Graphical abstract
Keywords: Hybrid coronary revascularization, Percutaneous coronary Intervention, Meta-analysis, Clinical outcomes
Abbreviations: CABG, coronary artery bypass grafting; CI, confidence interval; HCR, hybrid coronary revascularization; LAD, left anterior descending coronary artery; LITA, left internal thoracic artery; MACCE, major adverse cardiac and cerebrovascular events; MD, mean difference; MI, myocardial infarction; MVD, multivessel coronary artery disease; OR, odds ratio; PCI, percutaneous coronary intervention; PRISMA, Preferred Reporting Items for Systematic reviews Meta-Analyses; TVR, target vessel revascularization
Highlights
-
•
Hybrid coronary revascularization (HCR) for multivessel disease (MVD) is emerging.
-
•
This meta-analysis compared HCR to percutaneous coronary intervention (PCI)
-
•
Both procedures had comparable 30-day outcomes.
-
•
HCR had less myocardial infarction and target vessel revascularization at follow-up.
-
•
More randomized controlled trials comparing both approaches are needed.
Abstract
Background
Hybrid coronary revascularization (HCR) is an emerging approach for multivessel coronary artery disease (MVD) which combines the excellent long-term outcomes of surgery with the early recovery and reduced short-term complications of percutaneous coronary intervention (PCI). Here, we evaluated the effectiveness of HCR compared to PCI in patients with MVD.
Methods
A systematic database search in PubMed/MEDLINE, Embase, Scopus, and CENTRAL/CCTR was conducted by June 2021. Random-effects meta-analysis was performed, comparing major adverse cardiac and cerebrovascular events (MACCE) at 30 days and at latest follow-up between patients undergoing HCR versus PCI.
Results
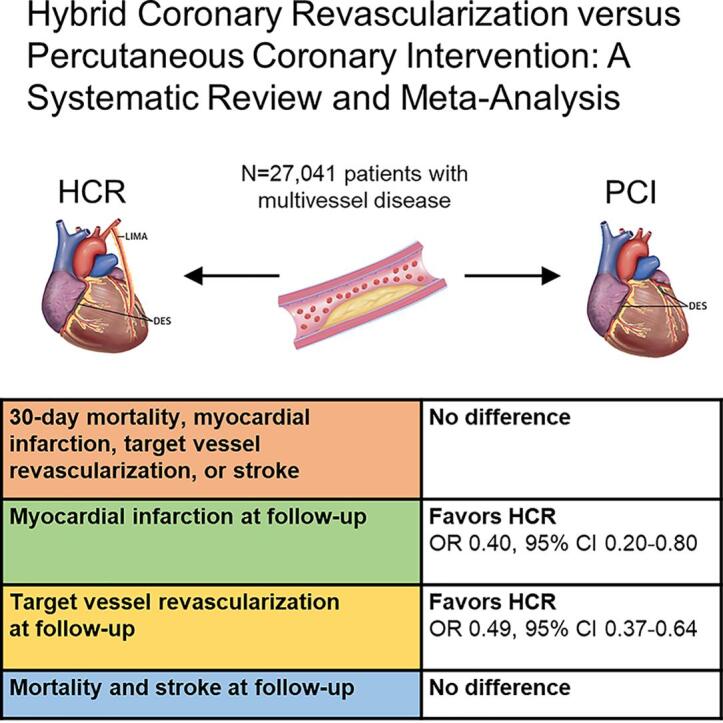
A total of 27,041 patients (HCR: 939 patients, PCI: 26,102 patients) were included from seven studies published between 2013 and 2021. At latest follow-up, HCR was associated with lower rates of myocardial infarction (OR 0.40, 95% CI 0.20–0.80, p = 0.010) and target vessel revascularization (OR 0.49, 95% CI 0.37–0.64, p < 0.001), while the difference for MACCE did not reach statistical significance (OR 0.46, 95% CI 0.20–1.05, p = 0.061). No differences were observed in terms of 30-day outcomes, nor rates of mortality or stroke at latest follow-up.
Conclusions
HCR might be a valid alternative to multivessel PCI, demonstrating a lower incidence of MI and TVR. Center experience, well-coordinated heart team discussions, and good patient selection likely remain essential to ensure optimal outcomes. Future comparative studies are required to define the optimal target population.
1. Introduction
Hybrid coronary revascularization (HCR) is an emerging treatment for multivessel coronary artery disease (MVD) which combines the excellent long-term outcomes of coronary artery bypass grafting (CABG) with the early recovery and reduced short-term complications of percutaneous coronary intervention (PCI) [1], [2]. Previous reports and meta-analyses have demonstrated favorable outcomes, showing lower need for blood transfusion, shorter length of stay, and faster recovery, while maintaining similar rates of major adverse cardiac and cerebrovascular events (MACCE) compared with conventional CABG [3], [4], [5], [6], [7].
So far, however, only a limited number of studies have directly compared HCR with multivessel PCI. The Hybrid Coronary Revascularization Trial (NCT03089398), which would have been the first to directly compare effectiveness of HCR and multivessel PCI with DES, was discontinued prematurely due to suboptimal enrolment [1]. The evidence comparing both strategies is therefore limited and their relative efficacy and safety remain unclear. The aim of the present meta-analysis was to compare MACCE at 30 days and at latest follow-up between HCR and multivessel PCI for the treatment of MVD.
2. Methods
2.1. Search strategy, eligibility criteria and study inclusion
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [8]. On June 25, 2021, a systematic literature search was performed in PubMed/MEDLINE, Embase, Scopus, Cochrane Controlled Trials Register (CENTRAL/CCTR), and reference lists of relevant articles. The detailed search terms that were used for this search are given in Supplementary Materials, Methods.
