Summary
Background
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a severe adverse event of SARS-CoV-2 vaccination. We describe the characteristics of patients reported in Germany based on the Brighton Collaboration (BC) case definition criteria for Thrombosis and Thrombocytopenia Syndrome (TTS) and focus on patients with complete anti-platelet factor 4 (PF4)-antibody laboratory work up.
Methods
The adverse drug reaction database of the Paul-Ehrlich Institute was queried for TTS cases following ChAdOx1 nCoV-19 vaccination from February 1, until May 21, 2021. Cases with reports from the Greifswald laboratory were analysed in detail.
Findings
PF4 antibody tests were available for 69 suspected TTS cases reported to the Paul-Ehrlich Institute, of whom 52 patients fulfilled the BC case definition; 37 (71%) women, 15 (29%) men, median age 46·0 years (interquartile range 31·0-60·3 years). Cerebral venous sinus thrombosis was confirmed in 37 (71%), (additional) multiple thromboses in 19 (37%) patients. Twelve patients died. Non-survivors showed lower platelet counts compared to survivors (median nadir 15,000/µL vs 49,000/µL; p<0·0001). Combined anti-PF4/heparin IgG ELISA and PF4-dependent platelet activation testing yielded sensitivity of 96% (95% confidence interval 87-100%) and specificity of 77% (50-93%) for TTS. Four patients with thrombocytopenia but without thrombosis presented with severe headache or cerebral bleeding, explaining the lower specificity.
Interpretation
VITT has high mortality and can present with isolated thrombocytopenia, severe headache, and bleeding. Demonstration of platelet activating anti-PF4 IgG has high sensitivity for TTS and captures a wider spectrum of clinically relevant VITT than the current BC case definition.
Funding
Deutsche Forschungsgemeinschaft: 374031971-TRR240; Domagk-Programm Universitätsmedizin Greifswald
Keywords: Vaccine, thrombosis, thrombocytopenia, Sars-CoV-2, TTS, VITT
Research in context.
Evidence before this study
Vaccine-induced immune thrombotic thrombocytopenia (VITT) was recognized from March 2021 after vaccination with the adenoviral vector-based vaccines ChAdOx1 nCoV-19, and Ad26nCoV.2S. Strongly reacting anti-platelet factor 4 (PF4) IgG antibodies, which activate platelets in presence of PF4 are found in VITT patients. Apart from VITT, the Thrombosis and Thrombocytopenia Syndrome (TTS) has been defined by the Brighton Collaboration for secondary data analysis following vaccination. We identified all cases with TTS reported in Germany to the federal agency Paul-Ehrlich Institute and provide an analysis of clinical and laboratory features of patients with TTS and confirmed positive PF4-antibody ELISA and functional tests.
Added value of this study
Our nation-wide analysis represents one of the largest cohorts of laboratory confirmed VITT patients. It consolidates that VITT is associated with a high mortality especially at low platelet counts <30,000/µL. It adds the information that a subgroup of patients with VITT presents with isolated thrombocytopenia, severe headache, and bleeding but no thrombosis.
Implications of all the available evidence
The Brighton Collaboration criteria of TTS do not cover all patients with VITT. Combined anti-PF4 IgG ELISA and PF4-enhanced functional testing results in high sensitivity and specificity for TTS and captures a wider spectrum of VITT compared to the Brighton Collaboration case definition.
Alt-text: Unlabelled box
Introduction
Pandemic coronavirus disease 2019 (Covid-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Vaccination against SARS-CoV-2 is key to control this pandemic. Since end of February 2021, venous thromboses at unusual sites including cerebral venous sinus thrombosis (CVST) and/or splanchnic vein thrombosis in combination with moderate to severe thrombocytopenia were observed in individuals approximately 5 to 30 days after vaccination with ChAdOx1 nCoV-19 COVID-19 Vaccine (Astra-Zeneca)1, 2, 3 and with the Covid-19 Vaccine Janssen (Ad26.COV2.S).4,5 We and others identified immunoglobulin G class platelet-activating antibodies directed against the platelet chemokine, platelet factor 4 (PF4; CXCL4), as the underlying cause.1, 2, 3
This adverse reaction to SARS CoV-2 vaccines has been named vaccine-induced immune thrombotic thrombocytopenia (VITT)2,3 as clinical definition for this syndrome, while US and European Union regulatory and public health authorities as well as the Brighton collaboration (BC) introduced the term - thrombosis with thrombocytopenia syndrome - (TTS).6 The BC developed an interim case definition of TTS for use in epidemiological studies based on clinical criteria: i) presence of thrombocytopenia defined as a platelet count below 150,000 per µL, and ii) new thrombosis. Depending on the methods to confirm thrombosis, three levels (1-3) of evidence are used. The case definition excludes patients with recent heparin exposure to avoid confusion with heparin-induced thrombocytopenia. Importantly, the combination of thrombocytopenia and thrombosis can occur in a variety of other clinical situations apart from VITT, such as pulmonary embolism which is unrelated to vaccination.7
