Abstract
Little information is available describing viral loads in body fluids other than blood. In addition, the suitability of commercially available assays for human immunodeficiency virus type 1 (HIV-1) RNA quantitation has not been evaluated in most nonblood fluids. We compared Organon Teknika's nucleic acid sequence-based amplification method (NASBA) and Roche's Amplicor HIV-1 Monitor (reverse transcriptase PCR [RT-PCR]) for quantitating HIV-1 RNA in cerebrospinal fluid (CSF), saliva, breast milk, seminal plasma, and cervical-vaginal lavage fluid (CVL). Saliva and breast milk frequently demonstrated some inhibition in the RT-PCR assay, similar to the inhibition previously described in seminal plasma. Inhibition of the RT-PCR assay was not observed with CSF or CVL, nor in any of the NASBA assays. When fluids from HIV-infected individuals were tested by RT-PCR and NASBA, 73 and 27% of CSF samples and 60 and 40% of breast milk specimens had detectable RNA, respectively. These differences were not statistically significant. In cross-sectional studies using RT-PCR to measure viral RNA in paired blood plasma and CSF samples, 71% of blood plasma samples and 42% of CSF samples were positive. A similar analysis using NASBA with paired blood plasma and CVL, saliva, or seminal plasma samples revealed 91% were blood plasma positive and 55% were CVL positive, 76% were blood plasma positive and 46% were saliva positive, and 83% were blood plasma positive and 63% were seminal plasma positive. NASBA worked fairly well to quantitate HIV-1 RNA from all fluids without apparent inhibition. RT-PCR performed well on CVL and CSF, frequently with greater sensitivity, although its use in other fluids appears limited due to the presence of inhibitors. These studies demonstrate that viral loads in nonblood fluids were generally lower than in blood.
Although human immunodeficiency virus (HIV) in the peripheral blood compartment has been the focus of considerable research over the past 15 years, much less work has been directed at HIV in nonblood compartments. Other compartments, such as the genital tract, nervous system, breast, and oral cavity, may be potential sanctuary sites harboring HIV and impacting both the transmission and pathogenesis of HIV infection. It is vital to investigate tissues and compartments other than blood for two important reasons. From a patient perspective, it is important to determine whether antiretroviral therapy can reduce viral load in nonblood compartments which can serve as potential reservoirs of viral replication, particularly in individuals whose systemic viral load has been substantially reduced via potent drug therapy (29, 30). From a public health perspective, it is critical to know the factors that contribute to the “infectiousness” of an individual in order to devise strategies to reduce the likelihood of transmission (21). Increased viral load in seminal plasma, cervical fluid, breast milk, and, potentially, saliva, likely contributes to increased transmission risks.
Previous studies quantifying HIV in other compartments have involved noncommercial assays, making comparisons between different studies problematic (10, 13, 15, 16, 23). Since it is often difficult to obtain specimens other than blood from patients, many of these studies have involved relatively few numbers of patients. There are three commonly used commercially available HIV RNA assays: Roche's Amplicor HIV-1 Monitor (reverse transcriptase PCR [RT-PCR]), Organon-Teknika's nucleic acid sequence-based amplification (NASBA), and Chiron's signal amplification assay (Quantiplex). The Roche Amplicor HIV-1 Monitor is an in vitro nucleic acid amplification test for the quantitation of HIV-1 RNA by using RT-PCR technology. NASBA, and its recently improved version, NucliSens HIV-1 QT, are isothermal NASBA assays for the quantitative determination of HIV-1 RNA. The NASBA-based assay incorporates a silica bead nucleic acid extraction process (1), and amplification is based on repeated transcription via T7 RNA polymerase. The Quantiplex assay relies on signal amplification through branched DNA, rather than target amplification. Both NASBA and RT-PCR require at least 0.2 ml of sample for most applications, whereas the Quantiplex requires at least 1 ml. Limited sample volumes of some body fluids may preclude the utility of the Quantiplex assay. Accordingly, in this study, we have evaluated only RT-PCR and NASBA for quantitating HIV-1 RNA in cerebrospinal fluid (CSF), seminal plasma, cervical vaginal lavage fluid (CVL), breast milk, and saliva.