Eligibility criteria were defined a priori, and studies were included if: (1) the population comprised patients with multivessel coronary artery disease (MVD); (2) there was an intervention group undergoing HCR; (3) there was a control group undergoing PCI; (4) 30-day outcomes (cumulative number of events 30 days after the procedure) or outcomes at latest follow-up (cumulative number of events ≥ 1 year after the procedure) were reported, including major adverse cardiac and cerebrovascular events (MACCE), or any of the individual components of each of these; and (5) studies were observational or randomized in nature. MACCE was defined as the composite of mortality, myocardial infarction (MI), target vessel revascularization (TVR), or stroke.
The following steps were taken: 1) identification of titles of records through databases searching, 2) removal of duplicates, 3) screening and selection of abstracts, 4) assessment for eligibility through full text articles, and 5) final inclusion in the study. Studies were selected by two independent reviewers (JVDE and SDG). Any discrepancies were resolved by consensus.
2.2. Endpoints, risk of bias, and statistical analysis
The outcomes were MACCE, and any of the individual components of these composite outcomes, at 30 days and at latest follow-up. Two independent reviewers extracted the data (JVDE and SDG). Any discrepancies were resolved by consensus. From each study, the following were extracted: first authors’ name, year of publication, country of origin, sample size, study design, characteristics of HCR, follow-up
Risk of bias of the selected studies was assessed independently by two reviewers (JVDE and SDG), using the Cochrane risk-of-bias tool for randomized trials (RoB 2) and the Risk Of Bias In Non-randomised Studies of Interventions (ROBINS-I), according to study design. Any discrepancies were resolved by consensus.
Odds ratios (OR) with 95% confidence interval (CI) and p-values for the crude endpoints were calculated. Chi-square test and I2 test were performed for assessment of statistical heterogeneity [9]. The OR were combined across the studies using a fixed-effects model, unless significant heterogeneity was observed; in that case, a random-effects models was used. Forest plots were created to represent clinical outcomes. Funnel plots were not appropriate for detecting publication bias since there were less than 10 studies.[10] All analyses were completed with R Statistical Software (version 4.0.5, Foundation for Statistical Computing, Vienna, Austria).
Ethical Approval: N/A
3. Results
3.1. Studies and participants
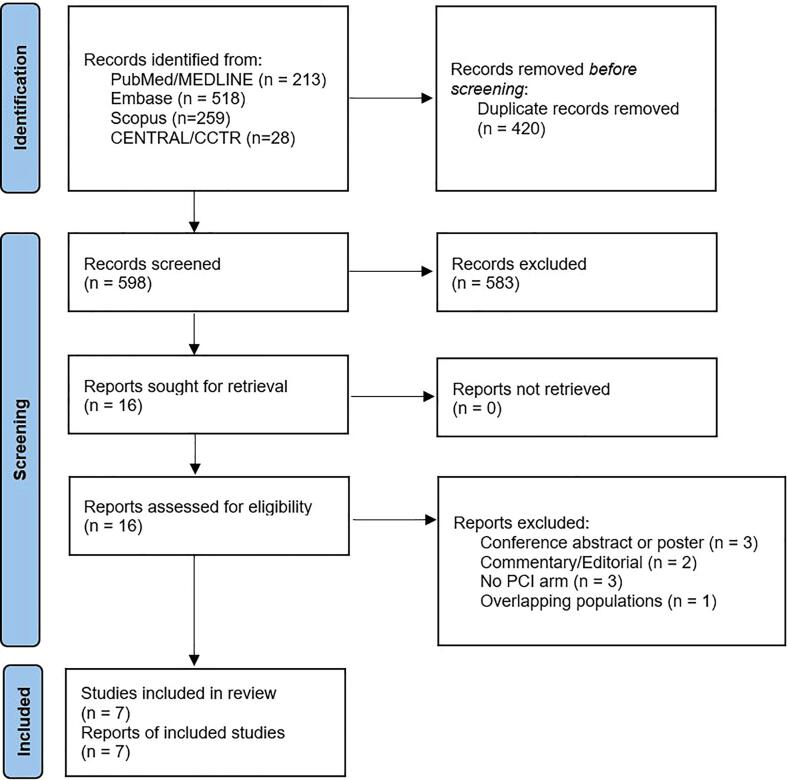
A total of 1018 citations were identified, of which 16 studies were potentially relevant and retrieved as full text. Seven publications [3], [11], [12], [13], [14], [15], [16] fulfilled our eligibility criteria (Fig. 1). Characteristics of each study and their patients are shown in Table 2, Table 3, Table 4. A total of 27,041 patients (HCR: 939 patients, PCI: 26,102 patients) were included from studies published from 2013 to 2021. Apart from one randomized controlled trial [12] and one observational study which used Cox proportional hazards methods to reduce selection bias [16], all studies were propensity score matched observational studies. The overall internal validity of the analysis was considered moderate risk of bias (Supplementary Materials, Tables S1 and S2).
Fig. 1.
Flow diagram of studies included in data search.
Table 2.