The TTS definitions may miss patients with platelet activating anti-PF4-antibodies with either thrombocytopenia before thrombosis develops, or with normal platelet counts and thrombosis. This may be highly relevant, because VITT shows striking similarities with the other diseases caused by anti-PF4 antibodies, namely heparin-induced thrombocytopenia8 and especially autoimmune heparin-induced thrombocytopenia.9 Those patients can present with isolated heparin-induced thrombocytopenia before onset of thrombosis, or with thrombosis and normal platelet counts.8 Notably, VITT can be confirmed by laboratory assays showing anti-PF4 IgG antibodies and PF4-dependent, FcγRIIa-receptor mediated platelet activation by sera of VITT patients.3,10, 11, 12, 13
Here we present the characteristics of all patients with clinical suspicion of VITT based on cases meeting the TTS-criteria, which were reported to the Paul-Ehrlich-Institute (PEI), the German regulatory body for vaccine safety. We put a special focus on patients for whom results of an anti-PF4/polyanion IgG ELISA and a functional test for PF4-dependent platelet activating antibodies from the Greifswald laboratory were available. We provide evidence that the criteria for case definition of TTS by the BC does not cover all patients with VITT and that diagnosis of VITT is improved by applying a combination of an anti-PF4 IgG ELISA and a functional washed platelet assay for PF4-dependent platelet activating antibodies, especially in patients not presenting with the typical combined symptoms of thrombocytopenia and thrombosis at unusual sites occurring 5-20 days after vaccination against SARS-CoV-2.
Methods
Ethics
The laboratory study has been approved by the ethics committee of the Universitätsmedizin Greifswald. Data protection was approved by the data protection officer of the PEI.
Data collection
Reports of patients with adverse reactions after vaccination were provided to the PEI by treating physicians as required by the German Infection Protection Act. This included hospitalized and non-hospitalized patients. A search strategy was applied to ensure identification of all relevant individual case safety reports using the Standardized Medical Dictionary for Regulatory Activities (MedDRA) using the Standardised MedDRA Queries14 (SMQs): Embolic and thrombotic events, vessel type unspecified and mixed arterial and venous; Embolic and thrombotic events, venous; Embolic and thrombotic events; Embolic and thrombotic events, arterial; Thrombophlebitis; and Hematopoietic thrombocytopenia. The search for thrombocytopenia included additional terms not captured by the SMQ: heparin-induced thrombocytopenia type II, autoimmune thrombocytopenia, heparin-induced thrombocytopenia test, idiopathic thrombocytopenia, heparin-induced thrombocytopenia NOS, heparin-induced thrombocytopenia, non-immune heparin-associated thrombocytopenia, spontaneous heparin-induced thrombocytopenia syndrome, autoimmune heparin-induced thrombocytopenia, immune-mediated thrombocytopenia, heparin-induced thrombocytopenia mimicking thromboembolic disorders, immune thrombocytopenia, primary immune thrombocytopenia, secondary immune thrombocytopenia, and persistent thrombocytopenia.
The PEI adverse drug reaction database was queried for case reports from Germany from February 1, until May 21, 2021. Results were manually reviewed by a physician for case reports of thrombosis with thrombocytopenia. Patients were assessed using the interim case definition of the BC i) presence of thrombocytopenia defined as a platelet count below 150,000 per µL, and ii) new thrombosis, with three levels of evidence depending on the methods to confirm thrombosis (level 1: Imaging, surgical, or pathology findings; Level 2: Clinical presentation with supporting imaging or laboratory findings (D-dimer); Level 3: Clinical presentation consistent with Thrombosis or Thromboembolism Event).6
Serological testing
Independently, blood samples were sent to the laboratory of the Department of Transfusion Medicine, University Medicine Greifswald at the discretion of the treating physicians for testing by anti-PF4/heparin IgG ELISA. Results were given in optical density (OD) units (reference range <0·50; weak reaction 0·5 to ≤1·0 units; strong reaction >1·0 units). Further, a functional washed platelet assay to detect PF4-dependent, platelet activating antibodies3 in the presence of PF4 (10µg/mL)15 was performed. Pseudonymised results were forwarded as follow up information to the PEI.
Statistical analysis
Descriptive data are presented as nominal values and percentages; medians and interquartile ranges (IQR) as indicated. The 95% confidence intervals were estimated using the exact Clopper-Pearson method. For comparison of binary variables (sex, CVST, and multiple thrombosis) between non-survivors and survivors, Fisher's exact test was applied (reporting the p-value as double the exact one-tailed probability, alpha=0·05). For comparison of continuous variables (age, time to onset, platelet count, and D-Dimer) between non-survivors and survivors, the non-parametric Wilcoxon Rank Sum test was used (Exact; in case of age: normal approximation; two-sided, alpha=0·05).
