Abstract
Infection by a single virus can evoke diverse immune responses, resulting in different neurological outcomes, depending on the host's genetic background. To study heterogenous viral response, we use Theiler's Murine Encephalomyelitis Virus (TMEV) to model virally induced neurological phenotypes and immune responses in Collaborative Cross (CC) mice. The CC resource consists of genetically distinct and reproducible mouse lines, thus providing a population model with genetic heterogeneity similar to humans. We examined different CC strains for the effect of chronic stage TMEV-induced immune responses on neurological outcomes throughout 90 days post infection (dpi), with a particular focus on limb paralysis, by measuring serum levels of 23 different cytokines and chemokines. Each CC strain demonstrated a unique set of immune responses, regardless of presence or absence of TMEV RNA. Using stepwise regression, significant associations were identified between IL-1α, RANTES, and paralysis frequency scores. To better understand these interactions, we evaluated multiple aspects of the different CC genetic backgrounds, including haplotypes of genomic regions previously linked with TMEV pathogenesis and viral clearance or persistence, individual cytokine levels, and TMEV-relevant gene expression. These results demonstrate how loci previously associated with TMEV outcomes provide incomplete information regarding TMEV-induced paralysis in the CC strains. Overall, these findings provide insight into the complex roles of immune response in the pathogenesis of virus-associated neurological diseases influenced by host genetic background.
Keywords: Chronic infection, Collaborative cross, Cytokine, Host response, IL-1 α, Paralysis, RANTES, TMEV, Viral infection
Abbreviations: Amyotrophic Lateral Sclerosis, (ALS); Multiple Sclerosis, (MS); Parkinson's disease, (PD); Theiler's murine encephalomyelitis virus, (TMEV); days post infection, (dpi); central nervous system, (CNS); Collaborative Cross, (CC); phosphate buffered saline, (PBS); plaque-forming units, (PFU); intraperitoneal, (IP); experimental autoimmune encephalitis, (EAE); receptor for IL-1 α, (Il1r1); Epstein-Barr Virus, (EBV); blood brain barrier, (BBB); Chromosome, (Chr)
Highlights
-
•
Paralysis resulting from long-term TMEV infection varies by host genetic background.
-
•
For CC mice, no single immune environment causes paralysis following TMEV infection.
-
•
IL-1A and RANTES significantly influence the progression of paralysis over time.
-
•
H2 haplotype alone did not predict viral presence or TMEV-induced paralysis.
-
•
Paralysis severity is influenced by multiple TMEV- and immune-related loci.
1. Introduction
Neurological diseases such as Amyotrophic Lateral Sclerosis (ALS) (Fang et al., 2015), epilepsy (Michael and Solomon 2012), Multiple Sclerosis (MS) (Steelman 2015), and Parkinson's disease (PD) (Woulfe et al., 2014) can, in some cases, stem from antecedent exposure to viral infections. Predisposing genetic risk factors for neurological dysfunction following viral infection will vary among individuals in a genetically diverse population, leading to a range of outcomes from complete lack of symptoms to death. Immune responses to neurotropic viruses can also vary among individuals depending on several factors including age, sex, psychological state, nutritional status, and genetic background. As a result, infection by a single virus can evoke diverse immune responses and neurological outcomes depending on the host's genetic background.
Theiler's murine encephalomyelitis virus (TMEV), a neurotropic single stranded RNA virus that belongs to the family Picornaviridae, is used to model human neurological conditions associated with prior viral infections (Lipton 1975, 1980; Procaccini et al., 2015). Depending on the genetic background of the mouse strain, TMEV infection can cause varied pathogenic responses that model heterogenous infections associated with human diseases such as ALS, epilepsy, MS, and PD (Brinkmeyer-Langford et al., 2017). In susceptible mouse strains such as SJL/J, intracranial inoculation of TMEV results in a biphasic disease, comprising an early acute phase lasting up to 14 days post-infection (dpi) followed by a late chronic phase after day 35 dpi (Oleszak et al., 2004; Steelman 2015). Early acute disease encompasses a mild encephalomyelitis with predominant neuronal degradation and replication of the virus in the central nervous system (CNS) (Murray et al., 1998; Oleszak et al., 1995). Susceptible strains are unable to clear the virus and a persistent CNS infection leads to progressive spinal cord atrophy and axonal loss causing disrupted motor coordination, limb paralysis, spasticity, and ataxia (DePaula-Silva, Tyler J. Hanak et al., 2017; McGavern 2000; Rodriguez et al. 1991). In contrast, some resistant mouse strains, such as C57BL/6, are able to clear the virus completely within 3 weeks post infection and do not develop severe demyelinating disease, but instead exhibit epileptic seizures within the first few days post-infection and hippocampal lesions (Bijalwan et al., 2019; Bröer et al., 2016; Gerhauser et al. 2007; Libbey et al., 2008; Stewart et al., 2009).
TMEV susceptibility and disease symptoms have been associated with several genetic risk factors (Tsunoda et al., 2016), and differences in immune response play a role in determining strain differences in viral load and clearance, or persistence in the chronic phase of the infection (Aubagnac et al. 1999; Mi et al., 2006). Cytokines play a major role in propagating antiviral responses even during the chronic phase of the infection. For example, in the CNS of TMEV-susceptible SJL mice, IL-1, IL-6, IL-10, IFN-γ, and TNF-α are expressed highly during the chronic stage of infection (Chang et al., 2000; Gilli et al. 2016).
Inbred strains of mice allow for phenotypic reproducibility and in-depth analyses of specific aspects of neurotropic viral infections. However, the limited genetic diversity present in common inbred strains does not allow representation of the heterogeneous phenotypic outcomes seen in human populations from viral infections. The Collaborative Cross (CC), a large panel of recombinant inbred mouse lines established from eight genetically diverse founder strains overcomes these genetic diversity limitations (Churchill et al., 2004; Threadgill et al. 2002; Zou et al., 2005). The founder strains (A/J, C57BL/6J, 129S1/SvImJ, NOD/LtJ, NZO/HlLtJ, CAST/EiJ, PWK/PhJ, and WSB/EiJ) were bred using a combinatorial funnel design to yield uniform genome wide genetic variation, via recombination and chromosomal assortment, randomized across a large, heterogeneous and reproducible population (Phillippi et al., 2014; Threadgill et al., 2011). The CC resource thus provides a model of human heterogeneity where each CC strain can be considered as a unique individual in a population, resulting in a phenotypically distinct and reproducible diverse outbred population. The CC is useful for characterizing how the genetic backgrounds of individuals contribute to diverse neurological responses after TMEV infection (Brinkmeyer-Langford et al., 2017).
In the current study, CC strains were used to evaluate neurological and immunological responses during the chronic phase of Theiler's virus infection, modeling the long-term outcomes of neurotropic viral infections in humans. These findings provide insight into the complex roles of immune response and viral clearance associated with persistence in the pathogenesis of virus-associated neurological diseases, such as in MS. We compare chronic immunological responses to viral persistence and long-term neurological outcomes. Ultimately, our findings will be useful for developing models of post-viral infection neurological conditions in humans.
2. Materials and methods
2.1. Ethics statement
All animal care protocols were approved by the Texas A&M University Laboratory Animal Care and Use Committee (AUP 2017-0082) and were compliant with NIH Guidelines for Care and Use of Laboratory Animals.
2.2. Mice
Female and male mice of 14 CC strains were randomly assigned to two separate groups (sham-infected and TMEV-infected) (Table 1). Strain and mouse numbers were dependent on the availability of our in-house breeding system and strain-specific fertility and fecundity.
Table 1.
Collaborative Cross strains investigated in this study.
| Strain | Sham | Infected | Total |
|---|---|---|---|
| CC002/Unc | 4 | 3 | 7 |
| CC005/TauUnc | 4 | 4 | 8 |
| CC006/TauUnc | 3 | 3 | 6 |
| CC011/UncJ | 4 | 4 | 8 |
| CC015/UncJ | 3 | 3 | 6 |
| CC017/UncJ | 3 | 2 | 5 |
| CC023/GeniUncJ | 5 | 2 | 7 |
| CC027/GeniUncJ | 4 | 4 | 8 |
|
CC032/GeniUncJ X CC013/GeniUncJ |
2 | 8 | 10 |
| CC036/UncJ | 2 | 4 | 6 |
| CC037/TauUnc | 3 | 4 | 7 |
|
CC041/TauUnc X CC012/GeniUncJ |
2 | 11 | 13 |
| CC043/GeniUncJ | 2 | 3 | 5 |
| CC046/Unc | 2 | 2 | 4 |
| Total Mice | 43 | 57 | 100 |
2.3. TMEV infection
Mice were anesthetized at 4 weeks of age by isoflurane inhalation (MWI, Meridian, ID). Anesthetized mice in sham-infected groups (22 females and 21 males) were injected intracerebrally with phosphate buffered saline (PBS) into the fenestra at a depth of ∼1.5 mm. Mice in TMEV-infected group (29 females and 28 males) were injected similarly with 5.0 × 104 plaque-forming units (PFU) of BeAn strain of TMEV (American Type Culture Collection [ATCC] VR 995, Manassas, VA) in 20 μl of PBS.
2.4. Qualitative neurological phenotyping
After infection, mice were evaluated twice daily for the first 2 weeks during the acute stage of infection, and once a week thereafter, during the chronic stage (14-90dpi). Individual limbs were observed for clinical signs of paralysis and scored on a scale of 0–4, with 0 consisting of mice being able to walk with no signs of weakness to 4 consisting of a lack of grip function and flaccid limb extension (Johnson et al., 2004; Lawley et al., 2021; McCarthy et al. 2012). Other qualitative phenotypes, such as piloerection, hunching, seizures, limb clasping, etc., were measured as described previously (Eldridge et al., 2020).
