Abstract
Objective
To evaluate the efficacy of repetitive transcranial magnetic stimulation (rTMS) in improving lower limb spasticity after stroke.
Methods
The PubMed, Web of Science, Cochrane Library, EMBASE, China National Knowledge Infrastructure (CNKI), China Biology Medicine (CBM) disc, China Science and Technology Journal Database (VIP), and Wanfang databases were searched online from their inception to May 2021 for randomized controlled trials (RCTs) involving repetitive transcranial magnetic stimulation for lower extremity spasticity after stroke. Valid data were extracted from the included literature, and the quality evaluation was conducted with the Cochrane Handbook for Systematic Reviews of Interventions along with the Physiotherapy Evidence Database scale (PE-Dro scale). The data that met the quality requirements were systematically analysed using Review Manager 5.4 software.
Results
A total of 554 patients from seven articles (nine studies) were quantitatively analysed. Outcomes included the Modified Ashworth Scale (MAS), Fugl–Meyer Assessment of Lower Extremity (FMA-LE), Modified Barthel Index (MBI), and Timed Up and Go (TUG), measured as the effect of rTMS compared with controls conditions after treatment. The systematic review showed that rTMS reduced MAS and increased MBI scores, respectively (SMD = −0.24, 95% CI [−0.45, −0.03], P = 0.02; MD = 6.14, 95% CI [−3.93,8.35], P < 0.00001), compared with control conditions. Low-frequency rTMS (LF-rTMS) significantly improved FMA-LE scores (SMD = 0.32, 95% CI [0.13, 0.51], P = 0.001). However, there was no significant difference in FMA-LE scores when using high-frequency rTMS (HF-rTMS) (P > 0.1) and in TUG times (P > 0.1) between the treatment and control groups.
Conclusions
rTMS was effective in improving spasticity and activities of daily living. LF-rTMS has positive clinical effects on enhancing motor function in patients who experience lower extremity spasticity after stroke. To better validate the above conclusions, more multicentre, high-quality, and double-blind randomized controlled trials are needed.
1. Introduction
Stroke is a common disease worldwide and causes serious disabilities for patients. More than two-thirds of stroke survivors develop poststroke sequelae that involve impairment of motor function, balance, gait, and activities of daily living [1, 2]. Poststroke spasticity (PSS) is a common motor dysfunction after stroke that clinically manifests as increased muscle tone, positive pathological signs, and tendon hyperreflexia [3], with a prevalence from 4% to 42.6% [2]. Current management of poststroke spasticity has shown that although drug therapy (such as botulinum toxin injection, oral baclofen, dantrolene, sodium, and tizanidine) is effective for improving spasticity and widely used in clinical practice, it had side effects and produced unsatisfied clinical effects such as muscle weakness [4]. Nondrug therapy, such as neuromuscular electrical stimulation and physical therapy, temporarily relieved poststroke spasticity and motor dysfunction. However, some of these interventions demand active participants to become involved, and the duration of the efficacy was relatively short [5, 6]. rTMS has been gradually applied in the clinical treatment of poststroke dysfunction due to its noninvasiveness and safety on the basis of conventional treatment of stroke sequelae. But most of the research focused on motor dysfunction, cognitive disorder, aphasia, and so on. There are few studies on the application of rTMS in poststroke spasticity, and the mechanism is unclear. What is more, rTMS has a significant impact on public acceptance due to the relatively high clinical costs and being excluded in the health insurance in some cities [7–9]. A recent meta-analysis explored the use of rTMS in stroke patients. Two meta-analyses published by McIntyre et al. [10] and Peng et al. [11] analysed the effect of rTMS in the rehabilitation of spasticity after stroke. However, they did not include RCTs for the treatment of lower limb spasticity after stroke, and some new RCTs have been published since then. Moreover, the efficacy of repetitive transcranial magnetic stimulation in improving lower limb spasticity after stroke remains unknown. As a result, the purpose of this study was to perform a systematic review of RCTs that explored the efficacy of rTMS in treating patients with lower limb spasticity after stroke.
2. Methods
2.1. Literature Search Strategy
We performed a search in the PubMed, Web of Science, Cochrane Library, EMBASE, CNKI, CBM, VIP, and Wanfang databases published up to May 2021. The search terms were “stroke” OR “hemiplegia” OR “cerebrovascular accident” OR “ischemic stroke” OR “hemorrhagic stroke” OR “CVA” OR “apoplexy” AND “repetitive transcranial magnetic stimulation” OR “rTMS” OR “transcranial magnetic stimulation” OR “TMS” AND “spasticity” AND “lower limb” OR “lower extremity”.
2.2. Inclusion and Exclusion Criteria
The relevant articles were selected based on the following eligibility criteria: (1) the involved patients were clinically diagnosed with lower limb spasticity after stroke by relevant examinations; (2) the experimental group used rTMS and traditional physical therapy, while the control group underwent traditional physical therapy plus sham rTMS (or only with traditional physical therapy); (3) the outcome measures included the MAS, FMA-LE, MBI, and TUG; and (4) the included articles were RCTs.
Articles meeting with the following criteria were excluded: (1) total sample size of fewer than 10 participants in each study; (2) study with incomplete data; (3) meta-analysis, case report, literature review, guidelines, dissertation, and others; and (4) non-RCTs.
