sulbactam group (p = 0.047) compared with the ceftriaxone group in the validated per-protocol population. 33
Multiple factors supported the superiority of combination therapy. For example, combination therapy can include two mechanisms of action so that the medications work at different sites of bacterial action, such as a beta-lactam that inhibits cell wall synthesis and a macrolide that inhibits protein synthesis. Moreover, macrolides reduce the adherence of S. pneumoniae to respiratory epithelial cells and show anti-inflammatory action by reducing the release of interleukin-8 and tumor necrosis factor-alpha.34,35
Adding a macrolide to a beta-lactam as empirical therapy was shown to reduce mortality in patients with pneumococcal pneumonia. 36 In our review, S. pneumoniae was commonly found in both macrolide groups, with 130 isolates in clarithromycin-based combinations and 80 isolates in the azithromycin-based group; additionally, 18 and 20 isolates from each group, respectively, were the atypical M. pneumoniae. In an open-label, prospective, nonrandomized study, patients received sequential intravenous ceftriaxone and oral amoxicillin–clavulanate with or without a macrolide, where the macrolide selection was either clarithromycin (n = 220) or azithromycin (n = 383). S. pneumoniae was the most frequently isolated organism among all the isolates with 27 and 35 patients, respectively, and M. pneumoniae was isolated from six and ten patients from each group, respectively. 37 Higher S. pneumoniae eradication rates with clarithromycin could be explained by the extent of antimicrobial activity. In in vitro studies, clarithromycin tended to have a lower minimum inhibitory concentration (MIC) compared with azithromycin for S. pneumoniae (0.015–16 mg/dL vs. 0.12–4 mg/dL). However, azithromycin had a lower MIC for H. influenzae (0.5–4 mg/dL vs. 8–16 mg/dL) and the atypical M. pneumoniae (0.00024–<0.01 mg/dL vs. 0.008–0.5 mg/dL) compared with clarithromycin. 38
The length of the hospital stay was one of the most common outcomes that was investigated in five studies.19–23 The average length for the hospital stay was 8.45 days in patients receiving beta-lactams in combination with azithromycin and 7.25 days for patients receiving beta-lactams in combination with clarithromycin. Compared with our findings, an open-label nonrandomized study by Sanchez et al. 37 showed that the mean length of the hospital stay was 7.4 days for an azithromycin-based regimen and 9.8 days for a clarithromycin-based regimen. In another study with a prospective observational design that compared clarithromycin with ceftriaxone (n = 209) to levofloxacin (n = 250), the length of the hospital stay was 6 and 5 days, respectively. 39 When the azithromycin–beta-lactam combination was compared with beta-lactam monotherapy in an observational study by Ito et al., 40 fewer hospitalization days (10 days) were found with the azithromycin–beta-lactam combination compared with monotherapy (12 days), but this difference was not statistically significant. Several factors could play a role in the hospitalization days including the patient’s condition, illness severity using the PSI score, patient characteristics, and initial antibiotic treatment. Menendez et al. 41 reported a hospital stay of 8 days in patients who received macrolide monotherapy, 8 days in patients who received third-generation cephalosporin combined with a macrolide, and 6 days in patients receiving amoxycillin–clavulanic acid and a macrolide compared with the initial antibiotic treatment. Thus, a shorter length of hospital stay is shown by patients who receive a macrolide–beta-lactam combination, but clarithromycin was not superior to azithromycin.
For the pharmacokinetic profiles, azithromycin has a lower incidence of drug–drug interactions compared with clarithromycin, which might influence the clinical decision when selecting the macrolide that is to be combined with a beta-lactam. For example, patients on theophylline, carbamazepine, or digoxin are at a high risk of reaching toxic levels when using clarithromycin because digoxin increases the plasma concentration of clarithromycin and azithromycin, whereas none of these interactions have been reported for azithromycin. 42 Additionally, a longer elimination half-life for azithromycin (68 hours) allowed for a single-dose daily regimen, while clarithromycin (half-life, 5–7 hours) is usually prescribed as a twice-daily regimen. 43
Conclusion
This systematic review and qualitative evidence synthesis reported inter-class macrolide differences. Both azithromycin and clarithromycin in combination with a beta-lactam have shown a significant clinical success rate at the end of therapy, with higher rates of a clinical cure at the end of therapy with an azithromycin-based regimen. However, if a shorter hospital stay is the main focus during the management of patients with CAP, then a clarithromycin-based combination would be the therapy choice. The susceptibility data for the concerned facility must be considered when deciding upon the treatment selection.