2.5. Serum collection and Euthanasia
Mice were euthanized at 90 dpi by intraperitoneal (IP) injection of Beuthansia (Schering-Plough Animal Health) and perfused with a 1x PBS solution through the left ventricle after blood samples were acquired for serum collection.
2.6. Cytokine assay
To evaluate long-term effects of TMEV, we measured cytokine and chemokine levels at the chronic stage of infection, using serum collected 90 dpi from genetically diverse mice. Bio-Plex Pro™ Mouse Cytokine 23-plex Assay kits (Bio-Rad, Hercules, CA) were used to determine the concentrations of 23 cytokines and chemokines in serum (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17 α, IFN-γ, CCL11 [Eotaxin], G-CSF, GM-CSF, CXCL1 [KC {keratinocyte-derived chemokine}], CCL2 [MCP-1 {monocyte chemotactic peptide 1}]), CCL3 [MIP-1α {macrophage inflammatory protein 1α}], CCL4 [MIP-1β], CCL5 [RANTES {regulated upon activation, normal T-cell expressed and secreted}], and TNF-α). Serum was thawed at room temperature before following the Bio-Plex Pro kit protocol. Instrument calibration was performed for each procedure and all samples were assayed in triplicate using Bio-Plex (Luminex) 200 system (Bio-Rad, Hercules, CA). Data was collected with the Bio-Plex Manager software program (Bio-Rad version 4.1.1).
2.7. RNA isolation and sequencing
TMEV is known to affect the thoracic spinal cord and hippocampus of infected mice, depending on mouse strain; therefore we collected RNA from these tissues at 90dpi to compare gene expression and presence of TMEV among the CC strains (Brinkmeyer-Langford et al., 2017; Gerhauser et al., 2019a; Lawley et al., 2021). RNA was extracted using Maxwell® 16 automated equipment with LEV simplyRNA tissue kit (Promega, Sunnyvale, CA). Samples were quantified with the Qubit Fluorometer (Life Technologies) with a broad range RNA assay and concentrations were normalized for library preparation. RNA quality was verified on the Agilent TapeStation with an RNA ScreenTape. Messenger RNA sequencing libraries were prepared and analyzed as described previously (Eldridge et al., 2020). For all samples, including those analyzed earlier (Eldridge et al., 2020), we used DESeq2 to identify Differentially Expressed Genes (DEG) between sex-matched infected and uninfected mice of each strain (Love et al. 2014). Next, to determine if TMEV RNA was present within the tissue, we measured the expression (fold change) of RNA encoding the TMEV polyprotein AA47930.1.
2.8. Statistics
GraphPad Prism version 9.0.2 for Mac (GraphPad Software, San Diego, CA) was used for nonparametric Mann-Whitney U tests when comparing paralysis frequency scores between sham and TMEV-infected mice, cytokine and chemokine levels among sham and TMEV-infected mice within the same CC strain, and between TMEV-infected CC strains and TMEV-infected SJL mice. All reported p values are based on two-tailed statistical tests with a significance level of 0.05.
Statistical analysis for stepwise regression was performed using R software (version 4.0.2) to determine relationships among cyto/chemokine measurements with paralysis progression scores.
We adapted the “stepwise” function in R to perform the stepwise regression using the progression score of the phenotype as the response and the sham-infected status as well as the twenty-three cytokine levels as covariates. An important parameter of the stepwise regression procedure is called alpha, which acts as a stopping criterion that prevents further variables from being included or excluded. Following (Li et al., 2018), we chose alpha to be 0.05 as the inclusion and exclusion criteria. The stepwise regression procedure involves a forward selection mechanism that starts with the intercept only model and proceeds according to the optimal stopping criterion to choose the final model.
3. Results
3.1. CC strains respond differently to TMEV infection resulting in paralysis
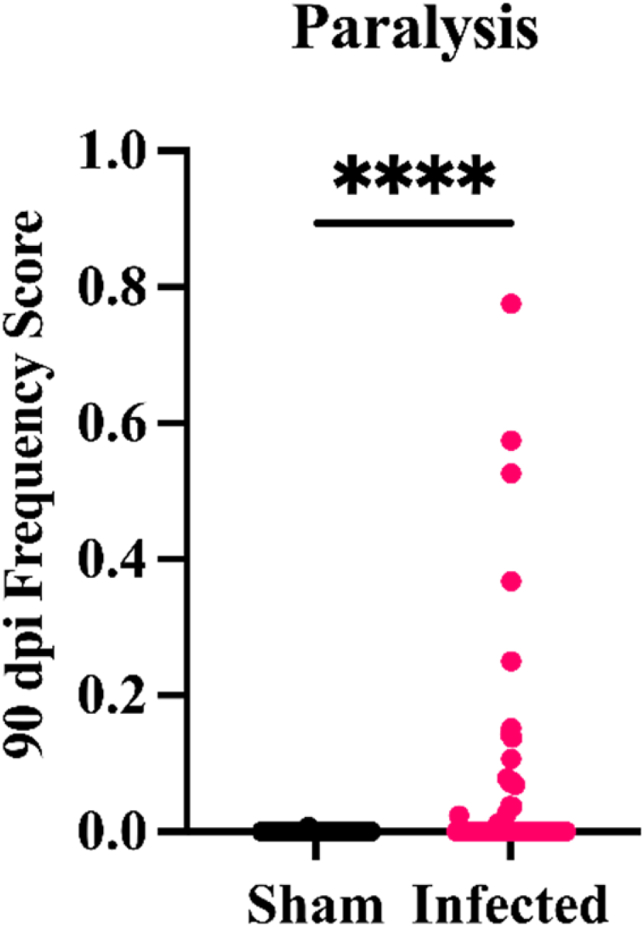
Over the course of 90 days, CC strains showed variable response to TMEV infection. Multiple phenotypes such as sickness, dystonia, reflex, weakness, and paralysis were observed throughout the infection (Eldridge et al., 2020). One of the most profound phenotypes observed within the infected CC strains was paralysis (Fig. 1).
Fig. 1.
Comparison between 90dpi sham and TMEV infected mice. P value was calculated for differences between control and infected mice (∗∗∗∗P < 0.0001).
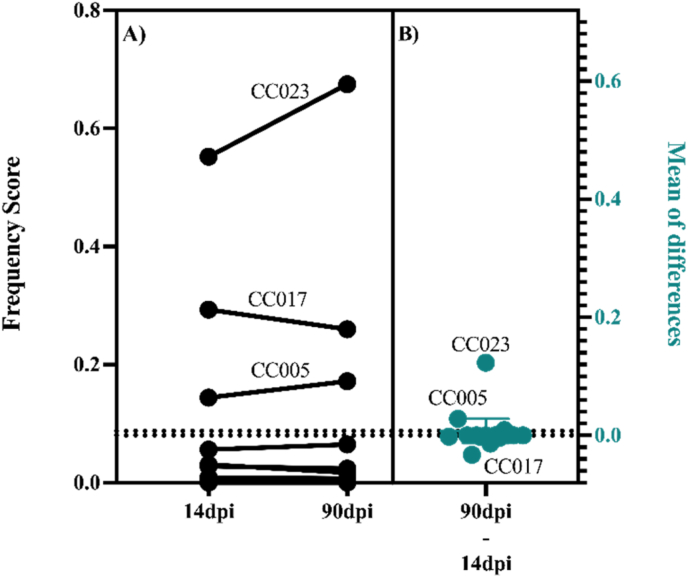
Eight out of the 14 studied strains developed paw or limb paralysis during the infection. Paralysis affected at least one mouse from each of the affected strains. However, for strains CC005, CC017, and CC023, all infected mice developed paralysis. In affected mice, paralysis was seen before the end of the acute phase with the exception of a single CC041xCC012 mouse. Limb paralysis was persistent and progressive for CC005 and CC023, worsening over the 90dpi period, with CC023 being the most severely affected. Contrastingly, the paralysis frequency score for CC017 slightly decreased between 14 and 90dpi, indicating symptom improvement (Fig. 2, panels A and B).
Fig. 2.
Proportions of days for which paralysis was observed over the 90-day infection period varied by strain. A) Frequency scores indicate the proportion of days for which paralysis was recorded by 14dpi and 90dpi. Each pair of connected points represents a different CC strain. The direction of the connecting line shows the relative increase or decrease in the paralysis frequency score between 14 and 90dpi. B) The mean differences between frequency scores at 90dpi and 14dpi are shown as individual dots for each CC strain.