Sensitivity and Specificity for the clinical diagnosis of TTS according to the BC case definition were calculated for the combined results of the anti-PF4/heparin IgG ELISA and the washed platelet assay for PF4-dependent platelet activating antibodies (either both positive or both negative; a result of ELISA positive and PF4-dependent platelet activating antibodies negative was considered “negative”). A secondary analysis was performed in which a strongly positive anti-PF4/heparin IgG ELISA (OD > 2·0) with PF4-dependent platelet activating antibodies negative was considered “positive”, if it was known that the sample was obtained post-intravenous immunoglobulin administration. Sensitivity was calculated as the proportion of TTS patients tested positive in the total group of TTS patients (= 50/52 or 52/52, respectively). Specificity was calculated as the proportion of non-TTS patients tested negative in the group of all non-TTS (= 13/17; Table 3). Statistical analyses were performed with SAS ®/STAT software, version 9·4, SAS System for Windows.
Table 3.
Results for anti-PF4/polyanion IgG and PF4-dependent platelet activating antibodies in the patient cohorts with thrombosis, thrombocytopenia, or both (TTS) after vaccination with ChAdOx1 nCov-19 (n=69).
| anti-PF4/heparin IgG ELISA (positive/tested) | heparin-dependent platelet activation (positive/tested) | PF4 dependent platelet activation (positive/tested) | |
|---|---|---|---|
| TTS patients | 52/52 (49 strongly, OD >1.0) |
13/50 | 50/52* |
| patients with thromboses without thrombocytopenia | 1/9 (1 weakly, OD <1.0) |
0/9 | 0/9 |
| patients with thrombocytopenia without thromboses | 4/8 (4 strongly, OD>1.0) |
1/8 | 4/8 |
Two patients with negative PF4 dependent platelet activation tests were pretreated with intravenous immunoglobulins (can inhibit the functional test).
Role of the funding source
The funders provided the financial resources to perform the experiments. Funders had no role in the study design, data collection, data analysis, interpretation and writing of the report.
Results
TTS cases reported to the PEI
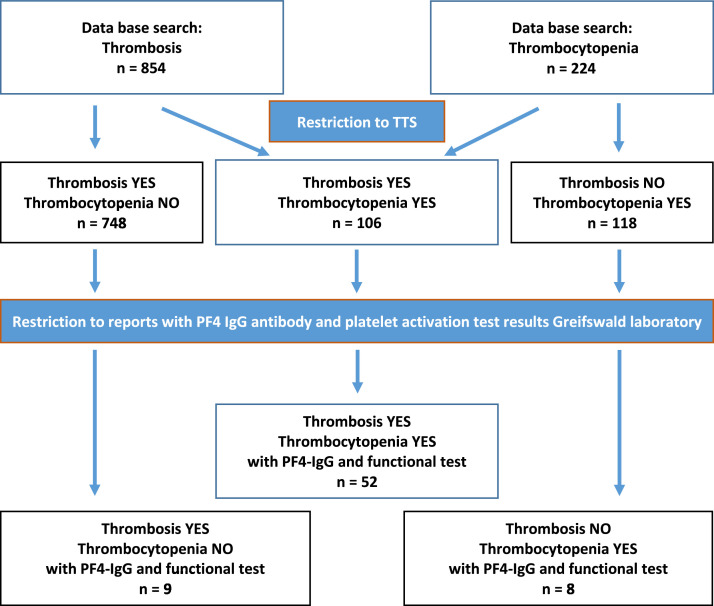
Within the study period, 854 patients with thrombosis and 224 patients with thrombocytopenia after vaccination with ChAdOx1 nCoV-19 COVID-19, were reported (Figure 1). Among patients with thrombosis, 748 had no documented thrombocytopenia; among patients with thrombocytopenia 118 had no documented thrombosis. For 106 individuals thrombosis and thrombocytopenia were reported; fulfilling the BC case definition criteria level 1 (n=73), 2 (n=7), and 3 (n=26)). Of these 106 patients, 73 were female (69%; median age 56·0 years; IQR 35·5-64·3) and 33 male (median age 54·0 years; IQR 33·0-63·0).
Figure 1.
Notified cases of thrombosis, thrombocytopenia, and TTS in Germany (01.02. - 21.05.2021) after ChAdOx-1 nCoV-19 vaccination.
The most frequent type of thrombosis was CVST, affecting 56/106 (53%) patients (42 women; 14 men). Among them 27/56 (48%) developed mostly secondary cerebral hemorrhage. Twenty-two (21%) patients presented with multiple thromboses; 24 (23%) with pulmonary embolism; 11 (10%) with splanchnic vein thromboses. Fatality rate was 21/106 (20%; [women 13/73 (18%); men 8/33 (24%)]) and in patients with CVST 14/56 (25%; [women 9/42 (21%); men 4/14 (29%)]).