Study characteristics.
| Author | Year | Country of origin | Total number of patients (HCR/PCI) | Study design | Staging strategy for HCR | Interval Surgery-PCI | Follow-up (months) | HCR surgical approach | Use of DES (PCI/HCR) |
|---|---|---|---|---|---|---|---|---|---|
| Hannan et al | 2021 | USA | 25,892 (335/25557) | RS | 2-stage | PCI within 60 days before or after surgery | 48.0 | Minimally Invasive CABG surgery in the LAD artery | NR |
| Basman et al | 2020 | USA | 200 (100/100) | RS, PSM | 2-stage | 4–6 weeks | 85.7 ± 1.4 | Robotic MIDCAB* | 100%/100% |
| Ganyukov et al | 2020 | Russia/Poland | 105 (52/53) | RCT | 2-stage | 3 days | 12.0 | Robotic MIDCAB* | 100%/100% |
| Qiu et al | 2019 | China | 94 (47/47) | RS, PSM | NR | NR | 59.0 | NR | 100%/100% |
| Repossini et al | 2018 | Italy | 175 (67/108) | RS, PSM | 2-stage | 1–4 weeks | 15.3 ± 2.7 | MIDCAB: LIMA Harvest through Anterior Left Mini-Thoracotomy; LIMA-LAD Anastomosis | NR |
| Puskas et al | 2016 | USA | 298 (200/98) | PS, PSM | 1-stage 12%, 2-stage 76%; Multi-stage 4%; 8% Surgery-only | NR | 17.6 | Robotic MIDCAB* 54%; TECAB 21%; MIDCAB 19%; Sternotomy 6% | 100%/100% |
| Shen et al | 2013 | China | 282 (141/141) | RS, PSM | 1-stage | NR | 36.0 | Robotic MIDCAB* | 100%/100% |
CABG, coronary artery bypass grafting; DES, drug-eluting stent; HCR, hybrid coronary revascularization; LAD, left anterior descending coronary artery; LIMA, left internal mammary artery; MIDCAB, minimally invasive direct coronary artery bypass; NR, not reported; PCI, percutaneous coronary intervention; PS, prospective; PSM, Propensity score matched; RCT, randomized controlled trial; RS, retrospective; TECAB, totally endoscopic coronary artery bypass.
*Robotic MIDCAB: Robotic-assisted LIMA harvest with LIMA-LAD anastomosis through mini-thoracotomy.
Table 3.
Characteristics of the study population (1).
| Age, years |
Female (%) |
Diabetes (%) |
Smoking (%) |
Hypertension (%) |
COPD (%) |
CKD (%) |
Previous MI (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | HCR | PCI | HCR | PCI | HCR | PCI | HCR | PCI | HCR | PCI | HCR | PCI | HCR | PCI | HCR | PCI |
| Hannan 2021 | NR | NR | 21.5 | 28.6 | 35.5 | 34.0 | NR | NR | NR | NR | 4.8 | 5.7 | 3.6 | 4.0 | 17.0 | 19.3 |
| Basman 2020 | 67.5 ± 10.1 | 67.8 ± 10.5 | 16 | 16 | 53 | 50 | NR | NR | NR | NR | 2 | 0 | 3 | 3 | 28 | 29 |
| Ganyukov 2020 | 62 ± 7.4 | 61.7 ± 7.7 | 25 | 30.2 | 17.3 | 20.7 | 46.1 | 47.2 | 65.4 | 67.9 | 7.7 | 11.3 | 1.9 | 5.7 | 51.9 | 58.5 |
| Qiu 2019 | 64.6 ± 8.3 | 65.0 ± 9.8 | 14.9 | 25.5 | 40.4 | 46.8 | 51.1 | 31.9 | 61.7 | 72.3 | 0 | 2.1 | NR | NR | 25.5 | 38.3 |
| Repossini 2018 | 68 0.0 ± 9.0 | 69.0 ± 11.0 | 22.7 | 24.8 | 26.8 | 30.8 | NR | NR | 66.4 | 64.7 | 19.3 | 19.9 | 16.5 | 17.9 | 28.2 | 29.5 |
| Puskas 2016 | 64.4 ± 11.8 | 63.9 ± 10.8 | 24 | 28.6 | 39.5 | 36.7 | NR | NR | NR | NR | 9 | 12.3 | NR | NR | 37 | 23.5 |
| Shen 2013 | 62.0 ± 9.9 | 61.7 ± 10.3 | 11.3 | 12.8 | 26.2 | 19.9 | 55.3 | 24.8 | 64.5 | 59.6 | 7.1 | 7.8 | 2.8 | 5.0 | 27.7 | 27.7 |
CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HCR, hybrid coronary revascularization; MI, myocardial revascularization; NR, not reported; PCI, percutaneous coronary intervention.
Table 4.
Characteristics of the study population (2).
| LVEF, % |
PVD (%) |
Prior CVA or TIA (%) |
SYNTAX score |
EuroSCORE |
TVD (%) |
LMD (%) |
Dyslipidemia (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | HCR | PCI | HCR | PCI | HCR | PCI | HCR | PCI | HCR | PCI | HCR | PCI | HCR | PCI | HCR | PCI |
| Hannan 2021 | NR | NR | 8.4 | 7.3 | 10.2 | 8.6 | NR | NR | NR | NR | 51.9 | 35.2 | 13.4 | 2.4 | NR | NR |
| Basman 2020 | 53.3 ± 11.0 | 54.5 ± 11.3 | 10 | 11 | 10 | 11 | 28.9 ± 10.6 | 22.1 ± 7.6 | NR | NR | NR | NR | NR | NR | NR | NR |
| Ganyukov 2020 | 56.2 ± 6.3 | 53.3 ± 9.9 | 30.8 | 30.2 | 7.7 | 5.7 | 19.4 ± 3.0 | 19.5 ± 2.7 | 1.71 ± 0.76 | 1.70 ± 0.79 | 48.1 | 43.4 | NR | NR | NR | NR |
| Qiu 2019 | 64.7 ± 5.4 | 64.1 ± 7.0 | 0.0 | 6.4 | 14.9 | 14.9 | 19.4 ± 3.6 | 19.8 ± 3.4 | 3.2 ± 1.8 | 3.0 ± 1.5 | NR | NR | NR | NR | 17 | 14.9 |
| Repossini 2018 | 51.8 ± 10.2 | 50.7 ± 10.7 | 19.9 | 23.4 | 15.3 | 12.9 | 29.5 ± 6.9 | 29.1 ± 6.5 | 3.4 ± 2.9 | 3.4 ± 3.2 | NR | NR | NR | NR | 48.9 | 47.8 |
| Puskas 2016 | NR | NR | 12.5 | 7.1 | 9.5 | 2 | 21.5 ± 9.5 | 15.8 ± 8.5 | NR | NR | 40 | 34.7 | 17.5 | 7.1 | NR | NR |
| Shen 2013 | 62.7 ± 7.1 | 61.2 ± 9.3 | 24.1 | 29.1 | 10.6 | 19.1 | 27.6 ± 7.9 | 26.0 ± 8.2 | 3.1 ± 2.3 | 3.5 ± 2.6 | NR | NR | 19.9 | 15.6 | 53.2 | 48.9 |
CVA, cerebrovascular accident; HCR, hybrid coronary revascularization; LMD, left main disease; LVEF, left ventricular ejection fraction; NR, not reported; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; TIA, transient ischemic attack; TVD, triple vessel disease.