2.3. Literature Screening and Data Extraction
Two researchers independently searched and screened the literature based on the above search strategy and removed the studies that did not meet the predefined criteria by reading the abstracts and full texts. Any inconsistencies between the two authors were resolved by discussion or in consultation with the third author. The following data were extracted: study characteristics (authors, year of publication, study design, sample sizes, age, and course of disease) and intervention details (intervention measures, treatment time, stimulated sites, treatment parameters, and outcome measures).
2.4. Literature Quality Evaluation
The quality of the included articles was evaluated by two authors using the Cochrane Handbook for Systematic Reviews of Interventions [12] and PE-Dro [13]. In cases of disagreement, a third person made the final decision. Three levels (level A, level B, and level C) were used to rank the quality of each study when using the former method [12]. Regarding the PE-Dro, 0–3 points indicated low quality, 4–7 points indicated medium quality, and 8–11 indicated high-quality [14].
2.5. Data Synthesis and Statistical Methods
The outcomes in both the treatment and control groups after the intervention period were extracted. The results were shown by the histogram in one study [15], and we estimated the results based on the X and Y axes and the corresponding parameters. A six-point scale (0, 1, 1+, 2, 3, and 4) was denoted as the MAS scale [16]. To quantify the score for analysis, we calculated 1+ as 1.5. If the results were not presented as the means and standard deviations, we calculated the original data using SPSS 25.0 [17] or the method of Wan et al. [18]. The data from the first phase for both groups were extracted in randomized controlled crossover studies [15, 19, 20]. If the variable between two groups in an article was only rTMS, we divided the one article into two studies [17, 21]. Quantitative analysis was performed using Review Manager version 5.4 by two authors. Concerning the continuous variables (excluding Hmax/Mmax), the mean difference (MD) or the standardized mean difference (SMD) with 95% CI were calculated for the outcome. The heterogeneity among the included studies was assessed by the χ2 test and Higgins I2 values. If there was clear heterogeneity (I2 > 50% or P < 0.1), a random effects model was used. Otherwise, a fixed effects model was applied.
3. Results
3.1. Characteristics of the Studies
A total of 113 entries were retrieved from Chinese and English databases, including 33 in Chinese and 80 in English (Figure 1); 50 duplicates were removed through EndNote X9; and 63 articles were screened. Then, 21 were excluded because the article type was not a clinical trial. A total of 42 full-text studies were obtained for eligibility. Then, 35 studies were rejected: 25 due to the population, 4 owing to the intervention, 3 because of the study design, and 3 because of the assessed outcome. Finally, seven articles with a total of 554 patients [15, 17, 19–23] were included. Two articles had two separate data sets [17, 21], and the others had one data set each. Table 1 shows the characteristics of the included articles. All the included studies were randomized controlled trials with quality level B, and three of them were crossover trials [15, 19, 20]. The risk of bias of the included RCT is shown in Figure 2. The total score on the PE-Dro was 51, with an average of 7.29. Two articles were of high quality [17, 19], and five were of medium quality (Table 2).
Figure 1.
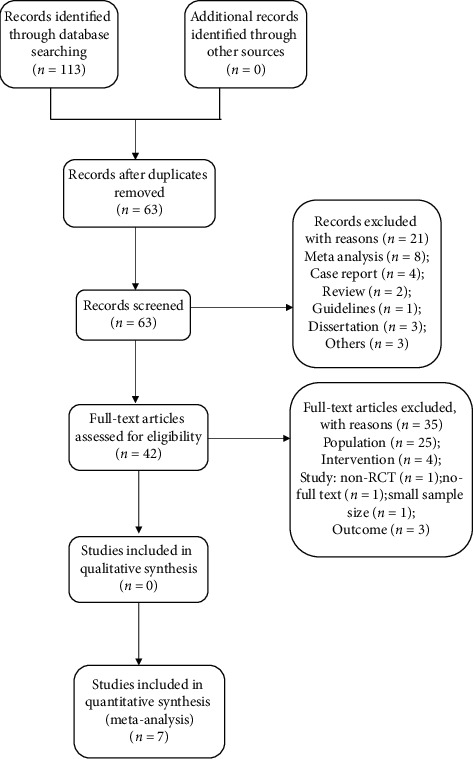
Flow diagram of study selection.
Table 1.
Characteristics of included articles.