3.2. The immune responses in CC strains during chronic TMEV infection are unlike “TMEV-susceptible” SJL mice
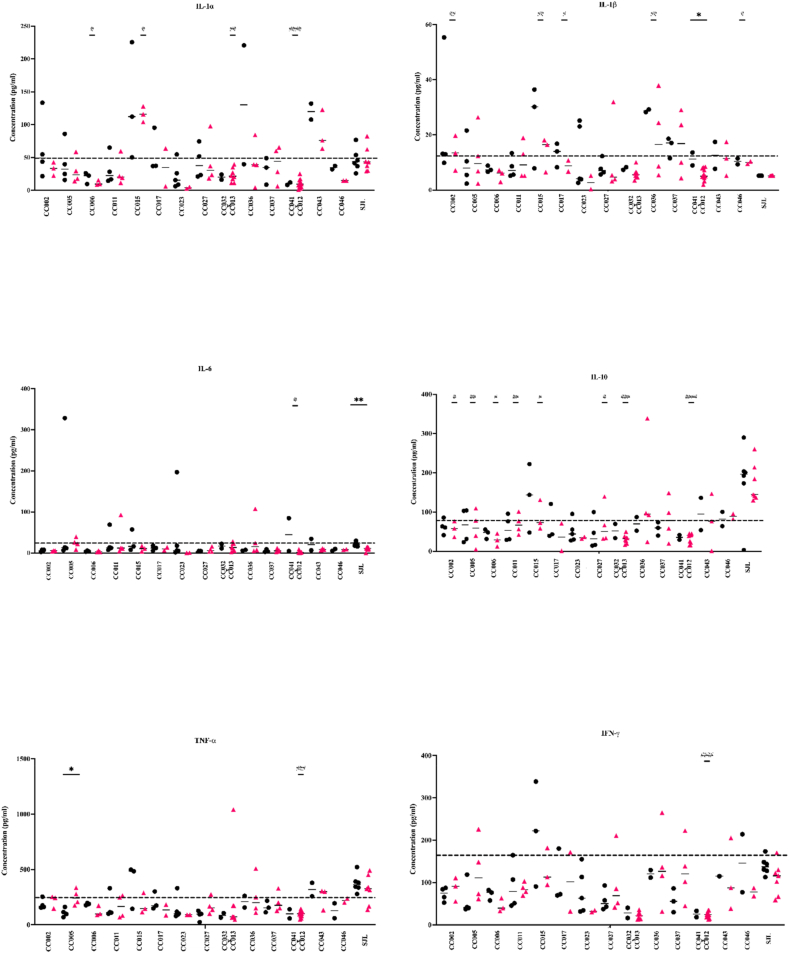
Cytokine and chemokine levels were measured for all 14 CC strains to identify potential connections between immune response and TMEV-induced paralysis at the chronic stage of infection. The TMEV 90dpi “immunological snapshots” differed amongst all infected CC strains. For each cyto/chemokine, serum levels were measured in sham and infected mice of the same strain to gauge strain-specific responses to TMEV infection (Fig. 3). Responses of SJL mice were considered as the prototype TMEV “susceptible” strain.
Fig. 3.
Cytokine protein levels were measured from serum collected at 90dpi. Six out of 23 cytokines and chemokines (IL-1α, IL-1β, IL-6, IL-10, TNF-α, and IFN-γ) are highlighted here to focus on the varied protein levels produced by the CC strains and SJL mice. Cytokine levels in CC mouse strains were often dissimilar to those measured for the TMEV susceptible strain, SJL. The dashed line (--) represents the total average cytokine concentration measurements for sham mice to determine a control baseline. Sham mice are represented by • and infected mice by▴. P values were calculated for differences between sham and infected mice from the same strain (∗) and between infected SJL and infected CC strains (#) using the Mann-Whitney U test. ∗/#P < 0.05 ∗∗/##P < 0.01 ###P < 0.001 ####P < 0.0001. IFN-γ outlier was removed from CC043 sham column due to its concentration value of 3447.03 pg/ml. Data for the other 17 cyto/chemokines evaluated can be found in supplemental A1.
Cytokine levels produced by the CC mouse strains were first compared to those produced by TMEV-infected SJL mice (Fig. 3). Most cyto/chemokines were produced at similar levels in SJL mice, regardless of infection status, although IL-6 and IFN-γ were produced at lower levels in the infected SJLs compared to sham. IL-6 concentration levels in SJL infected mice were significantly lower than those measured for sham-infected SJLs at 90dpi (p = 0.0012).
SJL cyto/chemokine levels were next compared with those of the CC strains which developed paralysis most consistently (CC005, CC017, and CC023). Significant differences compared to SJL mice included IL-1β levels of CC017 mice (p = 0.0278) and IL-10 levels of CC005 mice (p = 0.0061). Furthermore, CC005 infected mice produced TNF-α at significantly higher levels compared to CC005 sham (p = 0.0286), while no significant difference was found between sham and infected SJL mice. Interestingly, no significant differences were found for any cyto/chemokine levels measured for the severely paralyzed strain CC023 and those of SJLs. Infected CC023 cyto/chemokine levels were typically below the sham threshold line (defined here as the total average concentration for sham mice; Fig. 3).
The levels measured for the six cytokines shown in Fig. 3 were all statistically significantly different between SJL and at least one CC strain. Most notably, levels of cytokine IL-10 were significantly lower for eight out of 14 CC strains compared to SJLs. Infected CC041xCC012 produced IL-1β at significantly lower levels than sham-infected CC041xCC012 (p = 0.0256), while IL-1β levels were virtually the same for both infected and sham SJL mice. In summary, CC strains demonstrated an immunological heterogeneity in response to TMEV infection, unlike the responses of susceptible SJL mice.
3.3. IL-1α and RANTES levels are significantly correlated with degree of paralysis
We used stepwise regression to identify significant relationships between cyto/chemokine measurements and paralysis progression scores (calculated as the difference between how many limbs were paralyzed at 90dpi compared to 14dpi for mice of a given strain) for the 14 CC strains (Table 1). Of the 23 cyto/chemokines evaluated, IL-1α (p = 0.000174) and RANTES (p = 0.00628) were identified as being significantly associated with paralysis (Table 2). The regression model returned a p < 0.001, demonstrating the overall significance of the selected model describing the association between the selected cyto/chemokines and the progression score of paralysis.
Table 2.
Statistically significant associations between cytokine levels and paralysis. Associations were identified following stepwise regression analysis. B: Estimated coefficients of regression. SE: Standard Error.
| Variables | B | SE | p-value |
|---|---|---|---|
| IL-1α | −0.0120 | 0.0031 | 0.0002 |
| RANTES | 0.0080 | 0.0028 | 0.0063 |
3.4. Complex interactions, rather than simple associations, underlie paralysis in TMEV-infected mice
IL-1α and RANTES are under complex regulation. Consequently, the downstream effects of these proteins are also complicated and multifaceted. This is especially apparent across genetically diverse individuals such as mice of the CC strains. Rather than depending on a linear explanation for the roles of these two cyto/chemokines in relation to TMEV paralysis, a tiered perspective was developed using 1) haplotypes of genomic regions previously connected with TMEV pathogenesis and viral clearance or persistence, 2) levels of other cytokines and chemokines, and 3) TMEV-relevant gene expression to better understand the complex interactions of IL-1α and RANTES associated with paralysis scores.
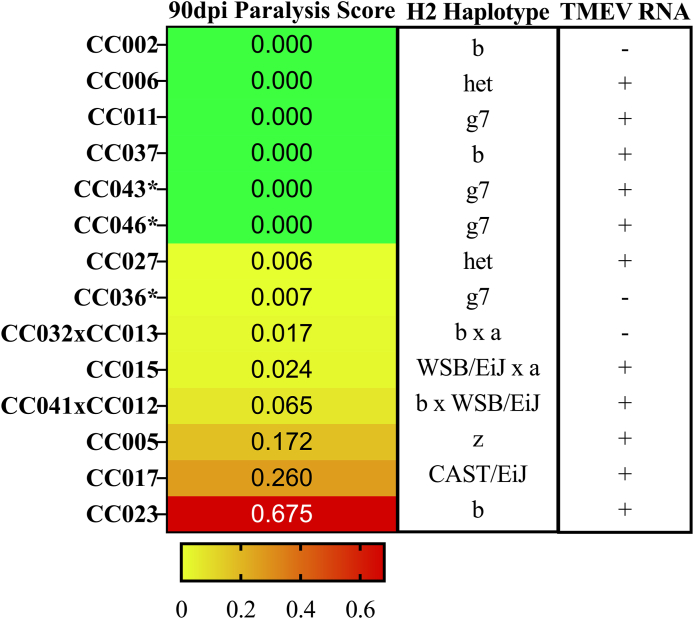
3.4.1. Haplotypes previously associated to TMEV pathogenesis and viral clearance/persistence
3.4.1.1. H2
The major histocompatibility region, known as H2 in mice, of many species contains genes that encode proteins involved in immune response. TMEV persistence and resulting phenotypes have previously been described in relation to H2 haplotypes (Clatch et al., 1985; Rodriguez and David 1985; Rodriguez et al. 1986).
H2 haplotypes were assigned for each strain used in this study based on the founder strains from which these haplotypes were inherited (Fig. 4). In keeping with previous observations (Brinkmeyer-Langford et al., 2017; Eldridge et al., 2020), H2 haplotype alone was not sufficient to explain TMEV persistence or phenotypic severity. However, strains with haplotype H2g7 showed substantially less paralysis, while those strains with H2z haplotypes had more severe paralysis. Heterozygosity of the H2 region was also more common in strains with less paralysis. Finally, the H2b haplotype – previously associated with TMEV resistance – was associated with reduced paralysis when inherited from the C57BL/6J founder, and greater paralysis when inherited from the 129S1/SvImJ founder.
Fig. 4.
CC strains and their respective H2 haplotypes and TMEVpresence/absence are categorized according to observed paralysis scores. CC strains are arranged by increasing paralysis frequency scores (green to red gradient). We identified the H2 haplotypes for each strain based on the founder strains from which the H2 complex was inherited, to demonstrate that H2 haplotype alone does not influence phenotypic severity. TMEV RNA presence was measured via relative levels of the TMEV polyprotein sequence AAA47930 within sham and infected mice. Those strains with detectable TMEV RNA within the CNS tissue are denoted with “+”; “-” denotes those strains with no detectable TMEV RNA. A complete RNA sequencing report of relative TMEV RNA levels are found in B1. ∗strains not included in (Eldridge et al., 2020). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4.1.2. Viral clearance or persistence in the chronic phase of infection
The expression of TMEV RNA from hippocampal and thoracic spinal cord tissue was evaluated at 90dpi to identify associations between viral persistence and paralysis. Fig. 4 shows presence or absence of TMEV RNA for each strain, using data from (Eldridge et al., 2020) plus new data for strains CC036, CC043, and CC046. While the strains could be grouped based on viral persistence or absence, both groups included mouse strains at opposite ends of the phenotypic spectrum (paralyzed vs non-paralyzed). For example, some strains with measurable levels of TMEV RNA at 90dpi included CC006, CC011, CC037, CC043, and CC046; these strains did not develop paralysis. These findings demonstrate that viral persistence or absence alone did not predict the presence or frequency of TMEV-induced paralysis.