3.2. Outcomes
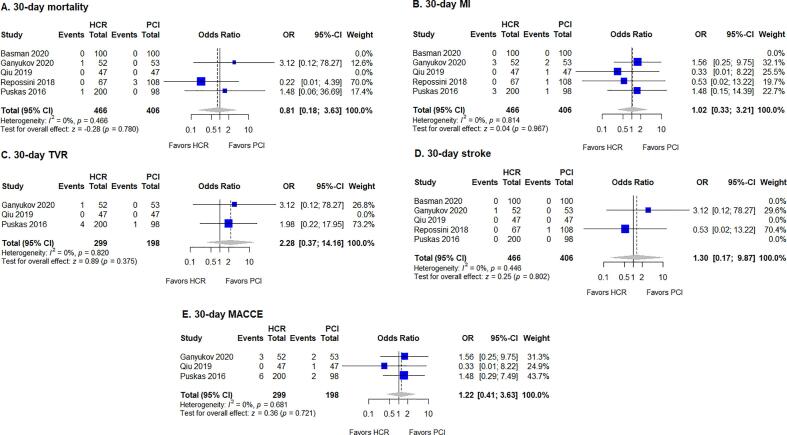
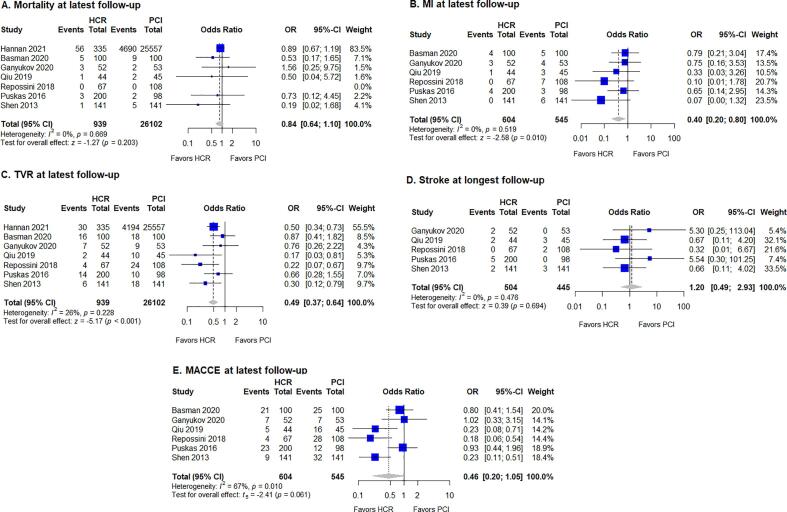
The results of the meta-analysis are summarized in Table 1. No significant differences between HCR and PCI were found for 30-day mortality, MI, TVR, stroke, or MACCE (Fig. 2). However, rates of outcomes at latest follow-up differed between treatment modalities. HCR was favored by lower rates of MI (fixed-effects model: OR 0.40, 95% CI 0.20–0.80, p = 0.010) and TVR (fixed-effects model: OR 0.49, 95% CI 0.37–0.64, p < 0.001) at latest follow-up (Fig. 3). No evidence of heterogeneity was found for the studies for MI (I2 = 0%, p = 0.519), while heterogeneity was low in studies for TVR (I2 = 26%, p = 0.228). The difference for MACCE at latest follow-up did not reach statistical significance (OR 0.46, 95% CI 0.20–1.05, p = 0.061), while there was evidence of significant heterogeneity of treatment effect among the studies for this outcome (I2 = 67%, p = 0.010). Finally, no significant differences were observed for mortality, stroke, or MACCE at latest follow-up.
Table 1.
Meta-analysis: summary of results.