TTS cohort with available anti PF4-IgG tests
Patient characteristics and clinical data
Of the 106 TTS cases identified by the PEI according to the BC case definition, in 52 (49%) laboratory results from Greifswald were available; 37 (71%) women, 15 (29%) men, median age 46·0 years (IQR 31·0 to 60·3 years). Their characteristics and clinical data are summarized in Tables 1 and 2. Fifty one out of 52 cases were classified as certain TTS (Level 1), one as likely TTS (Level 2; BC case definition). All cases were characterized as life threatening. CVST was confirmed in 37 (71%) patients, and multiple concomitant thromboses at different locations in 19 (37%) patients. Twelve patients died (23%, 10/12 had CVST, 7/12 had multiple thromboses).
Table 1.
Characteristics of 52 TTS patients with complete anti-PF4 antibody test results.
| Female |
Male |
|||||
|---|---|---|---|---|---|---|
| Age Category [years] | TTS (fatal) | CVST (fatal) | Multiple thromboses (fatal) | TTS (fatal) | CVST (fatal) | Multiple thromboses (fatal) |
| 18-39 | 10 (1) | 8 (1) | - | 10 (3) | 8 (2) | 5 (2) |
| 40-59 | 16 (4) | 11 (3) | 5 (1) | 2 (0) | 1 (0) | 1 (0) |
| 60+ | 11 (2) | 7 (2) | 5 (2) | 3 (2) | 2 (2) | 3 (2) |
| Total | 37 (7) | 26 (6) | 10 (3)* | 15 (5) | 11 (4) | 9 (4)* |
| Median Age [years]IQR | 4936-60 | 3629-48 | ||||
Abbr: TTS Thrombosis with thrombocytopenia; CVST cerebral venous sinus thrombosis; IQR interquartile range.
Definition of multiple thromboses: ≥ 2 thromboses in one patient at different sites and not linked to each other;
includes splanchnic thrombosis.
Table 2.
Clinical information of 52 TTS case reports with complete anti-PF4 antibody test results.
| Item | Measure | Non-survivors | Survivors | p-value |
|---|---|---|---|---|
| TTS | Total number | 12 | 40 | – |
| Platelet Count, Nadir [platelets/µl] | Median IQR |
15,000 10,000 - 18,000 |
49,000 27,000 - 64,000 |
<0·0001 |
| D-Dimer [mg/L] |
Median IQR number assessed |
24·8 20·0 – 36·0 9 |
30·7 17·0 – 35·0 27 |
0·75 |
| Age [years] | Median IQR |
49·0 35·5 – 62·0 |
44·5 29·0 – 59·5 |
0·45 |
| Sex [Female] | number [%] | 7 [58] | 30 [75] | 0·44 |
| CVST | number [%] | 10 [83] | 27 [68] | 0·50 |
| Multiple thrombosis | number [%] | 7 [58] | 12 [30] | 0·15 |
| Time to onset [days] | Median IQR |
9·0 7·5 – 11·0 |
10·0 7·0 – 12·0 |
0·86 |
Abbr: TTS Thrombosis with thrombocytopenia; CVST cerebral venous sinus thrombosis; IQR interquartile range.
Patients with a fatal outcome showed lower platelet counts compared to those who survived (median nadir 15,000 vs. 49,000; p <0.0001). D-dimer values ranged between 2·5 - 54 mg/L (median 29·8 mg/L; reference range <0·5 mg/L) and did not differ significantly between non-survivors and survivors (median 24·8 vs. 30·7 mg/L; p = 0·75). No significant differences were seen for sex and the incidences of CVST and multiple thrombosis between survivors and non-survivors in our study. Mortality neither differed significantly between CVST and non CVST-patients (27% vs 13%, p=0·50); nor between patients with multiple thromboses and those without (37% vs 15% p=0·15). Further, median time between vaccination and first onset of symptoms was 9·0 days (IQR 7·5 –11·5 days) and comparable between both groups (median 9·0 vs.10·0 days; p = 0·86; Table 2)
In 13 (25%) patients, first symptoms occurred between days four (n=2) and seven, in 33 (64%) in the second, and in 6 (12%) in the third week after vaccination. The most frequent first symptoms in patients with CVST were headache (25/37; 68%), nausea and vomiting (8/37; 22%), and convulsions (5/37; 14%). Treatment was heterogeneous. In addition to heparin, low molecular weight heparin, non-heparin anticoagulants and high dose intravenous immunoglobulins, patients received various other medications such as corticosteroids, eculizumab (to inhibit complement activation), and/or platelet transfusions. Twelve patients required craniectomy to reduce intracranial pressure (8 survived); in nine patients, catheter-assisted thrombectomy was performed (in one craniotomy and thrombectomy). One patient required splenectomy due to ruptured spleen and haemorrhagic shock.