| Author/year | Sample size | Mean age (y) | Course of disease (m) | Intervention | Treatment duration | Stimulated site | Treatment parameters | RCT type | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Treatment/control | |||||||||
| Chieffo et al., 2020 [19] | 6/6 | 58.67 ± 10.33/61.17 ± 8.70 | 41.50 ± 26.77/41.00 ± 26.54 | r + c/sham + c | 15 min/day, 11 sessions | Bilateral leg motor cortex | 20 Hz, 80%–90% RMT, H-coil |
Crossover trial | FMA-LE, MAS, 10-MT, 6MWT |
| Rastgoo et al., 2016 [15] | 7/7 | 54.60 ± 11.75/49.70 ± 11.00 | 30.2 ± 18.3/27.4 ± 20.1 | r/sham | 20 min/day, 5 days | Leg motor cortex of the unaffected hemisphere | 1 Hz, 90% RMT, eight coil |
Crossover trial | MMAS, FMA-LE, Hmax/Mmax, TUG |
| Yijie, 2018 [20] | 70/70 | 55.20 ± 11.50/51.30 ± 12.10 | 31.60 ± 11.5/51.3 ± 12.1 | r + R/sham + R | 20 min/day, 5 days | Contralateral cerebral cortex | 1 Hz, 90% RMT, eight coil |
Crossover trial | MAS, FMA-LE, Hmax/Mmax, TUG |
| Jing et al., 2018 [21] | 24/24 | 56.55 ± 13.11/57.33 ± 12.00 | 3.58 ± 2.44/4.01 ± 2.89 days | r + R/R | 15 min/day, 1 month | Primary motor cortex of the unaffected hemisphere | 1 Hz, 90% RMT | Factorial trial | MAS, FMA-LE, MBI, BBS |
| Jing et al., 2018 [21] | 24/24 | 56.21 ± 11.68/55.93 ± 13.88 | 4.33 ± 2.57/4.41 ± 2.69 days | r + B + R/B + R | 15 min/day, 1 month | Primary motor cortex of the unaffected hemisphere | 1 Hz, 90% RMT | Factorial trial | MAS, FMA-LE, MBI, BBS |
| Yang et al., 2015 [22] | 60/60 | 58.7 ± 3.5/59.2 ± 3.3 | 4.6 ± 1.2/4.3 ± 1.4 | r + R/r | 15 min/day, 5 days/week, 8 weeks | M1 of the affected hemisphere | 2 Hz, 90% RMT butterfly shaped coils |
Parallel trial | FMA, FAC, CSI, 10-MT |
| Hong et al., 2016 [17] | 20/20 | 62.18 ± 13.66/61.23 ± 14.24 | 3.98 ± 2.05/4.61 ± 2.50 days | r + R/R | 20 min/day, 6 days/week, 4 weeks | Primary motor cortex of the unaffected hemisphere | 1 Hz, 90% RMT circular coil |
Factorial trial | MAS, FMA-LE, MBI |
| Hong et al., 2016 [17] | 20/20 | 61.99 ± 15.02/60.89 ± 15.16 | 4.02 ± 3.17/4.35 ± 3.28 days | r + B + R/r + B | 20 min/day, 6 days/week, 4 weeks | Primary motor cortex of the unaffected hemisphere | 1 Hz, 90% RMT circular coil |
Parallel trial | MAS, FMA-LE, MBI |
| Huayao et al., 2019 [23] | 47/45 | 43.33 ± 9.18/44.33 ± 9.94 | ───── | r + F/sham + F | 20 min/day, 5 days/week, 4 weeks | M1 of the affected brain hemisphere | 1 Hz | Parallel trial | MAS, FMA-LE, MEP |
r: rehabilitation; sham: sham rTMS; c: cycling; B: BTX-A; R: rehabilitation; F: FES (functional electrical stimulation).
Figure 2.
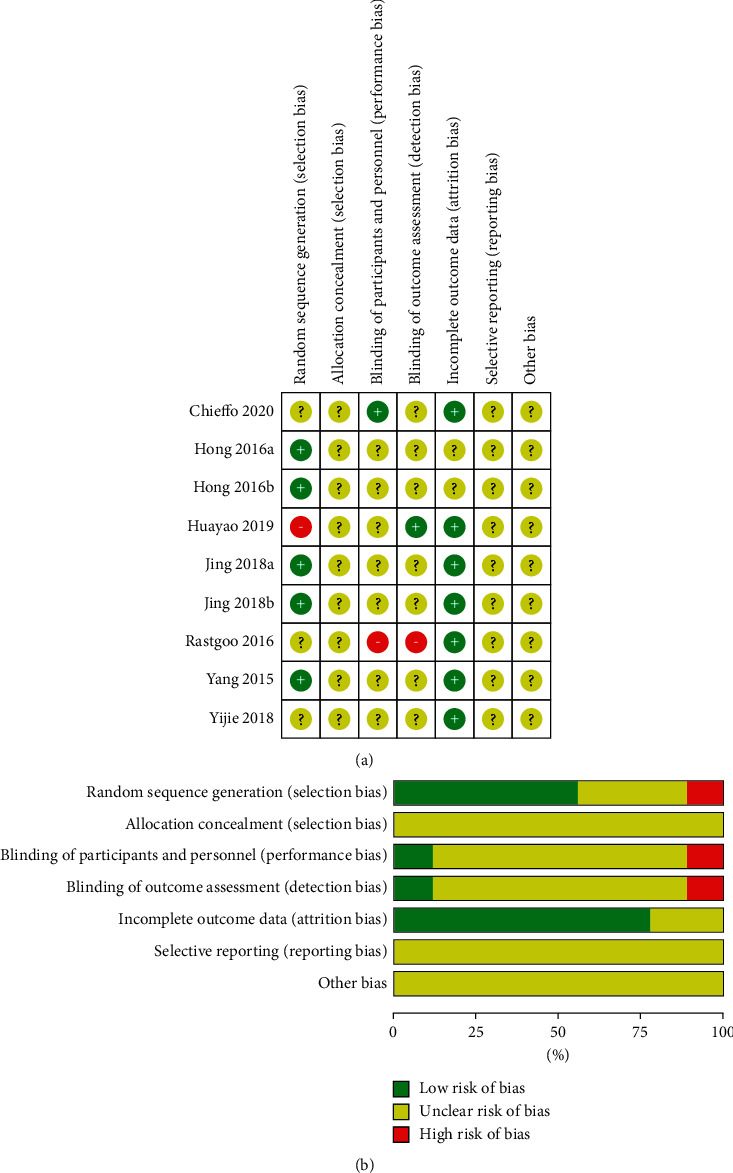
(a) Risk of bias graph. (b) Risk of bias summary.