| Effect size |
Heterogeneity |
||||||
|---|---|---|---|---|---|---|---|
| Outcome | Studies (n) | Patients (n) | OR | 95% CI | P-value | I2 (%) | P-value |
| 30-day outcomes (cumulative events at within 30 days after the procedure) | |||||||
| Mortality | 5 | 872 | 0.81 | 0.18–3.63 | 0.780 | 0 | 0.466 |
| MI | 5 | 872 | 1.02 | 0.33–3.21 | 0.967 | 0 | 0.814 |
| TVR | 3 | 497 | 2.28 | 0.37–14.16 | 0.375 | 0 | 0.820 |
| Stroke | 5 | 872 | 1.30 | 0.17–9.87 | 0.802 | 0 | 0.446 |
| MACCE | 3 | 497 | 1.22 | 0.41–3.63 | 0.721 | 0 | 0.681 |
| Outcomes at latest follow-up (cumulative events at > 1 year follow-up after the procedure) | |||||||
| Mortality | 7 | 27,041 | 0.84 | 0.64–1.10 | 0.203 | 0 | 0.669 |
| MI | 7 | 1,149 | 0.40 | 0.20–0.80 | 0.010 | 0 | 0.519 |
| TVR | 6 | 27,041 | 0.49 | 0.37–0.64 | <0.001 | 26 | 0.228 |
| Stroke | 5 | 949 | 1.20 | 0.49–2.93 | 0.694 | 0 | 0.476 |
| MACCE | 6 | 1,149 | 0.46 | 0.20–1.05 | 0.061 | 67 | 0.010 |
CI, confidence interval; MACCE, major adverse cardiac and cerebrovascular accidents; MI, myocardial infarction; OR, odds ratio; TVR, target vessel revascularization.
Fig. 2.
Forest plot for 30-day outcomes, with pooled odds ratio and conclusions plot for (A) mortality, (B) MI, (C) TVR, (D) stroke, and (E) MACCE. CI, confidence interval; HCR, hybrid coronary revascularization; MACCE, major adverse cardiac and cerebrovascular events; MI, myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; TVR, target vessel revascularization.
Fig. 3.
Forest plot for outcomes at latest follow-up, with pooled odds ratio and conclusions plot for (A) mortality, (B) MI, (C) TVR, (D) stroke, and (E) MACCE. CI, confidence interval; HCR, hybrid coronary revascularization; MACCE, major adverse cardiac and cerebrovascular events; MI, myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; TVR, target vessel revascularization.
4. Discussion
4.1. Summary of evidence
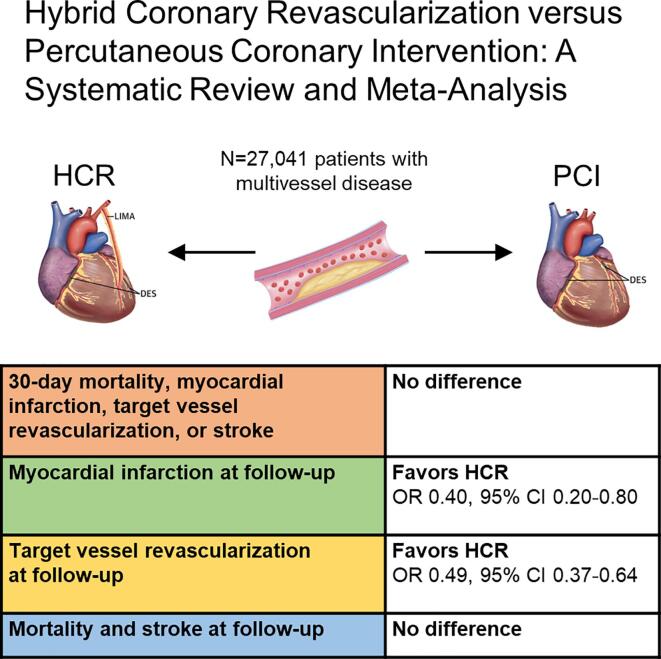
The main findings of this study are summarized in Fig. 4. To the best of our knowledge, this is the first systematic review with meta-analysis carried out to assess the results of HCR versus PCI to treat patients with MVD, providing additional value for the medical literature by showing that: 1) the immediate risk of mortality, MI, stroke, TVR and MACCE were not different between HCR and PCI; and 2) there was lower risk of MI and TVR during the follow-up for HCR in comparison with PCI.
Fig. 4.
Summary of the main findings of the meta-analysis. The 30-day risk of mortality, myocardial infarction, target vessel revascularization, or stroke were not different between HCR and PCI. However, There was a lower risk of myocardial infarction and target vessel revascularization during the follow-up for HCR in comparison with PCI. CI, confidence interval; HCR, hybrid coronary revascularization; OR, odds ratio; PCI, percutaneous coronary intervention.
4.2. HCR versus CABG
Before jumping into discussions about comparisons between outcomes of HCR and PCI, we should first ask ourselves if HCR is superior (or at least comparable) to CABG surgery, considered to be the best option for MVD. Two recent meta-analyses [6], [7] shed some light on this subject. Wang et al. [6] conducted a meta-analysis with 23 studies including 10,468 patients (2403 underwent HCR and 8065 patients underwent traditional CABG. Compared with CABG, HCR had a statistically significant lower risk of stroke, MACCE and blood transfusion, whereas no significant differences were detected in mortality, MI and TVR. However, long-term results showed no significant difference between the HCR and CABG techniques. Guan et al. [7] performed a systematic review and meta-analysis to compare clinical outcomes after HCR and minimally invasive coronary revascularization (including minimally invasive direct coronary artery bypass grafting and robotic-assisted coronary artery bypass grafting). Their study included 8 observational studies with 1084 cases of HCR and 2349 cases of minimally invasive coronary revascularization. The pooled analyses showed no statistically significant differences in terms of in-hospital death, MI, MACCE and long-term survival. Considering the two aforementioned studies, we can affirm that HCR is a feasible option for patients with MVD and non-inferior in comparison with CABG (actually, it presents lower risk of complications in the immediate postoperative period without compromising long-term results).