3.4.1.3. TMEV-related loci previously connected to differences in immune response and paralysis
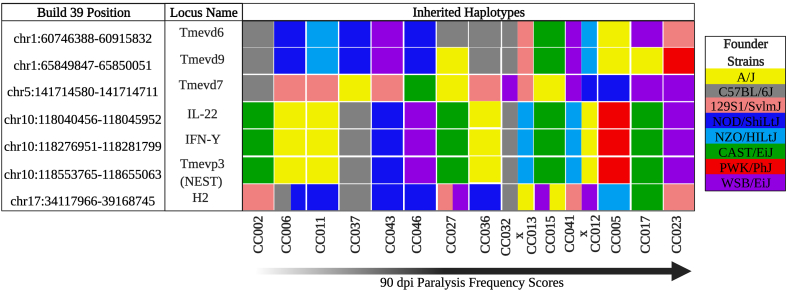
The TMEV quantitative trait loci Tmevp2 and Tmevp3 are located in close proximity on mouse Chromosome (Chr) 10 (Bihl et al. 1999) and have been associated with viral persistence. The genes Ifnγ and Il-22 are also located in this region, and key roles in TMEV persistence and pathogenesis/mortality have been suggested for these genes (Brahic and Bureau 1998; Fiette et al., 1995; Levillayer et al., 2007). Tmevp3 also contains a long non-coding RNA called NeST (nettoie Salmonella pas Theiler's [“cleanup Salmonella not Theiler's”]), which contributes to epigenetic regulation of Ifnγ (Gomez et al., 2013; Vigneau et al., 2003). Strains that inherited this region from the A/J or C57BL/6J founders showed below-average paralysis (Fig. 5). TMEV demyelination loci Tmevd6, Tmevd9, and Tmevd7 have previously been associated with TMEV-induced demyelinating disease, particularly in relation to sex effects and gait dysfunction (Butterfield et al., 2003). In the current study, strains carrying the NOD/ShiLtJ haplotype for Tmevd6 and Tmevd9, both located on mouse Chr 1 (Butterfield et al., 2003), experienced no paralysis throughout infection. Strains carrying the 129S1/SvImJ founder haplotype at Tmevd7 on Chr 5 showed little to no paralysis, but those strains with WSB/EiJ founder haplotype exhibited higher-than-average levels of paralysis.
Fig. 5.
TMEV-related loci and their link to CC founder strains. TMEV-related loci, sorted by position on the left, were inherited from different CC founder strains (color coded by strain as shown in the key on the far right). CC strains evaluated in this study are listed along the bottom in order of increasing paralysis levels (Created with BioRender.com). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4.2. Levels of other cytokines and chemokines in relation to IL-1α and RANTES
3.4.2.1. IL-1α
IL-1α is known to contribute to pathogenesis of experimental autoimmune encephalitis (EAE) (Jacobs et al., 1991; Mannie et al. 1987; Matsuki et al., 2006). Both IL-1α and IL-1β are connected with development of autoimmunity; specifically, a disruption of the balance between IL-1 and its receptor, IL-1Rα, can lead to a loss of self-tolerance (Matsuki et al., 2006). Much is known about IL-1β in relation to EAE autoimmunity and neuroinflammation (reviewed in (Hauptmann, 2020)), but the role(s) of IL-1α are less well-known.
Levels of IL-1α did not directly correlate with paralysis in the current study. Similarly, expression of the Il1α gene, and the gene encoding the receptor for IL-1α (Il1r1), did not correlate with each other, or with IL-1α levels, or with paralysis.
3.4.2.2. RANTES
During viral infection, the chemokine RANTES traffics macrophages to the CNS (Lane et al. 1999), and during chronic viral infection RANTES has been found to regulate CD8 T-cell responses (Crawford et al., 2011). RANTES did not correspond directly with TMEV disease severity in this study: RANTES protein levels were highest in CC005 and lowest in CC023, both strains with high levels of paralysis and persistent TMEV infection. Furthermore, CNS expression of Ccl5, the gene encoding RANTES, did not correlate with either paralysis, TMEV persistence, or RANTES serum protein levels.
3.4.2.3. Cytokines and chemokines regulated/influenced by IL-1A and/or RANTES
Higher-than-average paralysis scores correlated with lower-than-average levels of IL-6 in strains used in this study. Lower paralysis scores tended to be associated with relatively higher levels of IL-13, an immunoregulatory cytokine. IL-13 can inhibit production of IL-6 as well as IL-1α and RANTES, though these effects are likely to be governed by time point, tissue, and other aspects of the microenvironment. Also, lower-than-average levels of G-CSF were found in strains with higher paralysis levels. IL-1α acts by inducing G-CSF, which then coordinates with IL-6, to mobilize granulocytes and hematopoietic cells from the bone marrow to mediate the generation of effective immune responses against infections (Altmeier et al., 2016; Basu et al., 2008; Caldwell and Emerson 1995).
3.4.3. Expression of genes known to be relevant to TMEV infection
Differentially expressed genes located within H2, particularly previously implicated H2-D loci (Clatch et al., 1985; Lipton and Melvold 1984; Rodriguez and David 1985; Rodriguez et al., 1986), are associated with susceptibility/resistance to TMEV-induced demyelination. Interestingly, several class Ib genes (particularly H2-M5 and -Q3) and a nonclassical class II gene (H2-Ob) showed different expression patterns in the most paralyzed compared to the least paralyzed mouse strains (Appendix B). Expression of Class Ib loci in the brain has been previously associated with potential roles in development and neuronal function (Ohtsuka and Dalton 2008), while H2-Ob has been linked to responses to viral infections and autoimmunity (Denzin et al., 2017; Welsh et al., 2020).
Expression of Ifng and Il-22, as well as Ifngas1 (NeST locus), located within the Tmevp2/Tmevp3 QTL region on Chr 10, was not substantially different between strains with different levels of paralysis.
None of known genes located within the regions of Tmevd6, Tmevd9, or Tmevd7 were found to have significant expression differences (between infected and uninfected mice of a given strain, and/or between different strains), though many pseudogenes are present at each location. It is possible these loci contain regulatory elements that contribute collectively to TMEV outcomes. To better discern the roles of these loci in TMEV infection will require a larger sample size.
4. Discussion
The Collaborative Cross was used to determine profiles of systemic immune activation during chronic TMEV infection and to identify a hierarchical perspective for understanding cytokine interactions in the context of TMEV-induced paralysis. Over the course of the infection, mice in the current study developed disease profiles that varied according to their genetic backgrounds, similar to our previous findings (Brinkmeyer-Langford et al., 2017; Eldridge et al., 2020). Responses to the same viral infection vary according to a host's genetic background, with differences in immune response influencing the development of clinical symptoms.
The current study focused on the TMEV-induced paralysis phenotype (Fig. 1). Limb paralysis was most dominant in CC005, CC017, and CC023 strains with evidence of progressive paralysis (paralysis scores increased over the 90-day infection; Fig. 2) in the severely paralyzed strain CC023, followed by CC005. These findings were compared to those of SJL/J mice, which are susceptible to TMEV-induced paralysis and have been studied previously as a paradigm of viral-induced neurological disease (DePaula-Silva, Tyler J Hanak et al., 2017; Gerhauser et al., 2019b). While SJL/J mice exhibit loss of limb mobility at the late chronic stage of the infection, some CC strains developed paralysis starting from the acute phase of infection.
Aspects of the immune response were analyzed to identify factors influencing chronic paralysis. SJL/J mice were used as the prototype strain when evaluating immunological responses among the CC strains to determine if strains behaved similarly to the SJL/J model of MS. Initially, TMEV infection prompts a myriad of immunological responses, and clearance of TMEV from the CNS requires the migration of cells of the immune system to the site of infection, relying heavily on the action of cytokines and chemokines (Mi et al., 2004). These responses are active not only as part of the innate immune system, but can endure and have a role in the adaptive immune response, during the chronic stage of the infection.
In contrast to the SJL/J paradigm, little evidence of a dominant proinflammatory environment was observed in CC strains experiencing neurological dysfunction (Fig. 3). Despite evidence of CNS viral persistence in the SJL/J strain, most serum cytokine levels for infected SJL/J mice fell along the sham “threshold line,” indicating lack of cytokine induction at the chronic stage of injection. The two exceptions were IL-10 and TNF-α. IL-10 production is critical for tissue protection during viral infection by regulating and suppressing proinflammatory cytokine expression (Rojas et al., 2017). Failure to reduce inflammation has been accompanied by poor recovery of motor and sensory function (Siqueira et al., 2015). TNF-α plays a critical role as a proinflammatory cytokine for the formation and maintenance of granulomas, leukocyte trafficking, and other immune cell regulation (Atzeni and Sarzi-Puttini 2013; Steinman 1999). Data suggest that high levels of TNF-α in the spinal cord are involved in TMEV pathogenesis rather than protection (Katz-Levy et al., 2000). In the current study, SJL/J mice produced high levels of both IL-10 and TNF-α (Fig. 3). However, for all infected CC mice (except CC036), IL-10 was produced at levels below the sham threshold. Similarly, most CC strains produced TNF-α at levels below the sham threshold line, though infected CC005 mice produced TNF-α at levels significantly higher than sham-infected CC005.