We previously observed considerable inhibition of the RT-PCR when seminal plasma was assayed, but no inhibition with the NASBA assay (4). Therefore, as we began to study other body fluids, we initially tested a small number of samples from HIV-infected patients as well as specimens from uninfected individuals spiked with HIV to detect the presence of amplification inhibitors. After determining the better assay for each body fluid, a cross-sectional study was performed to quantitate HIV RNA in paired blood plasma and CSF samples, seminal plasma, CVL, or saliva samples.
(This study was presented in part at the 6th Conference on Retroviruses and Opportunistic Infections, Chicago, Ill., 31 January–4 February 1999 [abstr. 295].)
MATERIALS AND METHODS
Patients and samples.
HIV-1-infected patients participating in a variety of studies had blood and another body fluid obtained following informed consent. Breast milk and saliva samples were also obtained from consenting uninfected donors. The Institutional Review Board of the University of North Carolina at Chapel Hill approved all studies.
Whole blood for plasma was collected in either EDTA or acid-citrate-dextrose-anticoagulated tubes. CSF was collected in 10-ml sterile polypropylene tubes as described by Robertson et al. (19). Five milliliters of whole unstimulated saliva was collected in a sterile 50-ml centrifuge tube kept chilled on ice. Subjects abstained from eating or brushing their teeth for at least 30 min prior to saliva collection (25). Breast milk was expressed manually or with a breast pump into sterile containers (13). Semen was collected by masturbation without the use of lubricants or water (4). CVL samples were collected by bathing the cervical os with 10 ml of normal saline and collecting the fluid that pooled in the posterior fornix in a sterile 15-ml test tube (26).
All specimens were processed within 2 to 6 h of collection. Saliva and CVL samples were vortexed and then stored as 0.5- to 1.0-ml aliquots at −70°C until tested. All other specimens (blood, semen, breast milk, and CSF) were centrifuged at room temperature at 400 to 600 × g for 10 min. The lipid layer of the breast milk specimens was removed by using a wide-bore pipette and discarded. Cell-free supernatants for all specimens except saliva and CVL were carefully removed and stored in 0.5- to 1.0-ml aliquots at −70°C until tested. Because CVL is initially collected in approximately 10 ml of diluent and the CVL-specific volume is unknown, no dilution factor was calculated into the final results. In some instances, cultured primary HIV isolates from recently infected infants (∼ 5 × 106 HIV-1 RNA copies/ml) were added to cell culture medium or body fluids (saliva and breast milk) from uninfected individuals in virus spiking experiments to investigate the presence of amplification inhibitors.
HIV RNA assays.
The Roche Amplicor HIV-1 Monitor (Branchburg, N.J.) and Organon Teknika (Durham, N.C.) NASBA assays were performed by following the manufacturer's instructions. During the course of this investigation, Organon Teknika modified the NASBA assay to improve the sensitivity. Therefore, some of the data reported here were obtained with the older NASBA version, while other results were obtained with the more recent version, NucliSens HIV-1 QT. The two assays, however, have been reported to yield comparable results (T. D. Ly, B. Montes, J. E. Molkin, and M. Segondy, Abstr. 5th Conf. Retroviruses Opportunistic Infect., abstr. 309, 1998).
The Roche Monitor utilizes an internal quantitation standard (QS) which is incorporated into each individual sample at a known copy number. HIV-1 RNA levels in test specimens are determined by comparing the test HIV-1 signal to the QS signal for each sample. Maximum recovery of QS is observed when the second QS well has an optical density (OD) of 0.3 or greater. We defined partial inhibition as use of the first QS well with an OD of <1.0. Complete inhibition of amplification was defined as both QS wells with an OD of <0.3. The dynamic range for this assay is 400 to 750,000 HIV RNA copies/ml.
Quantitation in the NASBA or NucliSens assay is achieved by coamplification of the HIV-1 sample RNA together with internal calibrators (Qa, Qb, and Qc). No inhibition is observed when there is total recovery of the Qa, Qb, and Qc internal calibrators. Partial inhibition is exhibited with low recovery of Qa, Qb, or Qc as defined by the Organon Teknika software. No recovery of Qa, Qb, or Qc signifies complete inhibition of amplification. The upper limit of detection for both assays is 1.0 × 107 log HIV RNA copies/ml. The lower limit for the older NASBA assay is 1,000 HIV RNA copies/ml when the calibrators are diluted 1:10. The more sensitive NucliSens assay has a lower limit of 400 HIV RNA copies/ml. The NASBA and NucliSens assays can achieve greater sensitivity when the sample volume is increased. In this study, we took advantage of this property when testing the CVL and saliva samples, typically using 1 ml of specimen when available.