4.3. HCR versus PCI
In order to optimize outcomes, myocardial revascularization should: 1) offer minimal invasiveness with faster recovery, sternal-sparing incisions, absence of aortic manipulation and cardiopulmonary bypass to reduce complications; and 2) improve durability and patency to attain maximal survival. Consequently, a synergistic combination (rather than a dichotomous mindset) bringing together the best of both approaches may be considered to treat patients with MVD, thus, minimizing immediate risk and invasiveness while optimizing long-term outcomes. While standard HCR uses the left internal thoracic artery (LITA) to the left anterior descending (LAD) artery, advanced HCR uses both ITAs to the LAD and circumflex artery or diagonal branches. The other coronary arteries are treated with drug-eluting stents (DES). In this scenario, the long-term benefits of CABG have been largely associated with the durability of the LITA graft to the LAD [17]. Additionally, DESs compared with saphenous vein grafts (SVGs) seem to offer advantages in the long run [1]. The durability of SVGs are poor, with graft failure reaching 20% at 1 year and around 70% at 15 years [18], whereas current DESs provide long-term patency rates of 96% to 98% [19], [20].
Most detractors of HCR claim that, whereas PCI offers very low risks of immediate complications and more rapid recovery, CABG might have increased immediate complications such as stroke. Our findings revealed that this belief is, at least based on the available evidence published so far, unfounded when it comes to HCR. We observed no statistically significant differences between HCR and PCI at 30 days for the outcomes death, MI, stroke, TVR and MACCE, thus contradicting any claims of higher risks in the immediate postoperative period. The minimally invasive nature of HCR, the avoidance of cardiopulmonary bypass, and the opportunity to prevent manipulation of the aorta might explain why this procedure is associated with a low risk of perioperative stroke. Supporting the latter two points, a network meta-analysis of 37,720 from 13 studies reported off-pump CABG without manipulation of the aorta was the most effective treatment for decreasing the risk of stroke (OR 0.22, 95% CI 0.14–0.33 compared with conventional on-pump CABG) [21]. Regarding differences in procedural MI, we should note the emerging concern that rates after PCI and CABG might vary greatly with different definitions [22]. Furthermore, retrospective studies, as in the majority of the studies included in this meta-analysis, might underestimate the rate of MI as they are less likely to include serial troponin measurement. Illustrating this issue, Basman et al. [11] and Qiu et al. [13] defined procedural MI according to the Third Universal Definition of Myocardial Infarction while Ganyukov et al. [12] used the Fourth Universal Definition of Myocardial Infarction, and the three other studies did not specify. A re-analysis of the EXCEL trial revealed that the Third Universal Definition of Myocardial Infarction identified a relatively small number of procedural MI events after CABG that were strongly predictive of 5-year cardiovascular mortality, while a larger number of events identified after PCI were not [23]. Therefore, caution should be taken when interpreting this body of evidence, and future studies will be needed to determine which definition has the highest diagnostic accuracy and greatest prognostic utility.
At the longest follow-up, the pooled results revealed an advantage for HCR for the outcomes TVR and MI. A meta-analysis published by Sá et al. [24] had already showed that the HRs for MI and TVR were lower in the CABG group than those in the PCI group (all with P < 0.001), demonstrating the long-term benefit of CABG over PCI. Furthermore, the 5-year outcomes of the Hybrid Revascularization for Multivessel Coronary Artery Disease (HYBRID) trial which randomized 200 patients to either HCR or CABG, showed similar rates of long-term mortality, MI, TVR, stroke, and MACCE in both groups. [5] What we can affirm for the time being is that there is some evidence of better outcomes in the long-run favoring HCR in comparison with PCI, which is per se a very positive finding that points to the alignment of two well-established approaches (CABG and PCI) rather than competition between them.
4.4. Incorporating HCR into the interventional landscape for coronary revascularization
Having demonstrated the favorable outcomes after HCR in this meta-analysis, we should consider how this approach could be incorporated into the interventional landscape for coronary revascularization. While HCR has been around for two decades, its practice remains restricted to a limited number of specialized centers and remains reserved for a highly selected patient population. In an analysis of the Society of Thoracic Surgeons Adult Cardiac Surgery Database between July 2011 and March 2013, 950 HCR procedures were identified, representing 0.48% of the total CABG volume [25]. In line with this, most published data, including the studies in this meta-analysis, come from high-volume centers with experienced operators. However, it is likely that a similar volume-outcome relationship exists for HCR, as has previously been demonstrated for other technically challenging procedures such as off-pump CABG [26]. Clearly, the use of the robotic system for harvesting of the graft(s) and performance of the anastomosis through a mini-thoracotomy are technically demanding, and this has been reflected in the presence of learning curve to acquire experience with this procedure [27].
As HCR consists of two procedures, it is critical that all care providers of the same team are actively involved in planning and coordination of all cases. The importance of regular Heart Team meetings should be emphasized, and decision trees to guide the discussions have been proposed before [2]. A one-fits-all approach likely does not exist for coronary revascularization, so HCR should be considered and weighted against all other options. Of note, even emergent cases might still be eligible for HCR: in the acute phase, the culprit lesion is treated first, after which a Heart Team discussion is held to consider a delayed second procedure to achieve more complete revascularization. Eventually, good patient selection and individualized provision of care will likely help ensure optimal outcomes.
4.5. Limitations
Although we did our best to collect all the data available to carry out pooled analyses, there remains a scarcity of data regarding comparisons between HCR and PCI. Only one RCT has been published so far [12] and, although we set out to explore the longest follow-ups available, long-term data are almost non-existent. Another limitation is that there is not enough granularity of data available so as to know whether the MI and TVR events observed in the HCR group were due to new obstructions in coronaries that received an arterial graft or DES. It would be important to know whether this complication would be rather related to the stents (which would prompt us to study better this issue – maybe related to insufficient antiaggregation therapy) or related to the arterial graft (which might be owing to the quality of the anastomosis that is more challenging in HCR procedures carried out through minimally invasive or robotic platforms). A recent analysis of 303 HCR procedures in the New York’s cardiac surgery and PCI registries in 2010 to 2016 suggested that TVR was caused by both the LAD where CABG was performed (required in 12% at 6 years of follow-up), and in the left circumflex and right coronary arteries where PCI was performed (required in 15% at 6 years of follow-up) [28]. Finally, as discussed above, heterogeneity in the definition of endpoints and operator experience may impact the actual outcomes after HCR vs PCI.