Of note, in a 63-year-old male patient VITT may have persisted. Twelve days post vaccination (first dose) he developed pulmonary embolism and thrombocytopenia (26,000/µl). He was discharged from hospital at day 17 in stable condition, anticoagulated with therapeutic dose Factor Xa inhibitor. At day 22 he presented with headache and platelet count had increased to 82,000/µL. At that time, cranial computer tomography did not reveal signs of CVST. At day 27, he developed extensive CVST and cerebral haemorrhage (platelet count 71,000/µl).
PF4-antibody test results
All 52 cases classified as TTS according to the BC case definition tested positive for anti-PF4/heparin IgG. Forty-nine patients tested strongly positive with ODs >1 and three tested weakly positive in the ELISA (ODs between 0·5-1·0). Fifty patients tested also positive for PF4-dependent platelet activating antibodies in the functional assay. The two TTS cases who tested negative for PF4-dependent platelet activating antibodies had received body weight adjusted intravenous immunoglobulins two days before the blood sample was taken, which is known to inhibit the assay.16 In 13 (25%) patients, antibodies also activated platelets in the presence of heparin (and buffer).
For an additional 17 cases reported to the PEI with suspected VITT, antibody tests were available. These cases did not fulfill the criteria of the BC case definition for TTS (thrombosis without thrombocytopenia, n=9; thrombocytopenia without thrombosis, n=8) (Table 3). Of the nine cases with thromboses without thrombocytopenia one patient tested weakly positive for anti-PF4/polyanion IgG (OD < 1·0) but none tested positive for either heparin- or PF4-dependent platelet activating antibodies. Of the eight cases with isolated thrombocytopenia, four tested positive for anti-PF4/heparin IgG and for PF4-dependent platelet activating antibodies. As common features, these cases showed additional cerebral symptoms (2 had cerebral bleeding, 2 had severe headache and 3 of 4 had elevated D-Dimer values (in one case D-dimer values were not available due to sudden death).
The combination of a positive anti-PF4/polyanion IgG ELISA together with a positive functional washed platelet assay for PF4-dependent platelet activating antibodies results in a sensitivity of 96% (95% confidence interval: 87-100%) and a specificity of 77% (95% confidence interval: 50-93%) for TTS according to the BC case definition. If the patient was considered “positive” with a strongly positive anti-PF4/heparin ELISA (OD >2·0) despite a negative functional washed platelet assay due to known prior intravenous immunoglobulin administration, sensitivity increases to 100% (95% confidence interval 93-100%) while specificity remains unchanged.
Discussion
We report a large cohort of VITT-patients who meet the TTS criteria in a temporal relationship with ChAdOx1 nCoV-19 vaccination and for whom results of combined anti-PF4 IgG and PF4-dependent platelet activating antibody testing are available. Our cohort is representative including approximately half (49%) of all 106 TTS patients reported in Germany from the beginning of ChAdOx1 nCoV-19 vaccination in February until May 21, 2021. The young median age and strong female preponderance most likely reflect the vaccination recommendations in Germany. Until beginning of April 2021 ChAdOx1 nCoV-19 was recommended for certain occupational groups such as health care professionals and for persons below the age of 65.
VITT is a life-threatening adverse complication. Overall mortality was high in our cohort with 23%, and with 27% in patients with CVST. The risk of mortality was significantly increased when patients presented with a platelet count below 30,000/µL. This is in line with two recent reports showing 22% mortality in patients with VITT17 and 47% of patients with VITT and CVST in the United Kingdom.18 Treatment of patients had been very heterogeneous reflecting the uncertainty how to handle this new disease at the beginning. Importantly, we observed one patient with initial pulmonary embolism and thrombocytopenia with a potentially ongoing inflammatory process, leading to a new CVST after initial pulmonary embolism and who had discontinued anticoagulation. VITT patients with persistent or new occurrence of severe headache may require longer term monitoring of thrombocytopenia and/or D-dimer levels.
The first reports on VITT highlighted the clinical presentation of thrombosis and thrombocytopenia associated with a positive anti-PF4/heparin IgG ELISA.1, 2, 3, 4 Already in the first case series, we observed that these patients also present with PF4-dependent platelet activating antibodies.3 The finding of PF4-dependent platelet activating antibodies in a functional test is therefore the definite prove of VITT in symptomatic patients (in analogy to the laboratory diagnosis of heparin induced thrombocytopenia). We evaluate here the combination of clinical criteria, an anti-PF4/heparin IgG ELISA and a functional test for PF4-dependent, platelet-activating antibodies to diagnose VITT in a large series of patients. Our search revealed additional seven TTS cases with PF4/Heparin antibody results available from other laboratories.19,20 All showed a positive PF4/heparin IgG ELISA, in one patient also a positive PF4-dependent platelet activation test was reported. Due to differences in the methodology these cases were not considered in our detailed evaluation.