Table 2.
PE-Dro scale of the included study.
| Study | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chieffo et al., 2020 [19] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 9 |
| Rastgoo et al., 2016 [15] | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 7 |
| Yijie, 2018 [20] | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 6 |
| Jing et al., 2018 [21] | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Yang et al., 2015 [22] | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 7 |
| Hong et al., 2016 [17] | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Huayao et al., 2019 [23] | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
3.2. Effects of rTMS on Spasticity of the Lower Limbs in Stroke Patients
3.2.1. MAS
Five articles (seven studies) [17, 19, 21, 23] with 420 patients were included. The forest plot (Figure 3(a)) shows that statistical heterogeneity was not observed (I2 = 44%, P = 0.10). We used the fixed effects model because heterogeneity was not observed after two studies were [19, 21] excluded through sensitivity analysis (I2 = 0%, P = 0.78). The meta-analysis showed that rTMS had a significant beneficial effect on MAS scores in patients with lower limb spasticity after stroke (SMD = −0.24, 95% CI [−0.45, 0.03], P = 0.02) (Figure 3(b)).
Figure 3.
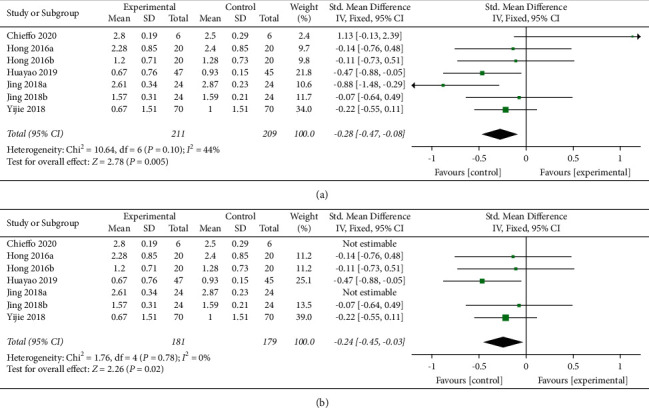
(a) Forest plot for MAS scores. (b) Forest plot for MAS scores when excluding two studies with high sensitivity.
3.3. Effects of rTMS on Spasticity of the Lower Limbs in Stroke Patients
3.3.1. FMA
A total of seven articles (nine studies) with 554 patients [15, 17, 19–23] presented effects on the FMA. Subgroup analysis based on low- and high-frequency indicated seven studies with LF-rTMS and two with HF-rTMS. The difference between groups among those using low-frequency rTMS showed a statistically significant effect on FMA scores (SMD = 0.32, 95% CI [0.13, 0.51], P = 0.001) with no statistical heterogeneity (I2 = 1%, P = 0.42). There was no statistical significance between the two groups in the studies using high-frequency rTMS (P = 0.72) (Figure 4).
Figure 4.
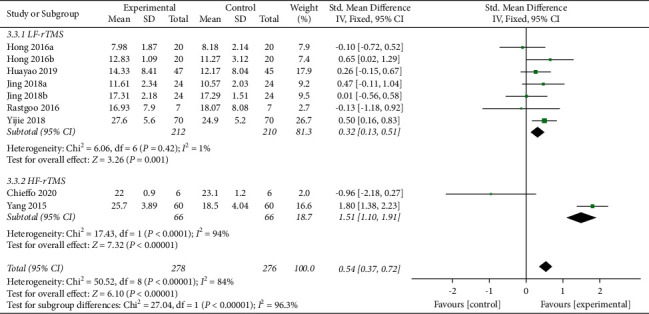
Forest plot of FMA.
3.4. Effects of rTMS on Spasticity of the Lower Limbs in Stroke Patients
3.4.1. MBI
Two articles (four studies) [17, 21] with 176 patients assessed this outcome. The random effects model was used with I2 = 40% and P = 0.17 (Figure 5(a)). Jing was the source of the heterogeneity after sensitivity analysis (Figure 5(b)). We found that there was a significant difference between the two groups (MD = 6.14, 95% CI [3.93, 8.35], P < 0.00001).
Figure 5.