5. Conclusions
In conclusion, HCR has proven to be a promising option with non-inferior results in the immediate postoperative period and better results at latest follow-up in comparison with contemporary PCI. Center experience, well-coordinated heart team discussions, and good patient selection likely remain essential to ensure optimal outcomes. These results encourage the conduction of new RCTs to expand the pool of reliable and comparative data and draw definitive conclusions on the optimal target population for HCR. Studies with better design and larger sample sizes are needed to advance this treatment option.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
J. Van den Eynde was supported by the Belgian American Educational.
Declaration of interest
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data used for the analyses presented in this meta-analysis will be made available from the corresponding author upon reasonable request.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100916.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Moreno P.R., Stone G.W., Gonzalez-Lengua C.A., Puskas J.D. The hybrid coronary approach for optimal revascularization: JACC review topic of the week. J. Am. Coll Cardiol. 2020;76(3):321–333. doi: 10.1016/j.jacc.2020.04.078. [DOI] [PubMed] [Google Scholar]
- 2.J. Van den Eynde, J. Bennett, K. McCutcheon, et al., Heart team 2.0: A decision tree for minimally invasive and hybrid myocardial revascularization. Trends Cardiovasc Med. Published online July 2020. doi: 10.1016/j.tcm.2020.07.005. [DOI] [PubMed]
- 3.J.D. Puskas, M.E. Halkos, J.J. DeRose, et al., Hybrid coronary revascularization for the treatment of multivessel coronary artery disease: a multicenter observational study, J. Am. Coll. Cardiol. 68(4) (2016) 356-365. doi: 10.1016/j.jacc.2016.05.032. [DOI] [PMC free article] [PubMed]
- 4.Harskamp R.E., Bagai A., Halkos M.E., Rao S.V., Bachinsky W.B., Patel M.R., de Winter R.J., Peterson E.D., Alexander J.H., Lopes R.D. Clinical outcomes after hybrid coronary revascularization versus coronary artery bypass surgery: a meta-analysis of 1,190 patients. Am. Heart J. 2014;167(4):585–592. doi: 10.1016/j.ahj.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Tajstra M., Hrapkowicz T., Hawranek M., Filipiak K., Gierlotka M., Zembala M., Gąsior M., Zembala M.O. Hybrid coronary revascularization in selected patients with multivessel disease. JACC Cardiovasc. Interv. 2018;11(9):847–852. doi: 10.1016/j.jcin.2018.01.271. [DOI] [PubMed] [Google Scholar]
- 6.Wang C., Li P., Zhang F., Li J., Kong Q. Is hybrid coronary revascularization really beneficial in the long term? Eur. J. Cardio-Thoracic Surg. 2021;00:1–9. doi: 10.1093/ejcts/ezab161. [DOI] [PubMed] [Google Scholar]
- 7.Guan Z., Zhang Z., Gu K., Wang H., Lin J., Zhou W., Wan F. Minimally invasive CABG or hybrid coronary revascularization for multivessel coronary diseases: which is best? A systematic review and meta-analysis. Heart Surg. Forum. 2019;22(6):E493–E502. doi: 10.1532/hsf.2499. [DOI] [PubMed] [Google Scholar]
- 8.M.J. Page, J.E. McKenzie, P.M. Bossuyt, et al., The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, BMJ. 2021;372. doi:10.1136/bmj.n71. [DOI] [PMC free article] [PubMed]
- 9.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.P. Macaskill, S.D. Walter, L. Irwig, A comparison of methods to detect publication bias in meta-analysis, Stat Med. 20(4) (2001) 641–654. doi:10.1002/sim.698. [DOI] [PubMed]
- 11.C. Basman, J.M. Hemli, M.C. Kim, et al., Long-term survival in triple-vessel disease: hybrid coronary revascularization compared to contemporary revascularization methods, J. Card Surg. Published online July 2020. doi: 10.1111/jocs.14891. [DOI] [PubMed]
- 12.Ganyukov V., Kochergin N., Shilov A., Tarasov R., Skupien J., Szot W., Kokov A., Popov V., Kozyrin K., Barbarash O., Barbarash L., Musialek P. Randomized clinical trial of surgical vs. percutaneous vs. hybrid revascularization in multivessel coronary artery disease: residual myocardial ischemia and clinical outcomes at one year-hybrid coronary revascularization versus stenting or surgery (HREVS) J. Interv. Cardiol. 2020;2020:1–11. doi: 10.1155/2020/5458064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu J., Zhu P., Liu Z., Xu H., Liu J., Zhao Q. Hybrid coronary revascularization versus off-pump coronary artery bypass grafting and percutaneous coronary intervention for the treatment of two-vessel coronary artery disease with proximal left anterior descending artery stenosis. J. Thorac. Dis. 2019;11(6):2402–2409. doi: 10.21037/jtd10.21037/jtd.2019.05.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Repossini A., Di Bacco L., Rosati F., Tespili M., Saino A., Ielasi A., Muneretto C. Hybrid coronary revascularization versus percutaneous strategies in left main stenosis: a propensity match study. J. Cardiovasc. Med. (Hagerstown). 2018;19(5):253–260. doi: 10.2459/JCM.0000000000000641. [DOI] [PubMed] [Google Scholar]