We found a very high sensitivity for TTS of 96% for the combination of a positive anti-PF4/polyanion IgG ELISA and the presence of PF4-dependent, platelet activating antibodies. The two cases missed had been pre-treated with high-dose intravenous immunoglobulin, which is known to inhibit the functional assay. If these post-intravenous immunoglobulin test results were considered “positive”, sensitivity would reach 100%. Laboratories need to be aware about this risk of false negative results and physicians should obtain blood samples for laboratory testing before treatment with intravenous immunoglobulin is started. Specificity of the test combination was 78% (72%, if all post-intravenous immunoglobulin test results were considered “positive”). The lower specificity was explained by four additional patients with thrombocytopenia but no thrombosis. Importantly, all four patients presented with severe cerebral symptoms. Among them two patients had cerebral bleeding; and elevated D-dimer levels further indicate a pro-thrombotic state. We cannot rule out that either sudden death or early anticoagulation has masked or prevented the occurrence of thromboses in these patients.
However, our data indicate that the interim BC case definition of TTS may not detect all patients with VITT. We identified patients with thrombocytopenia in the same range as observed in VITT patients and severe headache and/or cerebral bleeding (without documented thrombosis) who had high-titer PF4-dependent, platelet-activating anti-PF4 antibodies. While the interim BC case definition might be suitable for secondary data analysis, our data suggest that the spectrum of VITT may be expanded for primary data analysis including patients with thrombocytopenia and persistent clinical symptoms such as severe headache and/or sudden onset of severe hemorrhage—if patients test positive in a PF4/heparin ELISA and for PF4-dependent, platelet activating antibodies.
The Centre for Disease Control acknowledges the variety of clinical situations with TTS and suggests for tier 2 TTS definition the inclusion of a positive PF4/polyanion ELISA test21 to cover VITT patients more precisely. Nevertheless, the PF4/polyanion ELISA alone may not be specific enough for VITT. It had been positive in 5-8% of the vaccinated individuals without any adverse symptoms.22 In fact, one out of nine patients with thrombosis but no thrombocytopenia showed a weakly positive PF4/polyanion ELISA, but a negative functional test. On the other hand, thrombocytopenia is a major criterion for the TTS case definition. In all cases with a positive anti-PF4/heparin IgG ELISA and a positive PF4-dependent platelet activation test, thrombocytopenia was present. This underpins that VITT patients typically present with thrombocytopenia. Given the many similarities between heparin-induced thrombocytopenia and VITT, thrombotic events in VITT can also occur in the absence of thrombocytopenia23 as it is well established in heparin-induced thrombocytopenia patients.8
We propose to include the presence of anti-PF4/polyanion IgG and PF4-dependent platelet activating anti-PF4 antibodies into the definition of TTS to better target VITT patients for clinical diagnosis and research. This strongly parallels the recommendations for the diagnosis of the other clinically important disease caused by anti-PF4 antibodies, heparin-induced thrombocytopenia.24 We acknowledge that the diagnosis of VITT can be made without a functional assay, when patients present with the typical symptoms of thrombocytopenia, high D-dimer levels and thrombosis 5-20 days after vaccination with an adenoviral vector-based vaccine. However, the functional test is especially relevant for unusual presentations like early onset of thrombosis after vaccination with an adenoviral vector-based vaccine or suspected VITT after vaccination with mRNA vaccines. This may avoid misinterpretation of coincidental occurrence of thrombosis in the first weeks after vaccination. More difficult, washing of platelets requires expertise and skilled staff. Recently we introduced a whole blood flow cytometry-based assay, which seems to correlate reasonably with our washed platelet assay and might be easier to be established by routine laboratories.11
The strength of our study is that we were able to assess the complete cohort of patients with TTS in Germany and to focus on laboratory confirmed VITT cases. This excludes patients who developed TTS for other reasons and allowed to assess sensitivity and specificity of anti-PF4 testing. Our study is limited by the typical reporting bias of a spontaneous reporting system, because patients who may have had less severe VITT with asymptomatic thrombocytopenia without thrombosis are usually not detected. Along the same lines, the presence of a CVST would result in a high likelihood of inclusion whereas a less complex thrombotic event may have been overlooked and not reported. Finally, the specificity-analysis of our assays is limited because asymptomatic individuals are typically not reported to the national agency. To overcome this limitation, we can refer to an analysis of 281 health care workers vaccinated against Covid-19 without symptoms of VITT.22 None of them showed a positive result in the functional assay, including the 19 individuals with a positive anti-PF4/heparin IgG ELISA which corroborates the high specificity of our laboratory approach. It is possible, albeit unlikely that minor thrombotic events were overseen in the patients presenting without thrombotic event. Importantly, the presence of platelet activating anti-PF4 IgG in patients with cerebral symptoms is sufficient to establish the diagnosis of VITT (or “VITT without thrombosis”) and to start anticoagulation to avoid thrombotic complications, similar to what is recommended in heparin induced thrombocytopenia without thrombosis.24
The 106 TTS cases of our study were reported on the background of 9 million vaccinations with ChAdOx1 in Germany until the end of May 2021. The PEI database revealed six cases of suspected TTS after BNT162b2 (Biontech/Pfizer) and none after Covid-19 Vaccine Moderna (Moderna Biotech) in the same time period25 on the background of 42 million vaccinations with mRNA vaccines. In contrast, Burn et al. report that safety profiles of BNT162b2 and ChAdOx1 were similar and that no safety signals were seen for TTS in Spain.26 Further, only 1·7 thrombotic events per 100 000 participants were detected in a study of health care workers vaccinated with the vector-based Ad26.COV2.S in South Africa, and none of these cases was related to VITT.27 These various outcomes may reflect differences of the regional vaccination programs, between the vaccinated populations and the different approaches to collect the data. As VITT is a rare complication the investigated cohorts may also still be too small to detect any difference between the vaccines.