Clinical implications
The clarithromycin–beta-lactam combination regimen was associated with a shorter hospital stay.
Macrolides and azithromycin both have a relatively safe pharmacokinetic profile in terms of interactions.
The selection of an intra-class macrolide combination with a beta-lactam depends on clinical prognosis and the patient’s condition.
Few clinical trials have investigated the safety and efficacy profile of intra-class macrolides with beta-lactam.
Footnotes
Authorship statement: All the authors contributed equally to this research manuscript. The final manuscript has been read by all the authors, and all authors agreed to submit it for publication.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Jumana Al-Salloum https://orcid.org/0000-0003-1771-992X
Syed Wasif Gillani https://orcid.org/0000-0003-4327-2068
References
- 1.Ferreira-Coimbra J Sarda C andRello J.. Burden of community-acquired pneumonia and unmet clinical needs. Adv Ther 2020; 37: 1302–1318. Available from: /pmc/articles/PMC7140754/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO | Disease burden and mortality estimates [Internet]. WHO. World Health Organization; 2019. [cited 2021 Apr 25]. Available from: http: //www.who.int/healthinfo/global_burden_disease/estimates/en/
- 3.Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 2017; 65: 1806–1812. Available from: https://pubmed.ncbi.nlm.nih.gov/29020164/ [DOI] [PubMed] [Google Scholar]
- 4.ABCs 2018 Strep pneumoniae report | CDC [Internet]. [cited 2021 Apr 25]. Available from: https://www.cdc.gov/abcs/reports-findings/survreports/spneu18.html
- 5.Ynthia W, Hitney CG, Onica F, Arley MM, Ames Adler JH, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. Vol. 343. 1917. DOI: 10.1056/NEJM200012283432603 [DOI] [PubMed]
- 6.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. Am J Respir Crit Care Med 2019; 200: E45–E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vardakas KZ Trigkidis KK andFalagas ME.. Fluoroquinolones or macrolides in combination with β-lactams in adult patients hospitalized with community acquired pneumonia: a systematic review and meta-analysis. Clin Microbiol Infect 2017; 23: 234–241. Available from: 10.1016/j.cmi.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 8.Martin-Loeches I, Lisboa T, Rodriguez A, et al. Combination antibiotic therapy with macrolides improves survival in intubated patients with community-acquired pneumonia. Intensive Care Med 2010; 36: 612–620. Available from: https://link.springer.com/article/10.1007/s00134-009-1730-y [DOI] [PubMed] [Google Scholar]
- 9.Karhu J, Ala-Kokko TI, Ohtonen P, et al. Severe community-acquired pneumonia treated with β-lactam-respiratory quinolone vs. β-lactam-macrolide combination. Acta Anaesthesiol Scand 2013; 57: 587–593. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez F, Mensa J, Martínez JA, et al. Is azithromycin the first-choice macrolide for treatment of community-acquired pneumonia?. Clin Infect Dis 2003; 36: 1239–1245. Available from: https://academic.oup.com/cid/article/36/10/1239/307146 [DOI] [PubMed] [Google Scholar]
- 11.Ruhe J andMildvan D.. Does empirical therapy with a fluoroquinolone or the combination of a β-lactam plus a macrolide result in better outcomes for patients admitted to the general ward? Infect Dis Clin North Am 2013; 27: 115–132. [DOI] [PubMed] [Google Scholar]
- 12.Zarogoulidis P, Papanas N, Kioumis I, et al. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol 2012; 68: 479–503. [DOI] [PubMed] [Google Scholar]
- 13.Garin N, Genné D, Carballo S, et al. β-lactam monotherapy vs β-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med 2014; 174: 1894–1901. [DOI] [PubMed] [Google Scholar]