- 15.Shen L., Hu S., Wang H., et al. One-stop hybrid coronary revascularization versus coronary artery bypass grafting and percutaneous coronary intervention for the treatment of multivessel coronary artery disease: 3-Year follow-up results from a single institution. J. Am. Coll Cardiol. 2013;61(25):2525–2533. doi: 10.1016/j.jacc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Hannan E.L., Wu Y.F., Cozzens K., et al. Hybrid coronary revascularization vs. percutaneous coronary interventions for multivessel coronary artery disease. J. Geriatr. Cardiol. 2021;18(3):159–167. doi: 10.11909/j.issn.1671-5411.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farkouh M.E., Domanski M., Sleeper L.A., Siami F.S., Dangas G., Mack M., Yang M., Cohen D.J., Rosenberg Y., Solomon S.D., Desai A.S., Gersh B.J., Magnuson E.A., Lansky A., Boineau R., Weinberger J., Ramanathan K., Sousa J.E., Rankin J., Bhargava B., Buse J., Hueb W., Smith C.R., Muratov V., Bansilal S., King S., Bertrand M., Fuster V. Strategies for multivessel revascularization in patients with diabetes. N. Engl. J. Med. 2012;367(25):2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 18.Parang P., Arora R. Coronary vein graft disease: Pathogenesis and prevention. Can. J. Cardiol. 2009;25(2):e57–e62. doi: 10.1016/S0828-282X(09)70486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone G.W., Rizvi A., Newman W., Mastali K., Wang J.C., Caputo R., Doostzadeh J., Cao S., Simonton C.A., Sudhir K., Lansky A.J., Cutlip D.E., Kereiakes D.J. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl. J. Med. 2010;362(18):1663–1674. doi: 10.1056/NEJMoa0910496. [DOI] [PubMed] [Google Scholar]
- 20.Bangalore S., Toklu B., Patel N., Feit F., Stone G.W. Newer-generation ultrathin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease: meta-analysis of randomized trials. Circulation. 2018;138(20):2216–2226. doi: 10.1161/CIRCULATIONAHA.118.034456. [DOI] [PubMed] [Google Scholar]
- 21.Zhao D.F., Edelman J.J., Seco M., Bannon P.G., Wilson M.K., Byrom M.J., Thourani V., Lamy A., Taggart D.P., Puskas J.D., Vallely M.P. Coronary artery bypass grafting with and without manipulation of the ascending aorta: a network meta-analysis. J. Am. Coll Cardiol. 2017;69(8):924–936. doi: 10.1016/j.jacc.2016.11.071. [DOI] [PubMed] [Google Scholar]
- 22.Cutlip D.E. Procedural myocardial infarction: definitions everywhere, but not any that may fit. J. Am. Coll Cardiol. 2020;76(14):1640–1643. doi: 10.1016/J.JACC.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Gregson J., Stone G.W., Ben-Yehuda O., Redfors B., Kandzari D.E., Morice M.-C., Leon M.B., Kosmidou I., Lembo N.J., Brown W.M., Karmpaliotis D., Banning A.P., Pomar J., Sabaté M., Simonton C.A., Dressler O., Kappetein A.P., Sabik J.F., Serruys P.W., Pocock S.J. Implications of alternative definitions of peri-procedural myocardial infarction after coronary revascularization. J. Am. Coll Cardiol. 2020;76(14):1609–1621. doi: 10.1016/j.jacc.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Sá M.P.B.O., Perazzo Á.M., Saragiotto F.A.S., Cavalcanti L.R.P., Neto A.C.E.A., Campos J.C.S., Braga P.G.B., Rayol S.d.C., Diniz R.G.S., Sá F.B.C.A., Lima R.C. Coronary artery bypass graft surgery improves survival without increasing the risk of stroke in patients with ischemic heart failure in comparison to percutaneous coronary intervention: a meta-analysis with 54,173 patients. Brazilian J. Cardiovasc. Surg. 2019;34(4) doi: 10.21470/1678-9741-2019-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harskamp R.E., Brennan J.M., Xian Y., Halkos M.E., Puskas J.D., Thourani V.H., Gammie J.S., Taylor B.S., de Winter R.J., Kim S., O’Brien S., Peterson E.D., Gaca J.G. Practice patterns and clinical outcomes after hybrid coronary revascularization in the United States: an analysis from the society of thoracic surgeons adult cardiac database. Circulation. 2014;130(11):872–879. doi: 10.1161/CIRCULATIONAHA.114.009479. [DOI] [PubMed] [Google Scholar]
- 26.LaPar D.J., Mery C.M., Kozower B.D., Kern J.A., Kron I.L., Stukenborg G.J., Ailawadi G. The effect of surgeon volume on mortality for off-pump coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2012;143(4):854–863. doi: 10.1016/j.jtcvs.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 27.Van den Eynde J., Vaesen Bentein H., Decaluwé T., De Praetere H., Wertan M.C., Sutter F.P., Balkhy H.H., Oosterlinck W. Safe implementation of robotic-assisted minimally invasive direct coronary artery bypass: application of learning curves and cumulative sum analysis. J. Thorac. Dis. 2021;13(7):4260–4270. doi: 10.21037/jtd10.21037/jtd-21-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hannan E.L., Wu Y., Cozzens K., Sundt T.M., Girardi L., Chikwe J., Wechsler A., Smith C.R., Gold J.P., Lahey S.J., Jordan D. Hybrid coronary revascularization versus conventional coronary artery bypass surgery: utilization and comparative outcomes. Circ. Cardiovasc. Interv. 2020;13(10) doi: 10.1161/CIRCINTERVENTIONS.120.009386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for the analyses presented in this meta-analysis will be made available from the corresponding author upon reasonable request.