Statistical analysis.
Blood plasma and body fluid HIV RNA levels were log10 transformed prior to analysis. Fisher's exact test was used to compare the sensitivities of the two assays. Correlations of blood plasma with seminal plasma, CVL, and CSF viral loads were made by regression analysis with SigmaStat 2.0 software (SSPS, Inc., Chicago, Ill.).
RESULTS
Comparative performance of RT-PCR and NASBA-based HIV-1 RNA assays with different biological fluids.
CVL and CSF samples from infected individuals and breast milk and saliva from uninfected individuals spiked with HIV were tested for the presence of inhibitors in both HIV RNA assays (Table 1). Results from our previous analysis of seminal plasma (4) are provided for comparison. Partial inhibition was frequently observed when saliva (67% inhibited) and breast milk (38% inhibited) were assayed by RT-PCR, as evidenced by low recovery of the internal quantitation standard (QS) used for calculating viral load (Table 1). In comparison, 96% of the seminal plasma samples we tested earlier showed at least some inhibition and 20% demonstrated complete inhibition in the RT-PCR assay, as determined by low OD in the QS wells. CVL and CSF did not appear to contain inhibitors when tested in the RT-PCR assay. No inhibition was observed for any of the body fluids tested in the NASBA and NucliSens assays, confirming our previous results with seminal plasma (4).
TABLE 1.
CVL and CSF from HIV-infected patients and breast milk and saliva from uninfected individuals spiked with tissue culture-derived HIV-1 (primary isolates) and tested with RT-PCR and the NASBA and NucliSens HIV-1 RNA assaysa
| Body fluid (n samples) | No. of samples inhibited (%)
|
|
|---|---|---|
| Roche Monitor | NASBA | |
| Seminal plasma (25) | 24 (96) | 0 (0) |
| CVL (5) | 0 (0) | 0 (0) |
| Breast milk (8) | 3 (38) | 0 (0) |
| CSF (5) | 0 (0) | 0 (0) |
| Saliva (6) | 4 (67) | 0 (0) |
Recovery of the internal standards was used to gauge either partial or complete inhibition. Previously reported results from seminal plasma testing (4) are provided for comparison.
Although the RT-PCR assay frequently showed inhibition in some of the body fluids, it often displayed greater sensitivity than the NASBA or NucleiSens assay (Table 2). This was particularly evident in breast milk and CSF specimens, where the viral load was usually low (typically <104 copies/ml). The differences in sensitivity observed were not statistically significant, probably due to the small sample sizes.
TABLE 2.
CVL, breast milk, and CSF from infected patients tested for HIV RNA with RT-PCR and the NASBA and NucliSens HIV-1 RNA assaysa
| Body fluid (n samples) | Result by:
|
|||
|---|---|---|---|---|
| Roche Monitor
|
NASBA or NucliSens
|
|||
| % Detected | Range (log10 copies/ml) | % Detected | Range (log10 copies/ml) | |
| Seminal plasma (25) | 56 | <2.6–5.9 | 68 | <3–6.1 |
| CVL (5) | 100 | 2.4–4.3 | 100 | 2.1–4.6 |
| Breast milk (5) | 60 | <2.6–3.4 | 40 | <2.6–3.2 |
| CSF (11) | 73 | <2.6–4.8 | 27 | <2.6–4.5 |
Results were compared with previous data obtained from seminal plasma (4).
Comparison of viral loads in different biological compartments.