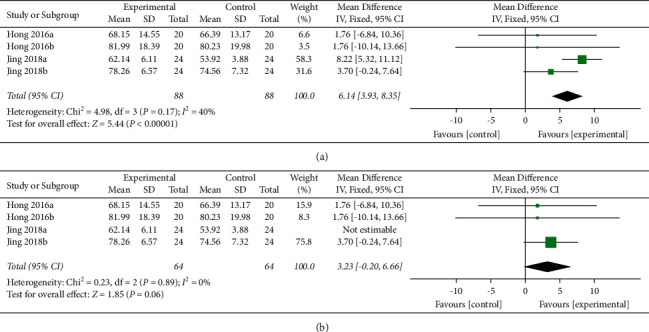
(a) Forest plot for MBI scores. (b) Forest plot for MBI scores after removing one study.
3.5. Effects of rTMS on Spasticity of the Lower Limbs in Stroke Patients
3.5.1. TUG Scores
Two studies [15, 20] showed that rTMS did not have a significant effect on MBI scores in the patients with lower limb spasticity (Figure 6).
Figure 6.
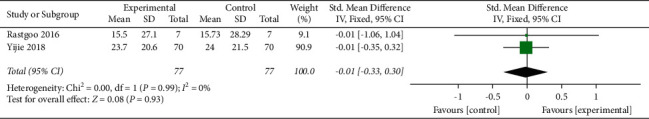
Forest plot for TUG times.
3.6. Others
The electrophysiological index Hmax/Mmax was described in two studies [15, 20] and showed that the index decreased in the treatment group without a significant difference compared with the control group.
The included articles reported adverse effects except for Hong [17]. Three studies [15, 20, 21] showed that patients had good tolerance to LF-rTMS in their studies. Chieffo et al. [19] reported transitory dizziness and muscle twitches in the shoulders of three patients, and they subsequently completed the remaining treatment after the intensity was decreased to 80% RMT. In another study, the patients had muscle pain and fatigue symptoms that were relieved after two to three days [21]. Huayao et al. [23] reported adverse effects without a significant difference between the two groups. In the treatment group, transitory headache was found in two patients, which diminished after suspension of the treatment [22].
4. Discussion
Velocity-dependent increases in muscle tone, hyperexcitable stretch reflexes, and hyperreflexia tendon jerks are often described as features of spasticity that appear in patients with stroke [24]. The reason for muscle spasticity in stroke patients is the hyperreflexia of the stretch reflex from the spine. The excitability and inhibitory imbalance of spinal descending fibres was considered the main cause of hyperreflexia of the stretch reflex. The disorder presents with high excitability of the reticular spine [25]. In addition, there has also been evidence that the vestibular spinal cord was less inhibitory [26], which reduces the inhibition of the spinal cord. Stroke patients with lower limb spasticity generally manifest hip adduction, knee extension, and ankle plantar flexion impacting the recovery of motor function and gait [27].
At present, the conventional treatment for lower limb spasticity after stroke includes drugs, motor therapy, and neuromuscular electrical stimulation [6]. However, the effect of conventional rehabilitation is limited. Thus some ways of complementary and alternative medicine are needed. Previous studies have shown that rTMS can be used to treat patients with lower limb spasticity after stroke with different results and unclear mechanisms. Naghdi et al. [28] reported that improvements in ankle plantar flexor and knee extensor spasticity were significant, but Hmax/Mmax showed no statistical improvement after five consecutive LF-rTMS sessions. Terreaux et al. [29] showed that 1 Hz rTMS reduced the excitability of the ankle plantar flexor reflex without modifying clinical signs of spasticity, but there was no change during 10-Hz rTMS. Because these studies were a nonrandomized controlled trial [28] or had a small sample size [29], our research group performed a systematic analysis including the most recent randomized controlled trials with more participants to further increase the quality of the included studies.
The results indicated that rTMS was effective in improving lower limb spasticity and activities of daily living; LF-rTMS had a positive influence on enhancing motor function in patients who experienced lower extremity spasticity after stroke, whereas HF-rTMS did not have a significant effect on motor function. Furthermore, rTMS did not have an additional significant effect on TUG times or Hmax/Mmax. McIntyre et al. [10] performed a systematic review regarding the use of rTMS in patients with spasticity after stroke and discovered that rTMS improved spasticity in the elbow, wrist, and finger flexors in uncontrolled pre-post studies, whereas there was no significant influence on spasticity in the wrist in two RCTs. Another meta-analysis by Xu et al. [11] was published in 2020 and reported no benefits on the use of rTMS for upper limb spasticity after stroke, which was different from our research.
Sensitivity analyses of the included studies found that the cause of high bias of risk in Jing and Yan [21] may have been the short duration of treatment and the poor baseline condition of the patients. Rastgoo [15] and Yijie [20] reported that the reason why gait function was improved in both the treatment and control groups after the treatment was that patients were similar regarding TUG measures (TUG refers to the time it takes for the subject to stand up after hearing the instruction, walk straight for three meters, and return to the sitting position with the quickest speed [30]). Few of them had barriers to walking. Electrophysiological changes (Hmax/Mmax) were [31] not accompanied by clinical improvement in spasticity in Rastgoo et al. [15], which was similar to the result of Dos Santos et al. [32]. Hmax/Mmax, which has been related to an increase in muscle tone in spasticity to a certain extent, is the ratio of the maximum amplitude of H reflexion and M wave recorded by surface electromyography. Hmax/Mmax does not completely reflect the excitability of neurons with measurement of error, and HSLP/MSLP was proposed as a better outcome [33]. HSLP/MSLP (the ratio of the slope of H and M-waves) was more sensitive to represent the excitability of motor neuron of the anterior horn of the spinal cord [34]. In addition, the reasons for unsynchronized changes were that the treatment times of rTMS were short with only five sessions and the relationship between Hmax/Mmax and MAS is not exact, which may not change with the change of MAS [35]. There were no serious adverse effects reported during the process of treatment. Five studies [15, 17, 20–22] followed up patients after therapy, and Jing [21] reported that FMA, MAS, and MBI scores were significantly different between the treatment and control groups after the 12-month follow-up. rTMS is based on the principle of electromagnetic induction and produces changes at the stimulated site and transsynaptically in distant cortical regions. Sustained physiological effects were a feature of rTMS, namely, long-term potential and long-term depression [36], while neuromuscular electrical stimulation had a short duration of the therapeutic effect without the above features [37], as previously mentioned.