An open question is, whether VITT is exclusively seen after application of vector-based vaccines. Apart from VITT, which is mediated by PF4 antibodies, other forms of TTS may occur after vaccination with mRNA-based vaccines and in Covid-19. No systematic study to date could show that persons vaccinated with mRNA vaccines develop anti-PF4-IgG mediated thrombotic thrombocytopenia, similar to what is seen with vector-based vaccines. We found only a low incidence of PF4-IgG antibodies in Covid-19 patients and these antibodies do not activate platelets in a functional assay,28 which is in contrast to the pattern seen in VITT. Hence, TTS seen after vaccination with mRNA-based vaccines or in Covid-19 may have different pathomechanisms and involve antibodies recognizing other epitopes.29 This must be addressed by future studies.
Given risk of Covid-19 infection and its complications, the benefits of vaccination against Covid-19 by far outweigh the risk of vaccine-related complications and the risk of an ongoing pandemic in many parts of the world. Our analysis characterizes a large cohort of laboratory confirmed VITT and underscores that this condition requires immediate attention and action by physicians. In this context, our analysis adds important data contributing to the safety of the world-wide vaccination campaign and supports ongoing efforts to fight the SARS-CoV-2 pandemic.
In conclusion, VITT is associated with a high mortality especially at platelet counts < 30,000/µL. A subgroup of patients with VITT present with isolated thrombocytopenia and severe headache. The case definition for TTS should be expanded accordingly. The presence of anti-PF4 IgG antibodies and PF4-dependent platelet activating antibodies has a very high sensitivity and specificity for VITT.
Acknowledgments
Contributors
TT, AG and BKS developed the concept and analysed patient data; KW, MF and GW collected clinical data from reported cases, analysed and verified the dataset; TT and LS collected all laboratory results of the Greifswald laboratory patients. All authors wrote the manuscript. All authors have critically revised and approved the final version of the manuscript.
Declaration of interests
Dr. Thiele reports grants from Deutsche Forschungsgemeinschaft, during the conduct of the study; personal fees, non-financial support and other from Bristol Myers Squibb, personal fees, non-financial support and other from Pfizer, personal fees from Bayer, personal fees, non-financial support and other from Chugai Pharma, non-financial support and other from Novo Nordisk, personal fees from Novartis, non-financial support and other from Daichii Sankyo, outside the submitted work;
Dr. Greinacher reports grants from Deutsche Forschungsgemeinschaft, grants from Deutsche Forschungsgemeinschaft, during the conduct of the study; personal fees from Aspen, grants from Ergomed, grants from Boehringer Ingelheim, personal fees from Bayer Vital, grants from Rovi, grants from Sagent, personal fees from Chromatec, personal fees from Instrumentation Laboratory, grants and personal fees from Macopharma, grants from Portola, grants from Biokit, personal fees from Sanofi-Aventis, grants from Fa. Blau Farmaceutics, grants from Prosensa/Biomarin, grants and other from DRK-BSD NSTOB, grants from DRK-BSD Baden-Würtemberg/Hessen, personal fees from Roche, personal fees from GTH e.V., outside the submitted work; In addition, Dr. Greinacher has a patent modified SARS CoV 2 vaccine pending.
Dr. Weisser, Dr. Funk, Dr. Weber, Dr. Schönborn and Dr. Keller-Stanislawski have nothing to disclose.
All conflict of interest statements are uploaded as separate files.
Acknowledgements
The work of the technologists Ulrike Strobel, Carmen Freyer, Katrin Stein, Ines Warnig, Ricarda Raschke, Jessica Fuhrmann, Nicole Lembke, Transfusion Medicine Greifswald, is highly appreciated.
Data sharing statement
Deidentified report forms collected for this study, including individual deidentified participant data will be made available to other researchers upon request with publication of this manuscript for a maximum of 5 years. Researchers need to provide a sound proposal which will need to be approved and to sign a data access agreement. Proposals should be directed to: andreas.greinacher@med.uni-greifswald.de
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2021.100270.
Contributor Information
Thomas Thiele, Email: thomas.thiele@med.uni-greifswald.de.
Brigitte Keller-Stanislawski, Email: brigitte.keller-stanislawski@pei.de.