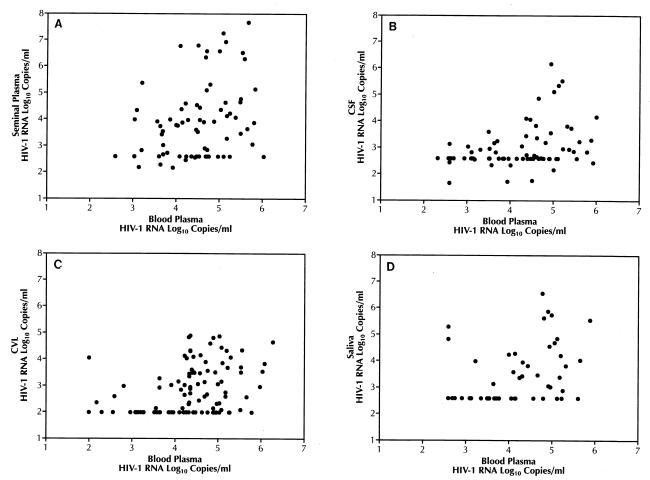
To determine the detection frequency and range of viral loads in different body fluids and to compare the association between blood plasma and body fluid viral loads, paired specimens from larger numbers of infected individuals were tested (Table 3). Seminal plasma and blood plasma were tested in the NASBA and NucliSens assays (n = 73; Fig. 1A). Paired CSF and blood samples were tested by RT-PCR (n = 102; Fig. 1B). Paired CVL and blood samples (n = 107; Fig. 1C) and paired saliva and blood samples were tested by the NucliSens assay (n = 59; Fig. 1D). In all cases, HIV-1 RNA was more frequently detected in the blood, with detection rates ranging from 71 to 97%. Detection rates in nonblood body fluids were typically lower, ranging from 42 to 77%. Although in most cases viral load was higher in blood than in the corresponding body fluid (Fig. 1B and C), there were clear cases of hyperproduction of HIV RNA in nonblood compartments relative to blood in a few individuals, especially in seminal plasma (Fig. 1A) and saliva (Fig. 1D).
TABLE 3.
Paired blood plasma and either CSF, seminal plasma, CVL, or saliva samples from HIV-infected patients assayed for HIV-1 RNA
| Fluid | Assay (n samples) | Result for:
|
|||
|---|---|---|---|---|---|
| Blood
|
Other body fluid
|
||||
| % Positive | Range (log10 copies/ml) | % Positive | Range (log10 copies/ml) | ||
| CSF | RT-PCR (102) | 71 | <2.6–6.0 | 42 | <2.6–6.2 |
| Seminal plasma | NASBA (73) | 97 | <2.6–6.0 | 77 | <2.6–7.7 |
| CVL | NucliSens (107) | 91 | <2.6–6.3 | 55 | <2.6–4.9 |
| Saliva | NucliSens (59) | 76 | 2.6–5.9 | 46 | <2.6–6.6 |
FIG. 1.
Correlation between blood plasma viral loads and viral loads from other body fluids. (A) Seminal plasma compared with blood plasma (R = 0.33, P < 0.001). (B) CSF compared with blood plasma (R = 0.49, P < 0.001). (C) CVL compared with blood plasma (R = 0.31, P < 0.001). (D) Saliva compared with blood plasma (R = 0.45, P < 0.0001).
DISCUSSION
HIV is known to be transmitted via semen, breast milk, cervical secretions (reviewed in reference 21), and, to a lesser degree, saliva (20). From a public health perspective, it is critical that we have the capability of accurately measuring viral loads in different body fluids in order to ultimately prevent viral transmission. Knowledge of the impact of highly active antiretroviral therapy on viral loads in different compartments is critical to the understanding of the pathogenesis of HIV infection.
Several publications provide comparative data among the three most widely used commercial HIV RNA assays for measuring blood plasma viral load: RT-PCR, NASBA or NucliSens, and Quantiplex (5, 14, 17, 22). In contrast, methods for evaluating HIV in nonblood compartments have yet to be standardized, making direct comparisons between studies difficult. The most extensively studied compartment is probably the male genital tract. Other investigators (3, 9) have now confirmed our initial finding (4) of inhibitors in seminal plasma which copurify with RNA in the Roche Monitor assay. These inhibitors, however, can be effectively removed by the silica bead isolation procedure (1) employed in NASBA-based assays.
Similar comparisons for other types of body fluids have rarely been published. Early studies used noncommercial assays for evaluating viral load in nonblood compartments (10, 13, 15, 16, 23). Results from noncommercial assays have seldom been compared with those from commercial kits and, when tested, usually involved only blood plasma as the body fluid evaluated (18). However, one recent study compared a noncommercial, quantitative-competitive RT-PCR assay with the Roche assay for the evaluation of viral load in blood and CVL (11). In that study, the RT-PCR-based assay appeared more sensitive than the commercial assay, although the issue of inhibitors was not discussed.