Cytokine responses varied for CC strains that developed limb paralysis, but remained near the average sham threshold line in Fig. 3. Interestingly, strains that did not develop paralysis produced a similar immunological response as paralyzed mice, regardless of viral presence or absence in the CNS. Furthermore, similarities in cytokine profiles did not translate to similarities in phenotype profiles. For example, CC023 mice developed spastic and flaccid limb paralysis and had the most severe disease symptoms overall. However, the cytokine levels measured for TMEV-infected CC023 mice were below the sham threshold line. In fact, the serum cytokine profile seen in CC023 is reminiscent of TMEV-resistant C57BL/6J mice, that experience seizures but not paralysis (Gerhauser et al., 2019a; Lipton 1975; Richards et al., 2011).
Viral presence had no correlative relationship with the paralysis seen at 90dpi. Twelve of the 14 CC strains in this study had detectable amounts of TMEV RNA present in CNS tissue (Table 2), but not all of these 12 strains developed paralysis. TMEV clearance was an inadequate predictor of paralysis severity at 90dpi.
Levels of TMEV RNA in the CNS at 90dpi were also not correlative with H2 haplotype, in line with previous observations (Eldridge et al., 2020). This finding contradicts previous data from SJL/J mice, which are characterized as TMEV-susceptible due to their severe disease profile and presence of high levels of TMEV RNA in CNS tissue (Melvold et al., 1987; Rodriguez et al., 1991; Trottier et al., 2004). None of the 14 CC strains used in this study share the SJL TMEV-susceptible H2 haplotype (H2s haplotype). Instead, the H2 haplotypes of the CC strains most susceptible to paralysis included Hz, HCAST/EiJ, and Hb. CC023 - the strain with the most severe paralysis - share the same haplotype (H2b) with TMEV-resistant strain C57BL/6J. While C57BL/6J mice are known to clear TMEV within the acute phase of infection (Jin et al., 2019; Richards et al., 2011; Tsunoda and Fujinami 2002), CC023 mice in this study continued to show evidence of persistent TMEV infection at 90dpi. Furthermore, despite being at opposite ends of the paralysis spectrum, CC002 and CC023 share the same H2 haplotype, derived from the same founder strain (129S1/SvImJ).
Along with H2 haplotypes, CNS expression of individual genes of the H2 complex was analyzed. Expression of the class Ib gene H2-M5 tended to be lowest in strains with higher paralysis scores, while another class Ib gene, H2-Q3, was expressed at lower levels in strains that were not paralyzed. The class Ib region of H2 has previously been associated with tissue- and developmental time point-specific expression (Ohtsuka and Dalton 2008). Brain development and plasticity are intimately tied to class I expression (Elmer and McAllister 2012), and response to CNS injury is dependent on expression of class I factors (Cartarozzi et al., 2019). Therefore, varying degrees of susceptibility to TMEV-induced paralysis could be influenced by variation in expression of these class I genes. Expression differences were observed in the H2-Ob gene with lower expression levels in strains with little to no paralysis. Prior studies have shown that H2-Ob genotype influenced the production of viral neutralizing antibodies regardless of overall H2 haplotype (Denzin et al., 2017); the development of autoimmunity has been associated with this locus (reviewed in Welsh et al., 2020). Therefore, strain-specific differences in the expression of this locus likely influence the degree of response to TMEV and potentially contribute to the paralysis-causing damage inflicted by the infection.
Potential immunological drivers of neurological phenotypes (e.g., paralysis) were identified using stepwise regression analysis, revealing significant associations between serum levels of IL-1α and RANTES and the severity of TMEV-induced paralysis. IL-1 is known for creating an inflammatory environment by regulating multiple immune processes (Dinarello 2009). There are two IL-1 isoforms produced, IL-1α and IL-1β, with the latter being the main isoform released (Kaneko et al., 2019). The roles of IL-1β have been extensively studied in stroke and EAE models (Matsuki et al., 2006; Salmeron et al., 2019; Sha and Markovic-Plese 2016), but IL-1α is less well-characterized. Despite considering IL-1α as a pro-inflammatory cytokine, it could have beneficial neuroprotective effects under appropriate conditions. For example, release of IL-1α by dying microglia has been identified as a DAMP to induce neuroinflammation; deletion of IL-1α resulted in increased levels of survival factor Tox3, correlating with oligodendrocyte survival after spinal cord injury (Bastien et al., 2015). However, IL-1α levels did not correspond linearly to paralysis scores: relatively low IL-1α levels were detected in both non-paralyzed and severely paralyzed strains. Given the myriad potential roles of IL-1α and other genes and proteins influenced by (and influencing) IL-1α in a genetically diverse background, a direct relationship between IL-1α level and paralysis was not anticipated. Further investigations are needed to evaluate relevant properties and mechanisms of IL-1α.
A significant association was also identified between serum levels of RANTES and progression of TMEV-induced paralysis. RANTES acts as a mediator for inflammatory infiltrates and T cell responses at sites of tissue damage. Studies have associated RANTES with early formation of plaques (HVAS et al., 1997; Simpson et al., 1998), spinal cord injury via activation of NF-κB signaling pathway (Wang et al., 2017), and leukocyte entry into the CNS (Lane et al., 2000), thus contributing to the pathogenesis of virus induced inflammation. In the acute stage of infection, RANTES amplifies the inflammatory process in an attempt to reduce viral presence in the CNS. Consequently, a sustained inflammatory environment has been thought to be enhanced by RANTES in EAE models (Glabinski et al. 2003; Glabinski et al. 1998). During the chronic stage of viral infection, RANTES plays a pivotal role by influencing CD4+ and CD8+ responses. In the absence of RANTES, CD8+ T cells become exhausted, hindering the ability to control the infection by introducing an immunological dysfunction within the host (Crawford et al., 2011). The exact mechanisms of proinflammatory effects modulated by RANTES during chronic infection remain unclear, but findings from previous studies suggest it plays a central role in inflammatory CNS disease.
Relevant expression levels of the many constituent proteins and genes of IL-1α and RANTES signaling pathways are expected to vary across genetically diverse CC strains. Potential cytokine associations were identified with the progression of paralysis over time, but these cytokines and chemokines are part of much larger synergistic relationships that vary from strain to strain.
Correlation between susceptibility to demyelination and IFN-γ upregulation of MHC Class II expression on murine cerebrovascular endothelial cells derived from SJL and CBA mice (both susceptible to TMEV-induced demyelination) was previously reported. CVE derived from TMEV-resistant strains (Balb/c mice) did not express MHC class II following IFN-γ treatment (Welsh et al., 1993). Also, Theiler's virus infection induced MHC class I expression in CVE derived from mice that are susceptible to TMEV-induced demyelination, but not in resistant CVE (Welsh et al., 1995). These effects may result in increased immune trafficking into the CNS, thereby impacting disease pathology. By reporting serum cytokine levels, systemic immune differences were compared rather than focusing only on the environment of the CNS.
Prior studies have shown that severe neurological deficits have been influenced by the degree of viral spreading within the CNS. For instance, variability of TMEV infection outcomes in CBA mice are related to viral spreading in defined structures in the brain leading to persistent infection and demyelination (Oliver et al. 1997). As supported in this study, cytokine and chemokine response is one of the many drivers in TMEV-related disease outcomes. For instance, IL-6 has been shown to have an important protection response for anterior horn neuronal injury (Pavelko et al., 2003). Further studies will be required to ascertain the mechanisms involved in the development of TMEV-induced paralysis.
With larger sample numbers and more data points collected at different spatial (i.e., CNS vs. serum) and temporal (i.e., acute vs. chronic infection) conditions, it will be possible to identify key drivers of neurological symptoms that follow viral infections. Future investigations will involve characterizing the immunological response during the acute phase of the infection to follow the cascade of events occurring between the innate and adaptive immune system. By identifying relevant immunological and genetic factors that contribute to neurological disease, an enhanced understanding of how the immune system influences susceptibility or resilience to specific virus-induced neurological phenotypes will be obtained.
5. Conclusion
In this study, we infected mice of both sexes representing 14 CC strains to characterize the long-term neurological and immunological responses caused by TMEV. Three strains (CC005, CC017, and CC023) exhibited pronounced paralysis by the chronic stage of infection. By evaluating the paralysis frequency scores (which reflect the duration and severity of the paralysis) in context with the production of cytokines and chemokines at 90 dpi, we determined that IL-1α and RANTES were significantly associated with the progression of paralysis. We also evaluated relationships between paralysis scores, H2 haplotypes, and expression levels of known TMEV-relevant genes, all previously implicated in TMEV susceptibility and pathogenesis. We were able to determine that long-term paralysis progression was the cumulative result of many interacting factors, and that the same outcome (in this case, paralysis) resulted from different sets of contributing influences. During late-stage TMEV infection, these complicated interactions were likely coordinated in part by levels of IL-1α and/or RANTES.