- 14.Lodise TP, Kwa A, Cosier L, et al. Comparison of β-lactam and macrolide combination therapy versus fluoroquinolone monotherapy in hospitalized veterans affairs patients with community-acquired pneumonia. Antimicrob Agents Chemother 2007; 51: 3977–3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gleason PP, Meehan TP, Fine JM, et al. Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia. Arch Intern Med 1999; 159: 2562–2572. Available from: https://jamanetwork.com/ [DOI] [PubMed] [Google Scholar]
- 16.Stahl JE, Barza M, Desjardin J, et al. Effect of macrolides as part of initial empiric therapy on length of stay in patients hospitalized with community-acquired pneumonia. Arch Intern Med 1999; 159: 2576–2580. Available from: https://jamanetwork.com/ [DOI] [PubMed] [Google Scholar]
- 17.Mufson MA andStanek RJ.. Bacteremic pneumococcal pneumonia in one American city: a 20-year longitudinal study, 1978–1997. Am J Med 1999; 107: 34S–43S. Available from: https://pubmed.ncbi.nlm.nih.gov/10451007/ [DOI] [PubMed] [Google Scholar]
- 18.Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia implications for practice guidelines. JAMA 1998; 279: 1452–1457. Available from: https://jamanetwork.com/ [DOI] [PubMed] [Google Scholar]
- 19.Lin TY, Lin SM, Chen HC, et al. An open-label, randomized comparison of levofloxacin and amoxicillin/clavulanate plus clarithromycin for the treatment of hospitalized patients with community-acquired pneumonia. Chang Gung Med J 2007; 30: 321–332. [PubMed] [Google Scholar]
- 20.Dean NC, Sperry P ,Wikler M, et al. Comparing gatifloxacin and clarithromycin in pneumonia symptoms resolution and process of care. Antimicrob Agents Chemother 2006; 50: 1164–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zervos M, Mandell LA, Vrooman PS, et al . Comparative efficacies and tolerabilities of intravenous azithromycin plus ceftriaxone and intravenous levofloxacin with step-down oral therapy for hospitalized patients with moderate to severe community-acquired pneumonia. Treat Respir Med 2004; 3: 329–336. [DOI] [PubMed] [Google Scholar]
- 22.Frank E, Liu J, Kinasewitz G, et al. A multicenter, open-label, randomized comparison of levofloxacin and azithromycin plus ceftriaxone in hospitalized adults with moderate to severe community-acquired pneumonia. Clin Ther 2002; 24: 1292–1308. [DOI] [PubMed] [Google Scholar]
- 23.Tamm M, Todisco T, Feldman C, et al. Clinical and bacteriological outcomes in hospitalised patients with community-acquired pneumonia treated with azithromycin plus ceftriaxone, or ceftriaxone plus clarithromycin or erythromycin: a prospective, randomised, multicentre study. Clin Microbiol Infect 2007; 13: 162–171. [DOI] [PubMed] [Google Scholar]
- 24.Rubio FG, Cunha CA, Lundgren FLC, et al. Intravenous azithromycin plus ceftriaxone followed by oral azithromycin for the treatment of inpatients with community-acquired pneumonia: an open-label, non-comparative multicenter trial. Braz J Infect Dis 2008; 12: 202–209. [DOI] [PubMed] [Google Scholar]
- 25.Rae N, Singanayagam A, Schembri S, et al. Oral versus intravenous clarithromycin in moderate to severe community-acquired pneumonia: an observational study. Pneumonia (Nathan) 2017; 9: 2. Available from: 10.1186/s41479-017-0025-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Method 2007; 7: 10. doi:10.1186/1471-2288-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dresser LD Niederman MS andPaladino JA.. Cost-effectiveness of gatifloxacin vs ceftriaxone with a macrolide for the treatment of community-acquired pneumonia. Chest 2001; 119: 1439–1448. Available from: 10.1378/chest.119.5.1439 [DOI] [PubMed] [Google Scholar]
- 28.Torres A, Muir J, Corris P, et al. Effectiveness of oral moxifloxacin in standard first-line therapy in community-acquired pneumonia. Eur Respir J 2003; 21: 135–143. [DOI] [PubMed] [Google Scholar]
- 29.Katz E, Larsen LS, Fogarty CM, et al. Safety and efficacy of sequential i.v. to p.o. moxifloxacin versus conventional combination therapies for the treatment of community-acquired pneumonia in patients requiring initial i.v. therapy. J Emerg Med 2004; 27: 395–405. Available from: https://pubmed.ncbi.nlm.nih.gov/15498622/ [DOI] [PubMed] [Google Scholar]