More recently, researchers have used the Roche Monitor assay to assess viral burden in body fluids, frequently without apparently checking for the presence of inhibitors (2, 12, 27). Yet other investigators have simply used the NASBA or NucliSens assay for all of their nonblood studies under the apparent assumption that there might be inhibitors to the RT-PCR method (7; A. Kovacs, P. Reichelderfer, and D. Wright, Abstr. Int. Conf. AIDS, abstr. 23488, 1998). In addition, most noncommercial assays use external standards for quantitation (10, 15). The lack of internal QSs in some of these assays may mask the presence of inhibitors. As a consequence, results derived from these assays with some types of body fluids may not be accurate. Our results suggest that breast milk and saliva samples frequently contain components which inhibit the Roche Monitor assay, although none of these specimens demonstrated complete inhibition, as was observed with some seminal plasma samples (4). Inhibitors were removed when the silica bead RNA extraction method was used, mirroring our earlier observations with seminal plasma (4). We detected no obvious inhibition when CVLs or CSFs were assayed with the Roche Monitor assay. Semba et al. (24), however, reported no inhibition when the Roche Monitor assay was used with breast milk specimens.
The Roche Monitor assay provided greater sensitivity, especially at low viral load levels. Five CSF specimens which had undetectable viral loads with the NASBA assay had viral loads of 620 to 950 copies/ml in the Roche assay. Although some degree of inhibition was observed with some breast milk specimens, three of five breast milk specimens from HIV-seropositive women were detectable in the Roche Monitor assay compared with two of five for the NASBA assay (Table 1). The highest viral load observed in these specimens was only 2,500 copies/ml. Greater sensitivity of the NASBA or NucliSens assay can be achieved by increasing the sample input volume, especially for cell-free specimens. However, care must be taken, because uncentrifuged specimens may contain enough cellular DNA to interfere with the Boom (1) isolation procedure (J. Schock and P. Vernazza, personal observation).
In cross-sectional studies, we found that viral loads in blood plasma were generally higher than those in corresponding body fluids. A high blood plasma viral load typically correlated with high viral loads in nonblood secretions. However, there were clear exceptions. Some men have been shown to excrete very high levels of HIV RNA in the seminal plasma (6, 28) and should be studied further to understand their potential role as transmitters. Similarly, a few women have extremely high cervical viral loads and may be more likely to transmit the virus either to sexual contacts or to their infants (8).
This study did not address differences in sample collection or processing of various body fluids that may also influence viral load. Collection of seminal plasma and CSF is straightforward, with few, if any variations in sample collection or processing methods among the various studies. HIV RNA in breast milk and saliva has been assayed as unprocessed (not centrifuged) or as cell-free supernatant. For this particular study, we used cell-free breast milk and whole, uncentrifuged saliva. In contrast, methods of sample collection in the female genital tract vary widely and have included endocervical wicks, cervical vaginal lavages, cervical swabs, and vaginal swabs (P. Reichelderfer, R. Coombs, D. Wright, D. Burns, and A. Kovacs for the WHS 001 Study Group, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. I-251, 1998). Unlike the other specimens, analysis of female genital secretions usually involves a dilution factor, due to the collection procedure, that is hard to estimate because the exact volume of the secretion in unknown. This study presented data on unfractionated CVL without correcting for dilution.
We conclude that HIV-1 RNA can be accurately detected by the NASBA- or NucliSens-based assay in all body fluids tested, while the presence of inhibitors limits the usefulness of RT-PCR. We also found that viral loads from nonblood sources are typically 1 to 2 logs lower than those from matched blood plasma samples. The existence of detectable loads in these body fluids, however, stresses the importance of evaluating the impact of therapeutic treatment regimens and candidate vaccines in both blood and nonblood fluids.
ACKNOWLEDGMENTS
This work was supported, in part, by grants DK49381 (M.S.C.), NS34243 (C.H.), DE12162 (D.C.S.), NS36518 (K.R.), and UNC CFAR P30 HD37260 (S.A.F. and D.C.S.).