In conclusion, synergistic relationships between immune response and genetic background determined long-term paralysis following viral infection of a human-relevant mouse model. These findings are novel in relation to previous paradigms of TMEV-induced paralysis and susceptibility, which were established using inbred mouse strains. Overall, these findings provide insight into the complex roles of immune response in relation to long-term virus-induced paralysis within a heterogeneous population.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Institutes of Health (NINDS) R01 NS103934. The Texas A&M Institute for Genome Sciences and Society (TIGSS) and The Texas A&M Center for Environmental Health Research (TiCER) are also acknowledged for providing support, technical assistance, and feedback.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2021.100395.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Altmeier Simon, Toska Albulena, Sparber Florian, Teijeira Alvaro, Halin Cornelia, LeibundGut-Landmann Salomé. “IL-1 coordinates the neutrophil response to C. Albicans in the oral mucosa” edited by R. C. May. PLoS Pathog. 2016;12(9) doi: 10.1371/journal.ppat.1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzeni F., Sarzi-Puttini P. Tumor necrosis factor. Brenner’s Encyclopedia of Genetics. 2013:229–231. [Google Scholar]
- Aubagnac S., Brahic M., Bureau J.F. Viral load and a locus on chromosome 11 affect the late clinical disease caused by Theiler's virus. J. Virol. 1999;73(10):7965–7971. doi: 10.1128/jvi.73.10.7965-7971.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien Dominic, Landete Victor Bellver, Lessard Martine, Vallières Nicolas, Champagne Mathieu, Takashima Akira, Tremblay Marie-Ève, Doyon Yannick, Lacroix Steve. IL-1α gene deletion protects oligodendrocytes after spinal cord injury through upregulation of the survival factor Tox3. J. Neurosci. : The Official Journal of the Society for Neuroscience. 2015;35(30):10715–10730. doi: 10.1523/JNEUROSCI.0498-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu Sunanda, Quilici Cathy, Zhang Hui-Hua, Grail Dianne, Ashley R., Dunn Mice lacking both G-CSF and IL-6 are more susceptible to Candida albicans infection: critical role of neutrophils in defense against Candida albicans. Growth Factors. 2008;26(1):23–34. doi: 10.1080/08977190801987513. [DOI] [PubMed] [Google Scholar]
- Bihl F., Brahic M., Bureau J.F. Two loci, Tmevp2 and Tmevp3, located on the telomeric region of chromosome 10, control the persistence of Theiler's virus in the central nervous system of mice. Genetics. 1999;152(1) doi: 10.1093/genetics/152.1.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijalwan M., Young C.R., Tingling J., Zhou X.J., Rimmelin A.R., Leibowitz J.L., Welsh C.J. Characterization of plaque-sized variants of daniel's (DA) strain in Theiler's virus-induced epilepsy. Sci. Rep. 2019;9(1):3444. doi: 10.1038/s41598-019-38967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Bureau J.F. Genetics of susceptibility to Theiler's virus infection. Bioessays : News and Reviews in Molecular, Cellular and Developmental Biology. 1998;20(8):627–633. doi: 10.1002/(SICI)1521-1878(199808)20:8<627::AID-BIES5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Brinkmeyer-Langford, Candice L., Rech Raquel, Amstalden Katia, Kochan Kelli J., Hillhouse Andrew E., Young Colin, Welsh C. Jane, Threadgill David W. Host genetic background influences diverse neurological responses to viral infection in mice. Sci. Rep. 2017;7(1):12194. doi: 10.1038/s41598-017-12477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer Sonja, Käufer Christopher, Haist Verena, Lin Li, Gerhauser Ingo, Anjum Muneeb, Bankstahl Marion, Baumgärtner Wolfgang, Löscher Wolfgang. Brain inflammation, neurodegeneration and seizure development following picornavirus infection markedly differ among virus and mouse strains and substrains. Exp. Neurol. 2016;279:57–74. doi: 10.1016/j.expneurol.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Butterfield Russell J., Roper Randall J., Rhein Dominic M., Melvold Roger W., Haynes Lia, Ma Runlin Z., Doerge R.W., Teuscher Cory. 2003. Sex-Specific Quantitative Trait Loci Govern Susceptibility to Theiler's Murine Encephalomyelitis Virus-Induced Demyelination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell Jerry, Emerson Stephen G. Interleukin-1α upregulates tumor necrosis factor receptors expressed by a human bone marrow stromal cell strain: implications for cytokine redundancy and synergy. Blood. 1995;86(9):3364–3372. [PubMed] [Google Scholar]
- Cartarozzi, Politti Luciana, Perez Matheus, Frank Kirchhoff, de Oliveira Alexandre Leite Rodrigues. Role of MHC-I expression on spinal motoneuron survival and glial reactions following ventral root crush in mice. Cells. 2019;8(5) doi: 10.3390/cells8050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. Rober, Zaczynska Ewa, Katsetos Christos D., Platsoucas Chris D., Oleszak Emilia L. Differential expression of TGF-β, IL-2, and other cytokines in the CNS of Theiler's murine encephalomyelitis virus-infected susceptible and resistant strains of mice. Virology. 2000;278(2):346–360. doi: 10.1006/viro.2000.0646. [DOI] [PubMed] [Google Scholar]
- Churchill Gary A., Airey David C., Allayee Hooman, Angel Joe M., Attie Alan D., Jackson Beatty William D. Beavis, Belknap John K., Bennett Beth, Wade Berrettini, Bleich Andre, Bogue Molly, Broman Karl W., Buck Kari J., Buckler Ed, Burmeister Margit, Chesler Elissa J., Cheverud James M., Clapcote Steven, Cook Melloni N., Cox Roger D., Crabbe John C., Crusio Wim E., Darvasi Ariel, Deschepper Christian F., Doerge R.W., Farber Charles R., Forejt Jiri, Gaile Daniel, Garlow Steven J., Geiger Hartmut, Howard Gershenfeld, Gordon Terry, Gu Jing, Gu Weikuan, de Haan Gerald, Hayes Nancy L., Heller Craig, Himmelbauer Heinz, Hitzemann Robert, Hunter Kent, Hsu Hui-Chen, Iraqi Fuad A., Ivandic Boris, Jacob Howard J., Jansen Ritsert C., Jepsen Karl J., Johnson Dabney K., Johnson Thomas E., Kempermann Gerd, Kendziorski Christina, Kotb Malak, Frank Kooy R., Llamas Bastien, Lammert Frank, Lassalle Jean-Michel, Lowenstein Pedro R., Lu Lu, Lusis Aldons, Manly Kenneth F., Marcucio Ralph, Matthews Doug, Medrano Juan F., Miller Darla R., Mittleman Guy, Mock Beverly A., Mogil Jeffrey S., Montagutelli Xavier, Morahan Grant, Morris David G., Mott Richard, Nadeau Joseph H., Nagase Hiroki, Nowakowski Richard S., O'Hara Bruce F., Osadchuk Alexander V., Page Grier P., Paigen Beverly, Paigen Kenneth, Palmer Abraham A., Pan Huei-Ju, Peltonen-Palotie Leena, Peirce Jeremy, Pomp Daniel, Pravenec Michal, Prows Daniel R., Qi Zhonghua, Reeves Roger H., Roder John, Rosen Glenn D., Schadt Eric E., Schalkwyk Leonard C., Seltzer Ze’ev, Shimomura Kazuhiro, Shou Siming, Sillanpää Mikko J., Siracusa Linda D., Snoeck Hans-Willem, Spearow Jimmy L., Svenson Karen, Tarantino Lisa M., Threadgill David, Toth Linda A., Valdar William, Pardo-Manuel de Villena Fernando, Warden Craig, Whatley Steve, Williams Robert W., Wiltshire Tim, Yi Nengjun, Zhang Dabao, Zhang Min, Zou Fei, Complex Trait Consortium The collaborative Cross, a community resource for the genetic analysis of complex traits. Nat. Genet. 2004;36(11):1133–1137. doi: 10.1038/ng1104-1133. [DOI] [PubMed] [Google Scholar]
- Clatch R.J., Melvold R.W., Miller S.D., Lipton H.L. Theiler's murine encephalomyelitis virus (TMEV)-Induced demyelinating disease in mice is influenced by the H-2D region: correlation with TEMV-specific delayed-type hypersensitivity. J. Immunol. 1985;135(2):1408–1414. [PubMed] [Google Scholar]
- Crawford Alison, Angelosanto Jill Marie, Nadwodny Kim Lynn, Blackburn Shawn D., John Wherry E. A role for the chemokine RANTES in regulating CD8 T cell responses during chronic viral infection. PLoS Pathog. 2011;7(7) doi: 10.1371/journal.ppat.1002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzin Lisa K., Khan Aly A., Virdis Francesca, Wilks Jessica, Kane Melissa, Beilinson Helen A., Dikiy Stanislav, Case Laure K., Roopenian Derry, Witkowski Michele, Chervonsky Alexander V., V Golovkina Tatyana. Neutralizing antibody responses to viral infections are linked to the non-classical MHC class II gene H2-Ob. Immunity. 2017;47(2):310–322. doi: 10.1016/j.immuni.2017.07.013. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaula-Silva Ana Beatriz, Hanak Tyler J., Libbey Jane E., Fujinami Robert S. Theiler's murine encephalomyelitis virus infection of SJL/J and C57BL/6J mice: models for multiple Sclerosis and epilepsy. J. Neuroimmunol. 2017;308:30–42. doi: 10.1016/j.jneuroim.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaula-Silva Ana Beatriz, Hanak Tyler J., Libbey Jane E., Fujinami Robert S. Theiler's murine encephalomyelitis virus infection of SJL/J and C57BL/6J mice: models for multiple Sclerosis and epilepsy. J. Neuroimmunol. 2017;308:30. doi: 10.1016/j.jneuroim.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello, Charles A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27(1):519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Eldridge Raena, Osorio Daniel, Amstalden Katia, Edwards Caitlin, Young Colin R., Cai James J., Konganti Kranti, Hillhouse Andrew, Threadgill David W., Jane Welsh C., Brinkmeyer-Langford Candice. Antecedent presentation of neurological phenotypes in the collaborative Cross reveals four classes with complex sex-dependencies. Sci. Rep. 2020;10(1):7918. doi: 10.1038/s41598-020-64862-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer Bradford M., Kimberley McAllister A. Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci. 2012;35(11):660–670. doi: 10.1016/j.tins.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Fang, Ingre Caroline, Roos Per, Kamel Freya, Piehl Fredrik. Risk factors for amyotrophic lateral Sclerosis. Clin. Epidemiol. 2015;7:181. doi: 10.2147/CLEP.S37505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiette Laurence, Aubert Christine, Mfiller Ulrike, Huang Sui, Aguet Michel, Brahic Michel, Jean-François Bureau Theiler's virus infection of 129Sv mice that lack the interferon alpha/beta or interferon gamma receptors. Tors. Journal of Experimental Medicine. 1995;(6):2069–2076. doi: 10.1084/jem.181.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhauser Ingo, Alldinger Susanne, Baumgärtner Wolfgang. Ets-1 represents a pivotal transcription factor for viral clearance, inflammation, and demyelination in a mouse model of multiple Sclerosis. J. Neuroimmunol. 2007;188(1–2):86–94. doi: 10.1016/j.jneuroim.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Gerhauser Ingo, Hansmann Florian, Ciurkiewicz Malgorzata, Löscher Wolfgang, Andreas Beineke. Facets of Theiler's murine encephalomyelitis virus-induced diseases: an update. Int. J. Mol. Sci. 2019;20(2) doi: 10.3390/ijms20020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhauser Ingo, Hansmann Florian, Ciurkiewicz Malgorzata, Löscher Wolfgang, Andreas Beineke. Facets of Theiler's murine encephalomyelitis virus-induced diseases: an update. Int. J. Mol. Sci. 2019;20(2) doi: 10.3390/ijms20020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilli Francesca, Li Libin, Pachner Andrew R. The immune response in the CNS in Theiler's virus induced demyelinating disease switches from an early adaptive response to a chronic innate-like response. J. Neurovirol. 2016;22(1):66–79. doi: 10.1007/s13365-015-0369-4. [DOI] [PubMed] [Google Scholar]
- Glabinski A.R., Bielecki B., Ransohoff R.M. Chemokine upregulation follows cytokine expression in chronic relapsing experimental autoimmune encephalomyelitis. Scand. J. Immunol. 2003;58(1):81–88. doi: 10.1046/j.1365-3083.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- Glabinski Andrzej R., Tuohy Vincent K., Ransohoff Richard M. Expression of chemokines RANTES, MIP-1α and GRO-α correlates with inflammation in acute experimental autoimmune encephalomyelitis. Neuroimmunomodulation. 1998;5(3–4):166–171. doi: 10.1159/000026333. [DOI] [PubMed] [Google Scholar]
- Gomez J. Antonio, Wapinski Orly L., Yang Yul W., Bureau Jean-François, Gopinath Smita, Monack Denise M., Chang Howard Y., Brahic Michel, Kirkegaard Karla. The NeST long NcRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell. 2013;152(4):743–754. doi: 10.1016/j.cell.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann Judith, et al. Interleukin-1 promotes autoimmune neuroinflammation by suppressing endothelial heme oxygenase-1 at the blood-brain barrier. Acta Neuropathol. 2020;140:549–567. doi: 10.1007/s00401-020-02187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvas J., McLean C., Justesen J., Kannourakis G., Steinman L., Oksenberg J.R., Bernard C.C.A. Perivascular T cells express the pro-inflammatory chemokine RANTES MRNA in multiple Sclerosis lesions. Scand. J. Immunol. 1997;46(2):195–203. doi: 10.1046/j.1365-3083.1997.d01-100.x. [DOI] [PubMed] [Google Scholar]
- Jacobs C.A., Baker P.E., Roux E.R., Picha K.S., Toivola B., Waugh S., Kennedy M.K. Experimental autoimmune encephalomyelitis is exacerbated by IL-1 alpha and suppressed by soluble IL-1 receptor. J. Immunol. 1991;146(9):2983–2989. [PubMed] [Google Scholar]
- Jin Leitzen, Goebbels, Nave, Baumgärtner, Hansmann Comparison of Theiler's murine encephalomyelitis virus induced spinal cord and peripheral nerve lesions following intracerebral and intraspinal infection. Int. J. Mol. Sci. 2019;20(20):5134. doi: 10.3390/ijms20205134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.R., Storts R., Welsh T.H., Welsh C.J.R., Meagher M.W. Social stress alters the severity of acute Theiler's virus infection. J. Neuroimmunol. 2004;148(1–2):74–85. doi: 10.1016/j.jneuroim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Kaneko Naoe, Kurata Mie, Yamamoto Toshihiro, Morikawa Shinnosuke, Masumoto Junya. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019;39(1):12. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz-Levy Y., Neville K.L., Padilla J., Rahbe S., Begolka W.S., Girvin A.M., Olson J.K., Vanderlugt C.L., Miller S.D. Temporal development of autoreactive Th1 responses and endogenous presentation of self myelin epitopes by central nervous system-resident APCs in Theiler's virus-infected mice. J. Immunol. 2000;165(9):5304–5314. doi: 10.4049/jimmunol.165.9.5304. [DOI] [PubMed] [Google Scholar]
- Lane T.E., Fox H.S., Buchmeier M.J. Inhibition of nitric Oxide synthase-2 reduces the severity of mouse hepatitis virus-induced demyelination: implications for NOS2/NO regulation of chemokine expression and inflammation. J. Neurovirol. 1999;5(1):48–54. doi: 10.3109/13550289909029745. [DOI] [PubMed] [Google Scholar]
- Lane T.E., Liu M.T., Chen B.P., Asensio V.C., Samawi R.M., Paoletti A.D., Campbell I.L., Kunkel S.L., Fox H.S., Buchmeier M.J. A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J. Virol. 2000;74(3):1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley Koedi S., Rech Raquel R., Elenwa Faith, Han Gang, Aracely A., Perez Gomez, Amstalden Katia, Welsh C. Jane, Young Colin R., Threadgill David W., Brinkmeyer-Langford Candice L. Host genetic diversity drives variable central nervous system lesion distribution in chronic phase of Theiler's murine encephalomyelitis virus (TMEV) infection. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0256370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levillayer F., Mas M., Levi-Acobas F., Brahic M., Bureau J.F. Interleukin 22 is a candidate gene for Tmevp3, a locus controlling Theiler's virus-induced neurological diseases. Genetics. 2007;176(3):1835–1844. doi: 10.1534/genetics.107.073536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Jing, Huo Xiaoxu, Cao Yun-Feng, Li Sai-Nan, Du Zuo, Shao Ping, Leng Junhong, Zhang Cuiping, Sun Xiao-Yu, Ronald C., Ma W., Fang Zhong-Ze, Yang Xilin. Bile acid metabolites in early pregnancy and risk of gestational diabetes in Chinese women: a nested case-control study. EBioMedicine. 2018;35:317–324. doi: 10.1016/j.ebiom.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey Jane E., Kirkman Nikki J., Smith Matthew C.P., Tanaka Tomoko, Wilcox Karen S., White H. Steve, Fujinami Robert S. Seizures following picornavirus infection. Epilepsia. 2008;49(6):1066–1074. doi: 10.1111/j.1528-1167.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- Lipton H.L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect. Immun. 1975;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H.L. Persistent Theiler's murine encephalomyelitis virus infection in mice depends on plaque size. J. Gen. Virol. 1980;46(1):169–177. doi: 10.1099/0022-1317-46-1-169. [DOI] [PubMed] [Google Scholar]
- Lipton H.L., Melvold R. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J. Immunol. 1984;132(4):1821–1825. [PubMed] [Google Scholar]
- Love Michael I., Huber Wolfgang, Anders Simon. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannie M.D., Dinarello C.A., Paterson P.Y. Interleukin 1 and myelin basic protein synergistically augment adoptive transfer activity of lymphocytes mediating experimental autoimmune encephalomyelitis in lewis rats. Journal of Immunology (Baltimore, Md. : 1950) 1987;138(12):4229–4235. [PubMed] [Google Scholar]
- Matsuki Taizo, Nakae Susumu, Sudo Katsuko, Horai Reiko, Iwakura Yoichiro. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int. Immunol. 2006;18(2):399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- McCarthy Derrick P., Richards Maureen H., Miller Stephen D. Mouse models of multiple Sclerosis: experimental autoimmune encephalomyelitis and Theiler's virus-induced demyelinating disease. Methods Mol. Biol. 2012;900:381–401. doi: 10.1007/978-1-60761-720-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern D.B. Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple Sclerosis. Brain. 2000;123(3):519–531. doi: 10.1093/brain/123.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvold R.W., Jokinen D.M., Knobler R.L., Lipton H.L. Variations in genetic control of susceptibility to Theiler's murine encephalomyelitis virus (TMEV)-Induced demyelinating disease. I. Differences between susceptible SJL/J and resistant BALB/c strains map near the T cell beta-chain constant gene on chromosome 6. Journal of Immunology (Baltimore, Md. : 1950) 1987;138(5):1429–1433. [PubMed] [Google Scholar]
- Mi W., Belyavskyi M., Johnson R., Sieve A., Storts R., Meagher M., Welsh C.J. Alterations in chemokine expression following Theiler's virus infection and restraint stress. J. Neuroimmunol. 2004;151(1–2):103–115. doi: 10.1016/j.jneuroim.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Mi W., Prentice T.W., Young C.R., Johnson R.R., Sieve A.N., Meagher M.W., Welsh C.J.R. Restraint stress decreases virus-induced pro-inflammatory cytokine MRNA expression during acute Theiler's virus infection. J. Neuroimmunol. 2006;178(1–2):49–61. doi: 10.1016/j.jneuroim.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Michael Benedict D., Solomon Tom. Seizures and encephalitis: clinical features, management, and potential pathophysiologic mechanisms. Epilepsia. 2012;53:63–71. doi: 10.1111/j.1528-1167.2012.03615.x. [DOI] [PubMed] [Google Scholar]