The mechanism by which rTMS improves spasticity is not clear. There are two commonly used models of rTMS: (1) a high-frequency (>1 Hz) facilitatory mode, which is applied to the affected brain region to increase cortical excitability and thus reduce spasticity and improve upper limb motor function, and (2) a low-frequency (≤1 Hz) inhibitory mode, which decreases excitability in the unaffected hemisphere and therefore reduces inhibition from the contralateral hemisphere to the ipsilateral hemisphere [31, 38, 39]. We concluded from the subgroup analysis that the LF-rTMS group showed a significant influence on lower limb motor function, while there were no benefits of HF-rTMS. The better therapy between high- or low-frequency stimulation has been controversial. Based on the guidelines written by Lefaucheur et al. [40], LF-rTMS over the contralesional hemisphere promoted poststroke recovery of motor function in chronic stroke patients. Owing to the limited studies we included, the curative effect of HF-rTMS remains to be discussed. Guo et al. [41] reported that the reorganization of the motor network was found with both HF-rTMS and LF-rTMS, and both improved motor recovery; HF-rTMS had more positive effects on the functional connectivity reorganization of the ipsilesional motor network. In addition to stimulation frequency, the stimulation parameters of rTMS also include stimulation duration and intensity. Further studies about parameters selection that make rTMS optimally effective need to be conducted.
The stimulated site included in this study was the leg motor cortex, which is associated with a deep position that is located on the inner side of the anterior central gyrus under a thick skull [42]. To ensure that rTMS has a good effect on the rehabilitation of patients with lower limb spasticity after stroke, the requirement for stimulation depth was quite high. In addition to the intensity of the rTMS used in the adopted studies (i.e., 80%–90% RMT), the rTMS coil itself also played a pivotal role in the effects of lower limb spasticity after stroke. The types of coils were different across studies included in our analysis: Rastgoo et al. [15] and Yijie [20] used a figure-8-shaped coil with higher focal stimulation and less white matter penetration. Chieffo et al. [19] found that the reason that the use of an H coil with deeper stimulation did not improve spasticity and walking function was that rTMS combined with cycling did not influence the functional networks involved in the coordination of gait and skilled walking. This was also considered the reason for the heterogeneity observed in this meta-analysis. Although the circular coil had deep penetration, the short duration of treatment with unconcentrated stimulation was the reason for the lack of improvements in lower limb MAS scores [17].
The limitations of this study are as follows: first, the risk of bias was slightly high. The three crossover trials included in this paper only contributed data from the first stage to this analysis; thus, the testing power was reduced. In addition, the affected region of the brain is a factor that influences spasticity after stroke, and subgroup analyses of the main muscle groups in the context of lower extremity spasticity were not conducted. The MAS, FMA, and MBI are semiquantitative indicators, and the evaluators may have introduced subjectivity when assessing them. More large-scale and high-quality randomized controlled trials with targeted and precise quantitative indicators as outcomes are expected in the future.
In summary, rTMS was effective in improving spasticity and activities of daily living in patients with lower limb spasticity after stroke. LF-rTMS had a positive effect on enhancing motor function.
Acknowledgments
This study was funded by the S&T Program of Hebei (2037727D).
Data Availability
All the data supporting this systematic review and meta-analysis are included in this study.
Disclosure
Yu Liu and Hong Li are equal contributors and co-first authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Yu Liu and Hong Li designed the work, analysed the data, and drafted the manuscript. Jun Zhang and Qing-qing Zhao screened the literature. Nan-Hao Mei assisted in screening the literature. Jiang Ma revised the work and agreed to be accountable for all aspects of the work.