REFERENCES
- 1.Boom R, Sol C J A, Salimans M M, Jansen C L, Wertheim-van Dillen P M E, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cinque P, Vago L, Ceresa D, Mainini F, Terrini M R, Vagani A, Torri W, Bossolasco S, Lazzarin A. Cerebrospinal fluid HIV-1 RNA levels: correlation with HIV encephalitis. AIDS. 1998;12:389–394. doi: 10.1097/00002030-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Coombs R W, Speck C E, Hughes J P, Lee W, Sampoleo R, Ross S O, Dragavon J, Peterson G, Hooten T M, Collier A C, Corey L, Koutsky L, Krieger J N. Association between culturable human immunodeficiency virus type 1 in semen and HIV-1 RNA levels in blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 4.Dyer J R, Gilliam B L, Eron J J, Grosso L, Cohen M S, Fiscus S A. Quantitation of human immunodeficiency virus type 1 RNA in cell free seminal plasma: comparison of NASBA and Amplicor reverse transcription-PCR amplification and correlation with quantitative culture. J Virol Methods. 1996;60:161–170. doi: 10.1016/0166-0934(96)02063-0. [DOI] [PubMed] [Google Scholar]
- 5.Dyer J R, Pilcher C D, Shepard R, Schock J, Eron J J, Fiscus S A. Comparison of NucliSens and Roche Monitor assays for quantitation of levels of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1999;37:447–449. doi: 10.1128/jcm.37.2.447-449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eron, J. J., L. Smeaton, S. Fiscus, J. Currier, J. Lennox, R. D'Aquila, M. Rogers, R. M. Gulick, and R. L. Murphy. The effects of antiretroviral therapy with protease inhibitor-containing regimens on HIV-1 levels in semen. J. Infect. Dis., in press. [DOI] [PubMed]
- 7.Farrar D J, Cu Uvin S, Caliendo A M, Costello S F, Murphy D M, Flanigan T P, Mayer K H, Carpenter C C J. Detection of HIV-1 RNA in vaginal secretions of HIV-1 seropositive women who have undergone hysterectomy. AIDS. 1997;11:1296–1297. doi: 10.1097/00002030-199710001-00004. [DOI] [PubMed] [Google Scholar]
- 8.Fiore J R, Lepera A, Di Stefano M, Saracino A, Favia A, Pastore G, Angarano G. Frequent cervicovaginal shedding of HIV-1 in asymptomatic, non-severely immunodeficient women. AIDS. 1999;13:626–627. doi: 10.1097/00002030-199904010-00016. [DOI] [PubMed] [Google Scholar]
- 9.Gupta P, Mellors J, Kingsley L, Riddler S, Singh M K, Shreiber S, Cronin M, Rinaldo C R. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J Virol. 1997;71:6271–6275. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamed K A, Winters M A, Holodniy M, Katzenstein D A, Merigan T C. Detection of human immunodeficiency virus type 1 in semen: effects of disease stage and nucleoside therapy. J Infect Dis. 1993;167:798–802. doi: 10.1093/infdis/167.4.798. [DOI] [PubMed] [Google Scholar]
- 11.Hart C E, Lennox J L, Pratt-Palmore M, Wright T C, Schinazi R F, Evans-Strickfaden T, Bush T J, Schnell C, Conley L J, Clancy K A, Ellerbrock T V. Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J Infect Dis. 1999;179:871–882. doi: 10.1086/314656. [DOI] [PubMed] [Google Scholar]
- 12.Iverson A K N, Larsen A R, Jensen T, Fuggar L, Balslev U, Wahl S, Gerstoft J, Mullins J I, Skinhøj P. Distinct determinants of human immunodeficiency virus type 1 RNA and DNA loads in vaginal and cervical secretions. J Infect Dis. 1998;177:1214–1220. doi: 10.1086/515266. [DOI] [PubMed] [Google Scholar]
- 13.Lewis P, Nduati R, Kreiss J K, John G C, Richardson B A, Mbori-Ngacha D, Ndinya-Achola J, Overbaugh J. Cell-free human immunodeficiency virus type 1 in breast milk. J Infect Dis. 1998;177:34–39. doi: 10.1086/513816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin H J, Myers L E, Yen-Lieberman B, Hollinger F B, Henrard D, Hooper C J, Kokka R, Kwok S, Rasheed S, Vahey M, Winters M A, McQuay L J, Nara P L, Reichelderfer P, Coombs R W, Jackson J B. Multicenter evaluation of methods for the quantitation of plasma HIV-1 RNA. J Infect Dis. 1994;170:553–562. doi: 10.1093/infdis/170.3.553. [DOI] [PubMed] [Google Scholar]