Appendix. Supplementary materials
References
- 1.Scully M, Singh D, Lown R, et al. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz NH, Sorvoll IH, Michelsen AE, et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.See I, Su JR, Lale A, et al. US Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N Engl J Med. 2021;384(20):1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://brightoncollaboration.us/wp-content/uploads/2021/05/TTS-Interim-Case-Definition-v10.16.3-May-23-2021.pdf (accessed August 2, 2021 ).
- 7.Warkentin TEC. In: Heparin-induced thrombocytopenia. Warkentin TEG, editor. Informa Health Care; London: 2012. A. Differential diagnosis of heparin-induced thrombocytopenia and scoring systems; pp. 77–109. A. [Google Scholar]
- 8.Greinacher A., CLINICAL PRACTICE Heparin-Induced Thrombocytopenia. N Engl J Med. 2015;373(3):252–261. doi: 10.1056/NEJMcp1411910. [DOI] [PubMed] [Google Scholar]
- 9.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. Journal of thrombosis and haemostasis: JTH. 2017;15(11):2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 10.Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopenia. Nature. 2021 doi: 10.1038/s41586-021-03744-4. [DOI] [PubMed] [Google Scholar]
- 11.Handtke S, Wolff M, Zaninetti C, et al. A flow cytometric assay to detect platelet-activating antibodies in VITT after ChAdOx1 nCov-19 vaccination. Blood. 2021;137(26):3656–3659. doi: 10.1182/blood.2021012064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachs UJ, Cooper N, Czwalinna A, et al. PF4-dependent immunoassays in patients with vaccine-induced immune thrombotic thrombocytopenia (VITT): results of an inter-laboratory comparison. Thromb Haemost. 2021 doi: 10.1055/a-1535-9002. [DOI] [PubMed] [Google Scholar]
- 13.Vayne C, Rollin J, Gruel Y, et al. PF4 Immunoassays in Vaccine-Induced Thrombotic Thrombocytopenia. N Engl J Med. 2021 doi: 10.1056/NEJMc2106383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.http://www.meddra.org/how-to-use/tools/smqs (accessed August 2, 2021).
- 15.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct Immune Globulin for Vaccine-Induced Thrombotic Thrombocytopenia. N Engl J Med. 2021 doi: 10.1056/NEJMoa2107051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavord S, Scully M, Hunt BJ, et al. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N Engl J Med. 2021 doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry RJ, Tamborska A, Singh B, et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: a multicentre cohort study. Lancet. 2021 doi: 10.1016/S0140-6736(21)01608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiede A, Sachs UJ, Czwalinna A, et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccine. Blood. 2021 doi: 10.1182/blood.2021011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf ME, Luz B, Niehaus L, Bhogal P, Bazner H, Henkes H. Thrombocytopenia and Intracranial Venous Sinus Thrombosis after "COVID-19 Vaccine AstraZeneca" Exposure. J Clin Med. 2021;10(8) doi: 10.3390/jcm10081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.https://www.cdc.gov/mmwr/volumes/70/wr/mm7017e4.htm. (accessed August 2, 2021 ).
- 22.Thiele T, Ulm L, Holtfreter S, et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 2021 doi: 10.1182/blood.2021012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walter U, Fuchs M, Grossmann A, et al. Adenovirus-Vectored COVID-19 Vaccine-Induced Immune Thrombosis of Carotid Artery: A Case Report. Neurology. 2021 doi: 10.1212/WNL.0000000000012576. [DOI] [PubMed] [Google Scholar]
- 24.Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360–3392. doi: 10.1182/bloodadvances.2018024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul-Ehrlich-Institute. Reports on suspected cases of adverse effects and vaccination complications following a vaccination for the protection against COVID-19 (reporting period 27 December 2020 to 31 May 2021) [German only] Available at: https://www.pei.de/EN/newsroom/dossier/coronavirus/coronavirus-content.html;jsessionid=2309214CB20790ADB168B096CEBA3CA0.intranet221?cms_pos=6. (accessed September 24, 2021 ).
- 26.Burn E, Roel E, Pistillo A, et al. Thromboembolic Events and Thrombosis With Thrombocytopenia After COVID-19 Infection and Vaccination in Catalonia, Spain. Available at http://dxdoiorg/102139/ssrn3886421 2021.
- 27.Takuva S, Takalani A, Garrett N, et al. Thromboembolic Events in the South African Ad26.COV2.S Vaccine Study. N Engl J Med. 2021;385(6):570–571. doi: 10.1056/NEJMc2107920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greinacher A, Selleng K, Mayerle J, et al. Anti-Platelet Factor 4 Antibodies Causing VITT do not Cross-React with SARS-CoV-2 Spike Protein. Blood. 2021 doi: 10.1182/blood.2021012938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Althaus K, Marini I, Zlamal J, et al. Antibody-induced procoagulant platelets in severe COVID-19 infection. Blood. 2021;137(8):1061–1071. doi: 10.1182/blood.2020008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.