References
- 1.Paul S. L., Srikanth V. K., Thrift A. G. The large and growing burden of stroke. Current Drug Targets . 2007;8(7):786–793. doi: 10.2174/138945007781077418. [DOI] [PubMed] [Google Scholar]
- 2.Wissel J., Manack A., Brainin M. Toward an epidemiology of poststroke spasticity. Neurology . 2013;80(3, Supplement 2):p. S13. doi: 10.1212/WNL.0b013e3182762448. [DOI] [PubMed] [Google Scholar]
- 3.Pundik S., McCabe J., Skelly M., Tatsuoka C., Daly J. J. Association of spasticity and motor dysfunction in chronic stroke. Annals of Physical and Rehabilitation Medicine . 2019;62(6):397–402. doi: 10.1016/j.rehab.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Foley N., Pereira S., Salter K., et al. Treatment with botulinum toxin improves upper-extremity function post stroke: a systematic review and meta-analysis. Archives of Physical Medicine and Rehabilitation . 2013;94(5):977–989. doi: 10.1016/j.apmr.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Chail A., Saini R. K., Bhat P. S., Srivastava K., Chauhan V. Transcranial magnetic stimulation: a review of its evolution and current applications. Industrial Psychiatry Journal . 2018;27(2):172–180. doi: 10.4103/ipj.ipj_88_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winstein C. J., Stein J., Arena R., et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke . 2016;47(6):e98–e169. doi: 10.1161/STR.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 7.Moslemi Haghighi F., Kordi Yoosefinejad A., Razeghi M., Shariat A., Bagheri Z., Rezaei K. The effect of high-frequency repetitive transcranial magnetic stimulation on functional indices of affected upper limb in patients with subacute stroke. Journal of Biomedical Physics and Engineering . 2021;11(2):175–184. doi: 10.31661/jbpe.v0i0.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dionísio A., Duarte I. C., Patrício M., Castelo-Branco M. The use of repetitive transcranial magnetic stimulation for stroke rehabilitation: a systematic review. Journal of Stroke and Cerebrovascular Diseases . 2018;27(1):1–31. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Fisicaro F., Lanza G., Grasso A. A., et al. Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Therapeutic Advances in Neurological Disorders . 2019;12:1756286419878317–1756286419878322. doi: 10.1177/1756286419878317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntyre A., Mirkowski M., Thompson S., Burhan A. M., Miller T., Teasell R. A systematic review and meta-analysis on the use of repetitive transcranial magnetic stimulation for spasticity poststroke. PM&R . 2018;10(3):293–302. doi: 10.1016/j.pmrj.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Xu P., Huang Y., Wang J., et al. Repetitive transcranial magnetic stimulation as an alternative therapy for stroke with spasticity: a systematic review and meta-analysis. Journal of Neurology . 2020;268:817–832. doi: 10.1007/s00415-020-10058-4.10058-4 [DOI] [PubMed] [Google Scholar]
- 12.Higgins J. P. T., Altman D. G., Gotzsche P. C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ . 2011;343:p. d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhogal S. K., Teasell R. W., Foley N. C., Speechley M. R. The PEDro scale provides a more comprehensive measure of methodological quality than the Jadad scale in stroke rehabilitation literature. Journal of Clinical Epidemiology . 2005;58(7):668–673. doi: 10.1016/j.jclinepi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Harvey L., Herbert R., Crosbie J. Does stretching induce lasting increases in joint ROM? a systematic review. Physiotherapy Research International . 2010;7(1):1–13. doi: 10.1002/pri.236. [DOI] [PubMed] [Google Scholar]
- 15.Rastgoo M., Naghdi S., Nakhostin Ansari N., et al. Effects of repetitive transcranial magnetic stimulation on lower extremity spasticity and motor function in stroke patients. Disability & Rehabilitation . 2016;38(19):1918–1926. doi: 10.3109/09638288.2015.1107780. [DOI] [PubMed] [Google Scholar]
- 16.Meseguer-Henarejos A. B., Sánchez-Meca J., López-Pina J. A., Carles-Hernández R. Inter-and intra-rater reliability of the Modified Ashworth Scale: a systematic review and meta-analysis. European Journal of Physical and Rehabilitation Medicine . 2017;54(4):576–590. doi: 10.23736/S1973-9087.17.04796-7. [DOI] [PubMed] [Google Scholar]
- 17.Hong W., Hua Y., Xiang M., et al. The effect of botulinum toxin type A combined with repetitive transcranial magnetic stimulation on spasticity of lower limbs in stroke patients. Chinese Journal of Rehabilitation Medicine . 2016;31(9):936–940. [Google Scholar]
- 18.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology . 2014;14(1):p. 135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chieffo R., Giatsidis F., Santangelo R., et al. Repetitive transcranial magnetic stimulation with H-coil coupled with cycling for improving lower limb motor function after stroke: an exploratory study. Neuromodulation: Journal of the International Neuromodulation Society . 2020;24:1–7. doi: 10.1111/ner.13228. [DOI] [PubMed] [Google Scholar]
- 20.Yijie C. Effects of repetitive transcranial magnetic stimulation on spasm and motor function of lower limbs in patints with stroke. Chongqing Medical Journal . 2018;47(25):3292–3298. [Google Scholar]
- 21.Jing T., Yan W. Long term efficacy and safety of repetitive transcranial magnetic stimulation combined with repeated injection of botulinum toxin type a in the treatment of spasticity of lower limb muscles spasm after stroke. Journal of Brain and Nervous Diseases . 2018;26(5):272–276. [Google Scholar]