- 15.Liuzzi G, Chirianni A, Clementi M, Bagnarelli P, Valenza A, Cataldo P T, Piazza M. Analysis of HIV-1 viral load in blood, semen, and saliva: evidence for different viral compartments in a cross-sectional and longitudinal study. AIDS. 1996;10:F51–F56. doi: 10.1097/00002030-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Rasheed S, Li Z, Xu D, Kovacs A. Presence of cell-free human immunodeficiency virus in cervicovaginal secretions is independent of viral load in the blood of human immunodeficiency virus-infected women. Am J Obstet Gynecol. 1996;175:122–130. doi: 10.1016/s0002-9378(96)70261-2. [DOI] [PubMed] [Google Scholar]
- 17.Revets H, Marissens D, De Wit S, Lacor P, Clumeck N, Lauwers S, Zissis G. Comparative evaluation of NASBA HIV-1 RNA QT, Amplicor-HIV Monitor, and QUANTIPLEX HIV RNA assay, three methods for quantification of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1996;34:1058–1064. doi: 10.1128/jcm.34.5.1058-1064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizzardini G, Trabattoni D, Saresella M, Piconi S, Lukwiya M, Declich S, Fabiani M, Ferrante P, Clerici M. Immune activation in HIV-infected African individuals. AIDS. 1998;12:2387–2396. doi: 10.1097/00002030-199818000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Robertson K, Fiscus S, Kapoor C, Robertson W, Schneider G, Shepard R, Howe L, Silva S, Hall C. CSF, plasma viral load and HIV associated dementia. J Neurovirol. 1998;4:90–94. doi: 10.3109/13550289809113485. [DOI] [PubMed] [Google Scholar]
- 20.Robinson E K, Evans B G. Oral sex and HIV transmission. AIDS. 1999;13:737–738. doi: 10.1097/00002030-199904160-00021. [DOI] [PubMed] [Google Scholar]
- 21.Royce R A, Sena A, Cates W, Cohen M S. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 22.Schuurman R, Descamps D, Weverling G J, Kaye S, Tijnagel J, Williams I, van Leeuwen R, Tedder R, Boucher C A B, Brun-Vezinet F, Loveday C. Multicenter comparison of three commercial methods for quantification of human immunodeficiency virus type 1. RNA in plasma. J Clin Microbiol. 1996;34:3016–3022. doi: 10.1128/jcm.34.12.3016-3022.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sei S, Stewart S K, Farley M, Mueller B U, Lane J R, Robb M L, Brouwer P, Pizzo P A. Evaluation of human immunodeficiency virus (HIV) type 1 RNA levels in cerebral spinal fluid and viral resistance to ZDV in children with HIV encephalopathy. J Infect Dis. 1996;174:1200–1206. doi: 10.1093/infdis/174.6.1200. [DOI] [PubMed] [Google Scholar]
- 24.Semba R D, Kumwenda N, Hoover D R, Taha T E, Quinn T C, Mtimavalye L, Biggar R J, Broadhead R, Miotti P G, Sokoll L J, van der Hoeven L, Chiphangwi J D. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180:93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 25.Shugars, D. C., G. D. Slade, L. L. Patton, and S. A. Fiscus. Oral and systemic factors associated with increased levels of human immunodeficiency virus type 1 RNA in saliva. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod., in press. [DOI] [PubMed]
- 26.Spear G T, Al-Harthi L, Sha B, Saarloos M N, Hayden M, Massad L S, Benson C, Roebuck K A, Glick N R, Landay A. A potent activator of HIV-1 replication is present in the genital tract of a subset of HIV-1 infected and uninfected women. AIDS. 1997;11:1319–1326. doi: 10.1097/00002030-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Staprans S, Marlowe N, Glidden D, Novakovic-Agopian T, Grant R M, Heyes M, Aweeka F, Deeks S, Price R W. Time course of cerebrospinal fluid responses to antiretroviral therapy: evidence for variable compartmentalization of infection. AIDS. 1999;13:1051–1061. doi: 10.1097/00002030-199906180-00008. [DOI] [PubMed] [Google Scholar]
- 28.Tachet A, Duliost E, Salmon D, De Almeida M, Rivalland S, Finkielsztejn L, Heard I, Jouannet P, Sicard D, Rouzioux C. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. AIDS. 1999;13:823–831. doi: 10.1097/00002030-199905070-00012. [DOI] [PubMed] [Google Scholar]
- 29.Vernazza P L, Troiani L, Flepp M J, Cone R W, Schock J, Roth F, Boggian K, Cohen M S, Fiscus S A, Eron J J The Swiss HIV Cohort Study. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. AIDS. 2000;14:117–121. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Dournadula G, Beumont M, Livornese L, Van Uitert B, Henning K, Pomerantz R J. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]