- Murray P.D., Pavelko K.D., Leibowitz J., Lin X., Rodriguez M. CD4(+) and CD8(+) T cells make discrete contributions to demyelination and neurologic disease in a viral model of multiple Sclerosis. J. Virol. 1998;72(9):7320–7329. doi: 10.1128/jvi.72.9.7320-7329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka S., Dalton S. Molecular and biological properties of pluripotent embryonic stem cells. Gene Ther. 2008;15(2):74–81. doi: 10.1038/sj.gt.3303065. [DOI] [PubMed] [Google Scholar]
- Oleszak Emilia L., Robert Chang J., Friedman Herman, Katsetos Christos D., Platsoucas Chris D. Theiler's virus infection: a model for multiple Sclerosis. Clin. Microbiol. Rev. 2004;17(1):174–207. doi: 10.1128/CMR.17.1.174-207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszak Emilia L., Kuzmak Jacek, Good Robert A., Platsoucas Chris D. Immunology of Theiler's murine encephalomyelitis virus infection. Immunol. Res. 1995;14(1):13–33. doi: 10.1007/BF02918495. [DOI] [PubMed] [Google Scholar]
- Oliver K.R., Brennan P., Fazakerley J.K. Specific infection and destruction of dopaminergic neurons in the substantia nigra by Theiler's virus. J. Virol. 1997;71(8):6179–6182. doi: 10.1128/jvi.71.8.6179-6182.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavelko Kevin D., Howe Charles L., Drescher Kristen M., Gamez Jeff D., Johnson Aaron J., Wei Tao, Ransohoff Richard M., Rodriguez Moses. Interleukin-6 protects anterior horn neurons from lethal virus-induced injury. J. Neurosci. : The Official Journal of the Society for Neuroscience. 2003;23(2):481–492. doi: 10.1523/JNEUROSCI.23-02-00481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillippi J., Xie Y., Miller D.R., Bell T.A., Zhang Z., Lenarcic A.B., Aylor D.L., Krovi S.H., Threadgill D.W., Pardo-Manuel de Villena F., Wang W., Valdar W., Frelinger J.A. Using the emerging collaborative Cross to probe the immune system. Gene Immun. 2014;15(1):38–46. doi: 10.1038/gene.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procaccini Claudio, De Rosa Veronica, Pucino Valentina, Formisano Luigi, Matarese Giuseppe. Animal models of multiple Sclerosis. Eur. J. Pharmacol. 2015;759:182–191. doi: 10.1016/j.ejphar.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards Maureen H., Getts Meghann Teague, Podojil Joseph R., Jin Young-Hee, Kim Byung S., Miller Stephen D. Virus expanded regulatory T cells control disease severity in the Theiler's virus mouse model of MS. J. Autoimmun. 2011;36(2):142–154. doi: 10.1016/j.jaut.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., David C.S. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. Journal of Immunology (Baltimore, Md. : 1950. 1985;135(3):2145–2148. [PubMed] [Google Scholar]
- Rodriguez Moses, Lindsley Mark D., Pierce Mabel L. Role of T Cells in resistance to Theiler's virus infection. Microb. Pathog. 1991;11(4):269–281. doi: 10.1016/0882-4010(91)90031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Moses, Pease Larry R., David Chella S. Immune-mediated injury of virus-infected oligodendrocytes A model of multiple Sclerosis. Immunol. Today. 1986;7(12):359–363. doi: 10.1016/0167-5699(86)90025-3. [DOI] [PubMed] [Google Scholar]
- Rojas José M., Avia Miguel, Martín Verónica, Noemí Sevilla IL-10: a multifunctional cytokine in viral infections. Journal of Immunology Research. 2017:1–14. doi: 10.1155/2017/6104054. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeron Kathleen E., Maniskas Michael E., Edwards Danielle N., Wong Raymond, Rajkovic Ivana, Trout Amanda, Rahman Abir A., Hamilton Samantha, Fraser Justin F., Pinteaux Emmanuel, Gregory J., Bix Interleukin 1 alpha administration is neuroprotective and neuro-restorative following experimental ischemic stroke. J. Neuroinflammation. 2019;16(1):222. doi: 10.1186/s12974-019-1599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Yonggang, Markovic-Plese Silva. Activated IL-1RI signaling pathway induces Th17 cell differentiation via interferon regulatory factor 4 signaling in patients with relapsing-remitting multiple Sclerosis. Front. Immunol. 2016;7:543. doi: 10.3389/fimmu.2016.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J.E., Newcombe J., Cuzner M.L., Woodroofe M.N. Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple Sclerosis lesions. J. Neuroimmunol. 1998;84(2):238–249. doi: 10.1016/s0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- Siqueira Mietto, Bruno Antje Kroner, Girolami Elizabeth I., Santos-Nogueira Eva, Zhang Ji, David Samuel. 2015. Development/plasticity/repair role of IL-10 in resolution of inflammation and functional recovery after peripheral nerve injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman Andrew J. Infection as an environmental trigger of multiple Sclerosis disease exacerbation. Front. Immunol. 2015;6:520. doi: 10.3389/fimmu.2015.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman Lawrence. Assessment of animal models minireview for MS and demyelinating disease in the design of rational therapy like paralysis, and then the animals would develop chronic neurologic disease, with relapsing episodes of paralysis followed by periods of clinical remission and ultimately permanent deficits. Many Animal Models of MS Feature Only Acute Attacks and Recovery, a Situation. 1999;24 [Google Scholar]
- Stewart Kerry-Ann A., Karen S., Wilcox, Fujinami Robert S., Steve White H. Theiler's virus infection chronically alters seizure susceptibility. Epilepsia. 2009;51(8):1418–1428. doi: 10.1111/j.1528-1167.2009.02405.x. [DOI] [PubMed] [Google Scholar]
- Threadgill D.W., Miller D.R., Churchill G.A., de Villena F.P.M. The collaborative Cross: a recombinant inbred mouse population for the systems genetic era. ILAR J. 2011;52(1):24–31. doi: 10.1093/ilar.52.1.24. [DOI] [PubMed] [Google Scholar]
- Threadgill David W., Hunter Kent W., Williams Robert W. Genetic dissection of complex and quantitative traits: from fantasy to reality via a community effort. Mamm. Genome. 2002;13(4):175–178. doi: 10.1007/s00335-001-4001-Y. [DOI] [PubMed] [Google Scholar]
- Trottier Mark, Schlitt Brian P., Kung Aisha Y., Lipton Howard L. Transition from acute to persistent Theiler's virus infection requires active viral replication that drives proinflammatory cytokine expression and chronic demyelinating disease. J. Virol. 2004;78(22):12480–12488. doi: 10.1128/JVI.78.22.12480-12488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda Ikuo, Fujinami Robert S. Inside-out versus Outside-in models for virus induced demyelination: axonal damage triggering demyelination. Springer Semin. Immunopathol. 2002;24(2):105–125. doi: 10.1007/s00281-002-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda Ikuo, Sato Fumitaka, Omura Seiichi, Fujita Mitsugu, Sakiyama Namie, Park Ah-Mee. Three immune-mediated disease models induced by Theiler's virus: multiple Sclerosis, seizures and myocarditis. Clinical & Experimental Neuroimmunology. 2016;7(4):330. doi: 10.1111/cen3.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau Soline, Rohrlich Pierre-Simon, Brahic Michel, Jean-François Bureau Tmevpg1, a candidate gene for the control of Theiler's virus persistence, could Be implicated in the regulation of gamma interferon. J. Virol. 2003;77(10):5632–5638. doi: 10.1128/JVI.77.10.5632-5638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Lingyun, Liu Changan, Jiang Liangliang, Wang Yuqing. vol. 10. 2017. (Aggravation of Spinal Cord Injury by CCL5 via Activating NK-ΚB Signaling Pathway). [Google Scholar]
- Welsh C.J., V Sapatino B., Rosenbaum B.A., Smith R. Characteristics of cloned cerebrovascular endothelial cells following infection with Theiler's virus. I. Acute infection. J. Neuroimmunol. 1995;62(2):119–125. doi: 10.1016/0165-5728(95)00093-2. [DOI] [PubMed] [Google Scholar]
- Welsh Jane, Bruno Sapatino, Rosenbaum Betty, Smith Roger, Scott Linthicum. Correlation between susceptibility to demyelination and interferon-γ induction of major histocompatibility complex class II antigens on murine cerebrovascular endothelial cells. J. Neuroimmunol. 1993;48(1):91–97. doi: 10.1016/0165-5728(93)90062-4. [DOI] [PubMed] [Google Scholar]
- Welsh Robin A., Song Nianbin, Foss Catherine A., Boronina Tatiana, Cole Robert N., Sadegh-Nasseri Scheherazade. Lack of the MHC class II chaperone H2-O causes susceptibility to autoimmune diseases. PLoS Biol. 2020;18(2) doi: 10.1371/journal.pbio.3000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woulfe John M., Gray Madison T., Gray Douglas A., Munoz David G., Middeldorp Jaap M. Hypothesis: a role for EBV-induced molecular mimicry in Parkinson's disease. Park. Relat. Disord. 2014;20(7):685–694. doi: 10.1016/j.parkreldis.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Zou Fei, Gelfond Jonathan A.L., Airey David C., Lu Lu, Manly Kenneth F., Williams Robert W., Threadgill David W. Quantitative trait locus analysis using recombinant inbred intercrosses. Genetics. 2005;170(3):1299–1311. doi: 10.1534/genetics.104.035709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.