- 22.Yang Y., Lijie H., Xiguo C., Liushuan C., Baoyan Q. Effect of repetitive transcranial magnetic stimulation on limb function recovery in stroke patients with lower limb spasm. Chinese Journal of Physical Medicine and Rehabilitation . 2015;37(8):602–603. [Google Scholar]
- 23.Hua-yao H., Hou-Wei D., Chao C., Yi-xian Z., Qing-fa C. Rehabilitative effect of low-frequency rTMS combined FES on lower limb spasm and motor function in patients with subacute ischemic stroke. Chinese Journal of Cardiovascular Rehabilitation Medicine . 2019;28(2):134–138. [Google Scholar]
- 24.Li S., Francisco G. E. New insights into the pathophysiology of post-stroke spasticity. Frontiers in Human Neuroscience . 2015;9:p. 192. doi: 10.3389/fnhum.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanhoe C. B., Reistetter T. A. Spasticity: the misunderstood part of the upper motor neuron syndrome. American Journal of Physical Medicine and Rehabilitation . 2004;83(10 Suppl):S3–S9. doi: 10.1097/01.phm.0000141125.28611.3e. [DOI] [PubMed] [Google Scholar]
- 26.Miller D. M., Klein C. S., Suresh N. L., Rymer W. Z. Asymmetries in vestibular evoked myogenic potentials in chronic stroke survivors with spastic hypertonia: evidence for a vestibulospinal role. Clinical Neurophysiology . 2014;125(10):2070–2078. doi: 10.1016/j.clinph.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thibaut A., Chatelle C., Ziegler E., Bruno M.-A., Laureys S., Gosseries O. Spasticity after stroke: physiology, assessment and treatment. Brain Injury . 2013;27(10):1093–1105. doi: 10.3109/02699052.2013.804202. [DOI] [PubMed] [Google Scholar]
- 28.Naghdi S., Ansari N. N., Rastgoo M., Forogh B., Jalaie S., Olyaei G. A pilot study on the effects of low frequency repetitive transcranial magnetic stimulation on lower extremity spasticity and motor neuron excitability in patients after stroke. Journal of Bodywork and Movement Therapies . 2015;19(4):616–623. doi: 10.1016/j.jbmt.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Terreaux L., Gross R., Leboeuf F., et al. Benefits of repetitive transcranial magnetic stimulation (rTMS) for spastic subjects: clinical, functional, and biomechanical parameters for lower limb and walking in five hemiparetic patients. Science World Journal . 2014;2014 doi: 10.1155/2014/389350.389350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Podsiadlo D., Richardson S. The timed “up & go:” a test of basic functional mobility for frail elderly persons. Journal of the American Geriatrics Society . 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi M., Pascual-Leone A. Transcranial magnetic stimulation in neurology. The Lancet Neurology . 2003;2(3):145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 32.Dos Santos R. B. C., Galvão S. C. B., Frederico L. M. P., et al. Cortical and spinal excitability changes after repetitive transcranial magnetic stimulation combined to physiotherapy in stroke spastic patients. Neurological Sciences . 2019;40(6):1199–1207. doi: 10.1007/s10072-019-03765-y. [DOI] [PubMed] [Google Scholar]
- 33.Ansari N. N., Naghdi S. The effect of Bobath approach on the excitability of the spinal alpha motor neurones in stroke patients with muscle spasticity. Electromyography & Clinical Neurophysiology . 2007;47(1):29–36. [PubMed] [Google Scholar]
- 34.Phadke C. P., Robertson C. T., Condliffe E. G., Patten C. Upper-extremity H-reflex measurement post-stroke: reliability and inter-limb differences. Clinical Neurophysiology . 2012;123(8):1606–1615. doi: 10.1016/j.clinph.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Ansari N. N., Naghdi S. The effect of Bobath approach on the excitability of the spinal alpha motor neurones in stroke patients with muscle spasticity. Electromyography & Clinical Neurophysiology . 2007;47(1):29–36. [PubMed] [Google Scholar]
- 36.Hoogendam J. M., Ramakers G. M. J., Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimulation . 2010;3(2):95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Lin Z., Yan T. Long-term effectiveness of neuromuscular electrical stimulation for promoting motor recovery of the upper extremity after stroke. Journal of Rehabilitation Medicine . 2011;43(6):506–510. doi: 10.2340/16501977-0807. [DOI] [PubMed] [Google Scholar]
- 38.Corti M., Patten C., Triggs W. Repetitive transcranial magnetic stimulation of motor cortex after stroke. American Journal of Physical Medicine & Rehabilitation . 2012;91(3):254–270. doi: 10.1097/phm.0b013e318228bf0c. [DOI] [PubMed] [Google Scholar]
- 39.Hoyer E. H., Celnik P. A. Understanding and enhancing motor recovery after stroke using transcranial magnetic stimulation. Restorative Neurology and Neuroscience . 2011;29(6):395–409. doi: 10.3233/rnn-2011-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lefaucheur J.-P., Aleman A., Baeken C., et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018) Clinical Neurophysiology . 2020;131(2):474–528. doi: 10.1016/j.clinph.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Guo Z., Jin Y., Bai X., et al. Distinction of high- and low-frequency repetitive transcranial magnetic stimulation on the functional reorganization of the motor network in stroke patients. Neural Plasticity . 2021;2021 doi: 10.1155/2021/8873221.8873221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth Y., Zangen A., Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. Journal of Clinical Neurophysiology . 2002;19(4):361–370. doi: 10.1097/00004691-200208000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting this systematic review and meta-analysis are included in this study.