- 30.Brown RB, Iannini P, Gross P, et al. Impact of initial antibiotic choice on clinical outcomes in community-acquired pneumonia: analysis of a hospital claims-made database. Chest 2003; 123: 1503–1511. Available from: https://pubmed.ncbi.nlm.nih.gov/12740267/ [DOI] [PubMed] [Google Scholar]
- 31.Waterer GW Somes GW andWunderink RG.. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med 2001; 161: 1837–1842. Available from: https://pubmed.ncbi.nlm.nih.gov/11493124/ [DOI] [PubMed] [Google Scholar]
- 32.Dudas V, Hopefl A, Jacobs R, et al. Antimicrobial selection for hospitalized patients with presumed community-acquired pneumonia: a survey of nonteaching US community hospitals. Ann Pharmacother 2000; 34: 446–452. Available from: https://pubmed.ncbi.nlm.nih.gov/10772428/ [DOI] [PubMed] [Google Scholar]
- 33.Hamao N, Ito I, Konishi S, et al. Comparison of ceftriaxone plus macrolide and ampicillin/sulbactam plus macrolide in treatment for patients with community-acquired pneumonia without risk factors for aspiration: an open-label, quasi-randomized, controlled trial. BMC Pulm Med 2020; 20: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaul R, Mcgeer A, Norrby-Teglund A, et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome-A comparative observational study and the national. Clin Infect Dis 1999; 28: 800–807. Available from: https://academic.oup.com/cid/article/28/4/800/401565. DOI: https://doi.org/10.1086/515199 [DOI] [PubMed] [Google Scholar]
- 35.Takizawa H, Desaki M, Ohtoshi T, et al. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med 1997; 156: 266–271. Available from: https://pubmed.ncbi.nlm.nih.gov/9230759/ [DOI] [PubMed] [Google Scholar]
- 36.Martínez JA, Horcajada JP, Almela M, et al. Addition of a macrolide to a β-lactam-based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin Infect Dis 2003; 36: 389–395. Available from: https://academic.oup.com/cid/article/36/4/389/437900 [DOI] [PubMed] [Google Scholar]
- 37.Sánchez F, Mensa J, Martínez JA, et al. Is azithromycin the first-choice macrolide for treatment of community-acquired pneumonia?. Clin Infect Dis 2003; 36: 1239–1245. Available from: https://academic.oup.com/cid/article/36/10/1239/307146 [DOI] [PubMed] [Google Scholar]
- 38.Blondeau JM. Update on the use of the macrolides for community-acquired respiratory tract infections. Therapy 2006; 3: 619–650. DOI: https://doi.org/ 10.1586/14750708.3.5.619 [Google Scholar]
- 39.Querol-Ribelles JM, Tenías JM, Querol-Borrás JM, et al. Levofloxacin versus ceftriaxone plus clarithromycin in the treatment of adults with community-acquired pneumonia requiring hospitalization. Int J Antimicrob Agents 2005; 25: 75–83. [DOI] [PubMed] [Google Scholar]
- 40.Ito A, Ishida T, Tachibana H, et al. Azithromycin combination therapy for community-acquired pneumonia: propensity score analysis. Nature 2019; 9. 10.1586/14750708.3.5.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menéndez R, Cremades MJ, Martínez-Moragón E, et al. Duration of length of stay in pneumonia: Influence of clinical factors and hospital type. Eur Respir J 2003; 22: 643–648. [DOI] [PubMed] [Google Scholar]
- 42.McKenna S, Evans G. and the Canadian Infectious Disease Society Antimicrobial Agents Committee. Macrolides: a Canadian infectious disease society position paper. Can J Infect Dis 2001; 12: 218–231. Available from: moz-extension://49819bed-5a00-c648-8809-d741f41bf993/enhanced-reader.html?pdf=https%3A%2F%2Fbrxt.mendeley.com%2Fdocument%2Fcontent%2Ff76c08c2-3aae-3cce-9a17-b48b6cec9de5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LeBel M. Pharmacokinetic properties of clarithromycin: a comparison with erythromycin and azithromycin. Can J Infect Dis 1993; 4: 148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]